Abstract
Objective
Obesity-associated WAT inflammation is characterized by the accumulation and local activation of macrophages (MΦs), and recent data from mouse studies suggest that macrophages are modifiers of adipocyte energy metabolism and mitochondrial function. As mitochondrial dysfunction has been associated with obesity and the metabolic syndrome in humans, herein we aimed to delineate how human macrophages may affect energy metabolism of white adipocytes.
Methods
Human adipose tissue gene expression analysis for markers of macrophage activation and tissue inflammation (CD11c, CD40, CD163, CD206, CD80, MCP1, TNFα) in relationship to mitochondrial complex I (NDUFB8) and complex III (UQCRC2) was performed on subcutaneous WAT of 24 women (BMI 20–61 kg/m2). Guided by these results, the impact of secreted factors of LPS/IFNγ- and IL10/TGFβ-activated human macrophages (THP1, primary blood-derived) on mitochondrial function in human subcutaneous white adipocytes (SGBS, primary) was determined by extracellular flux analysis (Seahorse technology) and gene/protein expression.
Results
Stepwise regression analysis of human WAT gene expression data revealed that a linear combination of CD40 and CD163 was the strongest predictor for mitochondrial complex I (NDUFB8) and complex III (UQCRC2) levels, independent of BMI. IL10/TGFβ-activated MΦs displayed high CD163 and low CD40 expression and secreted factors that decreased UQCRC2 gene/protein expression and ATP-linked respiration in human white adipocytes. In contrast, LPS/IFNγ-activated MΦs showed high CD40 and low CD163 expression and secreted factors that enhanced adipocyte mitochondrial activity resulting in a total difference of 37% in ATP-linked respiration of white adipocytes (p = 0.0024) when comparing the effect of LPS/IFNγ- vs IL10/TGFβ-activated MΦs.
Conclusion
Our data demonstrate that macrophages modulate human adipocyte energy metabolism via an activation-dependent paracrine mechanism.
Keywords: Cytokines, Oxidative phosphorylation, Glycolysis, Cellular metabolism
Highlights
-
•
CD40:CD163 ratio in human WAT is proportional to OXPHOS expression.
-
•
CD40:CD163 ratio is high in LPS/IFNγ- and low in IL10/TGFβ-activated MΦs.
-
•
Opposing action on adipocyte bioenergetics by LPS/IFNγ- vs IL10/TGFβ-activated MΦs.
1. Introduction
White adipose tissue (WAT) plays a major role in energy homeostasis by storing nutrient energy, releasing fatty acids as bioenergetic substrates, and secreting endocrine mediators and factors [1], [2], [3]. Due to low mitochondrial content and activity as compared with brown adipocytes or myotubes, oxidative energy metabolism in WAT has been neglected for a long time. However, accumulating evidence suggests that mitochondrial activity is crucial for a normal WAT function since mitochondria are important for lipid storage [4], [5] and secretory functions [6], [7], [8]. This relevance has been further corroborated by clinical studies showing strong associations of decreased mitochondrial content and oxygen consumption of WAT/adipocytes with metabolic complications such as insulin resistance, type 2 diabetes and cardiovascular diseases [9], [10], [11], [12], [13].
One key hallmark in the development of obesity-associated metabolic disorders is the chronic, low-grade inflammation of WAT [14], [15]. Central players in obesity-associated inflammation and its comorbidities such as insulin resistance are adipose tissue macrophages (ATMΦs). They increase in number during the progression of obesity and subsequently become the most abundant cell type in WAT besides adipocytes and preadipocytes [15], [16]. Although macrophages are mainly studied in the context of pathologies, ATMΦs execute important physiological functions such as metabolic adaption to cold, stress, and exercise [17], [18]. Additionally, inflammation appears to be crucial for healthy WAT expansion and remodeling processes [19]. Notably, it has been suggested that the obesity-associated inflammation of human WAT may compromise mitochondrial function [16], [20], [21], [22]. These findings indicate an important role for the immune system in controlling WAT mitochondrial function, strongly suggesting the need to understand the global effects of macrophages on the bioenergetics of adipocytes, in particular in humans. To elucidate the crosstalk and functional interactions between macrophages and adipocytes, it is pivotal to identify cytokine-mediated pathways as potential novel therapeutic targets to modify WAT mitochondrial function in metabolic disorders.
In WAT of obese humans, several different ATMΦ subtypes have been identified expressing macrophage activation markers not only of the M1 spectrum but also the M2 spectrum [23], [24], [25], [26]. CD40, related to the M1 spectrum, displays upregulated gene expression by pro-inflammatory stimuli such as LPS and IFNγ [27]. The amount of CD40+-macrophages is higher in obese WAT and decreases after weight loss induced by gastric surgery [28]. As a co-stimulatory factor, CD40 plays a crucial role in adipose tissue inflammation in mice [29], [30], [31] and has been suggested as a promising target in obesity-associated insulin resistance [32], [33]. CD163, a monocyte/macrophage specific marker related to the M2 spectrum [34], is increased by IL10 [35], in obese human WAT [22], [26], and mRNA levels associate with insulin-resistance [36], [37].
In the present study, we performed adipose tissue gene expression analysis on CD40 and CD163 together with other known obesity-associated WAT inflammation and macrophage activation markers (CD11c, CD80, CD206, MCP1, TNFα) in relation to mitochondrial markers of the electron transport chain (ETC; NDUFB8 and UQCRC2) to gain insights into potential relevant connections of inflammation and mitochondrial bioenergetics in vivo. We performed quantitative analysis of respirometry and extracellular acidification measurements of human adipocytes (SGBS and primary cells) after exposure to microenvironments (conditioned media) created by either LPS/IFNγ- or IL10/TGFβ-activated human THP1, or primary macrophages (MΦs).
2. Materials and methods
2.1. Materials
Cell culture media and supplements were from Thermo Fisher Scientific (Invitrogen, Waltham, MA, USA). Human recombinant cytokines for macrophage activation were purchased from Peprotech (Rocky Hill, NJ, USA). All other chemicals and reagents were obtained from Sigma Aldrich (St. Louis, MO, USA) if not otherwise stated.
2.2. Experimental subjects and study approval
Human subcutaneous adipose tissue samples (mamma, abdominal) were obtained from 24 Caucasian women undergoing plastic surgery (cohort A: n = 16 (only abdominal), cohort B: n = 8 (mamma and abdominal)). Mean age of patients was 40 years (range 21–73 years) and their mean BMI was 31 kg/m2 (range 20–61 kg/m2). All procedures were performed in accordance with the Declaration of Helsinki guidelines and approved by the ethics committee of the University of Ulm. Patients gave a written informed consent in advance.
2.3. Macrophage culture and conditioned media
THP1 cells (purchased from ATCC, Manassas, VA, USA) tested for mycoplasma contamination were cultured and differentiated as reported previously [38], [39] with the following modifications. THP1 cells (1 × 106/ml) were differentiated by incubation with 100 ng/ml PMA for 24 h in growth medium. After differentiation, adherent THP1 cells were stimulated with 10 ng/ml LPS plus 10 ng/ml IFNγ (LPS/IFNγ-activated THP1) or 10 ng/ml IL10 and 1 ng/ml TGFβ (IL10/TGFβ-activated THP1). PMA and all cytokines were added to a control dish not containing THP1 cells. After 24 h, control (cell-free), LPS/IFNγ-activated THP1 and IL10/TGFβ-activated THP1 containing dishes were washed thoroughly with PBS and serum-free medium was added. After 24 h, conditioned media (CM) and control media (cell-free, CF) were collected and cleared by centrifugation. Cells were collected for RNA analysis. Human CD14+ blood monocytes from 4 healthy donors were purchased from Zenbio (Research Triangle Park, NC, USA) and differentiated with 10 ng/ml CSF1 for 7 days to adherent macrophages (MΦ) before same stimulation as for THP1 cells was performed. After 48 h, cells were thoroughly washed, and serum-free medium was added. After 24 h, LPS/IFNγ-activated MΦ-CM, IL10/TGFβ-activated MΦ-CM and control media were collected and cleared by centrifugation.
2.4. Adipocyte culture
SGBS adipocytes were cultured and differentiated for 10 days as published [40]. Human subcutaneous fat biopsies were obtained during gastric sleeve surgery from obese donors (11 for RNA analysis and 3 for energetic pathway studies) after an overnight fast. Preadipocytes were isolated and differentiated in vitro to adipocytes as previously described [41]. The study adhered to The Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants gave informed written consent to the study, and the study protocol was approved by the local ethics board. On day 10 of adipogenic differentiation, primary human cells or SGBS adipocytes were stimulated for 48 h with cell-free control media or CM of activated THP1 or primary MΦs (LPS/IFNγ-activated THP1-CM, IL10/TGFβ-activated THP1-CM or LPS/IFNγ-MΦ-CM, IL10/TGFβ-MΦ-CM) followed by RNA, protein and bioenergetic analysis.
2.5. Gene expression analysis
Total RNA was prepared using RNeasy Lipid tissue kit (Qiagen, Hilden, Germany). After cDNA synthesis (Superscript-II Reverse Transcriptase, Invitrogen) expression of specific genes was analyzed by real-time-PCR using SYBR® Green (Invitrogen) and the ViiA™ 7 Dx Instrument (Applied Biosystems, Foster City, CA, USA). Specific primers were obtained from Sigma (Sequences are available upon request). The mRNA levels of genes were normalized to Hypoxanthine-Phosphoribosyl-Transferase (HPRT) using ΔCt and if applicable to control group by ΔΔCt method.
2.6. Analysis of surface markers
THP1 cells after activation with LPS/IFNγ or IL10/TGFβ were harvested on ice, incubated with Fc block (Miltenyi, Bergisch-Gladbach, Germany) followed by incubation with primary antibodies against CD40 (clone 5C3, FITC, eBioscience) or CD163 (clone GHI/61.1, vioblue, Miltenyi, PE, eBioscience), CD11c (Miltenyi, clone MJ4-27G12.4.6, FITC), CD80 (Miltenyi, clone 2D10, PE) or CD206 (Miltenyi, clone DCN228, FITC) in the dark (4 °C). After 30 min, cells were washed and analyzed by flow cytometry (MACSQuant VYB, Miltenyi). Mean fluorescence intensity (MFI) was analyzed using MACSQuantify software (Miltenyi), normalized to MFI of corresponding isotype control.
