Abstract
Objective
The fatty acid receptor 1 (FFAR1/GPR40) mediates fatty acid-dependent augmentation of glucose-induced insulin secretion (GIIS) in pancreatic β-cells. Genetically engineered Ffar1-knockout/congenic mice univocally displayed impaired fatty acid-mediated insulin secretion, but in vivo experiments delivered controversial results regarding the function of FFAR1 in glucose homeostasis and liver steatosis. This study presents a new coisogenic mouse model carrying a point mutation in Ffar1 with functional consequence. These mice reflect the situations in humans in which point mutations can lead to protein malfunction and disease development.
Methods
The Munich N-ethyl-N-nitrosourea (ENU) mutagenesis-derived F1 archive containing over 16,800 sperms and corresponding DNA samples was screened for mutations in the coding region of Ffar1. Two missense mutations (R258W and T146S) in the extracellular domain of the protein were chosen and homozygote mice were generated. The functional consequence of these mutations was examined in vitro in isolated islets and in vivo in chow diet and high fat diet fed mice.
Results
Palmitate, 50 μM, and the FFAR1 agonist TUG-469, 3 μM, stimulated insulin secretion in islets of Ffar1T146S/T146S mutant mice and of wild-type littermates, while in islets of Ffar1R258W/R258W mutant mice, these stimulatory effects were abolished. Insulin content and mRNA levels of Ffar1, Glp1r, Ins2, Slc2a2, Ppara, and Ppard were not significantly different between wild-type and Ffar1R258W/R258W mouse islets. Palmitate exposure, 600 μM, significantly increased Ppara mRNA levels in wild-type but not in Ffar1R258W/R258W mouse islets. On the contrary, Slc2a2 mRNA levels were significantly reduced in both wild-type and Ffar1R258W/R258W mouse islets after palmitate treatment. HFD feeding induced glucose intolerance in wild-type mice. Ffar1R258W/R258W mutant mice remained glucose tolerant although their body weight gain, liver steatosis, insulin resistance, and plasma insulin levels were not different from those of wild-type littermates. Worth mentioning, fasting plasma insulin levels were lower in Ffar1R258W/R258W mice.
Conclusion
A point mutation in Ffar1 abrogates the stimulatory effect of palmitate on GIIS, an effect that does not necessarily translate to HFD-induced glucose intolerance.
Keywords: FFAR1/GPR40, Free fatty acids, Insulin secretion, ENU-mutated Ffar1, FFAR1 deficient mice, High fat diet
Abbreviations: CD, regular chow diet; ENU, N-ethyl-N-nitrosourea; FFAR1, free fatty acid receptor 1; GIIS, glucose-induced insulin secretion; GLP-1, glucagon like peptide-1; Glut-2, glucose transporter 2; GTT, glucose tolerance test; HEK-EM 293 cells, human embryonic kidney macrophage scavenger receptor-expressing (TRH-R) cells; HEPES, 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid; HFD, high fat diet; ipITT, intraperitoneal insulin tolerance test; PAX6, paired box protein, also known as aniridia type II protein (AN2) or oculorhombin; Ppara/Ppard, peroxisome proliferator activated receptor α/δ genes; Slc2a2, solute carrier family 2 member 2 gene; TAK875 and TUG-469, FFAR1 agonists
Highlights
-
•
Generation of mice carrying point mutations in Ffar1 using ENU.
-
•
FFAR1 point mutation R258W abrogates fatty acid-induced insulin secretion.
-
•
Dysfunctional FFAR1 inhibits the development of diet-induced glucose intolerance.
1. Introduction
Free fatty acid receptor-1 (FFAR1, formerly GPR40) promotes long chain fatty acid-mediated augmentation of glucose-induced insulin secretion (GIIS) [1], [2], [3]. In humans and rodents, high expression of FFAR1 is restricted to pancreatic and gastric endocrine cells, while expression in other tissues, including brain, is much lower [1], [2], [4], [5]. These features make FFAR1 an attractive drug target for the treatment of insufficient insulin secretion, which is the ultimate cause for the onset of hyperglycemia and type-2 diabetes mellitus [6], [7]. Until today, multiple agonists have been generated and tested for their efficacy to treat hyperglycemia in humans [6]. Although FFAR1 agonists counteract glucose intolerance in mice and humans, the beneficial effect of these new therapeutic drugs is still a matter of debate [8], [9]. Thus, the promising drug TAK875 was discontinued after clinical phase III due to its liver toxicity. Confirming this side effect, FFAR1-deficient mice are protected against diet-induced liver steatosis [10]. This observation prompted the investigation of FFAR1-antagonists as therapeutic tools against fatty liver disease.
In addition, different FFAR1 agonists exert their effects through different cellular pathways. Thus, fatty acids stimulate insulin secretion mainly via Gq proteins, while TAK875 stimulation is mediated by β-arrestin-2 [11]. An additional, but indirect, stimulatory effect of FFAR1-agonists on insulin secretion is caused by the activation of FFAR1 expressed in intestinal endocrine cells which leads to GLP-1 secretion [12].
Several transgenic and knockout/congenic mouse models have been generated in order to assess the role of FFAR1 for proper insulin secretion and maintenance of glucose homeostasis. The results obtained with three different receptor knockout mouse models were not consistent. The protection against high fat feeding-induced fatty liver and glucose intolerance, as observed by Steneberg and colleagues, could not be reproduced using other Ffar1 KO mouse models [10], [13], [14]. Such differences may be explained by undesirable side effects generated by insertion of exogenous DNA, deletion of non-coding regions with specific functions, e.g. microRNA, and the additional role of the Ffar1 promoter for the expression of FFAR2 (GPR43) and FFAR3 (GPR41) [15], [16]. Congenic mice differ not only in the ablated gene but also in a flanking segment on either side of the ablated locus [17]. Furthermore, a complete deletion of a protein may generate a compensatory up-regulation of other proteins. To circumvent such problems, we searched for a coisogenic mouse model with a minimal genetic alteration producing a maximal effect. Using site-directed mutagenesis, several point mutations in Ffar1 with functional consequences have been identified, including R258 [18], [19]. We screened the Munich ENU-mutagenesis-derived F1 sperm and corresponding DNA archive for point mutations in Ffar1. The archive comprises more than 16,800 samples from individual F1-mutagenized mice on the C3HeB/FeJ genetic background [20], [21]. Two mouse models carrying point mutations in the coding region of Ffar1 are presented in this study of which the R258W mutation prevents the stimulation of insulin secretion by palmitate and the FFAR1 agonist TUG-469.
