Abstract
Objective
Upon activation, G protein coupled receptors (GPCRs) associate with heterotrimeric G proteins at the plasma membrane to initiate second messenger signaling. Subsequently, the activated receptor experiences desensitization, internalization, and recycling back to the plasma membrane, or it undergoes lysosomal degradation. Recent reports highlight specific cases of persistent cyclic AMP generation by internalized GPCRs, although the functional significance and mechanistic details remain to be defined. Cyclic AMP generation from internalized Glucagon-Like Peptide-1 Receptor (GLP-1R) has previously been reported from our laboratory. This study aimed at deciphering the molecular mechanism by which internalized GLP-R supports sustained cyclic AMP generation upon receptor activation in pancreatic beta cells.
Methods
We studied the time course of cyclic AMP generation following GLP-1R activation with particular emphasis on defining the location where cyclic AMP is generated. Detection involved a novel GLP-1 conjugate coupled with immunofluorescence using specific endosomal markers. Finally, we employed co-immunoprecipitation as well as immunofluorescence to assess the protein–protein interactions that regulate GLP-1R mediated cyclic AMP generation at endosomes.
Results
Our data reveal that prolonged association of G protein α subunit Gαs with activated GLP-1R contributed to sustained cyclic AMP generation at Rab 5 endosomal compartment.
Conclusions
The findings provide the mechanism of endosomal cyclic AMP generation following GLP-1R activation. We identified the specific compartment that serves as an organizing center to generate endosomal cyclic AMP by internalized activated receptor complex.
Keywords: GLP-1 receptor, Rab5, Cyclic AMP, Insulin secretion, Gαs, Beta arrestin-1, Pancreatic beta cells
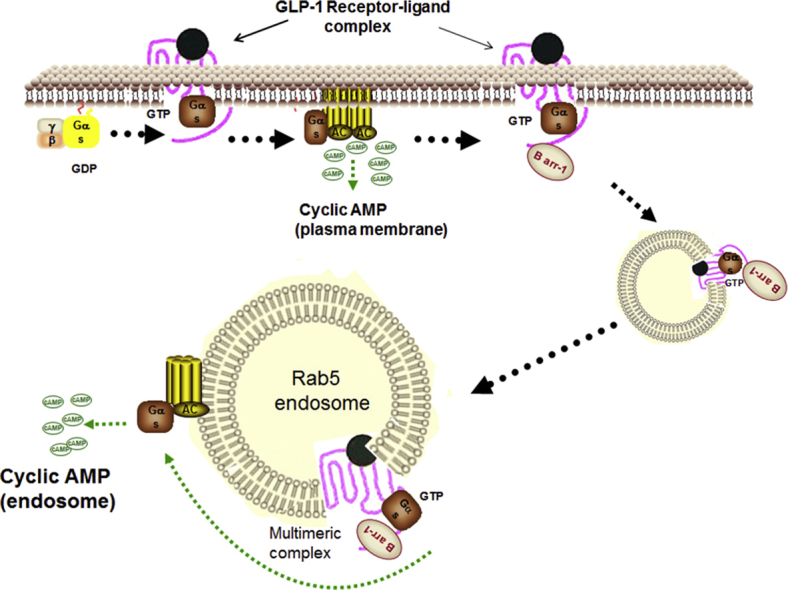
Graphical abstract
Highlights
-
•
Prolonged association of Gαs with activated GLP-1 R contributes to sustained c AMP generation in pancreatic beta cells.
-
•
Beta arrestin-1 association with the receptor does not attenuate GLP-1R mediated cyclic AMP generation.
-
•
Rab5A endosomes serve as a niche for sustained endosomal cyclic AMP generation upon GLP-1 receptor activation.
1. Introduction
G protein coupled-receptor (GPCR) signaling is initiated through agonist mediated stabilization of the receptor in active conformation resulting in association with hetero trimeric G proteins, which upon guanine nucleotide exchange initiates diverse cellular processes [1], [2]. In the canonical pathway of GPCR activation, association of GPCR with heterotrimeric G proteins takes place exclusively at the plasma membrane [2], [3]. This interaction is terminated by a series of events involving phosphorylation of GPCRs by G protein receptor kinases (GRKs) and recruitment of β arrestins which sterically hinder the association of GPCR with G protein α subunit to desensitize and internalize the receptor [4], [5], [6]. The establishment of the recent model of sustained signaling by a number of GPCRs [7], [8], [9], [10], [11], [12], [13], [14], which continue signaling after initial agonist activation necessitates reassessment of the role of these regulatory proteins in mediating the GPCR response.
Endosomal localization of Galpha S subunit (Gαs) and its role in endosomal receptor trafficking and signaling is well documented [11], [15], [16], [17], [18]. Gαs has been reported to be activated and signal from the intracellular compartment following cell-surface activation and internalization of parathyroid hormone receptor (PTHR) [19], β2-adrenergic receptor (β2AR) [11] and vasopressin type 2 receptor (V2R) [16]. Single–particle electron microscopy analysis revealed simultaneous association of G protein α subunit and beta arrestin at the C terminal tail of Class B GPCRs forming megaplexes to provide the physical basis of interaction among these GPCR regulatory proteins [20] in regulating receptor internalization as well as endogenous cyclic AMP formation. In addition, Rosciglione et al. reported interaction of Gαs with ESCRT (endosomal sorting complex required for transport), highlighting its role in cellular trafficking of GPCRs [21].
GLP-1R mediated generation of cyclic AMP had previously been reported from our laboratory [22]. The internalized activated receptor complex was associated with Bodipy Forskolin, and pharmacological inhibition of internalization was found to attenuate GLP-1R mediated cyclic AMP generation in BRIN-BD11 pancreatic beta cells [22]. In the present study, we report the mechanism by which GLP-1R activation resulted in the generation of cyclic AMP from endosomes. Our data reveal that GLP-1R remained associated with Gαs after internalization of the receptor, which, in turn, contributes to sustained cyclic AMP generation upon GLP-1R activation.
2. Materials & methods
2.1. Reagents
BRIN-BD11 was procured from European Collection of Cell Cultures (ECACC cat no: 10033003). RPMI Glutamax, Lipofectamine 2000, penicillin-streptomycin, gentamycin and sodium pyruvate were purchased from Thermo-Scientific, the mRFP-Rab5A was a gift from Ari Helenius (Addgene plasmid # 14437) [23], Myc-Rab5A:S34N was a gift from Qing Zhong (Addgene plasmid # 28044) [24], Gαs-GFP was a kind gift from Marc Rasenick [25] and beta arrestin-1 plasmid, was a gift from Robert Lefkowitz (Addgene plasmid # 14687&42196) [26]. Rabbit monoclonal antibody against Rab5 was purchased from Cell Signaling Technology, MA, USA; mouse monoclonal antibody against Gαs (Gαs/olf (sc-365855)) was purchased from Santacruz Biotech, Texas, USA; anti rabbit secondary antibody coupled to Alexa 594 (Life Technologies) was purchased from Thermo-Fischer Scientific and anti-mouse secondary antibody coupled to FITC was purchased from Sigma. Exendin-4 was obtained from Prof. DiMarchi's laboratory. All other chemicals used in this study were of reagent grade.
2.2. Cell culture
BRIN-BD11 pancreatic beta cells were cultured at 37° Celsius with 5% CO2 in RPMI media supplemented with 10% heat inactivated Fetal Bovine Serum (FBS), 1 mM Sodium pyruvate, 10 μg/ml Gentamycin, 100 units/ml Penicillin, and 100 μg/ml Streptomycin following the protocol published earlier from our laboratory [22].
2.3. Time course of GLP-1R – mediated cyclic AMP generation
A time-course of cyclic AMP generation was carried out in BRIN-BD11 pancreatic beta cells using Exendin-4 (100 nM), Liraglutide (10 nM) and GLP-S-S tetramethyl rhodamine conjugate (1 μM) using direct cyclic AMP ELISA kit (Enzo Life Sciences-ADI-900-163). The cells were seeded in 24 well plates and prior to ligand treatment, they were incubated with Kreb Ringer's Buffer (KRB) containing 0.2% BSA for 1 h. Cells were treated for with ligands for different time points at indicated concentrations in KRB media containing IBMX (200 μM). After treatment, the media was removed and fresh KRB media containing IBMX (200 μM) was added. At respective time points, the cells were lysed with the addition of 0.1N HCl and processed for cyclic AMP estimation through ELISA determination, following kit protocol.