2.7. Immunological detection of OXPHOS components
Cells were lysed (30 min at 4 °C), cleared by centrifugation, and protein concentrations were determined using BCA protein assay (Pierce). 15–30 μg protein lysate were separated on a 4–12% Bis–Tris gel (Invitrogen) and blotted onto a Nitrocellulose Membrane using iBlot (Invitrogen). Membrane was blocked for 1 h in Odyssey Blocking Buffer (LiCor, Lincoln, NE USA) followed by incubation with primary antibodies. IRDye® secondary antibodies (LiCor and Abcam, Cambridge, England) were used and signals were detected using the Odyssey Sa (LiCor). Following antibodies were used: MitoProfile® Total OXPHOS Human WB Antibody Cocktail (abcam #ab110411), human CD40 (abcam #13545), IL10 (#ab133575), IL6 (Cell Signaling, #12153), β-actin (abcam), α-Tubulin (LiCor) and β-tubulin (Santa Cruz, Dallas, TX, USA and abcam) were used as loading controls. Original Blot for Figure 2 and for the cut bands shown in Figure 3B are presented in Appendix A (page 7).
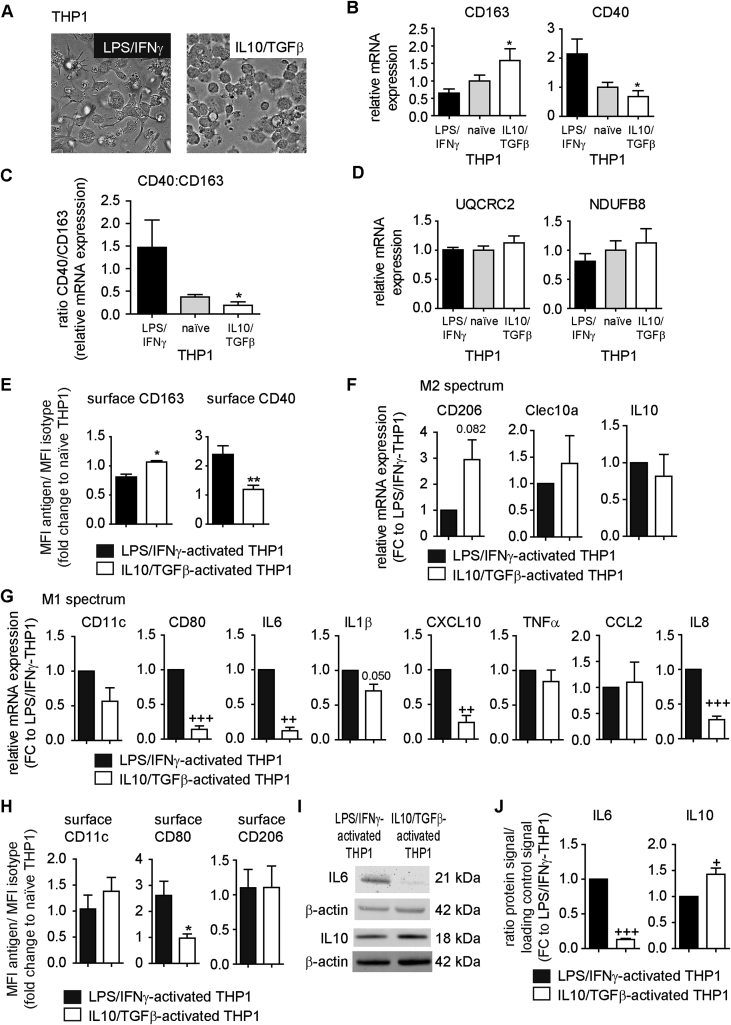
Figure 2.
Effects of LPS/IFNγ and IL10/TGFβ on the expression of CD40 and CD163 in THP1 cells. THP1 cells were cultured and activated with LPS/IFNγ or IL10/TGFβ as described in Methods. (A) Morphological changes detected by bright field microscopy (one representative experiment shown). (B) mRNA expression of CD163 and CD40 analyzed with ΔΔCT method using naïve THP1 macrophages as Calibrator (n = 4). (C) CD163 and CD40 mRNA expression presented as ratio. (D) mRNA expression of UQCRC2 and NDUFB8 analyzed with ΔΔCT method using naïve THP1 macrophages as Calibrator (n = 4). (E) Surface expression of CD163 and CD40 was analyzed by flow cytometry. Data are presented as MFI antigen/MFI isotype and expressed as fold change to naïve THP1 cells (n = 5–9). (B–E) *p < 0.05 vs. LPS/IFNγ-activated THP1; **p < 0.01 vs. LPS/IFNγ-activated THP1. (F, G) mRNA expression of indicated genes analyzed with ΔCT method. Data are presented as fold change to LPS/IFNγ-activated THP1 cells within each experiment and are mean + SEM (n = 4). ++p < 0.01 vs. 1; +++p < 0.001 vs. 1 (one-sample t-test). (H) Surface expression of CD11c, CD80 and CD206 was analyzed by flow cytometry. Data are presented as MFI antigen/MFI isotype and expressed as fold change to naïve THP1 cells (n = 5). *p < 0.05 vs. LPS/IFNγ-activated THP1. (I) Protein lysates were prepared from LPS/IFNγ- or IL10/TGFβ-activated THP1 cells and analyzed by western blot using antibodies for IL6, IL10 and β-actin. One representative experiment is shown. (Original blots are presented in Appendix A). (J) Quantification of signals normalized to β-actin and presented as fold change to LPS/IFNγ-activated THP1 cells within each experiment. Data are mean + SEM (n = 3–4). +p < 0.05 vs. 1; +++p < 0.001 vs. 1 (one-sample t-test).
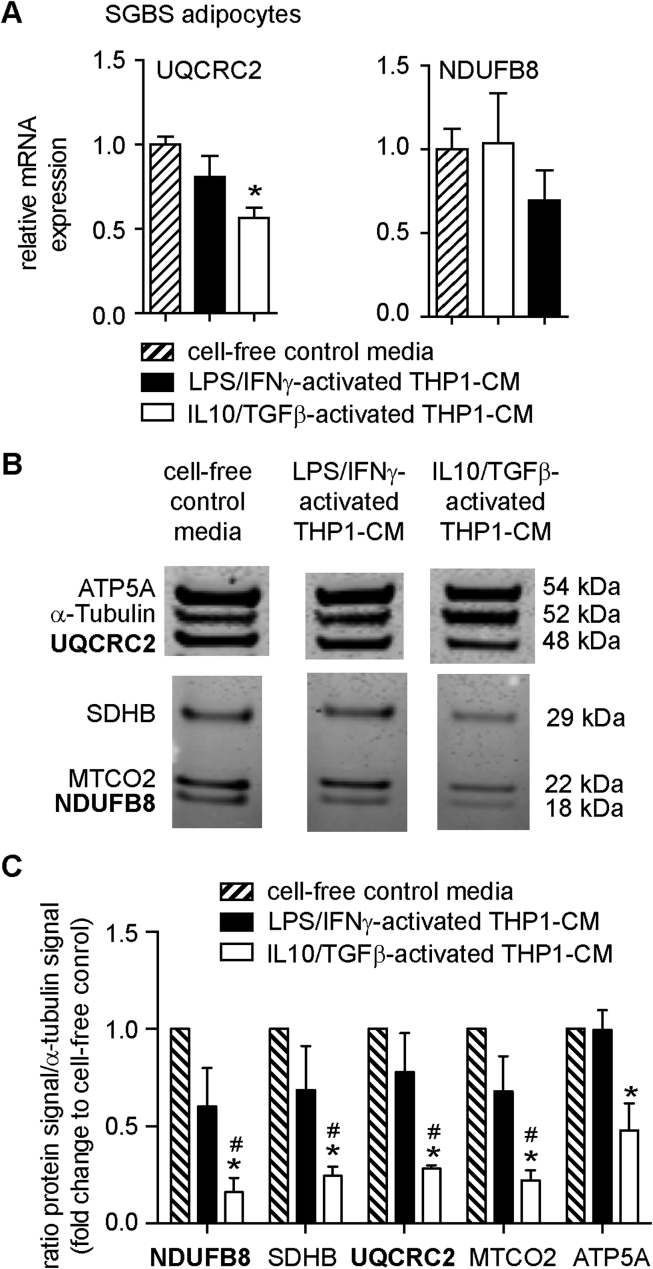
Figure 3.
Effects of LPS/IFNγ- and IL10/TGFβ-activated THP1-CM on the expression of UQCRC2 and NDUFB8 in human SGBS adipocytes. Differentiated SGBS cells were treated with either cell-free control media, LPS/IFNγ-activated THP1-CM or IL10/TGFβ-activated THP1-CM for 48 h as described in Methods. (A) mRNA expression of UQCRC2 and NDUFB8 were analyzed with ΔΔCT using SGBS cells exposed to cell-free control media as Calibrator. Data are the mean + SEM of 4 independent experiments performed in duplicates. (B) Protein lysates were prepared from SGBS adipocytes exposed to LPS/IFNγ- or IL10/TGFβ-activated THP1-CM for 48 h and analyzed by western blot using an antibody cocktail for OXPHOS components, including UQCRC2 and NDUFB8. One representative experiment is shown. Lanes were run on the same gel but were noncontiguous indicated by gap. (Original blot is presented in Appendix A). (C) Quantification of signals, normalized to α-tubulin and shown as fold change to SGBS cells treated with cell-free control media (n = 3). Data are presented as the mean + SEM. *p < 0.05 vs. LPS/IFNγ-activated THP1-CM; #p < 0.05 vs. cell-free control media.