2. Materials, animals and methods
2.1. Materials
TUG-469, a specific FFAR1 agonist, was a kind gift of Trond Ulven, Southern University of Denmark, Odense M, Denmark. All other materials, unless otherwise stated, were from Sigma–Aldrich (Deisenhofen, Germany) and of analytical grade.
2.2. Generation of mice
ENU mutagenesis was performed as described previously [22]. Briefly, male C3HeB/FeJ mice were treated weekly by one 90 mg/kg ENU-injection for three consecutive weeks. First generation F1 mice were phenotyped, and male mice were cryo-archived by their sperm and spleen-derived DNA samples. The DNA archive was screened for alleles of interest using a LightScanner® device originally from Idaho Technology Inc. (distributed by Bioke, Leiden, Netherlands). In vitro fertilization, fusing sperm of mutated F1 mice and mating with wild-type C3HeB/FeJ mice were performed as described elsewhere [23]. During maintenance the mutant mice were repeatedly backcrossed to wild-type C3HeB/FeJ mice in order to eliminate unwanted ENU mutations. Mice were kept under a 12 h light/dark cycle and had ad libitum access to chow diet (CD) and water. High fat diet (HFD) containing 45% fat/kcal from lard and soybean (Research Diets D12451; New Brunswick; NJ; USA) was fed for 8 weeks starting at age of 4 weeks. Mouse holding and handling were done according to the federal animal welfare guidelines and the state ethics committee and approved by the governments of Upper Bavaria and Baden-Württemberg.
2.3. Glucose and insulin tolerance tests
Blood glucose concentrations were measured after intra-peritoneal injection of 2 g glucose/kg body weight (ipGTT) or of intra-peritoneal injection of 1 unit/kg body weight insulin (ipITT) in mice fed CD or HFD for 8 weeks. Before GTT, mice were fasted overnight. For determination of plasma insulin levels blood samples were collected in heparinized capillary from tail vein. Blood glucose was measured with a glucometer. During oral glucose tolerance test (oGTT), 2 g glucose/kg body weight was administered via gavage in overnight fasted animals. The tests were performed with the same animals keeping an interval of 1 week between the tests. Plasma insulin and glucagon were measured using ELISA kits (Mercodia, Sweden). Plasma leptin and resistin were quantified using a ProcartaPlex™ immunoassay (Luminex™ xMAP technology, Invitrogen).
2.4. Isolation of islets and insulin secretion
Mouse islets were isolated via collagenase digestion (1 mg/ml collagenase, Serva, Heidelberg, Germany) and thereafter purified by handpicking. Islets were cultured overnight in RPMI 1640 medium containing 11 mM glucose supplemented with 10% FCS, 10 mM HEPES, 2 mM L-glutamine, and 1 mM Na-pyruvate without antibiotics. Thereafter, islets were washed twice and pre-incubated for 1 h at 37 °C with Krebs Ringer buffer (KRB) containing (in mM): 135 NaCl, 4.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 5 NaHCO3, 2.8 glucose, 10 HEPES, and 5 g/l bovine serum albumin (fatty acid free, low endotoxin, Sigma, Deisenhofen, Germany), pH 7.4. Subsequently, islets were incubated in fresh KRB containing 0.5 g/l bovine serum albumin supplemented with test substances as indicated for 1 h at 37 °C. Palmitate was added from a stock solution of 50 mM in DMSO. Secreted insulin and islet insulin content after insulin extraction in acid ethanol (1.5% [vol/vol] HCl/75% [vol/vol] ethanol) were measured via radioimmunoassay (Millipore, Biotrend Chemikalien GmbH, Germany).
2.5. Semiquantitative analysis of cellular mRNA levels
Islets were isolated and directly lysed in RNA lysis buffer (Macherey–Nagel, Düren, Germany, Figure 1C), or islets were cultured before lysis in medium supplemented with 10% FCS ± 600 μM palmitate. Palmitate, from a stock solution of 100 mM in DMSO, was coupled to FCS at a final concentration of 6 mM before addition to the culture medium. 50–200 islets were collected for total RNA-isolation using the commercial RNeasy kit (Qiagen, Hilden, Germany). Residual DNA was removed using on column DNAse treatment (Qiagen, Hilden, Germany). Eluted RNA was transcribed into cDNA using Oligo(dT)12–18 as primer (Roche Diagnostics GmbH, Mannheim, Germany).
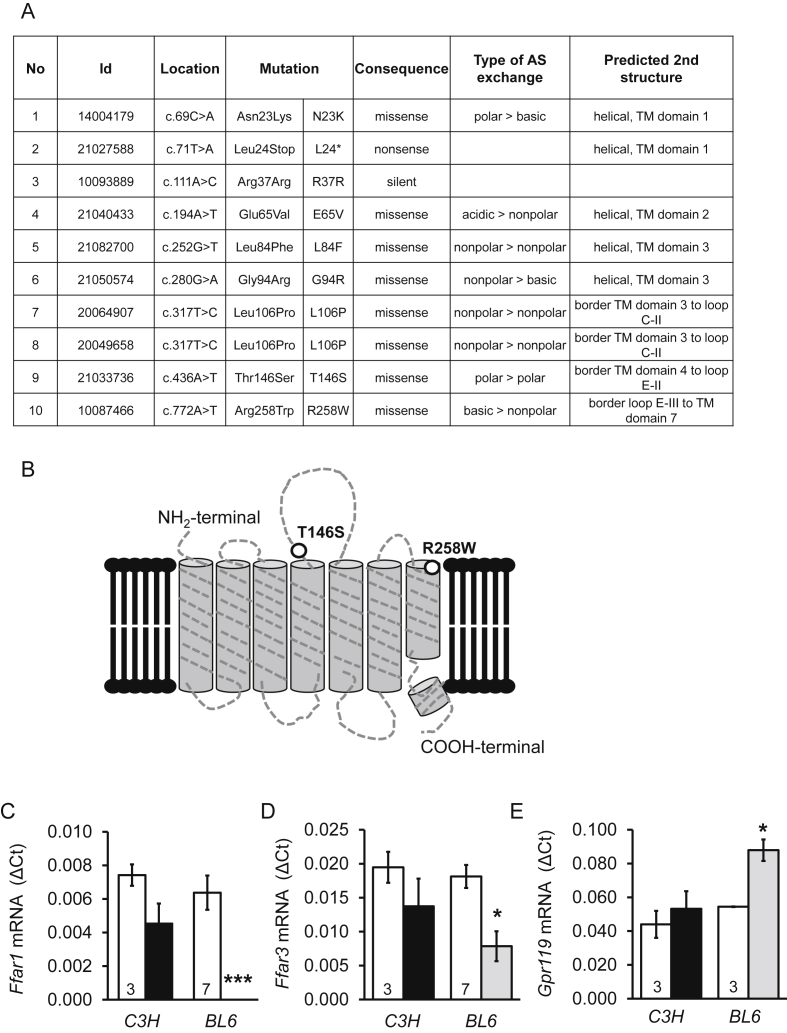
Figure 1.