2.4. CRE luciferase reporter assay
GLP-1R mediated signaling was assessed by cyclic AMP responsive element (CRE) Luciferase reporter assay following the method of Fortin et al. [27] with modifications. The cells were grown in 70 mm dish until they attain 70% confluence. A cAMP responsive element-luciferase reporter plasmid encoding the luciferase reporter gene under the control of minimal promoter and six tandem repeats of CRE transcriptional response element (CRE6X-luc) [22] and a beta galactosidase plasmid were transiently transfected in 1:1 ratio using Lipofectamine 2000, following manufacturer's protocol. Four hours post transfection, cells were transferred to 96-well Cell Bind plates (Corning) at a density of 50,000 cells per well. After 24 h, cells were treated with and without appropriate GLP-1R agonist in complete medium for another 4 h. The medium was then aspirated, cells were lysed, and luciferase activity was measured using Steadylite plus reagent (Perkin Elmer Life and Analytical Science, Waltham, MA). Correction for inter-well variability of transfection was carried out through β-galactosidase assay by addition of 2-nitrophenyl-beta galacto pyranoside (Sigma). After incubation for 15 min at 37 °C, substrate cleavage was quantified by measuring optical density at 405 nm in ELISA plate reader (Perkin Elmer, USA), and the corresponding values were used to normalize luciferase activity. The data were expressed as fold luciferase activity increase on agonist treatment over untreated control, which is considered as basal.
2.5. Immunocytochemical staining and confocal microscopy
The GLP-1 receptor-ligand internalization was conducted following the method of Kuna et al. [22]. Briefly, BRIN-BD11 cells were co-transfected with GLP-1R-GFP and beta arrestin-1 RFP plasmid using Lipofectamine 2000 (cat# 11668019, Thermo Fisher) and plated in six-well plates containing 22-mm-diameter glass coverslips. Sixty hours after transfection, cells in coverslips were incubated with Exendin-4 (100 nM) in 200 μL of Krebs-HEPES buffer for 60 min at 4 °C in the dark. Cells were then washed in phosphate buffer saline (PBS) and incubated at 37 °C for the desired time period in complete medium, after which they were fixed in 4% paraformaldehyde, mounted in Vectashield mounting medium (Vector Laboratories), and imaged using a Zeiss LSM 710 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany) equipped with krypton-argon laser sources. Pinhole diameter was maintained at 1 airy unit. Image acquisition was carried out using a 63× oil immersion objective lens with 2× optical zoom using the Zenlite 2011 program. To study the association between activated receptor complex and Gαs, the GLP-1Tmr (1 μM) was internalized following similar protocol and at different time points, cells fixed in 4% paraformaldehyde, permeabilized with 0.1% (w/v) Triton X-100 in PBS, incubated with Gαs antibody overnight and counterstained with goat anti mouse secondary antibody coupled to FITC. To evaluate the distribution of GLP-1R GFP and Gαs, BRIN-BD11 cells were transfected with GLP-1R-GFP using Lipofectamine 2000 and plated in six-well plates containing 22-mm-diameter glass coverslips. Sixty hours after transfection, cells in coverslips were incubated with Exendin-4 (100 nM) in 200 μL of Krebs-HEPES buffer for 60 min at 4 °C in the dark. The cells were then washed in PBS and incubated at 37 °C for the desired time period in complete medium after which they were fixed in 4% paraformaldehyde, permeabilized with 0.1% (w/v) Triton X-100 in PBS, incubated with Gαs antibody overnight, and counterstained with goat anti mouse secondary antibody coupled to Alexa 594. Association of Rab5 with Gαs and to GLP-1R-GFP was carried out following similar methods.
2.6. Image quantification
Image quantification was carried out in ImageJ (JACoP plugin) and co-localization was measured by Pearson Correlation Coefficient (PC) using the formula:
where Ch1 = channel 1 (green), Ch2 = channel 2 (red); values indicating co-localization (0.5–1.0); values indicating absence of co-localization (−1.0 to 0.5).
2.7. Cross-linking and co-immunoprecipitation
BRIN-BD11-GLP-1R GFP stable cell lines were grown to 85% confluency in 100 mm dishes. Cells washed with PBS were treated with or without Exendin-4 for 30 min in KRB media, and the cells were subjected to PBS wash post treatment to remove the excess ligand. Cells were then treated with an amine-reactive cross-linker Dithiobis (succinimydyl) propionate (DSP) (2 mM) for 30 min and the reaction was stopped by the addition of 10 mM Tris–EDTA pH 7.5 following which they were lysed with lysis buffer (25 mM NaCl, 10 mM HEPES, 1% Digitonin and 0.1% NP-40) containing protease and phosphate inhibitor cocktail (Merck cat: 539134 and cat No: 524625). Lysates were precleared on treatment with agarose beads and, after preclearing, the supernatant was incubated with GFP trap A beads (chromotek) overnight at 4° Celsius with nutation. Beads were then collected on centrifugation at 1000 rpm, boiled in 2× sample buffer and the supernatant was used for western blotting.
2.8. Insulin secretion assay
Insulin secretion studies from BRIN-BD11 cells were carried out following the method of Asalla et al. [28] using a Millipore Rat/Mouse Insulin ELISA kit (cat no. EZRMI-13K). In brief, the cells were seeded in 24-multiwell plates at a density of 1 × 105 cells/well and cultured overnight in complete medium. Prior to the insulin release experiment, the cells were pre-treated with 300 μL of Krebs-Ringer bicarbonate (KRB) buffer [115 mM NaCl, 4.7 mM KCl, 1.28 mM CaCl2, 1.2 mM KH2P04, 1.2 mM MgSO4, 10 mM NaHCO3, 0.1% (wt./vol) BSA, pH 7.4] without any glucose supplementation for 60 min. Glucose-stimulated insulin release was measured in the presence of indicated concentrations of glucose and GLP-1 agonists for 30 min. At the end of stimulation, the medium was collected and cleared by centrifugation. The cell lysates were quantified for protein concentration to normalize insulin secretion data. Ten microliters of cell supernatant was used for the ELISA. The insulin release was measured as nanograms per μg protein and expressed as fold over basal insulin secretion.
2.9. Total internal reflection fluorescence microscopy
Total Internal Reflection Fluorescence microscopy (TIRFm) on fixed cells were carried out following the method of Bogan et al. [29] with modifications. Insulin GFP has been cloned as pre-pro insulin into pCDNA3-EGFP plasmid (Addgene: 13031) with GFP at C-terminus. BRIN-BD11 cells in KRB buffer at 37 °C was treated with 100 nM Exendin-4 in presence of 11 mM glucose for 10 min, fixed and imaged with Carl Zeiss TIRF system Axiovert 200 inverted microscope fitted with TIRFm lens (Carl Zeiss objective part no: 1084-514; Plan Fluor 100× oil/1.45 NA), and detected with Axiocam HRm monochrome CCD camera with Axiovision software. The depth of the evanescent field was adjusted manually.