2.8. Energetic pathway studies
SGBS cells or primary human preadipocytes were differentiated in XF96-PS plates (Seahorse Bioscience, North Billerica MA, USA). After stimulation for 48 h as described in Section 2.4, cells were washed with XF assay medium containing 5 mM glucose (pH adjusted to 7.5), incubated for 1 h in 37 °C air incubator in fresh XF assay medium containing 5 mM glucose and subjected to respiratory and extracellular acidification analysis as previously described [42]. The following injections and final concentrations were used: oligomycin (1 μg/ml), the chemical uncoupler FCCP (0.5 μM), rotenone (4 μM), antimycin A (2 μM) and 2-deoxy-glucose (100 mM). Seahorse data were normalized to DAPI or Quant-iT™ PicoGreen® dsDNA Kit (Invitrogen) as a surrogate for cell number per well.
2.9. Cytokine profiling of macrophage-conditioned media
Human cytokine antibody arrays (#ab133996, Abcam) with LPS/IFNγ-activated THP1-CM and IL10/TGFβ-activated THP1-CM (n = 5) were performed according to the manual and adapted for Odyssey System (LiCor) according to manufacturer's recommendation. Quantification, background correction and normalization of fluorescent signals were performed with ImageJ software using the Protein Array Analyzer tool set [43].
2.10. Statistics
To test for group differences shown in the figures, either unpaired t-test (two-tailed), Mann-Whitney-U test, if data failed normal distribution, or one-sample t-test to the value 1 were performed to compare two groups. One-way ANOVA (post-hoc: Bonferroni, Tukey) or ANOVA on ranks, if data failed normal distribution (posthoc: Dunn's), were performed to compare more than two groups. p < 0.05 was considered statistically significant. For gene expression analysis in human WAT biopsies, logarithmic-transformation was performed before statistical comparisons, as data failed normal distribution (D'Agostino Pearson test). Stepwise regression (backwards elimination) and multiple linear regressions were performed. To account for the two cohorts (year of tissue processing and analysis), we included this factor (cohort) besides BMI as a cofactor in the multiple linear regression model. All statistical tests were performed using Sigma Plot 12.0 (Systat Software, Inc., San Jose, California, USA) or R version 3.1.1.
3. Results
3.1. Gene expression ratio of CD40 and CD163 correlates with the expression of mitochondrial ETC components in human WAT
To investigate the link between MΦs and mitochondrial function in the WAT of human subjects, we analyzed gene expression of mitochondrial ETC (electron transport chain) components, i.e. NDUFB8, encoding a Complex I subunit, and UQCRC2, encoding Complex III Core protein 2, in whole WAT samples of 24 female donors with a wide range of BMIs (20–61 kg/m2) in relation to the gene expression of macrophage markers and inflammation mediators (CD11c, CD40, CD80, CD206, CD163, TNFα, and MCP1). Backward stepwise regression analysis revealed that the expression levels of mitochondrial genes UQCRC2 and NDUFB8 can be predicted by the combination of CD40 and CD163. In contrast, CD206, TNFα, CD11c, CD80, MCP1, and the BMI of the donor did not significantly impact this association (Table 1).
Table 1.
Identification of factors related to obesity-associated inflammation influencing UQCRC2 and NDUFB8 gene expression in whole human WAT samples.
| Dependent variable | Variables in model | p | Coefficient |
|---|---|---|---|
| NDUFB8 | CD163 | 0.059 | −0.162 |
| CD40 | <0.001 | 0.414 | |
| UQCRC2 | CD163 | 0.009 | −0.233 |
| CD40 | <0.001 | 0.404 |
Relative gene expression of UQCRC2, NDUFB8, CD40, CD206, CD163, CD11c, CD80, TNFα, and MCP1 was determined in whole subcutaneous WAT samples of 24 women (BMI 20–61 kg/m2). Data were log-transformed and stepwise regression analysis using backward elimination was performed using either NDUFB8 or UQCRC2 as dependent variable and CD40, CD206, CD163, CD11c, CD80, TNFα, MCP1, and BMI as independent variables. Results are presented for the final model where all non-significant variables (CD206, CD11c, CD80, TNFα, MCP1, and BMI) were eliminated.
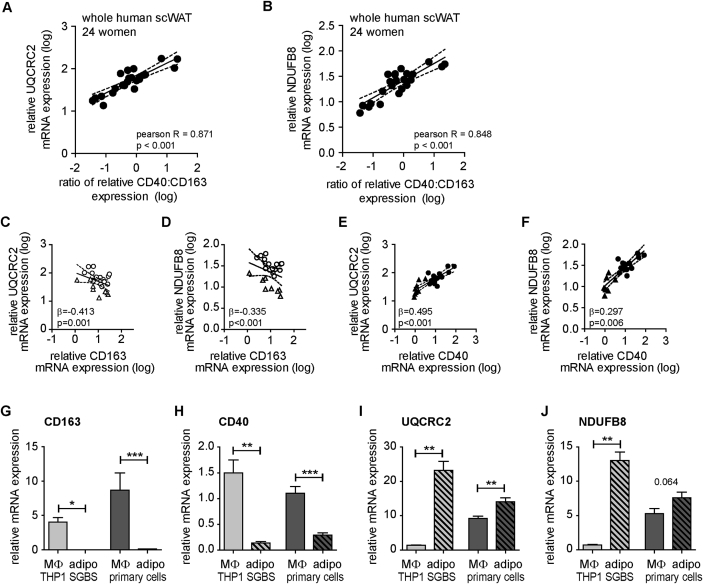
The relation of CD40 and CD163 with UQCRC2 and NDUFB8 is illustrated by plotting the ratio of CD40 and CD163 gene expression against UQCRC2 (Figure 1A) or NDUFB8 (Figure 1B) levels. Multiple linear regression analysis, revealed BMI-independent negative associations of UQCRC2 (Figure 1C) and also of NDUFB8 with CD163 (Figure 1D), contrasting strong BMI-independent positive association of UQCRC2 (Figure 1E) and NDUFB8 (Figure 1F) with CD40.
Figure 1.
Correlations of CD40 and CD163 with UQCRC2 and NDUFB8 expression in whole human WAT and their expression in macrophages versus adipocytes. (A)–(F) Total RNA of human subcutaneous adipose tissue (2 cohorts, total n = 24, BMI 20–61 kg/m2) was prepared and reversely transcribed. Expression levels of OXPHOS components (NDUFB8, UQCRC2), CD40, and CD163 were measured with qPCR and normalized to HPRT (ΔCt). Data have been log-transformed to meet assumption of normal distribution. Pearson R or coefficient β and p values are given in the graphs. Pearson correlation analysis was performed for the ratio of the relative mRNA expression of CD40 to CD163 with either NDUFB8(A) or UQCRC2(B). Multiple linear regressions (with BMI and cohort (cohort A: circle, cohort B: triangle) as independent variable) were performed for the relative mRNA expression of either UQCRC2(C) or NDUFB8(D) with CD163 and for the relative mRNA expression of either UQCRC2(E) or NDUFB8(F) with CD40. p-values and coefficient β for the multiple linear regression model including BMI and cohort as cofactors are presented in the graphs. (G)–(J) THP1 cells, primary (blood-derived) macrophages, SGBS adipocytes and primary adipocytes were cultured as described in Methods. mRNA expression of CD163, CD40, UQCRC2, and NDUFB8 was determined by qPCR and normalized to HPRT (ΔCt) and are the mean + SEM (n = 4–11). Ct values normalized to macrophages (THP1 or primary) without prior normalization to housekeeping gene are presented in Figure S2. *p < 0.05; **p < 0.01; ***p < 0.001.
We observed similar association with CD163 and CD40 when we analyzed two additional ETC components (ATP5A (Complex V) and SDHB (Complex II)) in 16 WAT samples (Figure S1A–D).
3.2. CD40 and CD163 are predominantly expressed in macrophages; UQCRC2 and NDUFB8 are predominantly expressed in adipocytes
While CD163 is expressed exclusively on cells of the monocyte-macrophage cell lineage [34], CD40 expression was reported in several cell types including human adipocytes [44]. To determine whether adipocytes with high UQCRC2 expression co-express high levels of CD40, we examined primary human subcutaneous adipocytes of 11 donors. We report that CD40 mRNA levels neither associate significantly with UQCRC2 (p = 0.722, Pearson R = 0.121) nor with NDUFB8 (p = 0.996, Pearson R = −0.002) in cultured primary adipocytes, strongly suggesting that associations found in whole human WAT samples are not attributable to adipocytes. Notably, comparing CD163 (Figure 1G) and CD40 (Figure 1H) mRNA levels in adherent, macrophage-like THP1 cells, SGBS adipocytes, primary human macrophages and adipocytes (Figure 1G–J) revealed higher expression in THP1 and primary macrophages. Conversely, UQCRC2 (Figure 1I) and NDUFB8 (Figure 1J) mRNA levels were higher in SGBS and primary adipocytes than in THP1 and primary macrophages. Supplementary Figure S2 displays the data without normalization to housekeeping genes but to THP1 (Figure S2A) or primary macrophages (Figure S2B) in order to exclude potential impact of cell-type specific housekeeper regulation. Higher expression of genes coding for electron transport in human adipocytes as compared to the stromal-vascular fraction which contains preadipocytes and immune cells including macrophages has been reported previously [45]. Furthermore, western blot analysis revealed barely detectable CD40 protein levels in SGBS and primary adipocytes, and higher levels of UQCRC2 and NDUFB8 in SGBS adipocytes vs. THP1 cells and primary macrophages vs. primary adipocytes (Figure S3).
Thus the observed correlation of CD40 and CD163 with UQCRC2 and NDUFB8 in human whole WAT may point to a macrophage-adipocyte interaction with macrophages modulating mitochondrial bioenergetics of adipocytes within the tissue.