Generation of mice with mutations in Ffar1. (A) Mutations in Ffar1 induced by ENU in mice. Sperm of mice carrying the mutation 9 and 10 were chosen for in vitro fertilization and generation of mutant mice. (B) Extracellular locations of the mutations 9 and 10 in FFAR1. Note that R258 is located in the agonist-binding domain of the receptor. (C, D, E) Relative mRNA levels in freshly isolated islets from Ffar1R258W/R258W (black bars) and Ffar1 KO mice (gray bars) compared to their respective wild-type mice expressed as means ± SEM. The number of mice is given in the respective columns. Rps13 was used as housekeeping gene.
PCR was performed using the LightCycler 480 Probes Master system (Roche Diagnostics GmbH, Mannheim, Germany). Quantification was performed by the 2−(ΔCT) method relative to the housekeeping gene Rps13. Specific primers used were: for mFfar1 up: 5′-CATCACTCTGCCCCTGAAG-3′ down: 5′-AAGGCAAAGACTGGGCAGA-3′, probe #50; for mFfar2 up: 5′-AAAGGAGCTGACAGGGGTTC-3′ down: 5′-GCAAGTTCAGGGGTTTCTTCT-3′, probe #82; for mFfar3 up: 5′-GTGCACTCACAAGGACTCTCC-3′ down: 5′-AAATTCGGGGTTTATGAGAGG-3′, probe #12; for mFfar4 up: 5′-TTGGTGTTGAGCGTCGTG-3′ down: 5′-CCAGCAGTGAGACGACAAAG-3′, probe #45; for mGpr119 up: 5′-TTCACTTCAATCCTCCTCCTTC-3′ down: 5′-TGCATGTTCTTGAGAGAAGTCC-3′, probe #72; for mGLP1-R up: 5′-GGACAACTGGGTCAAGCATT-3′, down: 5′-CTTTTCTCCCCTCATGGACA-3′, probe #12; for mIns2 up: 5′-GAAGTGGAGGACCCACAAGT-3′, down: 5′-AGTGCCAAGGTCTGAAGGTC-3′ probe #32; for mPpara up: 5′-CACGCATGTGAAGGCTGTAA-3′, down: 5′-CAGCTCCGATCACACTTGTC-3′, probe #41; for mPpard up: 5′-ATGGGGGACAGAACACAC-3′, down: 5′-GGAGGAATTCTGGGAGAGGT-3′, probe #11; for mRps13 up: 5′-TGCTCCCACCTAATTGGAAA-3′, down: 5′-CTTGTGCACACAACAGCATTT-3′, probe #110; for mSlc2a2 up: 5′-TCTGCTACTGCTCTTCTGTCCA-3′, down: 5′-GGTGACATCCTCAGTTCCTCTTA-3′, probe #45.
2.6. Oil red staining and measurement of liver triglyceride content
For oil red staining, liver cryosections (20 μm thick) were fixed with 4% formalin in phosphate-buffered saline and dehydrated in 100% propylene glycol. Staining was carried out with 0.5% oil red in propylene glycol for 15 min and hematoxylin was used as counterstaining. For the assessment of triglyceride concentrations, cryoconserved liver samples were homogenized in phosphate-buffered saline containing 1% Triton-X100 (20 μl buffer/mg tissue) using a TissueLyser (Qiagen, Hilden, Germany). Triglyceride content in the lysates was measured by a fully automatic enzymatic method on an ADVIA Chemistry XPT system (Siemens Healthcare GmbH, Erlangen, Germany).
2.7. Statistical analysis
All data were examined using ANOVA with Tukey's multiple comparisons test as post-hoc test. The level of significance was set to p < 0.05.
3. Results
3.1. Generation of mice with point mutations in Ffar1
Mutations of Ffar1 were selected from the F1 repository of ENU mutated mice. Ten point mutations were detected in the coding region of Ffar1 (Figure 1A). We chose two missense mutations (T146S and R258W) in the extracellular domain of the receptor for generation of mutant mice strains (Figure 1B). Of note, the amino acid R258 is located in the agonist binding domain [19].
Heterozygous mutant mice of both lines developed normally under chow diet and did not display any metabolic phenotype regarding body weight gain, glucose and insulin tolerance as well as fasting and fed plasma insulin concentrations (data not shown). Therefore, homozygous mice (Ffar1R258W/R258W and Ffar1T146S/T146S) were generated for further analysis.
The expression of Ffar1 and the adjacent genes Ffar2 and Ffar3 were estimated by RT-PCR in freshly isolated islets from wild-type (C3HeB/FeJ) and homozygote mutant mice and compared to the mRNA levels of Ffar1(−/−) and respective wild-type (C57/BL6) mouse islets [10]. As shown in Figure 1C–E, mRNA levels of Ffar1, Ffar3, and Gpr119 were not significantly different between C3HeB/FeJ and FfarR258W/R258W mutant mouse islets. In contrast, in Ffar1 deficient mice, Ffar3 mRNA levels were significantly lower and Gpr119 mRNA higher than in the respective wild-type mouse islets. The mRNA levels of Ffar2 and Ffar4 (GPR120) were 1000- and 50-times lower than Ffar1 mRNA levels, respectively, and no differences were detected (data not shown).
3.2. R258W mutation of Ffar1 prevents FFAR1-dependent stimulation of insulin secretion in isolated mouse islets
As suggested by the use of Ffar1 knockout mice, FFAR1 contributes to palmitate-dependent augmentation of insulin secretion. Therefore, the functional relevance of the mutations was analyzed in isolated islets (Figure 2). FFAR1 was activated by a physiological agonist, palmitate, and a synthetic agonist, TUG-469 [24]. The concentrations of palmitate, 50 μM, and TUG-469, 3 μM, were adapted to the low concentration of albumin (0.05%) since albumin interferes with agonist (TUG-469)-receptor interaction [1].