2.10. Synthesis of tetra-methyl rhodamine labeled GLP-1 with disulfide linkage (GLP-1 S-S Tmr)
The GLP-1 peptide was synthesized by standard fluorenylmethoxycarbonyl (Fmoc)-based solid phase peptide synthesis. The following sequence (HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRGC) was assembled on a 0.1 mmol Rink amide 4-methylbenzhydrylamine (MBHA) resin (Midwest Biotech) using an Applied Biosystems 433A automated peptide synthesizer. The synthesis utilized 20% piperidine in N-methyl-2-pyrrolidone (NMP) for N-terminal amine deprotection and diisopropylcarbodiimide/6-Cl-HOBt for amino acid coupling. A methyltrityl (Mtt) protected C-terminal cysteine was incorporated as the initiating GLP-1 residue. The side-chain was selectively removed from the peptidyl-resin by 1% trifluoroacetic acid (TFA), 2% triisopropylsilane (TIS) in dichloromethane (DCM) for 30 min after full length phase synthesis. The peptidyl-cysteine was activated with 5 equivalents of 2, 2′-dithiobis (5-nitropyridine) (DTNP) in dimethylformamide (DMF)/pyridine (1:1, v:v) for 1 h. Subsequently, the peptide resin was treated with 2 equivalents of 2-aminoethanethiol hydrochloride in dimethylformamide (DMF)/pyridine (1:1, v:v) for 1 h. The resin was filtered, washed with DMF several times, and then 1 equivalent NHS-rhodamine (∼50 mg, Thermo-Fisher) in 2–3 ml DMF was added with 0.1 equivalent of diisopropylethylamine (DIEA). The reaction was conducted in the dark for 10 h. The modified peptide resin was filtered, washed with DMF several times, and followed with a DCM wash. Once dry, the resin was treated with a TFA cleavage cocktail containing TFA/anisole/TIS/H2O (85:5:5:5) for 2 h at room temperature to release the disulfide linked GLP-1/rhodamine conjugate from the resin. The liberated and fully de-protected conjugate was precipitated and washed with chilled diethyl-ether. The crude conjugate was dissolved in 15% aqueous acetonitrile containing 15% acetic acid and purified by preparative reversed-phase HPLC, utilizing a linear gradient of acetonitrile in 0.1% TFA on an axia-packed Phenomenex Luna C18 column (250 × 21.20 mm). After analyzing the collected fractions by analytical HPLC and mass spectrometry, the desired fractions were combined and lyophilized to yield 9 mg of final peptide. The HPLC purity of the purified pool was greater than 95% and ESI mass spectral analysis confirmed a molecular mass of 3948.3 (theoretical mass of 3946.1).
2.11. Statistical analysis
The data are expressed as mean ± SEM. For assessing the significance of differences Student's t test (unpaired) has been used.
3. Results
3.1. GLP-1R mediated cyclic AMP generation persists after its association with beta arrestin-1
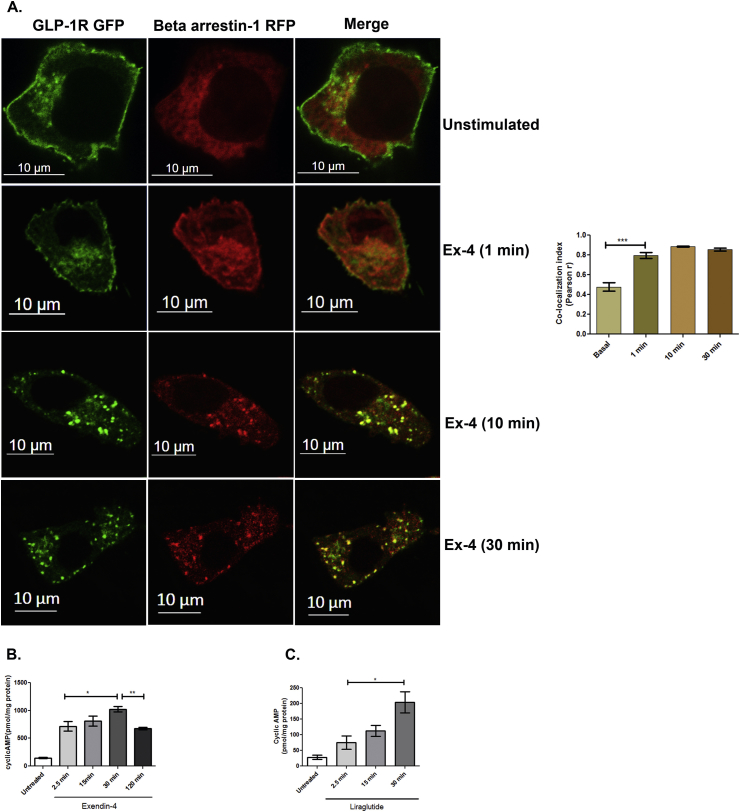
Sustained cyclic AMP generation by internalized activated receptor is most often documented with class B GPCRs. Paradoxically, beta arrestin, which is known to desensitize the receptor [26], [30], establishes a stable interaction with many Class B GPCRs [20]. Association of beta arrestin-1 with GLP-1R had previously been reported and ablation of beta arrestin expression reduces GLP-1R mediated cyclic AMP generation and glucose stimulated insulin secretion in pancreatic beta cells [31]. However, the mechanism of this regulation of GLP-1R function by beta arrestin-1 was incompletely understood. We initiated this study with the assessment of the temporal and spatial association of GLP-1R with beta arrestin-1 in BRIN-BD11 pancreatic beta cells. As Figure 1 shows, in unstimulated cells, GLP-1R GFP is present primarily at the plasma membrane while beta arrestin-1 RFP shows diffused presence at cytoplasm with no significant co-localization (Pearson's correlation coefficient, r, 0.4775 ± 0.04.) At 1 min after stimulation with Exendin-4, beta arrestin-1 RFP was found to associate with GLP-1R GFP at the plasma membrane (Pearson's correlation coefficient, r, 0.7975 ± 0.02). The association of GLP-1R GFP with beta arrestin-RFP appears as punctate dots 10 min after internalization (Pearson's correlation coefficient r, 0.885 ± 0.005), which persisted till 30 min after internalization (Pearson's correlation coefficient, r, 0.856 ± 0.017), signifying a prolonged association of the protein with the receptor following activation with orthosteric ligand.
Figure 1.
Time course analysis of GLP-1R GFP and beta arrestin-1RFP association in context of the cyclic AMP generation on receptor activation: A. BRIN-BD11 pancreatic beta cells were transfected with GFP-tagged GLP-1R and Beta arrestin-1RFP, stimulated with 100 nM Exendin-4 for 1 min, 10 min, and 30 min, after which they are fixed and visualized by confocal microscopy. Images are representive of three independent experiments n = 15 cells/each time point B. Time course of GLP-1R mediated cyclic AMP generation on activation with Exendin-4 (n = 3). C. Time course of GLP-1R mediated cyclic AMP generation on activation with Liraglutide (10 nM), (n = 3). The data is expressed as mean ± SEM (*p < 0.05; **p < 0.01, n = 3).
Beta arrestin-1 is a known mediator of GPCR endocytosis, which, in canonical pathway of GPCR activation, desensitizes the receptor and attenuates the signaling. In the present study, we evaluated whether GLP-1R mediated cyclic AMP generation is compromised upon association of beta arrestin-1 with the receptor. Cultured pancreatic beta cells were treated with 100 nM GLP-1R agonist Exendin-4 or 10 nM Liraglutide, and 5 min post treatment, the excess ligand was washed away with KRB buffer. The cyclic AMP was measured after 2.5, 15, 30, and 120 min after KRB wash. As Figure 1B shows, GLP-1R mediated cyclic AMP generation on 100 nM Exendin-4 treatment was increased from 715.5 ± 88.0 pmol/mg protein (2.5 min treatment) to 1023 ± 47.7 pmol/mg protein (30 min treatment, (p < 0.05), n = 3), which decreased to 679.1 ± 23.2 pmol/mg protein (p < 0.05, n = 3) 2 h after the internalization of the receptor following activation with the ligand. Similarly, a significant increase of cyclic AMP from 75.19 ± 20.92 pmol/mg protein (2.5 min time point) to 204.0 ± 33.21 pmol/mg protein (p < 0.05, n = 3) was observed 30 min after internalization of the receptor following the treatment with liraglutide (10 nM) indicating sustained generation of cyclic AMP generation post internalization of the activated receptor (Figure 1C). The data indicate that the association of beta arrestin-1 with receptor does not impede GLP-1R mediated cyclic AMP generation on receptor activation in BRIN-BD11 pancreatic beta cells.