3.3. Activating macrophages with LPS/IFNγ or IL10/TGFβ controls CD40 and CD163 levels
To study potential effects of macrophages with different CD40 and CD163 expression levels on adipocyte mitochondrial function, we activated THP1 and primary MΦs using cytokines. First, to exclude bias by donor–donor variations, we established an in vitro model system, the THP1 cells, a human monocytic cell line, which can be differentiated in vitro to macrophage-like cells, and the SGBS cell strain, a commonly used model for human, insulin-sensitive adipocytes [38], [39], [46]. Previous studies suggest induction of CD163 by IL10 and reduction by LPS and IFNγ [35], whereas CD40 expression is induced by IFNγ and LPS and repressed by TGFβ [27]. In our study, we confirmed altered morphology of THP1 macrophages upon activation with LPS plus IFNγ or IL10 plus TGFβ (Figure 2A) [47], [48]. LPS/IFNγ-activated THP1 cells were fibroblastoid with multiple filopodia potentially reflecting their function for engulfing and phagocytosis (Figure 2A). In contrast, IL10/TGFβ-activated THP1 cells displayed mainly a round cell shape (Figure 2A). Importantly, activation of THP1 cells led to differential expression of CD40 and CD163. LPS/IFNγ-activated THP1 cells showed high CD40 and low CD163 mRNA levels as compared to the IL10/TGFβ-activated THP1 cells (Figure 2B), resulting in a significantly, 7.9-fold higher ratio of CD40:CD163 for LPS/IFNγ-activated THP1 cells as compared to IL10/TGFβ-activated THP1 cells (Figure 2C). No significant differences were found for UQCRC2 and NDUFB8 mRNA (Figure 2D) between the IL10/TGFβ-activated THP1 cells and LPS/IFNγ-activated THP1 cells. Flow cytometry demonstrated higher CD40 surface expression on LPS/IFNγ-activated THP1 cells (Figure 2E) whereas CD163 was expressed more highly on IL10/TGFβ-activated THP1 cells (Figure 2E). To further characterize the LPS/IFNγ- vs. IL10/TGFβ-activated THP1 cells, we analyzed genes related to M2 activation spectrum (Figure 2F) and genes related to the pro-inflammatory M1 spectrum (Figure 2G). Additionally, we determined surface expression of CD80, CD11c, and CD206 (Figure 2H) and analyzed IL6 and IL10 protein expression (Figure 2I and J). These data indicate that LPS/IFNγ-activated THP1 cells are activated towards the M1 spectrum of macrophage polarization, whereas IL10/TGFβ-activated THP1 cells tend towards the M2 spectrum. From these two macrophage populations, which differed in their CD40 and CD163 mRNA and protein expression, we collected the supernatant (IL10/TGFβ-activated THP1-CM, LPS/IFNγ-activated THP1-CM; CM = conditioned media) to test their global secretome for effects on mitochondrial function of human adipocytes.
3.4. The microenvironment of IL10/TGFβ-activated THP1 macrophages decreases mitochondrial ATP production in human SGBS adipocytes
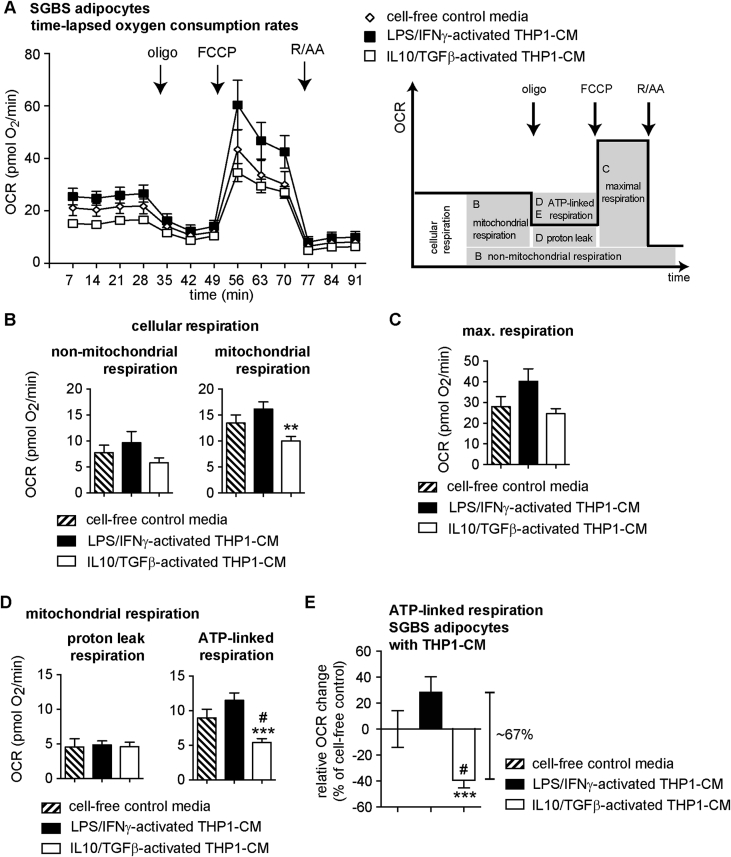
SGBS adipocytes were treated for 48 h with 10% IL10/TGFβ-activated THP1-CM or 10% LPS/IFNγ-activated THP1-CM followed by analysis of UQCRC2 and NDUFB8 mRNA levels. UQCRC2 mRNA was significantly decreased in SGBS adipocytes after treatment with IL10/TGFβ-activated THP1-CM, but not with LPS/IFNγ-activated THP1-CM (Figure 3A). No significant differences in NDUFB8 mRNA levels were detectable under the two treatment regimens (Figure 3A). Determination of the protein abundance of UQCRC2 and NUDBF8 with an antibody cocktail for 5 OXPHOS (oxidative phosphorylation) complexes demonstrated that all five OXPHOS complexes, including UQCRC2 and NDUFB8, were significantly less abundant after exposing SGBS adipocytes to IL10/TGFβ-activated THP1-CM (Figure 3B and C). To determine whether THP1-CM-induced changes of OXPHOS expression impose functional changes in mitochondrial energy metabolism of human SGBS adipocytes, we measured real-time plate-based oxygen consumption rates (OCRs) of differentiated SGBS cells that were incubated with LPS/IFNγ-activated THP1-CM or IL10/TGFβ-activated THP1-CM. When OCR measurements were analyzed in absolute values (Figure 4A), we found that the resting cellular respiration (control: 21.2 pmol O2/min) decreased by 5.5 pmol O2/min (∼26%) in adipocytes that had been exposed to the IL10/TGFβ-activated THP1 microenvironment. Resting cellular respiration demonstrated a trend towards an increase by 4.4 pmol O2/min (∼21%) when human adipocytes were exposed to the LPS/IFNγ-activated THP1-CM (Figure 4A). Specific mitochondrial inhibitors (oligomycin (oligo), rotenone (R), antimycin A (AA)), and chemical uncoupler (FCCP) were sequentially added to the cells to enable experimental partitioning of cellular respiration into distinct modules of oxidative phosphorylation (Figure 4A) as described previously [42]. Mitochondrial respiration was significantly reduced only in response to IL10/TGFβ-activated THP1-CM with no changes in non-mitochondrial respiration (Figure 4B). Since maximal substrate oxidation capacity showed no significant reduction (Figure 4C), we assume that neither IL10/TGFβ-activated THP1-CM nor LPS/IFNγ-activated THP1-CM compromised substrate delivery or oxidation. Importantly, ATP-linked respiration reporting mitochondrial ATP synthesis rates of adipocytes that had been exposed to IL10/TGFβ-activated THP1-CM, was significantly lower compared to control medium (∼39%, Figure 4D and E) and to LPS/IFNγ-activated THP1-CM (Figure 4D and E). SGBS cells exposed to LPS/IFNγ-activated THP1-CM tended to ∼28% increase in ATP-linked respiration (Figure 4E). Neither IL10/TGFβ-activated THP1-CM nor LPS/IFNγ-activated THP1-CM modulated proton leak respiration rates (Figure 4D). As ATP production and consumption are balanced in intact cells, the increased ATP-linked respiration in adipocytes exposed to LPS/IFNγ-activated THP1-CM strongly suggests additional ATP demand, thus contrasting decreased mitochondrial ATP turnover in response to IL10/TGFβ-activated THP1-CM.
Figure 4.
Effects of LPS/IFNγ- and IL10/TGFβ-activated THP1-CM on mitochondrial bioenergetics of human SGBS adipocytes. Differentiated SGBS cells were treated with either cell-free control media, LPS/IFNγ-activated THP1-CM or IL10/TGFβ-activated THP1-CM for 48 h as described in Methods. (A) Oxygen consumption rates over time (OCRs) of SGBS adipocytes treated for 48 h with indicated THP1-CM using a XF96 extracellular flux analyzer. OCRs were recorded and analyzed as described in Methods. Scheme shows the section of the time trace corresponding to each module. (B) Cellular respiration was dissected into non-mitochondrial (left) and mitochondrial (right) respiration using the OXPHOS complex inhibitors rotenone (R) and antimycin A (AA). (C) Maximal respiration can be obtained after addition of chemical uncoupler FCCP. (D) Mitochondrial respiration was further dissected into proton leak (left) and ATP-linked respiration (right) using the ATP-synthase inhibitor oligomycin (oligo). (E) ATP-linked respiration presented as relative change in OCR in percent of cell-free control. Respirometry data were normalized to cell number (DAPI signal). Data are presented as the means ± SEM of 4 independent experiments each performed with 6–8 wells/condition. **p < 0.01 vs. LPS/IFNγ-activated THP1-CM; ***p < 0.001 vs. LPS/IFNγ-activated THP1-CM; #p < 0.05 vs. cell-free control media.