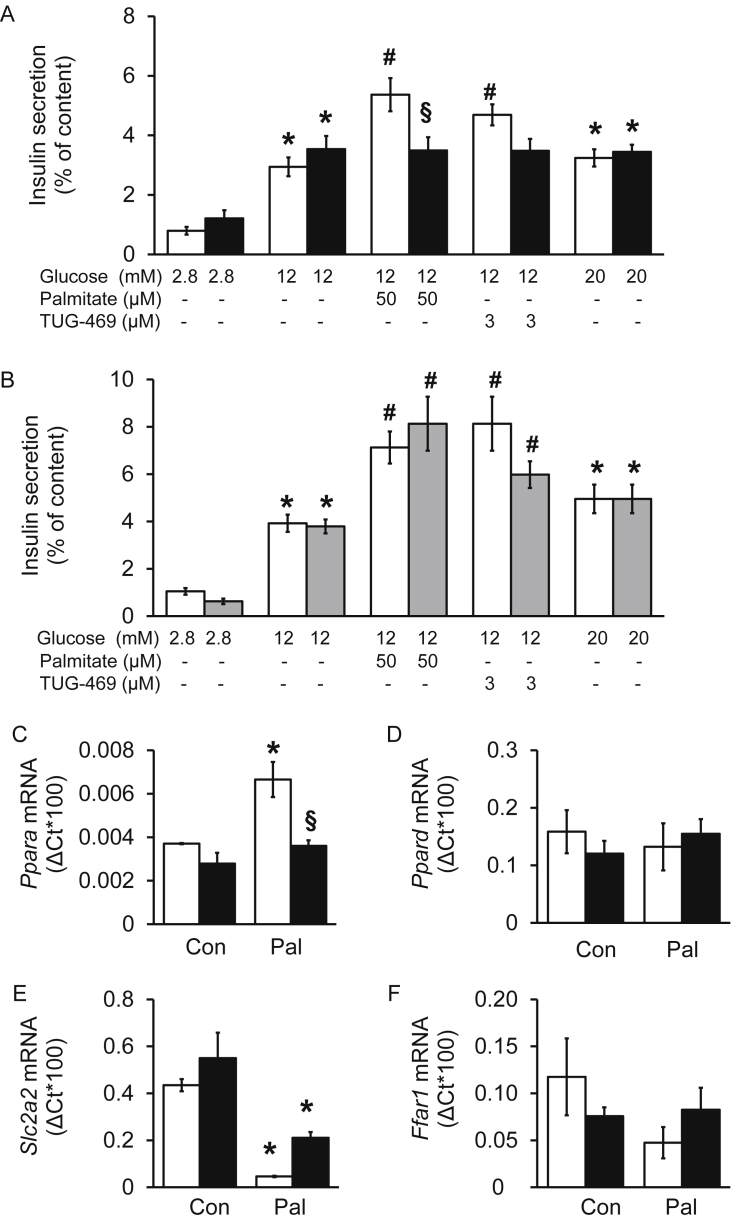
Figure 2.
Mutation R258W but not T146S of FFAR1 abrogates palmitate- and TUG-469-induced stimulation of insulin secretion and the effect of palmitate on Ppara mRNA levels. (A, B) Insulin secretion of isolated islets from (A) wild-type littermates (white bars) and Ffar1R258W/R258W mice (black bars) as well as (B) wild-type littermates (white bars) and Ffar1T146S/T146S mice (gray bars) measured after 1 h static incubation with substances as indicated. Results are presented as means ± SEM of n = 3–5 independent experiments. (C–F) Isolated islets from wild-type (white bars) and Ffar1R258W/R258W mice (black bars) were cultured under control condition or exposed to palmitate, 600 μM for 24 h. Relative mRNA levels are expressed as means ± SEM of n = 3–4 independent experiments. Rps13 was used as housekeeping gene. * denotes significance vs respective 2.8 mM glucose or Control (Con). # denotes significance vs. 12 mM glucose, § denotes significance between genotypes.
In islets of wild-type littermates of both mutant mouse strains (Ffar1R258W/R258W, Figure 2A and Ffar1T146S/T146S, Figure 2B), palmitate, 50 μM, or TUG-469, 3 μM, significantly augmented insulin secretion in the presence of 12 mM glucose. In mouse islets carrying the R258W mutation the effects of palmitate and TUG-469 on insulin secretion were abrogated (Figure 2A), while the mutation T146S had no impact on fatty acid- or agonist-induced insulin secretion (Figure 2B). Neither mutation (T146S; R258W) affected glucose-stimulated insulin secretion or insulin content (46.8 ± 7.9 and 53.4 ± 8.2 ng insulin/islet of wild-type and Ffar1R258W/R258W mice, respectively). These data reveal that mice with the R258W mutation represent a model with dysfunctional FFAR1, while the T146S mutation has no functional consequence. Therefore, further analyses were performed with Ffar1R258W/R258W mice only.
3.3. R258W mutation of Ffar1 prevents palmitate-induced up-regulation of peroxisome proliferator-activated receptor α (Ppara) mRNA levels in isolated mouse islets
To further investigate the specificity of the R258W mutation in FFAR1, palmitate-induced changes in gene expression were analyzed. Previous works reported the involvement of FFAR1 in activation of peroxisome proliferator-activated receptor α (Ppara) [10]. When isolated islets were exposed to palmitate (600 μM, adapted to the albumin concentration in culture medium) for 24 h, Ppara mRNA levels were augmented in wild-type islets (Figure 2C). This effect was absent in islets of Ffar1R258W/R258W mice, confirming that the mutation impairs receptor function. The effect is specific for Ppara, in that Ppard mRNA levels remained unchanged (Figure 2D). Interestingly, palmitate significantly reduced mRNA levels of Slc2a2 (Glut-2 gene) in wild-type and mutant islets, indicating that this effect is FFAR1-independent (Figure 2E). Neither the R258W mutation nor palmitate altered the mRNA levels of Ffar1, Glp1r, and Ins2 (Figure 2F,D, data not shown).
In freshly isolated, non-cultured, wild-type islets from CD and HFD fed mice, Ffar1 mRNA levels were 5-fold lower after high fat feeding (ΔCt*1000: 0.9 ± 0.2, n = 6 vs 4.5 ± 1.2, n = 5; HFD vs CD, respectively) while Slc2a2 mRNA levels remained unchanged (ΔCt*1000: 48.2 ± 7.9, n = 6 vs 57.7 ± 2.1, n = 6; HFD vs CD, respectively). Ppara mRNA levels appearing at >35 cycles were 10-times lower than Ffar1 mRNA levels, which made the quantification unreliable.