3.2. Cyclic AMP generation by internalized GLP-1 receptor following activation takes place at endosomes
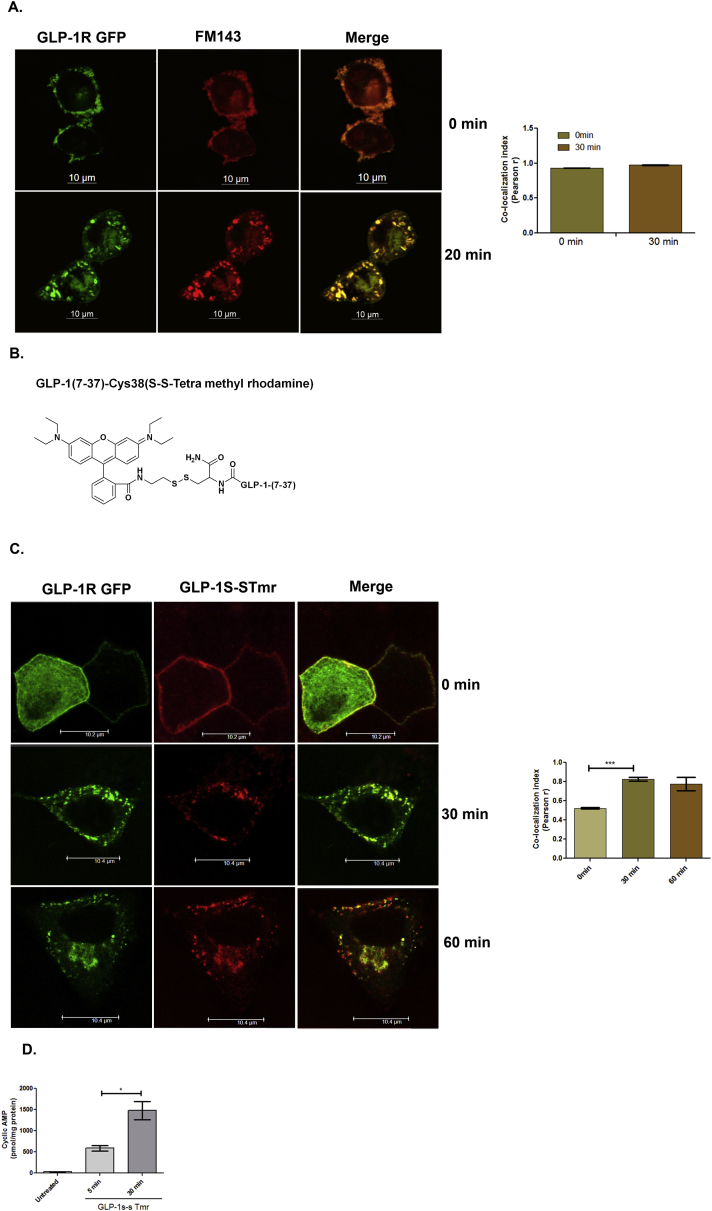
We next assessed the dynamics of internalization using the fluorescent styryl dye FM1-43 [32], [33] to visualize endocytosed vesicle at specific time points. As Figure 2A (upper panel) reveals, the GLP-1R GFP and the fluorescent dye FM1-43 was co-localized at the plasma membrane at the initiation time point (Pearson's r 0.93 ± 0.002). Twenty minutes post activation with the orthosteric agonist Exendin-4, a complete association between FM1-43-stained vesicles and the GLP-1R GFP was observed in cytoplasm as punctate yellow dots (Pearson's r 0.973 ± 0.002, Figure 2A lower panel) indicating that treatment with the orthosteric agonist causes GLP-1R internalization in BRIN-BD11 pancreatic beta cells.
Figure 2.
Internalized GLP-1R generates cyclic AMP from endosomes: A. BRIN-BD11 pancreatic beta cells were transfected with GFP-tagged GLP-1R and, following stimulation with Exendin-4, the endosomes were stained with FM-143; cells were fixed at 0 min and after 20 min and visualized by confocal microscopy. B. Chemical structure of GLP-1-SS-Tmr. C. BRIN-BD11 pancreatic beta cells were transfected with GFP-tagged GLP-1R and stimulated with GLP-1 S-S Tmr (1 μM) for 0 min, 30 min, and 60 min, after which fixed and visualized by confocal microscopy. Images are representative of 3 independent experiments (n = 15 cells for each time point). D. Time course of GLP-1R mediated cyclic AMP generation on activation with GLP-1S-S Tmr (1 μM). The data are expressed as mean ± SEM (*p < 0.05; n = 3).
We also assessed the receptor-ligand internalization with GLP-1S-S-tetra methyl Rhodamine, in which we tagged the fluorophore to the GLP-1 carboxy terminus with a disulphide linker that has no effect on GLP-1 potency (Figure 2B). The rationale for tagging with disulphide linker is to determine the environment of the ligand after internalization to the cell interior. Since cytosol has a reducing environment and endosomes are essentially non-reducing, the yellow co-localized dots would be visible only if the ligand is internalized at endosomes. As the data show at 0 min, coefficient of co-localization as measured by Pearson's r was 0.523 ± 0.007. At 30 min after internalization, rhodamine remained associated with GLP-1R GFP (Pearson's r 0.8263 ± 0.019), and the association persisted until 60 min following internalization (Pearson's r 0.777 ± 0.069) (Figure 2C) which implies that the linker is not degraded and as a consequence not exposed to the reducing environment of the cytoplasm. This result signifies that the activated receptor is internalized at endosomes following its entry to the cell interior with the receptor being oriented in the endocytic vesicle so that the ligand-binding amino terminus faces the lumen of the vesicle.
We then evaluated the time course of cyclic AMP generation on treatment with GLP-1S-S tetra methyl rhodamine (chemical structure shown in Figure 2B). As Figure 2D shows, GLP-1R mediated cyclic AMP generation was increased from 586.9 ± 64.3 pmol/mg protein (measured 5 min after internalization) to 1477 ± 210.9 pmol/mg protein (p < 0.05) which is measured 30 min after internalization, indicating a significant increase of cyclic AMP generation after internalization of the receptor.
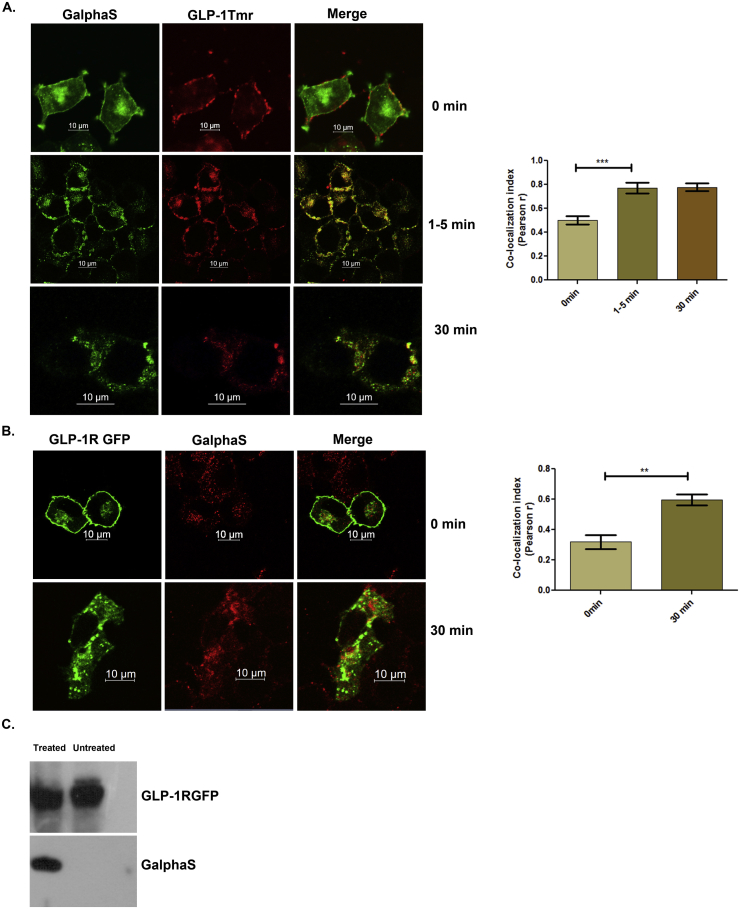
3.3. Internalized GLP-1 receptor following activation associates with Gαs
Association of internalized GLP-1 receptor with Bodipy Forskolin that labels adenylate cyclase has been previously reported [22]. Since Gαs associates with the third intracellular loop of Class B GPCRs [34], we explored the kinetics of GLP-1R association with Gαs in cultured pancreatic beta cells. As Figure 3A reveals, at 0 min, GLP-1Tmr is on the plasma membrane, whereas Gαs is at plasma membrane as well as cytoplasm (Pearson r 0.5030 ± 0.034). At 1–5 min after activation, the majority of GLP-1 tetramethyl rhodamine conjugate (GLP-1Tmr) co-localized with Gαs at the plasma membrane (Pearson's r 0.77 ± 0.043). Thirty minutes after activation, the association persisted as punctate dots in the cytoplasm to signify a prolonged association of G protein subunit with activated GLP-1 receptor (Pearson's r 0.77 ± 0.033). A significant co-localization of GLP-1R GFP with Gαs was also observed 30 min post activation with the GLP-1R agonist Exendin-4 (Pearson's r 0.598 ± 0.036), which was absent in unstimulated cells (Pearson's r 0.319 ± 0.045), (Figure 3B). We evaluated the association through co-immunoprecipitation with GFP trap beads following cross-linking with DSP cross-linkers. As Figure 3C shows, Gαs was found to be coimmunopreciptated with GLP-1R GFP 30 min post treatment with Exendin-4 revealing a prolonged association of the protein with the receptor following activation with ligand.