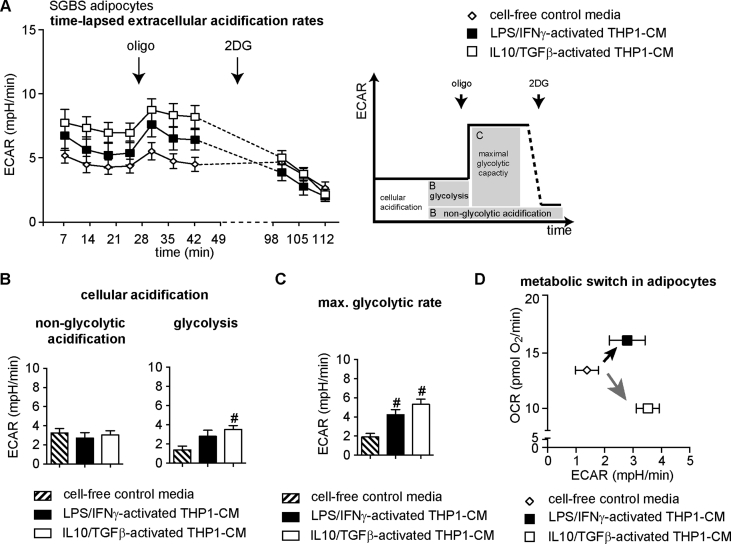
3.5. IL10/TGFβ-activated THP1 cells switch human SGBS adipocytes from oxidative phosphorylation towards glycolysis (Warburg-like effect)
A decrease in ATP-linked respiration, as found for IL10/TGFβ-activated THP1-CM treated adipocytes, may indicate lower ATP demand. However, ATP demand is complemented and immediately compensable by glycolytic ATP production. To investigate whether glycolytic pathways compensate decreased mitochondrial ATP production in IL10/TGFβ-activated THP1-CM treated adipocytes, extracellular acidification rates (ECARs) of SGBS adipocytes were assessed simultaneously to OCRs (Figure 5A). Adipocytes treated with IL10/TGFβ-activated THP1-CM tended to higher extracellular acidification values as compared to control adipocytes (p = 0.099, Figure 5A). 2-deoxy glucose (2DG) injection allowed correction for non-glycolytic acidification, which was not significantly different (Figure 5B). Basal glycolytic rates (basal – 2 DG) were significantly higher in adipocytes exposed to IL10/TGFβ-activated THP1-CM (Figure 5B), providing higher glycolytic ATP output. Next, oligomycin was injected to inhibit mitochondrial ATP production and to induce a compensatory increase in glycolytic rates to estimate glycolytic capacity as described previously [42]. We found significantly higher capacity for glycolysis in adipocytes treated with both CMs as compared to control medium (Figure 5C). Intriguingly, LPS/IFNγ-activated THP1-CM and IL10/TGFβ-activated THP1-CM altered cellular energy metabolism of human white adipocytes in a distinct manner. Simultaneous assessment of glycolytic and respiratory flux and the calculation of their ratio demonstrated a metabolic switch in adipocytes (Figure 5D): LPS/IFNγ-activated THP1-CM provoked a higher ATP demand/turnover (Figure 5D, black arrow) that induced both, glycolytic and mitochondrial fluxes. In contrast, the IL10/TGFβ-activated THP1-CM induced glycolytic pathways while reducing oxidative mitochondrial ATP production, thus switching adipocyte metabolism to a Warburg-like effect (Figure 5D, gray arrow).
Figure 5.
Effects of LPS/IFNγ- and IL10/TGFβ-activated THP1-CM on glycolytic activity of human SGBS adipocytes. SGBS adipocytes (d10) were treated with indicated conditioned-media (THP1-CM) and analyzed after 48 h with a XF96 extracellular flux analyzer, as described in Methods. Extracellular acidification rates (ECARs) were recorded simultaneously to OCR from Figure 4. (A) Time-resolved ECAR of adipocytes treated with either cell-free control media, LPS/IFNγ-activated THP1-CM or IL10/TGFβ-activated THP1-CM. Data after FCCP and rotenone/antimycin A injection were not used for ECAR analysis and are depicted as dashed lines. Scheme shows the section of the time trace corresponding to each module. (B) By inhibiting glycolysis with 2-deoxy-glucose (2DG; 100 mM), cellular acidification was dissected into non-glycolytic acidification (left) and acidification due to glycolysis (right). (C) By inhibiting mitochondrial ATP synthesis with oligomycin (oligo), the compensatory glycolytic capacity was obtained. (D) Glycolytic acidification rates plotted against mitochondrial respiration rates (c.f. Figure 4B). All data are normalized to cell number (DAPI signal) and are presented as the means ± SEM of 4 independent experiments each performed in 6–8 wells/condition. #p < 0.05 vs. cell-free control media.
3.6. The microenvironment of primary human macrophages (MΦs) activated with LPS/IFNγ increases mitochondrial activity of human SGBS and primary adipocytes
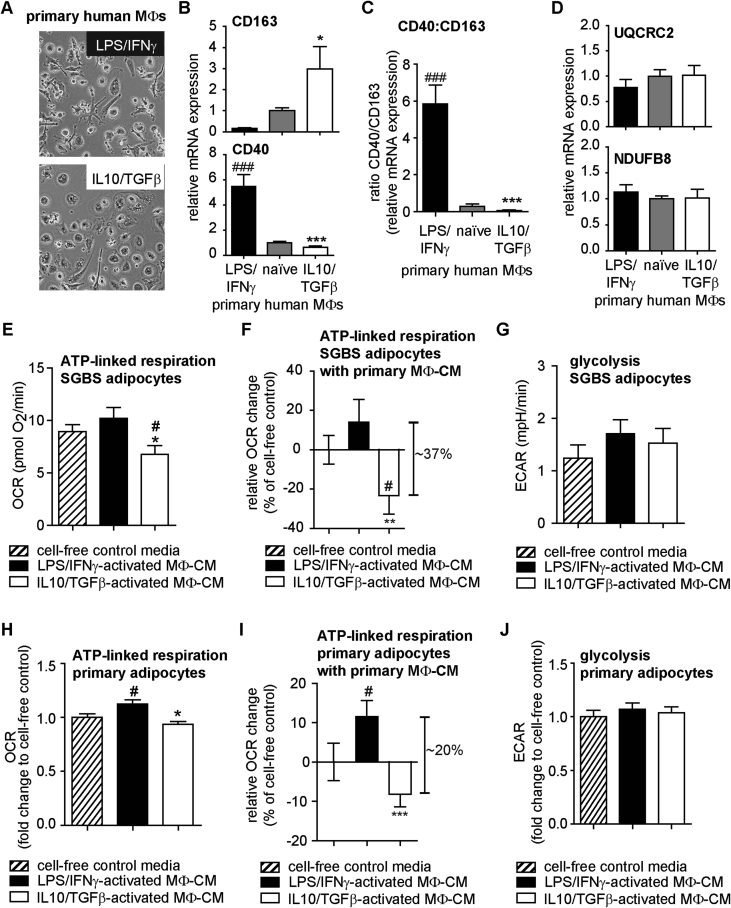
Next, we validated the global effects of CM from THP1 cells on human SGBS adipocytes by using primary CD14+ blood monocytes from healthy donors differentiated in vitro to macrophages (MΦs). Subsequently, MΦs were activated like THP1 cells with LPS/IFNγ or IL10/TGFβ. Primary MΦs (Figure 6A) displayed similar morphological changes as THP1 cells (c.f. Figure 2A). CD40 mRNA levels were upregulated upon LPS/IFNγ activation, whereas CD163 mRNA levels were down-regulated (Figure 6B). In contrast, CD163 was up-regulated upon IL10/TGFβ activation and CD40 was down-regulated (Figure 6B). Thus, LPS/IFNγ-activated primary MΦ had a significantly higher CD40/CD163 ratio as compared with IL10/TGFβ-activated primary MΦ (Figure 6C). No significant changes in UQCRC2 and NDUFB8 mRNA levels were detected (Figure 6D).
Figure 6.
Effects of LPS/IFNγ- and IL10/TGFβ-activated primary MΦ-CM on bioenergetics of human SGBS and primary adipocytes. Primary human CD14+ monocytes from 4 healthy donors were cultured, differentiated to macrophages (naïve MΦs) and activated with LPS/IFNγ or IL10/TGFβ as described in Methods. (A) Morphological changes detected by bright field microscopy (one representative experiment shown). (B) mRNA expression of CD163 and CD40 analyzed with ΔΔCT method using naïve MΦs as Calibrator (n = 4). (C) CD163 and CD40 mRNA expression presented as ratio. (B, C) *p < 0.05 vs. LPS/IFNγ-activated MΦ, ***p < 0.001 vs. LPS/IFNγ-activated MΦ, ###p < 0.001 vs. naïve MΦs. (D) mRNA expression of UQCRC2 and NDUFB8 analyzed with ΔΔCT method using naïve THP1 macrophages as Calibrator (n = 4). (E–G) SGBS adipocytes were treated with either cell-free control media, LPS/IFNγ-activated or IL10/TGFβ-activated MΦ-CM collected from 3 different donors for 48 h as described in Methods and analyzed as described in Figure 4, Figure 5. Data are normalized to cell number (DNA content) and are the mean + SEM of 5 independent experiments each performed in 2–8 wells condition. OCR and ECAR traces vs time are shown in Appendix A (Figure S4B and C). (E) ATP-linked respiration of SGBS adipocytes after treatment for 48 h with indicated MΦ-CM. (F) ATP-linked respiration of SGBS adipocytes presented as relative change in OCR in percent of cell-free control. (G) ECARs due to glycolysis of SGBS adipocytes after treatment for 48 h with indicated MΦ-CM. (H–J) Human primary subcutaneous adipocytes were treated with either cell-free control media, LPS/IFNγ-activated or IL10/TGFβ-activated MΦ-CM from 3 different donors as described in Methods. Bioenergetic profile was analyzed as described in Figure 4, Figure 5. Data are presented as fold change to the mean rates of cell-free control for each donor and are the mean + SEM of experiments from 3 adipocyte donors performed in 6–8 wells per condition and donor. OCR and ECAR traces vs time are shown in Appendix A (Figure S4E and F) (H) ATP-linked respiration of human primary adipocytes after treatment for 48 h with indicated MΦ-CM. (I) ATP-linked respiration of human primary adipocytes presented as relative change in OCR in percent of cell-free control. (J) ECARs due to glycolysis of human primary adipocytes after treatment for 48 h with indicated MΦ-CM. (E)–(J) *p < 0.05 vs. LPS/IFNγ-activated MΦ-CM; **p < 0.01 vs. LPS/IFNγ-activated MΦ-CM; ***p < 0.001 vs. LPS/IFNγ-activated MΦ-CM; #p < 0.05 vs. cell-free control media.