These observations substantiate that the R258W mutation specifically inhibits FFAR1 function.
3.4. Ffar1R258W/R258W mice are protected against HFD-induced glucose intolerance in spite of insulin resistance and fatty liver
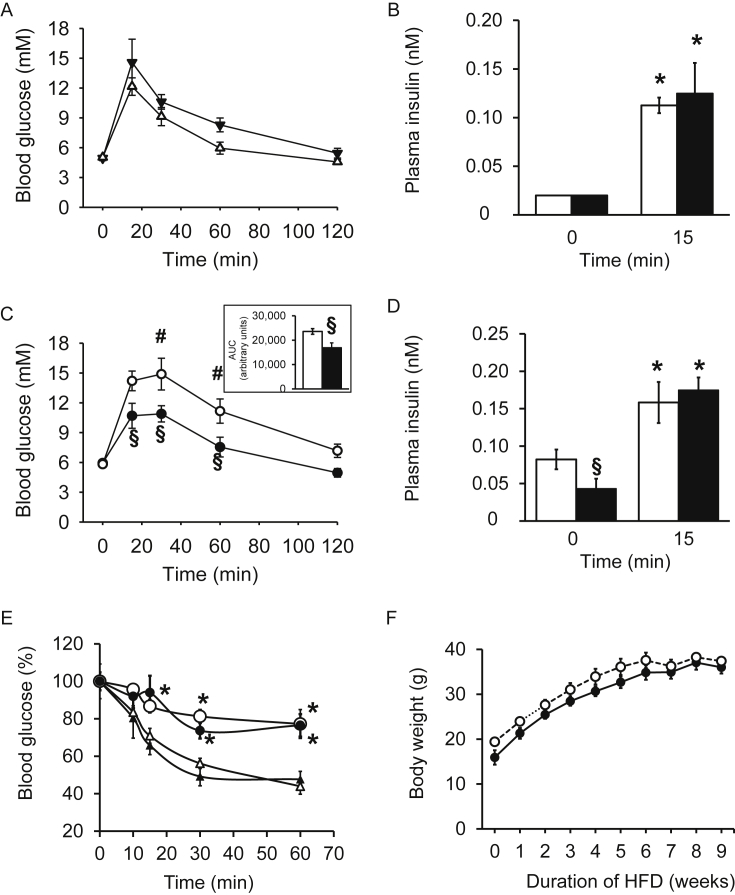
In regular CD fed wild-type and Ffar1R258W/R258W mutant mice, fasting blood glucose and plasma insulin levels were identical (Figure 3A,B). During ipGTT, the rise in blood glucose and plasma insulin was also not significantly different between wild-type and mutant mice. As expected, after HFD wild-type male mice but, surprisingly, not Ffar1R258W/R258W mutant mice, became glucose intolerant (Figure 3C). Due to significantly lower fasting insulin levels of Ffar1R258W/R258W mice, the increase in plasma insulin during ipGTT was 4-fold in mutant mice compared to 2-fold in wild-type mice (Figure 3D). The Ffar1 mutation protected against HFD-induced glucose intolerance, although wild-type and mutant mice developed a similar insulin resistance upon high fat feeding (Figure 3E). During high fat feeding, the mice became overweight regardless of their genotype (Figure 3F). Body weights of 12 weeks old wild-type mice were 26.5 ± 0.4 g (n = 4) and 34.1 ± 1.6 g (n = 5, p < 0.05) after CD and HFD feeding, respectively. The corresponding weights of Ffar1R258W/R258W mutant mice were 27.0 ± 0.5 g (n = 3) and 35.0 ± 2.0 g (n = 4, p < 0.05).
Figure 3.
R258W mutation in FFAR1 protects against HFD-induced glucose intolerance. (A and C) Blood glucose and (B and D) plasma insulin concentrations during ipGTT of wild-type (white symbols and bars) and Ffar1R258W/R258W littermates (black symbols and bars) after (A and B) CD and (C and D) HFD feeding expressed as means ± SEM, n = 4–6 (CD) and 6–8 (HFD) male mice. * denotes significance vs respective 0 min time point. # denotes significance between HFD and CD of wild-type mice at the same time point; § significance between genotypes at the same condition. (E) Blood glucose in wild-type (white symbols) and Ffar1R258W/R258W (black symbols) mice during ipITT after CD (triangles, n = 4–6) and HFD (circles, n = 6–8). (F) Body weight gain during high fat feeding.
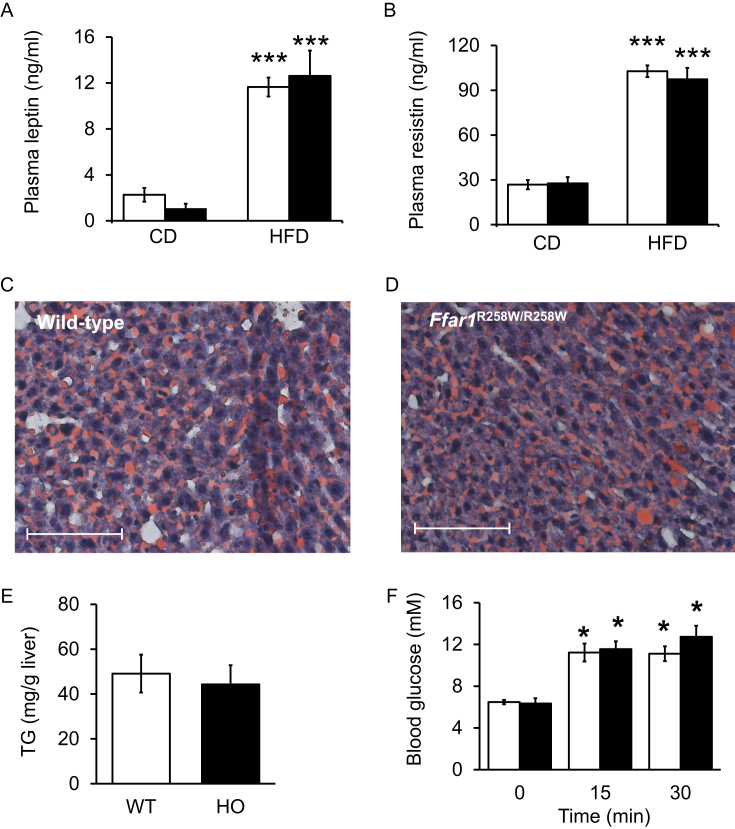
In agreement, plasma leptin and resistin were significantly higher in mice fed HFD compared to CD regardless of the genotype (Figure 4A,B). Furthermore, liver steatosis was detectable after HFD in both wild-type and mutant mice and the mean hepatic triglyceride content was not significantly different (Figure 4C–E).