Figure 3.
Association of Gαs with activated GLP-1 receptor. A. BRIN-BD11 pancreatic beta cells were treated with 1 μM GLP-1Tmr for 0 min, 1–5 min, and 30 min, after which fixed and immunostained with Gαs mouse monoclonal antibody, counterstained with goat anti-mouse IgG coupled with FITC and visualized by confocal microscopy. The figure is representative of three independent experiments (n = no. of cells assessed for each time point = 15). B. BRIN-BD11 pancreatic beta cells were transfected with GFP-tagged GLP-1R and treated with 100 nM Exendin-4 for 0 min and 30 min, after which fixed and immunostained with Gαs mouse monoclonal antibody, counterstained with goat anti-mouse IgG coupled with Alexa 594, and visualized by confocal microscopy The data are representative of three independent experiments (n = no. of cells assessed for each time point = 11). C. Co-immunoprecipitation with GLP-1R GFP in presence or absence of 100 nM Exendin-4. The data are representative of three independent co-immunoprecipitation experiments (n = 3).
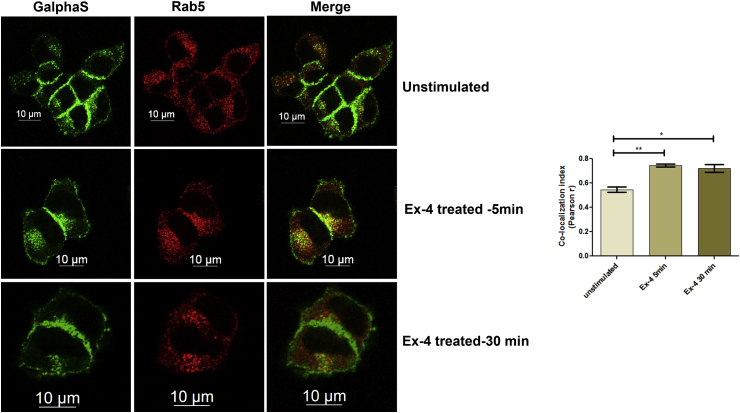
3.4. Gαs co-localizes with Rab5 endosomes on exendin-4 treatment
We next assessed the intracellular localization of Gαs in cultured pancreatic beta cells. Previous published literature has reported the presence of Gαs in Rab5 endosomes [7]. In unstimulated cells, we observed Gαs at plasma membrane and as punctate dot in cytoplasm. Rab5 was present exclusively at cytoplasm and the correlation coefficient between the two proteins as determined by Pearson's r was 0.54 ± 0.022 (Figure 4, upper panel). Five minutes after stimulation with Exendin-4, co-localization of Gαs and Rab5 was observed as yellow punctate dots in cytoplasm (Pearson's R 0.745 ± 0.011) (Figure 4, middle panel), which persisted till 30 min after the treatment with ligand (Pearson's r 0.72 ± 0.03) (Figure 4, lower panel), indicating at the dynamism of the association of Gαs with Rab5 endosomes following Exendin-4 treatment.
Figure 4.
Intracellular distribution of Gαs on Exendin-4 treatment in pancreatic beta cells. BRIN-BD11 pancreatic beta cells were treated with Exendin-4 (100 nM) 0 min, 5 min, and 30 min after which they were fixed and immunostained with Gαs mouse monoclonal antibody and Rab5 rabbit monoclonal antibody, counterstained with goat anti-mouse IgG coupled with FITC and goat anti rabbit IgG coupled with Alexa 594, and visualized by confocal microscopy. The data are representative of three independent experiments (n = no. of cells assessed for each time point n = 21).
3.5. GLP-1 receptor, following orthosteric activation, localizes at Rab5 endosomes
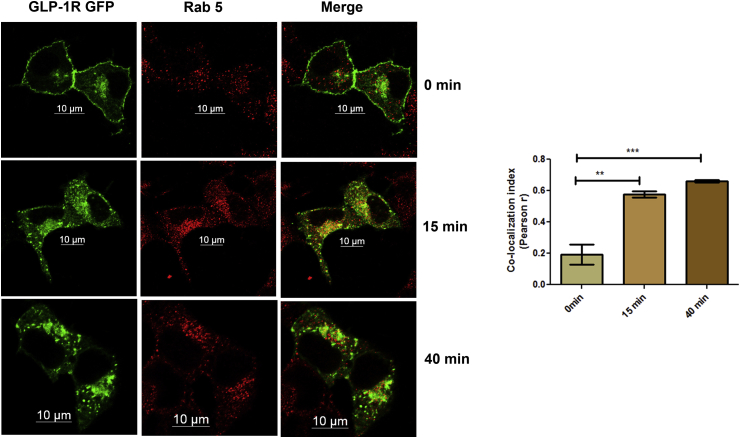
We next conducted a time-course analysis to study the co-localization of GLP-1R GFP with Rab5 endosomes after the treatment with Exendin-4. As Figure 5 shows, at the 1 min time point, GLP-1R GFP was on the plasma membrane while Rab5 was visible as punctate dots in the cytoplasm showing no association between the two proteins (Figure 5 top panel), (Pearson's r 0.19 ± 0.06). At 15 min, a partial co-localization was observed between GLP-1R GFP and endogenous Rab5 protein, which appears as yellow punctate dots, (Figure 5 middle panel) (Pearson's r 0.57 ± 0.01). The association of GLP-1R GFP (green) with Rab5 endosomes (red) as evident by yellow punctate dots at cytoplasm (Pearson's r 0.66 ± 0.009) persisted until 40 min post internalization highlighting the temporal and spatial association of activated GLP-1 receptor with Rab5 endosomes.
Figure 5.
Association of GLP-1 receptor ligand complex with Rab5 endosomes. BRIN-BD11 pancreatic beta cells were transfected with GFP-tagged GLP-1R and, following stimulation with 100 nM Exendin-4 for 0 min, 15 min, and 40 min, the cells were fixed, immunostained with Rab5 rabbit monoclonal antibody, counterstained with goat anti rabbit secondary antibody, and visualized by confocal microscopy. The data are representative of three independent experiments (n = no. of cells assessed for each time point n = 11).