Exposing SGBS adipocytes to the LPS/IFNγ-activated primary MΦ-CM tended to higher ATP-linked respiration when compared with cell-free control media and led to significantly increased ATP-linked respiration as compared to IL10/TGFβ-activated primary MΦ-CM (Figure 6E and F). IL10/TGFβ-activated primary MΦ-CM significantly reduced ATP-linked respiration of SGBS adipocytes (∼23% as compared with cell free control, Figure 6F). We did not find a robust increase in glycolysis when SGBS adipocytes were exposed to LPS/IFNγ-activated primary MΦ-CM (p = 0.21) or when they were exposed to IL10/TGFβ-activated primary MΦ-CM (p = 0.44) (Figure 6G). Importantly, exposing primary adipocytes from three obese donors to primary MΦ-CM altered their mitochondrial activity: primary adipocytes that had been exposed to LPS/IFNγ-activated primary MΦ-CM demonstrated increased ATP-linked respiration as compared to cell-free control CM exposure (∼12%, Figure 6H and I). Furthermore, primary adipocytes showed reduced ATP-linked respiration after exposure to IL10/TGFβ-activated primary MΦ-CM (∼8%, Figure 6I) resulting in a significant ∼20% total difference in ATP-linked respiration when comparing the two primary MΦ-CM (Figure 6I). No changes of glycolytic rates in primary adipocytes of obese donors were detected with CM from primary MΦs (Figure 6J).
In summary, we report an unexpected contribution of IL10/TGFβ-activated human macrophages (characterized by low CD40/CD163 expression) to reduced mitochondrial activity of human white adipocytes. In contrast, LPS/IFNγ-activated MΦs increased ATP-linked respiration. These opposing effects were reflected at the gene expression level in whole human WAT: a low CD40:CD163 ratio was linked to low UQCRC2 and NDUFB8 expression and a high CD40:CD163 ratio was linked to high UQCRC2 and NDUFB8 gene levels.
4. Discussion
The present study provides evidence that microenvironments derived from MΦs have distinct effects on the energy homeostasis of human white adipocytes by modulating mitochondrial activity. Importantly, we report that IL10/TGFβ-activated human MΦs secrete factors that reduce mitochondrial activity and the gene/protein levels of UQCRC2 of human white adipocytes. Our data suggest an unexpected involvement of IL10/TGFβ-activated MΦs (usually classified as anti-inflammatory or M2) in the obesity-associated WAT dysfunction of humans impacting mitochondrial activity that is important for healthy WAT function including adipogenic differentiation, adipokine secretion, lipolysis, and lipogenesis [5], [6], [49], [50], [51]. Our functional analysis of mitochondrial bioenergetics is consistent with the association between high CD163 (a marker induced by IL10 in macrophages [35]) and low CD40 with low OXPHOS expression in vivo. In contrast LPS/IFNγ-activated MΦs increase mitochondrial activity, specifically ATP-linked respiration in human white adipocytes. In our in vitro model, there is no apparent regulation of UQCRC2 and NDUFB8 mRNA levels (Figure 3, Figures 3A, S4A and D). We may assume that maximal mitochondrial OXPHOS activity will adapt to changes in cellular ATP demand, which, in turn, will be reflected at a molecular level in vivo with levels of CD40 and CD163 mRNA linked to levels of OXPHOS components (NDUFB8, UQCRC2).
Our results suggest that inflammation may serve as an indicator of mitochondrial function in human WAT. In a study investigating the transcriptome of monozygotic twins discordant for obesity, genes clustering to mitochondrial pathways (in particular subunits of complex I and complex III) are significantly downregulated in the heavier co-twins in both adipocytes and adipose tissue [52]. In the same study, CD163 was one of the most upregulated genes in the WAT of the heavy twin [52], which is in line with our observation of negative association between CD163 and UQCRC2 expression. Other recent reports show obesity-associated increase in soluble CD163 (sCD163) and CD163+-macrophages in adipose tissue [53]. Furthermore, there is a correlation between sCD163 in the plasma and its mRNA expression in human WAT associating with insulin resistance [36], [37]. A transcriptome study with >800 obese donors detected high levels of CD163 among other inflammation markers and low expression of genes involved in TCA cycle and β-oxidation in visceral WAT of obese donors with high HOMA-IR as compared with obese donors with low HOMA-IR [22]. As the cleaved form of CD163 (sCD163) is easily detectable in the blood of patients, clinical trials are testing whether sCD163 can serve as a predictor for unhealthy (insulin-resistant) versus healthy (insulin-sensitive) obesity (http://clinicaltrials.gov/show/NCT01463449). Importantly, CD40 can also be cleaved from the cell surface and may be detected as soluble CD40 (sCD40) in the blood of patients [54]. For its ligand CD154, higher levels of sCD154 have already been detected in obese patients [55], [56], and several studies show an important function of the CD40-CD154 system in obesity and type 2 diabetes [33], [56], [57], [58]. Our present data indicate that CD163 and/or CD40 may serve as markers for the metabolic state (mitochondrial activity) of WAT. Further research is required to investigate CD163 and the CD40-CD154 system as important markers in humans that report how the inflammatory state modulates the energy metabolism of tissues and if the correlations between macrophage and mitochondrial markers could be used as endogenous biomarkers discriminating metabolically unhealthy (insulin resistant, high inflammation) versus metabolically healthy (insulin sensitive, low inflammation) obesity.
Insulin resistance is linked to the accumulation of MΦs in WAT, which associates with obesity in both, humans and mice [15], [16], [59]. The exact spatiotemporal activation of adipose tissue macrophages (ATMΦs), however, is not fully understood. Different obese mouse models demonstrate an increase in pro-inflammatory MΦ markers and a decrease in anti-inflammatory MΦ markers [15], [16], [60]. However, the expression of some, commonly defined as anti-inflammatory, MΦ markers such as IL10 and CD206 are also increased in WAT of obese mice after several weeks (>12 weeks) on a high-fat diet [61], [62]. In particular, human studies often show, in addition to pro-inflammatory markers, an increase in anti-inflammatory MΦ markers in WAT of obese subjects [23], [26], [63], [64]. When performing single correlation analysis of the relative mRNA levels of macrophage/inflammation markers in WAT with the BMI of the donors in our cohort, we found that CD80 (p = 0.033, Pearson R = 0.437) as well as CD206 (p = 0.003, Pearson R = 0.586) associated with the BMI. Our analyses focus on subcutaneous WAT from women. For comparisons with the literature, however, one should consider that sex- and depot-differences have been described for the inflammatory and metabolic profile of WAT [59], [64], [65], [66], [67], [68]. However, sex and depot position do not fully explain the above-mentioned contradictory data on ATMΦ subtypes during obesity. MΦs in WAT have a high phenotypic plasticity and are subjected to dynamic, phase-wise (initiation, propagation and remodeling) changes during the development of obesity and metabolic disorders [69], [70], [71], [72], [73]. Accumulating evidence suggests that samples taken from obese patients represent a snapshot of remodeling processes in obese WAT, which are characterized by MΦs displaying both, markers that are categorized as pro-inflammatory (M1) as well as markers commonly classified as anti-inflammatory (M2) [74]. Dissecting the different spatiotemporal phenotypes of human ATMΦs including their secreted cytokines, chemokines, and other factors will provide further insights into their role in WAT metabolism and dysfunction.
ATMΦs not only contribute to obesity-associated metabolic dysfunctions, e.g. insulin resistance but also exert physiological roles and beneficial effects on WAT homeostasis, e.g. healthy lipid storage [17], [19], [75], [76]. Ablating tissue-resident ATMΦ (anti-inflammatory M2) in mice [77] or reducing pro-inflammatory signals in murine WAT [19] impairs healthy WAT expansion leading to adverse ectopic lipid storage in other organs, seen as hepatic steatosis. In line with these observations, ATMΦs appear to be important for buffering excess lipid mobilization during fasting-induced WAT lipolysis [78], [79] and overnutrition [80]. Notably, during overnutrition, ATMΦs are not shifted into the classic M1 spectrum. Recently, ATMΦs have also been implicated in cold adaptions and exercise in mice. IL4-activated MΦs are part of an anti-inflammatory signaling cascade contributing to cold-induced browning and recruitment of beige adipocytes in WAT [17], [18], [75], [81], [82], [83], although the underlying mechanisms are still controversially debated [84].
In the present study, we focused on the effects of LPS/IFNγ- and IL10/TGFβ-activated macrophages on white adipocytes bioenergetics. It is reasonable to argue that not only LPS/IFNγ- and IL10/TGFβ-activated macrophages impact the mitochondrial function in WAT. For example, there are other macrophage subtypes and importantly, other immune cells such as T cells in WAT which also express CD40 [30], [56]. Other macrophage subtypes, T cells and B cells are yet missing in our present study and require further investigations as they potentially contribute to the connection between inflammation-induced dysfunction of human WAT and metabolic disorders such as insulin resistance [85], [86]. With our experimental setup, we have not yet addressed the direct or indirect contribution of one or more of these cell types to the association of CD40 and CD163 with UQCRC2 expression in vivo. Our data, however, show that IL10/TGFβ- and LPS/IFNγ-activated MΦs are able to regulate mitochondrial function of human white adipocytes, thus contributing at least partly to mitochondrial function in human WAT, in particular in the context of obesity-associated increase of macrophage content.
Our study provides results supporting a global, activation-dependent paracrine effect of human macrophages on white adipocyte mitochondrial function, with opposing effects when comparing LPS/IFNγ-activated MΦ-CM with IL10/TGFβ-activated MΦ-CM. This provides an important tool to identify factors/pathways that can be explored as novel targets modulating mitochondrial activity in obese WAT. Using cytokine arrays covering 23 cytokines, we only identify two significantly altered chemokines. CCL7 and CCL8 are significantly more abundant in the LPS/IFNγ-activated THP1-CM, whereas the increased abundance of GM-CSF in IL10/TGFβ-activated THP1-CM did not reach significance (Figure S5A–D). Testing the effects of CCL7 and CCL8 on the bioenergetic profile of adipocytes revealed no robust effect on ATP-linked respiration and glycolysis (Figure S5E and F), but additional effects cannot be formally excluded at this stage. Identification of the secreted factors awaits further studies using sensitive approaches such as non-targeted secretome analysis and/or metabolomics. Recently, an increase in the oxidative phenotype of human-induced pluripotent stem-derived adipocytes by an intermediate metabolite (i.e. lactate) has been shown [87]. Upon activation, macrophages change their metabolic profile [88] and their profile of lipid mediators [89], [90], indicating that these macrophage-released factors are additional candidates for the modulatory effects of macrophages on mitochondrial activity in white adipocytes.