Figure 4.
R258W mutation of FFAR1 did not influence HFD-stimulation of adipocyte hormone release nor liver fat accumulation. Fasting plasma (A) leptin and (B) resistin concentrations after CD (n = 4–6) and HFD (n = 6–8) feeding of wild-type (white bars) and Ffar1R258W/R258W mice (black bars). Liver fat droplets (red staining) in (C) wild-type and (D) Ffar1R258W/R258W liver tissue sections counterstained with hematoxylin. Scale bars, 100 μm. (E) Mean hepatic TG of HFD fed wild-type (WT, white bar) and Ffar1R258W/R258W (HO, black bar) mice. (F) Plasma glucose concentrations before (0 min) and 15 and 30 min after an oral glucose load (n = 6–8). Data are expressed as means ± SEM. *** denotes significance to the respective plasma concentration of CD fed mice; * significant to respective 0 min.
Finally, when HFD-fed mice were subjected to an oral glucose tolerance test, the glucose excursions were not significantly different between the genotypes (Figure 4F). Also, fasting plasma glucagon levels were 2.8 ± 0.3 pM and 3.8 ± 0.7 pM in wild-type and mutant mice fed CD, respectively. After HFD, plasma glucagon concentrations dropped under the detection level.
In conclusion, the Ffar1R258W/R258W mutation generated a functional phenotype, i.e. the repression of fatty acid-induced insulin secretion along with minimal genetic modification. The unexpected protection against HFD-induced impaired glucose tolerance suggests an unmasking of a glucose lowering mechanism in the mutant mouse.
4. Discussion
This study presents mice with a missense point mutation in R258 of FFAR1 that has functional consequences. Firstly, in islets of Ffar1R258W/R258W mice, both the physiological agonist palmitate and the synthetic agonist TUG-469 were unable to augment GIIS. Secondly, palmitate-mediated increase of Ppara mRNA levels was abrogated. The findings that FFAR1 mediates FFA effects on insulin secretion and Ppara mRNA are consistent with previous observations [1], [3], [10]. The loss of function of FFAR1 in Ffar1R258W/R258W mouse islets was not accompanied by a change of Ffar1 mRNA levels, indicative of a normal expression of the non-functional receptor. Whether protein trafficking to the plasma membrane remains unaltered needs further experimental evidence. HEK-EM 293 cells that overexpress R258A-mutated FFAR1 exhibit an unaltered receptor abundance at the plasma membrane and an abrogation of FFAR1-agonist GW9508-induced Ca2+-flux when compared to cells expressing wild-type receptors [19]. In contrast, in isolated islets of Ffar1(−/−) mice, Ffar1 mRNA was undetectable. That the deletion affected the expression of adjacent genes is suggested by concomitant reduction of Ffar3 mRNA levels. The functional consequence of the reduction of Ffar3 and the increase of Gpr119 mRNA levels is unknown. In respect to undesirable side effects, the mutant mouse represents a more reliable model.
The functional impact of Ffar1R258W/R258W became visible only in homozygous mice, while heterozygous mice did not develop any phenotype revealing a recessive character of the mutation (data not shown). Furthermore, GIIS was unaffected. Comparably, in humans, mutations (single nucleotide polymorphisms) in FFAR1 link to minor, but significant metabolic changes [24].
The Ffar1R258W/R258W mouse model provided new insight into FFAR1-dependent and -independent effects of palmitate. Thus, in contrast to the FFAR1-mediated effect on Ppara mRNA, the palmitate-induced reduction of Slc2a2 mRNA levels was independent of functional FFAR1. An effect of palmitate on Glut-2 expression has been previously reported but the underlying signaling pathways remained unexplored [25]. However, HFD did not alter Slc2a2 mRNA levels, suggesting that the in vitro observation may not translate to the in vivo situation and, consequently, does not link to HFD-induced glucose intolerance. Chronic stimulation of G-protein coupled receptors, including FFAR1, is known to induce a downregulation of receptors and receptor function [26]. The exposure of wild-type islets to palmitate for 24 h was not sufficient for a significant reduction of Ffar1 mRNA levels. Nevertheless, after 8 weeks HFD feeding Ffar1 mRNA levels were reduced 5-fold indicating that chronic stimulation may attenuate receptor function.
The improved glucose tolerance of Ffar1R258W/R258W mice on HFD was unexpected. In combination with similar fasting blood glucose levels, the significant lower fasting plasma insulin of mutant mice compared to wild-type mice is indicative of improved insulin sensitivity. However, peripheral insulin resistance assessed with ipITT was not different between wild-type and mutant mice. The degree of liver steatosis was also independent of the expression of a functional FFAR1. Indeed, any change of liver steatosis and insulin resistance can only be attributed to an indirect effect of FFAR1, since the receptor is not expressed in rodent liver, muscle and adipose tissue (Refs. [1], [27]; data not shown).
The lower basal plasma insulin levels of Ffar1R258W/R258W compared to wild-type mice could be attributed to FFAR1 deficiency, because fatty acids are increased after overnight fasting and blood glucose levels were elevated, i.e. at 6 mM. In view of similar HFD-induced insulin resistance and liver steatosis, the significantly higher glucose excursions in wild-type mice during ipGTT cannot be explained by β-cell dysfunction only. It is more likely that additional, insulin-independent factors regulating blood glucose levels, e.g. via the regulation of hepatic glucose production, account for differences in glucose tolerance between wild-type and FFAR1 mutant mice.
An increased sympathetic tone and the hormone glucagon are the main glucose mobilizing factors [28], [29]. Single-cell transcriptome analysis of human islet cells suggests the expression of Ffar1 not only in β-cells but also in α-cells [30]. Moreover, analysis of rat α-cells indicates that FFAR1 expression is under the control of PAX6 [31]. At least in rodents, long chain fatty acids stimulate glucagon secretion at low glucose, i.e. under hypoglycemic condition [32]. However, there was no significant difference in plasma glucagon levels of CD-fed mutant and wild-type mice under fasting conditions. In HFD-fed mice, glucagon levels were much lower than in CD-fed mice and unfortunately under the detection level. In view of stable glucagon levels in humans during FFAR1-agonist administration and the lack of FFAR1-dependent stimulation of glucagon secretion in isolated human and rat islets at high glucose, it seems unlikely that FFAR1-dependent glucagon secretion inducing hepatic glucose mobilization accounts for higher glucose levels during a glucose load [33], [34]. Recently, evidence was presented that FFAR1 deficient mice display higher noradrenaline levels in brain [35]. The effects of changes in sympathetic nervous function during fat-rich feeding on glucose homeostasis in FFAR1-deficient mice require further studies.