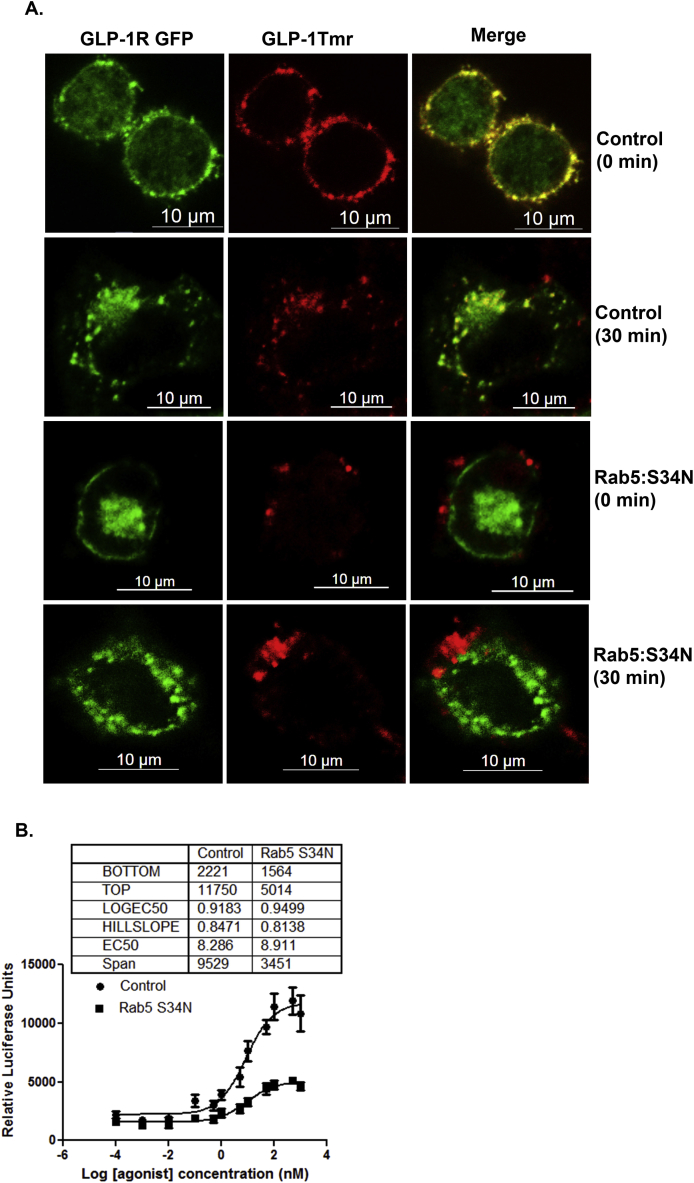
3.6. Expression of Rab5:S34N mutant hinders receptor internalization and attenuates GLP-1R mediated cyclic AMP generation
Rab5 GTPases are known to play an essential role in receptor internalization and vesicle traffic [35]. Expression of dominant-negative mutant Rab5:S34N impairs β2 adrenergic receptor internalization in human embryonic kidney (HEK) cells [36]. In the present study, we explored the impact of the expression of GDP bound Rab5:S34N inactive mutant on GLP-1 receptor-ligand traffic and receptor-mediated cyclic AMP generation. As shown in Figure 6A, in control cells, at 0 min time point, both the receptor (green) and the ligand (red) appear at the plasma membrane, and the merger appears as yellow rim on the plasma membrane. Thirty minutes after internalization, activated receptor appeared as yellow dots at cytoplasm. The data are similar to what we reported on GLP-1R internalization in our previous papers ([22], [28]) In contrast, in Rab5:S34N mutant expressing cells, at 0 min time point, GLP-1R GFP appears as green rim where as GLP-1Tmr appears as red aggregate in patches. We do not see internalization of the GLP-1-Tmr 30 min post treatment indicating impedence in internalization of the activated receptor. Pancreatic beta cells expressing Rab5:S34N mutant also exhibited reduced GLP-1R mediated cyclic AMP generation. As the dose response curve (Figure 6B) shows, the maximum response as measured by GLP-1R mediated CRE luciferase activity was reduced from 11,970 ± 1523 to 502.3 ± 306.2 relative luciferase units (n = 3, p < 0.05) in Rab5:S34N mutant expressing cells in comparison to control cells expressing empty vector plasmid. The data indicate that hindrance in internalization of activated receptor due to the expression of dominant negative Rab5:S34N mutant protein in cultured pancreatic beta cells reduces GLP-1R mediated cyclic AMP generation.
Figure 6.
Expression of Rab5:S34N mutant hinders the internalization of GLP-1 receptor following orthosteric activation and blunts the receptor mediated cyclic AMP generation. A. BRIN-BD11 pancreatic beta cells were transfected with or without Rab5:S34N mutant and 48 h post transfection assessed for GLP-1Tmr internalization at 0 min and 30 min. The data are representative of three independent experiments (n = no. of cells assessed for each time point n = 23). B. BRIN-BD11 pancreatic beta cells were transfected with Rab5:S34N mutant and, 24 h post transfection, assayed for GLP-1R mediated cyclic AMP generation as determined by Cre-Luciferase assay. The data are presented as a 4-parameter logistic curve analyzed in Graph pad prism (version 6.0), and each data point is assessed in duplicates Dose response curve represents mean ± SEM of 3 independent experiments.
3.7. Rab5 overexpression, hinders internalization, GLP-1R mediated cyclic AMP generation and GSIS
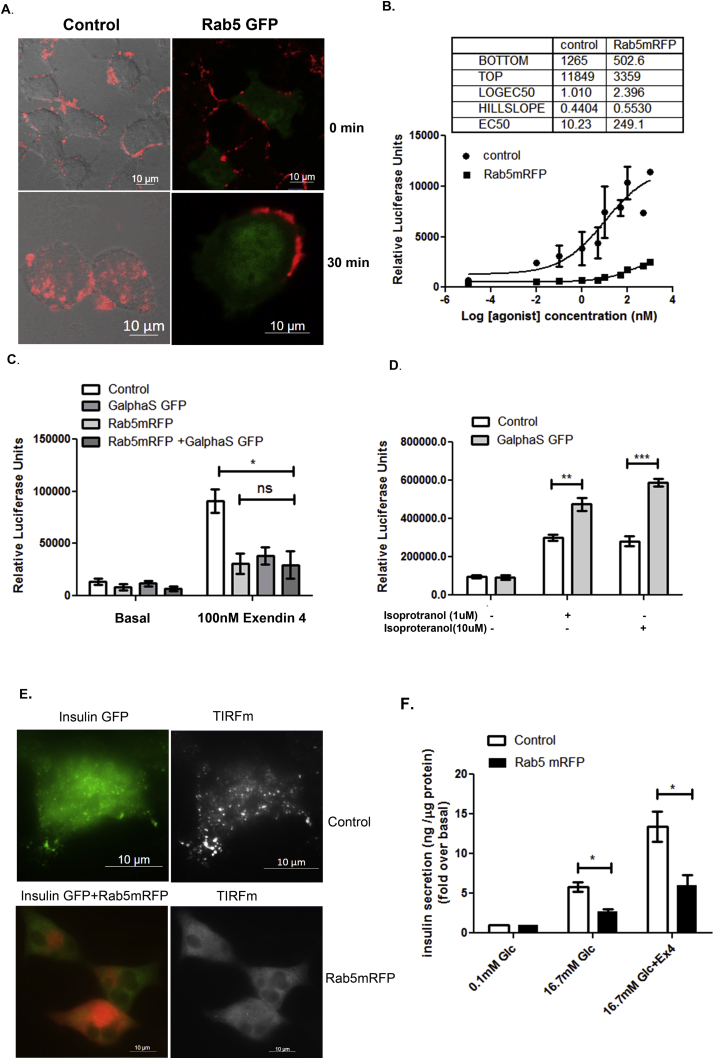
We next explored whether enhanced Rab5 expression had any effect on GLP-1R trafficking and function in cultured pancreatic beta cells. Surprisingly, we observed that overexpression of Rab5 alone causes accumulation of GLP-1Tmr at the cell periphery (Figure 7A), which resulted in a dose dependent decrease of GLP-1R mediated cyclic AMP generation (Figure 7B). As shown in Figure 7C, Rab5 mRFP expressing cells and control cells transfected with empty vector show comparative cyclic AMP in unstimulated condition as measured by CRE-luciferase assay. However, GLP-1R mediated cyclic AMP generation, as measured on treatment with 100 nM Exendin-4, was found to be decreased from 90,820 ± 11,110 relative luciferase units (RLU) to 30,940 ± 9780 RLU (p < 0.05, n = 3) in Rab5mRFP expressing cells in comparison to control cells expressing empty vector plasmid. Rasenick et al. reported that expression of Gαs GFP enhanced cyclic AMP generation in cyc-S49 lymphoma cells [25]. In alignment with the published data, Gαs GFP was found to enhance isoproterenol mediated cyclic AMP generation in human embryonic kidney (HEK) cells as shown by Cre luciferase assay (Figure 7D). In contrast, expression of Gαs GFP causes reduction of GLP-1R mediated cyclic AMP generation from 90,820 ± 11,110 RLU to 38,350 ± 7983 (p < 0.05, n = 3) in BRIN-BD11 pancreatic beta cells, (Figure 7C). In addition, expression of Gαs GFP in Rab5mRFP expressing cells does not improve the GLP-1R response signifying that a proper stoichiometry of interacting proteins is necessary to sustain GLP-1R mediated endosomal cyclic AMP generation (Figure 7C). We next compared the glucose stimulated insulin secretion (GSIS) in Rab5mRFP expressing BRIN-BD11 cells and control BRIN-BD11 cells. Total internal reflection fluorescence microscopy (TIRFm) reveals a compromised docking and fusion of GFP labeled insulin vesicle in Rab5mRFP expressing BRIN-BD11 pancreatic beta cells (Figure 7E). We assessed GSIS using insulin ELISA as well (Figure 7F). As the data shows, in control cells, GSIS at 16.7 mM glucose is enhanced by 5.817 ± 0.617 (n = 3) fold over basal insulin secretion at 0.1 mM concentration glucose, which is further potentiated to 13.43 ± 1.92 fold (n = 3, p < 0.05) when treated with 100 nM Exendin-4. In Rab5mRFP expressing BRIN-BD11 cells there is a significant decrease of GSIS to 2.754 ± 0.255 fold (n = 3) as compared to cells expressing empty vector. Exendin-4 treatment could potentiate GSIS in Rab5mRFP expressing to 6.054 ± 1.286 (n = 3), which was found to be significantly reduced as compared to pancreatic beta cells expressing empty vector plasmid only (Figure 7F).