Taken together, we report an unexpected, direct action of IL10/TGFβ-activated macrophages on decreased mitochondrial gene expression and function of human white adipocytes, which is reflected in whole human WAT samples by the association of UQCRC2 and NDUFB8 gene expression levels with low CD40:CD163 ratio. Our two cohorts only consist of subcutaneous WAT of female donors. It will be important to determine if a similar link is present in WAT of male donors, or possibly in visceral WAT. Notably, our data suggest that human white adipocytes in different inflammatory microenvironments demonstrate differential metabolic profiles (total difference in ATP-linked respiration of adipocytes exposed to primary MΦ-CM: ∼37% for SGBS cells (Figure 6F), ∼20% for adipocytes from obese donors (Figure 6I)). As metabolic unhealthy (insulin-resistant) obesity [91] may be characterized by different activated ATMΦs (e.g. activation marker CD163 [22], [53]), the contribution of the macrophage-created environments on adipocytes energy metabolism in particular in the context of obesity and in relation to metabolic complications such as diabetes, liver damage, and cardiovascular disease, should be further explored as it offers the potential to identify new subgroups of unhealthy obese patients.
Acknowledgments
This work was funded (in part) by the Helmholtz Alliance ICEMED (Imaging and Curing Environmental Metabolic Diseases), the Network Fund of the Helmholtz Association and the German Center for Diabetes Research (DZD), and by grants from the Deutsche Forschungsgemeinschaft to SMH (DFG: SFB1123-A4), to PFP (DFG: Fi 1700/5-1), and to DT (DFG: TE 912/2-1). DT was supported by a grant from the Ministry of Science, Research and the Arts of Baden-Württemberg (Boehringer Ingelheim University Ulm Biocenter (BIU) Az: 32-7533.-6-10/15/5).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.07.008.
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 2.Scherer P.E. Adipose tissue from lipid storage compartment to endocrine organ. Diabetes. 2006;55(6):1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 3.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaaman M., Sparks L.M., van Harmelen V., Smith S.R., Sjölin E., Dahlman I. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007;50(12):2526–2533. doi: 10.1007/s00125-007-0818-6. [DOI] [PubMed] [Google Scholar]
- 5.Kusminski C.M., Scherer P.E. Mitochondrial dysfunction in white adipose tissue. Trends in Endocrinology & Metabolism. 2012;23(9):435–443. doi: 10.1016/j.tem.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh E.H., Park J.-Y., Park H.-S., Jeon M.J., Ryu J.W., Kim M. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56(12):2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- 7.Wang C.-H., Wang C.-C., Huang H.-C., Wei Y.-H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS Journal. 2013;280(4):1039–1050. doi: 10.1111/febs.12096. [DOI] [PubMed] [Google Scholar]
- 8.Szkudelski T., Nogowski L., Szkudelska K. Short-term regulation of adiponectin secretion in rat adipocytes. Physiological Research. 2011;60(3):521. doi: 10.33549/physiolres.931971. [DOI] [PubMed] [Google Scholar]
- 9.Yin X., Lanza I.R., Swain J.M., Sarr M.G., Nair K.S., Jensen M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. The Journal of Clinical Endocrinology and Metabolism. 2014;99(2):E209–E216. doi: 10.1210/jc.2013-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinonen S., Buzkova J., Muniandy M., Kaksonen R., Ollikainen M., Ismail K. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64(9):3135–3145. doi: 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- 11.Pietiläinen K.H., Naukkarinen J., Rissanen A., Saharinen J., Ellonen P., Keränen H. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Medicine. 2008;5(3):e51. doi: 10.1371/journal.pmed.0050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer B., Schöttl T., Schempp C., Fromme T., Hauner H., Klingenspor M. Inverse relationship between body mass index and mitochondrial oxidative phosphorylation capacity in human subcutaneous adipocytes. American Journal of Physiology. Endocrinology and Metabolism. 2015;309(4):E380–E387. doi: 10.1152/ajpendo.00524.2014. [DOI] [PubMed] [Google Scholar]
- 13.Yehuda-Shnaidman E., Buehrer B., Pi J., Kumar N., Collins S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes. 2010;59(10):2474–2483. doi: 10.2337/db10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York, N.Y.) 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 15.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of Clinical Investigation. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Y., Nguyen K.D., Odegaard J.I., Cui X., Tian X., Locksley R.M. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao R.R., Long J.Z., White J.P., Svensson K.J., Lou J., Lokurkar I. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wernstedt Asterholm I., Tao C., Morley T.S., Wang Q.A., Delgado-Lopez F., Wang Z.V. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metabolism. 2014;20(1):103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bondia-Pons I., Ryan L., Martinez J.A. Oxidative stress and inflammation interactions in human obesity. Journal of Physiology and Biochemistry. 2012;68(4):701–711. doi: 10.1007/s13105-012-0154-2. [DOI] [PubMed] [Google Scholar]
- 21.Hahn W.S., Kuzmicic J., Burrill J.S., Donoghue M.A., Foncea R., Jensen M.D. Proinflammatory cytokines differentially regulate adipocyte mitochondrial metabolism, oxidative stress, and dynamics. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(9):E1033–E1045. doi: 10.1152/ajpendo.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qatanani M., Tan Y., Dobrin R., Greenawalt D.M., Hu G., Zhao W. Inverse regulation of inflammation and mitochondrial function in adipose tissue defines extreme insulin sensitivity in morbidly obese patients. Diabetes. 2013;62(3):855–863. doi: 10.2337/db12-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fjeldborg K., Pedersen S.B., Møller H.J., Christiansen T., Bennetzen M., Richelsen B. Human adipose tissue macrophages are enhanced but changed to an anti-inflammatory profile in obesity. Journal of Immunology Research. 2014;2014:1–10. doi: 10.1155/2014/309548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P., Lu M., Nguyen M.T.A., Bae E.J., Chapman J., Feng D. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. Journal of Biological Chemistry. 2010;285(20):15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wentworth J.M., Naselli G., Brown W.A., Doyle L., Phipson B., Smyth G.K. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima S., Koh V., Kua L.-F., So J., Davide L., Lim K.S. Accumulation of CD11c+CD163+ adipose tissue macrophages through upregulation of intracellular 11β-HSD1 in human obesity. The Journal of Immunology. 2016 doi: 10.4049/jimmunol.1600895. [DOI] [PubMed] [Google Scholar]
- 27.Benveniste E.N., Nguyen V.T., Wesemann D.R. Molecular regulation of CD40 gene expression in macrophages and microglia. Brain, Behavior, and Immunity. 2004;18(1):7–12. doi: 10.1016/j.bbi.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Aron-Wisnewsky J., Tordjman J., Poitou C., Darakhshan F., Hugol D., Basdevant A. Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. The Journal of Clinical Endocrinology & Metabolism. 2009;94(11):4619–4623. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 29.Guo C.-A. 2013. Genetic deficiency of CD40 in mice exacerbates metabolic manifestations of diet-induced obesity: a dissertation. [Google Scholar]
- 30.Yi Z., Stunz L.L., Bishop G.A. CD40-mediated maintenance of immune homeostasis in the adipose tissue microenvironment. Diabetes. 2014;63(8):2751–2760. doi: 10.2337/db13-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris D.L., Oatmen K.E., Mergian T.A., Cho K.W., DelProposto J.L., Singer K. CD40 promotes MHC class II expression on adipose tissue macrophages and regulates adipose tissue CD4+ T cells with obesity. Journal of Leukocyte Biology. 2016;99(6):1107–1119. doi: 10.1189/jlb.3A0115-009R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatzigeorgiou A., Seijkens T., Zarzycka B., Engel D., Poggi M., van den Berg S. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(7):2686–2691. doi: 10.1073/pnas.1400419111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berg S.M., Seijkens T.T.P., Kusters P.J.H., Zarzycka B., Beckers L., den Toom M. Blocking CD40-TRAF6 interactions by small-molecule inhibitor 6860766 ameliorates the complications of diet-induced obesity in mice. International Journal of Obesity. 2015;39(5):782–790. doi: 10.1038/ijo.2014.198. [DOI] [PubMed] [Google Scholar]
- 34.Backé E., Schwarting R., Gerdes J., Ernst M., Stein H. Ber-MAC3: new monoclonal antibody that defines human monocyte/macrophage differentiation antigen. Journal of Clinical Pathology. 1991;44(11):936–945. doi: 10.1136/jcp.44.11.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buechler C., Ritter M., Orsó E., Langmann T., Klucken J., Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. Journal of Leukocyte Biology. 2000;67(1):97–103. [PubMed] [Google Scholar]
- 36.Kračmerová J., Rossmeislová L., Kováčová Z., Klimčáková E., Polák J., Tencerová M. Soluble CD163 is associated with CD163 mRNA expression in adipose tissue and with insulin sensitivity in steady-state condition but not in response to calorie restriction. The Journal of Clinical Endocrinology and Metabolism. 2014;99(3):E528–E535. doi: 10.1210/jc.2013-3348. [DOI] [PubMed] [Google Scholar]
- 37.Sørensen L.P., Parkner T., Søndergaard E., Bibby B.M., Møller H.J., Nielsen S. Visceral obesity is associated with increased soluble CD163 concentration in men with type 2 diabetes mellitus. Endocrine Connections. 2015;4(1):27–36. doi: 10.1530/EC-14-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keuper M., Dzyakanchuk A., Amrein K.E., Wabitsch M., Fischer-Posovszky P. THP-1 macrophages and SGBS adipocytes – a new human in vitro model system of inflamed adipose tissue. Frontiers in Endocrinology. 2011;2:89. doi: 10.3389/fendo.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keuper M., Blüher M., Schön M.R., Möller P., Dzyakanchuk A., Amrein K. An inflammatory micro-environment promotes human adipocyte apoptosis. Molecular and Cellular Endocrinology. 2011;339(1–2):105–113. doi: 10.1016/j.mce.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Fischer-Posovszky P., Newell F.S., Wabitsch M., Tornqvist H.E. Human SGBS cells – a unique tool for studies of human fat cell biology. Obesity Facts. 2008;1(4):184–189. doi: 10.1159/000145784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berti L., Irmler M., Zdichavsky M., Meile T., Böhm A., Stefan N. Fibroblast growth factor 21 is elevated in metabolically unhealthy obesity and affects lipid deposition, adipogenesis, and adipokine secretion of human abdominal subcutaneous adipocytes. Molecular Metabolism. 2015;4(7):519–527. doi: 10.1016/j.molmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keuper M., Jastroch M., Yi C.-X., Fischer-Posovszky P., Wabitsch M., Tschop M.H. Spare mitochondrial respiratory capacity permits human adipocytes to maintain ATP homeostasis under hypoglycemic conditions. The FASEB Journal. 2014;28(2):761–770. doi: 10.1096/fj.13-238725. [DOI] [PubMed] [Google Scholar]