During ipGTT, plasma insulin concentrations increased to a similar level in wild-type and Ffar1R258W/R258W mice, reflecting a β-cell glucose-responsiveness independent of FFAR1 function. Indeed, during ipGTT, plasma fatty acid concentrations decline and, therefore, it is unlikely that FFAR1 contributes to insulin secretion during ipGTT [36].
Glucose homeostasis is further regulated by incretins, and FFAR1-agonists increase incretin release in rodents [12], [37], [38]. In contrast to the significantly different plasma glucose levels at 30 min after ip glucose administration, 30 min after an oral glucose load, plasma glucose levels were not significantly different between wild-type and Ffar1R258W/R258W mice. GLP-1 secretion is stimulated by FFAR1 from the vascular but not from the luminal site, making it unlikely that FFAR1 is activated and augments incretin secretion during an oral glucose load when plasma fatty acids decline [36], [38]. Plasma glucose homeostasis is maintained via an interaction of many organs, which generate a large variety of metabolic regulators. Only a detailed analysis of the individual players and the reciprocal influences will give an explanation why Ffar1R258W/R258W mice are protected against diet induced glucose intolerance.
This study introduces a mouse model carrying the point mutation R258W in Ffar1, which abolishes the stimulation of insulin secretion in response to long chain fatty acids. The minimal genetic alteration mirrors the human situation and has the advantage over conventional knockout/congenic mouse models. It also circumvents side effects generated by viral constructs, the removal of additional non-coding regions within the deleted gene, and changes in protein–protein interactions such as receptor G-protein coupling due to complete abrogation of a receptor protein.
Disclosure statement
This study was supported by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.).
Author contributions
GKHP, HUH, MHA, and SU designed the study, SS, SM, and GKHP established the mouse models, SS, GK, FG, TS, and SM generated and analyzed the mouse strains and performed and analyzed the in vivo experiments. GK, FG, MH, ELG, MP, and SU performed the in vitro experiments, analyzed data, and wrote the manuscript. All authors approved the final version.
Acknowledgments
We thank Sandra Hoffmann (Helmholtz Zentrum München, German Research Center for Environmental Health (GmbH)), Andreas Mayer (Helmholtz Zentrum München, German Research Center for Environmental Health (GmbH)), Elisabeth Metzinger (University Hospital of Tübingen), Heike Runge (University Hospital of Tübingen), Birgit Schreiner (University Hospital of Tübingen) and Ulrike Schmidt (Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Center Munich at the University of Tübingen (IDM), Partner in the DZD) for excellent technical help.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 2.Briscoe C.P., Tadayyon M., Andrews J.L., Benson W.G., Chambers J.K., Eilert M.M. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. Journal of Biological Chemistry. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro H., Shachar S., Sekler I., Hershfinkel M., Walker M.D. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochemical and Biophysical Research. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 4.Tomita T., Masuzaki H., Noguchi M., Iwakura H., Fujikura J., Tanaka T. GPR40 gene expression in human pancreas and insulinoma. Biochemical and Biophysical Research. 2005;338:1788–1790. doi: 10.1016/j.bbrc.2005.10.161. [DOI] [PubMed] [Google Scholar]
- 5.Tomita T., Masuzaki H., Iwakura H., Fujikura J., Noguchi M., Tanaka T. Expression of the gene for a membrane-bound fatty acid receptor in the pancreas and islet cell tumours in humans: evidence for GPR40 expression in pancreatic beta cells and implications for insulin secretion. Diabetologia. 2006;49:962–968. doi: 10.1007/s00125-006-0193-8. [DOI] [PubMed] [Google Scholar]
- 6.Poitout V., Lin D.C. Modulating GPR40: therapeutic promise and potential in diabetes. Drug Discovery Today. 2013;18:1301–1308. doi: 10.1016/j.drudis.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Naik H., Vakilynejad M., Wu J., Viswanathan P., Dote N., Higuchi T. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of the GPR40 agonist TAK-875: results from a double-blind, placebo-controlled single oral dose rising study in healthy volunteers. Journal of Clinical Pharmacology. 2012;52:1007–1016. doi: 10.1177/0091270011409230. [DOI] [PubMed] [Google Scholar]
- 8.Gowda N., Dandu A., Singh J., Biswas S., Raghav V., Lakshmi M.N. Treatment with CNX-011-67, a novel GPR40 agonist, delays onset and progression of diabetes and improves beta cell preservation and function in male ZDF rats. BMC Pharmacology Toxicology. 2013;14:28. doi: 10.1186/2050-6511-14-28. http://www.biomedcentral.com/2050-6511/14/28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaku K., Enya K., Nakaya R., Ohira T., Matsuno R. Long-term safety and efficacy of fasiglifam (TAK-875), a G-protein-coupled receptor 40 agonist, as monotherapy and combination therapy in Japanese patients with type 2 diabetes: a 52-week open-label phase III study. Diabetes, Obesity and Metabolismes. 2016;18:925–929. doi: 10.1111/dom.12693. [DOI] [PubMed] [Google Scholar]
- 10.Steneberg P., Rubins N., Bartoov-Shifman R., Walker M.D., Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metabolism. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Mancini A.D., Bertrand G., Vivot K., Carpentier E., Tremblay C., Ghislain J. Beta-arrestin recruitment and biased agonism at free fatty acid receptor 1. Journal of Biological Chemistry. 2015;290:21131–21140. doi: 10.1074/jbc.M115.644450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauge M., Vestmar M.A., Husted A.S., Ekberg J.P., Wright M.J., Di Salvo J. GPR40 (FFAR1) – combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Molecular Metabolism. 2015;4:3–14. doi: 10.1016/j.molmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latour M.G., Alquier T., Oseid E., Tremblay C., Jetton T.L., Luo J. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan H., Hoos L.M., Liu L., Tetzloff G., Hu W., Abbondanzo S.J. Lack of FFAR1/GPR40 does not protect mice from high-fat diet-induced metabolic disease. Diabetes. 2008;57:2999–3006. doi: 10.2337/db08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahar H.K., Veprik A., Rubins N., Naaman O., Walker M.D. GPR41 gene expression is mediated by internal ribosome entry site (IRES)-dependent translation of bicistronic mRNA encoding GPR40 and GPR41 proteins. Journal of Biological Chemistry. 2012;287:20154–20163. doi: 10.1074/jbc.M112.358887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osokine I., Hsu R., Loeb G.B., McManus M.T. Unintentional miRNA ablation is a risk factor in gene knockout studies: a short report. PLoS Genetics. 2008;4:e34. doi: 10.1371/journal.pgen.0040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Ledesma A.M., Desai A.N., Bolivar V.J., Symula D.J., Flaherty L. Two new behavioral QTLs, Emo4 and Reb1, map to mouse Chromosome 1: congenic strains and candidate gene identification studies. Mammalian Genome. 2006;17:111–118. doi: 10.1007/s00335-005-0107-y. [DOI] [PubMed] [Google Scholar]