Figure 7.
Rab5 overexpression causes aggregation of ligand at cell periphery and blunts GLP-1R mediated cyclic AMP generation and GSIS. A. BRIN-BD11 pancreatic beta cells were transfected with Rab5 and 48 h post transfection assessed for localization of GLP-1Tmr (1 μM) at 0 min and 30 min post treatment with ligand. The data are representative of three independent experiments (n = no. of cells assessed for each time point n = 29). In control panel, immunofluorescence image is merged with differential interference contrast (DIC) image of the same cell. B. BRIN-BD11 pancreatic beta cells were transfected with Rab5-mRFP plasmid and, 24 h post transfection, assayed for GLP-1R mediated cyclic AMP generation as determined by Cre-Luciferase assay. The data are presented as a 4-parameter logistic curve analyzed in Graph pad prism (version 6.0) and each data point is assessed in duplicates. The dose response curve represents mean ± SEM of 3 independent experiments. C. Effect of Gαs GFP on GLP-1R mediated cyclic AMP generation in mRFP-Rab5 expressing BRIN-BD11 pancreatic beta cells. Gαs GFP and mRFP-Rab5 separately as well as in combination was transfected to Brin-BD11 pancreatic beta cells and 24 h post transfection GLP1R mediated cyclic AMP generation was determined by Cre-Luciferase assay. Data are shown as mean ± SEM (*p < 0.05; **p < 0.01, n = 3). D. Effect of Gαs GFP on isoproterenol induced cyclic AMP generation in Human Embryonic Kidney 293 (HEK293) cells. HEK293 cells were transfected with Gαs GFP and 24 h post transfection isoproterenol induced cyclic AMP generation was measured by Cre-Luciferase assay. Data are shown as mean ± SEM (*p < 0.05, n = 3). E. Total internal reflection fluorescence microscopy to assess docking and fusion of insulin vesicle at plasma membrane in control and Rab5mRFP expressed cells on treatment with Exendin-4 for 10 min in presence of 16.7 mM glucose. Insulin is tagged with GFP while rab5 is tagged with mRFP. Images are representative of 3 independent experiments (n = 9 cells for each experiment). F. GLP-1R mediated potentiation of GSIS in control versus Rab5-mRFP transfected BRIN-BD11 pancreatic beta cells. Control and Rab5-mRFP transfected BRIN-BD11 cells were treated with GLP-1R agonist Exendin-4 (Ex-4) in presence of 0.1 mM and 16.7 mM glucose, and insulin secretion was measured as ng insulin/μg protein for a period of 30 min and expressed as fold over basal secretion. Insulin secretion from untreated cells in presence of 0.1 mM glucose is considered as basal. Data are expressed as mean ± SEM of 3 independent experiments (*p < 0.05).
4. Discussion
GLP-1R previously has been reported to interact with beta arrestin-1 [31], which causes desensitization of the receptor [26]. Recent data have reported sustained second messenger signaling as a special attribute of Class B GPCRs. More importantly, the report of simultaneous association of beta arrestin and Gαs with Class B GPCR forming megaplexes involving beta arrestin as well as Gαs has provided a new concept of non-canonical pathway in Class B GPCR internalization and sustained cyclic AMP generation [20].
Silencing of beta arrestin-1 and consequent reduction of GLP-1R mediated cyclic AMP generation and glucose stimulated insulin secretion has previously been reported by Sonoda et al. [31]. This observation was prior to the development of the concept of sustained GPCR signaling from endosomes and as such the significance of this observation remained underappreciated. In this study, we describe the sustained association of GLP-1R with beta arrestin-1. We also carried out the analysis of cyclic AMP generation at the time point when beta arrestin-1 was associated with the activated receptor. As the data reveal, at 2.5, 10, and 30 min post internalization, when the receptor remained associated with beta arrestin-1, there is a continuous enhancement of GLP-1R mediated cyclic AMP generation. This indicates that the association of the receptor with beta arrestin-1 following activation does not hinder the GLP-1R mediated cyclic AMP generation. We obtained similar results with liraglutide and Exendin-4 which imply sustained receptor mediated cyclic AMP generation as a characteristic feature of orthosteric agonism of GLP-1 receptor.
To study the mechanism in more detail, we employed a novel peptide based approach in which GLP-1 is tagged with tetramethyl rhodamine through a disulphide linker (GLP-1S-S Tmr) to explore the niche of cyclic AMP generation at the cell interior. Since cytoplasm has a reducing environment and endosomes are non-reducing, we reasoned that the stability of the conjugate would determine its location post internalization of the receptor. As our data show, GLP-1-S-S Tmr appeared as punctate dots after entering the cell interior, reflecting its non-reducing milieu, which implies that the ligand is internalized at a non-reducing endosomal environment following activation of the receptor. The data are further corroborated by co-localization of the activated receptor with FM1-43, the fluorescent styryl dye that stains endosomes [37]. Association of GLP-1 receptor to Rab5 endosomes also supports its endosomal localization following activation.