- 43.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poggi M., Jager J., Paulmyer-Lacroix O., Peiretti F., Gremeaux T., Verdier M. The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia. 2009;52(6):1152–1163. doi: 10.1007/s00125-009-1267-1. [DOI] [PubMed] [Google Scholar]
- 45.Cancello R., Henegar C., Viguerie N., Taleb S., Poitou C., Rouault C. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 46.Wabitsch M., Brenner R.E., Melzner I., Braun M., Möller P., Heinze E. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. International Journal of Obesity. 2001;25(1):8. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- 47.Ploeger D.T., Hosper N.A., Schipper M., Koerts J.A., Rond S., de Bank R.A. Cell plasticity in wound healing: paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Communication and Signaling. 2013;11(1):29. doi: 10.1186/1478-811X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassol E., Cassetta L., Rizzi C., Alfano M., Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. The Journal of Immunology. 2009;182(10):6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Marsboom G., Toth P.T., Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One. 2013;8(10):e77077. doi: 10.1371/journal.pone.0077077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson-Fritch L., Burkart A., Bell G., Mendelson K., Leszyk J., Nicoloro S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Molecular and Cellular Biology. 2003;23(3):1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Pauw A., Tejerina S., Raes M., Keijer J., Arnould T. Mitochondrial (Dys)function in adipocyte (De)differentiation and systemic metabolic alterations. The American Journal of Pathology. 2009;175(3):927–939. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heinonen S., Muniandy M., Buzkova J., Mardinoglu A., Rodríguez A., Frühbeck G. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia. 2016:1–13. doi: 10.1007/s00125-016-4121-2. [DOI] [PubMed] [Google Scholar]
- 53.Sporrer D., Weber M., Wanninger J., Weigert J., Neumeier M., Stögbauer F. Adiponectin downregulates CD163 whose cellular and soluble forms are elevated in obesity. European Journal of Clinical Investigation. 2009;39(8):671–679. doi: 10.1111/j.1365-2362.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 54.Contin C., Pitard V., Itai T., Nagata S., Moreau J.-F., Déchanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-α-converting enzyme implications for CD40 signaling. Journal of Biological Chemistry. 2003;278(35):32801–32809. doi: 10.1074/jbc.M209993200. [DOI] [PubMed] [Google Scholar]
- 55.Unek I.T., Bayraktar F., Solmaz D., Ellidokuz H., Sisman A.R., Yuksel F. The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people. Clinical Medicine & Research. 2010;8(2):89–95. doi: 10.3121/cmr.2010.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf D., Jehle F., Michel N.A., Bukosza E.N., Rivera J., Chen Y.C. Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation. 2014;129(23):2414–2425. doi: 10.1161/CIRCULATIONAHA.113.008055. [DOI] [PubMed] [Google Scholar]
- 57.Chatzigeorgiou A., Phieler J., Gebler J., Bornstein S., Chavakis T. CD40L stimulates the crosstalk between adipocytes and inflammatory cells. Hormone and Metabolic Research. 2013;45(10):741–747. doi: 10.1055/s-0033-1348221. [DOI] [PubMed] [Google Scholar]
- 58.Neubauer H., Setiadi P., Günesdogan B., Pinto A., Börgel J., Mügge A. Influence of glycaemic control on platelet bound CD40–CD40L system, P-selectin and soluble CD40 ligand in Type 2 diabetes. Diabetic Medicine. 2010;27(4):384–390. doi: 10.1111/j.1464-5491.2010.02957.x. [DOI] [PubMed] [Google Scholar]
- 59.Cancello R., Tordjman J., Poitou C., Guilhem G., Bouillot J.L., Hugol D. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55(6):1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 60.Lumeng C.N., DelProposto J.B., Westcott D.J., Saltiel A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaul M.E., Bennett G., Strissel K.J., Greenberg A.S., Obin M.S. Dynamic, m2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet-induced obesity in mice. Diabetes. 2010;59(5):1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujisaka S., Usui I., Bukhari A., Ikutani M., Oya T., Kanatani Y. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bourlier V., Zakaroff-Girard A., Miranville A., De Barros S., Maumus M., Sengenes C. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117(6):806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 64.Spencer M., Yao-Borengasser A., Unal R., Rasouli N., Gurley C.M., Zhu B. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. American Journal of Physiology. Endocrinology and Metabolism. 2010;299(6):E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahlin S., Sjöholm K., Jacobson P., Andersson-Assarsson J.C., Walley A., Tordjman J. Macrophage gene expression in adipose tissue is associated with insulin sensitivity and serum lipid levels independent of obesity: macrophage markers in adipose tissue. Obesity. 2013;21(12):E571–E576. doi: 10.1002/oby.20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White U.A., Tchoukalova Y.D. Sex dimorphism and depot differences in adipose tissue function. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease. 2014;1842(3):377–392. doi: 10.1016/j.bbadis.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nookaew I., Svensson P.-A., Jacobson P., Jernås M., Taube M., Larsson I. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. The Journal of Clinical Endocrinology and Metabolism. 2013;98(2):E370–E378. doi: 10.1210/jc.2012-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kralova Lesna I., Kralova A., Cejkova S., Fronek J., Petras M., Sekerkova A. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. Journal of Translational Medicine. 2016;14 doi: 10.1186/s12967-016-0962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amengual-Cladera E., Lladó I., Proenza A.M., Gianotti M. High-fat diet feeding induces a depot-dependent response on the pro-inflammatory state and mitochondrial function of gonadal white adipose tissue. British Journal of Nutrition. 2013;109(03):413–424. doi: 10.1017/S0007114512001171. [DOI] [PubMed] [Google Scholar]
- 70.Amengual-Cladera E., Lladó I., Gianotti M., Proenza A.M. Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolism. 2012;61(8):1108–1117. doi: 10.1016/j.metabol.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 71.Surmi B.K., Hasty A.H. Macrophage infiltration into adipose tissue. Future Lipidology. 2008;3(5):545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chinetti-Gbaguidi G., Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Current Opinion in Lipidology. 2011;22(5):365–372. doi: 10.1097/MOL.0b013e32834a77b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris D.L., Singer K., Lumeng C.N. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(4):341–346. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of Pathology. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 75.Brestoff J.R., Kim B.S., Saenz S.A., Stine R.R., Monticelli L.A., Sonnenberg G.F. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu P.-S., Lin Y.-W., Burton F.H., Wei L.-N. Injecting engineered anti-inflammatory macrophages therapeutically induces white adipose tissue browning and improves diet-induced insulin resistance. Adipocyte. 2015;4(2):123–128. doi: 10.4161/21623945.2014.981438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Satoh T., Kidoya H., Naito H., Yamamoto M., Takemura N., Nakagawa K. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495(7442):524–528. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 78.Kosteli A., Sugaru E., Haemmerle G., Martin J.F., Lei J., Zechner R. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. Journal of Clinical Investigation. 2010;120(10):3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fitzgibbons T.P., Czech M.P. Emerging evidence for beneficial macrophage functions in atherosclerosis and obesity-induced insulin resistance. Journal of Molecular Medicine. 2016;94(3):267–275. doi: 10.1007/s00109-016-1385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu X., Grijalva A., Skowronski A., van Eijk M., Serlie M., Ferrante A. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metabolism. 2013;18(6):816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu D., Molofsky A.B., Liang H.-E., Ricardo-Gonzalez R.R., Jouihan H.A., Bando J.K. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science (New York, N.Y.) 2011;332(6026):243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee M.-W., Odegaard J.I., Mukundan L., Qiu Y., Molofsky A.B., Nussbaum J.C. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1–2):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen K.D., Qiu Y., Cui X., Goh Y.P.S., Mwangi J., David T. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer K., Ruiz H.H., Jhun K., Finan B., Oberlin D.J., van der Heide V. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nature Medicine. 2017;23(5):623–630. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McLaughlin T., Liu L.-F., Lamendola C., Shen L., Morton J., Rivas H. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014 doi: 10.1161/ATVBAHA.114.304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishimura S., Manabe I., Takaki S., Nagasaki M., Otsu M., Yamashita H. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metabolism. 2013;18(5):759–766. doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 87.Carrière A., Jeanson Y., Berger-Müller S., André M., Chenouard V., Arnaud E. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63(10):3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- 88.Kelly B., O'Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Research. 2015;25(7):771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dalli J., Serhan C.N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masoodi M., Kuda O., Rossmeisl M., Flachs P., Kopecky J. Lipid signaling in adipose tissue: connecting inflammation & metabolism. Biochimic Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids. 2015;1851(4):503–518. doi: 10.1016/j.bbalip.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 91.Stefan N., Häring H.-U., Hu F.B., Schulze M.B. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. The Lancet Diabetes & Endocrinology. 2013;1(2):152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.