- 18.Guo S., Zhang J., Zhang S., Li J.A. Single amino acid mutation (R104P) in the e/DRY motif of GPR40 impairs receptor function. PLoS One. 2015;10:e0141303. doi: 10.1371/journal.pone.0141303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sum C.S., Tikhonova I.G., Neumann S., Engel S., Raaka B.M., Costanzi S. Identification of residues important for agonist recognition and activation in GPR40. Journal of Biological Chemistry. 2007;282:29248–29255. doi: 10.1074/jbc.M705077200. [DOI] [PubMed] [Google Scholar]
- 20.Hrabĕ de Angelis M.H., Flaswinkel H., Fuchs H., Rathkolb B., Soewarto D., Marschall S. Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nature Genetics. 2000;25:444–447. doi: 10.1038/78146. [DOI] [PubMed] [Google Scholar]
- 21.van Buerck L., Schuster M., Rathkolb B., Sabrautzki S., Hrabĕ de Angelis M.H., Wolf E. Enhanced oxidative stress and endocrine pancreas alterations are linked to a novel glucokinase missense mutation in ENU-derived Munich Gck(D217V) mutants. Molecular and Cellular Endocrinology. 2012;362:139–148. doi: 10.1016/j.mce.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Aigner B., Rathkolb B., Klempt M., Wagner S., Michel D., Klaften M. Generation of N-ethyl-N-nitrosourea-induced mouse mutants with deviations in hematological parameters. Mammalian Genome. 2011;22:495–505. doi: 10.1007/s00335-011-9328-4. [DOI] [PubMed] [Google Scholar]
- 23.Marschall S., Huffstadt U., Balling R., Hrabĕ de Angelis M. Reliable recovery of inbred mouse lines using cryopreserved spermatozoa. Mammalian Genome. 1999;10:773–776. doi: 10.1007/s003359901090. [DOI] [PubMed] [Google Scholar]
- 24.Wagner R., Kaiser G., Gerst F., Christiansen E., Due-Hansen M.E., Grundmann M. Reevaluation of fatty acid receptor 1 as a drug target for the stimulation of insulin secretion in humans. Diabetes. 2013;62:2106–2111. doi: 10.2337/db12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busch A.K., Cordery D., Denyer G.S., Biden T.J. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic beta-cell function. Diabetes. 2002;51:977–987. doi: 10.2337/diabetes.51.4.977. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt J., Liebscher K., Merten N., Grundmann M., Mielenz M., Sauerwein H. Conjugated linoleic acids mediate insulin release through islet G protein coupled receptor FFA1/GPR40. Journal of Biological Chemistry. 2011;286:11890–11894. doi: 10.1074/jbc.C110.200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teutsch C.A., Panse M., Grundmann M., Kaiser G., Kostenis E., Häring H.U. Detection of free fatty acid receptor 1 expression: the critical role of negative and positive controls. Diabetologia. 2014;57:776–780. doi: 10.1007/s00125-014-3161-8. [DOI] [PubMed] [Google Scholar]
- 28.Gosmain Y., Masson M.H., Philippe J. Glucagon: the renewal of an old hormone in the pathophysiology of diabetes. Journal of Diabetes. 2013;5:102–110. doi: 10.1111/1753-0407.12022. [DOI] [PubMed] [Google Scholar]
- 29.Shimazu T., Minokoshi Y. Systemic glucoregulation by glucose-sensing neurons in the ventromedial hypothalamic nucleus (VMH) Journal of the Endocrine Society. 2017;1:449–459. doi: 10.1210/js.2016-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segerstolpe A., Palasantza A., Eliasson P., Andersson E.M., Andreasson A.C., Sun X. Single-cell transcriptome profiling of human pancreatic islets in Health and type 2 diabetes. Cell Metabolism. 2016;24:593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gosmain Y., Cheyssac C., Masson M.H., Guérardel A., Poisson C., Philippe J. Pax6 is a key component of regulated glucagon secretion. Endocrinology. 2012;153:4204–4215. doi: 10.1210/en.2012-1425. [DOI] [PubMed] [Google Scholar]
- 32.Hong J., Abudula R., Chen J., Jeppesen P.B., Dyrskog S.E., Xiao J. The short-term effect of fatty acids on glucagon secretion is influenced by their chain length, spatial configuration, and degree of unsaturation: studies in vitro. Metabolism. 2005;54:1329–1336. doi: 10.1016/j.metabol.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Yashiro H., Tsujihata Y., Takeuchi K., Hazama M., Johnson P.R., Rorsman P. The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. Journal of Pharmacology and Experimental Therapeutics. 2012;340:483–489. doi: 10.1124/jpet.111.187708. [DOI] [PubMed] [Google Scholar]
- 34.Araki T., Hirayama M., Hiroi S., Kaku K. GPR40-induced insulin secretion by the novel agonist TAK-875: first clinical findings in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2012;14:271–278. doi: 10.1111/j.1463-1326.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 35.Aizawa F., Nishinaka T., Yamashita T., Nakamoto K., Kurihara T., Hirasawa A. GPR40/FFAR1 deficient mice increase noradrenaline levels in the brain and exhibit abnormal behavior. Journal of Pharmacological Sciences. 2016;132:249–254. doi: 10.1016/j.jphs.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Tang C., Ahmed K., Gille A., Lu S., Gröne H.-J., Tunaru S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nature Medicine. 2015;21:173–176. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 37.Edfalk S., Steneberg P., Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen L.W., Kuhre R.E., Janus C., Svendsen B., Holst J.J. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiological Reports. 2015;3(9):e12551. doi: 10.14814/phy2.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]