We next assessed the cyclic AMP generation by GLP-1S-S Tmr at time points when it was observed at endosomes. As the data show, there is a gradual, significant increase of GLP-1R mediated cyclic AMP generation from 5 to 30 min indicating that receptor upon activation continues cyclic AMP generation when internalized at endosomes. The washing of the ligand following activation rules out the possibility of engagement of unbound ligand with newly synthesized receptor that appeared at the plasma membrane. Prolonged association of Gαs with internalized receptor following activation implies its role beyond signaling at the plasma membrane. To decipher the role of Gαs in regulating GLP-1R trafficking and second messenger signaling, we determined its intracellular location. As our data show, Gαs was located at the plasma membrane as well as at cytoplasm in unstimulated cells having no association with the Rab5 endosomal compartment. On treatment with Exendin-4 for 5 and 30 min we observed a partial overlap between Gαs and Rab5 endosomes, indicating at the dynamic nature of the interaction following treatment with the GLP-1R agonist. GLP-1R-Gαs association was assessed through fluorescence microscopy using antibody against endogenous Gαs and GLP-1 conjugated with fluorescent marker rhodamine. The data show sustained association of Gαs with activated GLP-1R. Similar prolonged association was observed between Gαs and GLP-1R GFP on treatment of BRIN-BD11 pancreatic beta cells with Exendin-4. We conducted co-immunoprecipitation studies after cross-linking the receptor and interacting proteins with DSP, with or without Exendin-4 treatment. As our data show, Gαs was found to be associated with ligand 30 min post internalization indicating the prolonged association of the G protein subunit with the activated receptor. Localization of internalized GLP-1R in Rab5 endosomal compartment was found to be temporally and spatially regulated. We explored the consequence of this association by expression of GDP bound inactive Rab5:S34N mutant which has previously been reported to hinder internalization of β2 adrenergic receptor [36]. As our data show, the expression of Rab5:S34N mutant inhibited the internalization of GLP-1 receptor–ligand complex resulting in a significant decrease in GLP-1R mediated cyclic AMP generation. We also evaluated the consequence of Rab5 overexpression on GLP-1R mediated cyclic AMP generation. As observed under fluorescent microscopy, Rab5 overexpression causes aggregation of ligand at the cell periphery, which is associated with a significant decrease in GLP-1R mediated cyclic AMP generation. Co-expression of Rab5 and Gαs-GFP could not restore GLP-1R mediated cyclic AMP generation, which implies that receptor internalization, trafficking at endosomes, and a proper stoichiometry of interacting partners play important roles in mediating GLP-1R stimulated endosomal cyclic AMP generation in pancreatic beta cells.
Alteration of Rab5 expression has a profound influence on GSIS as well. As our data show, overexpression of Rab5 alters both GSIS and GLP-1R mediated potentiation of insulin secretion indicating the pleiotrophic effect of Rab5 in regulating GSIS in cultured pancreatic beta cells.
Collectively, our results suggest that cyclic AMP generation by internalized GLP-1R takes place at the Rab5 endosomal compartment, where prolonged association of Gαs with the internalized receptor following orthosteric activation contributes to the sustained endosomal cyclic AMP generation that likely supports GSIS in pancreatic beta cells.
Author contributions
SBG and RSK performed the experiments with the help of SB; NRC contributed to TIRFm experiments. SBG and RSK contributed equally to the manuscript. RD designed the GLP-1 conjugate peptide, guided the synthesis, helped developing the concept, and edited the manuscript. PM designed the project, supervised the whole study, and wrote the manuscript.
Acknowledgements
Shravan Babu Girada is supported by Senior Research Fellowship from Indian Council of Medical Research (3/1/3/ /JRF-2011/HRD-146(61060)); Ramya Sri Kuna is supported by Senior Research Fellowship sponsored by Council of Scientific and Industrial Research (SRF (09/985/029/2014-EMR-1)). The work has been supported by DST (SR/S1/OC-30/2010) (funding agencies in India) and institutional research grant provided to Prasenjit Mitra. Corresponding Author takes the responsibility of all the data submitted.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Gilman A.G. G proteins: transducers of receptor-generated signals. Annual Review of Biochemistry. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nature Reviews Molecular Cell Biology. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 3.Lamb T.D. Gain and kinetics of activation in the G-protein cascade of phototransduction. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(2):566–570. doi: 10.1073/pnas.93.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liggett S.B. Phosphorylation barcoding as a mechanism of directing GPCR signaling. Science Signaling. 2011;4(185):pe36. doi: 10.1126/scisignal.2002331. [DOI] [PubMed] [Google Scholar]
- 5.Lohse M.J., Hoffmann C. Arrestin interactions with G protein-coupled receptors. Handbook of Experimental Pharmacology. 2014;219:15–56. doi: 10.1007/978-3-642-41199-1_2. [DOI] [PubMed] [Google Scholar]
- 6.Irannejad R., Tsvetanova N.G., Lobingier B.T., von Zastrow M. Effects of endocytosis on receptor-mediated signaling. Current Opinion in Cell Biology. 2015;35:137–143. doi: 10.1016/j.ceb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calebiro D., Nikolaev V.O., Gagliani M.C., de Filippis T., Dees C., Tacchetti C. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biology. 2009;7(8):e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nature Chemical Biology. 2009;5(6):428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 9.Vilardaga J.P., Romero G., Friedman P.A., Gardella T.J. Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm. Cellular and Molecular Life Sciences. 2011;68(1):1–13. doi: 10.1007/s00018-010-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werthmann R.C., Volpe S., Lohse M.J., Calebiro D. Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells. The FASEB Journal. 2012;26(5):2043–2048. doi: 10.1096/fj.11-195248. [DOI] [PubMed] [Google Scholar]
- 11.Irannejad R., Tomshine J.C., Tomshine J.R., Chevalier M., Mahoney J.P., Steyaert J. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495(7442):534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gidon A., Al-Bataineh M.M., Jean-Alphonse F.G., Stevenson H.P., Watanabe T., Louet C. Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nature Chemical Biology. 2014;10(9):707–709. doi: 10.1038/nchembio.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilardaga J.P., Jean-Alphonse F.G., Gardella T.J. Endosomal generation of cAMP in GPCR signaling. Nature Chemical Biology. 2014;10(9):700–706. doi: 10.1038/nchembio.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsvetanova N.G., von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nature Chemical Biology. 2014;10(12):1061–1065. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Dyke R.W. Heterotrimeric G protein subunits are located on rat liver endosomes. BMC Physiology. 2004;4:1. doi: 10.1186/1472-6793-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinstein T.N., Yui N., Webber M.J., Wehbi V.L., Stevenson H.P., King J.D., Jr. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. Journal of Biological Chemistry. 2013;288(39):27849–27860. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsvetanova N.G., Irannejad R., von Zastrow M. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. Journal of Biological Chemistry. 2015;290(11):6689–6696. doi: 10.1074/jbc.R114.617951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng B., Lavoie C., Tang T.D., Ma P., Meerloo T., Beas A. Regulation of epidermal growth factor receptor degradation by heterotrimeric Galphas protein. Molecular Biology of the Cell. 2004;15(12):5538–5550. doi: 10.1091/mbc.E04-06-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrandon S., Feinstein T.N., Castro M., Wang B., Bouley R., Potts J.T. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nature Chemical Biology. 2009;5(10):734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomsen A.R., Plouffe B., Cahill T.J., Shukla A.K., Tarrasch J.T., Dosey A.M., Kahsai A.W. GPCR-G protein-beta-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166(4):907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosciglione S., Thériault C., Boily M.O., Paquette M., Lavoie C. Galphas regulates the post-endocytic sorting of G protein-coupled receptors. Nature Communications. 2014;5:4556. doi: 10.1038/ncomms5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuna R.S., Girada S.B., Asalla S., Vallentyne J., Maddika S., Patterson J.T. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic beta-cells. American Journal of Physiology - Endocrinology And Metabolism. 2013;305(2):E161–E170. doi: 10.1152/ajpendo.00551.2012. [DOI] [PubMed] [Google Scholar]
- 23.Vonderheit A., Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biology. 2005;3(7):e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q., Westphal W., Wong K.N., Tan I., Zhong Q. Rubicon controls endosome maturation as a Rab7 effector. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(45):19338–19343. doi: 10.1073/pnas.1010554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J.Z., Rasenick M.M. Real-time visualization of a fluorescent G(alpha)(s): dissociation of the activated G protein from plasma membrane. Molecular Pharmacology. 2002;61(2):352–359. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
- 26.Luttrell L.M., Lefkowitz R.J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. Journal of Cell Science. 2002;115(Pt 3):455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 27.Fortin J.P., Schroeder J.C., Zhu Y., Beinborn M., Kopin A.S. Pharmacological characterization of human incretin receptor missense variants. Journal of Pharmacology and Experimental Therapeutics. 2010;332(1):274–280. doi: 10.1124/jpet.109.160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asalla S., Girada S.B., Kuna R.S., Chowdhury D., Kandagatla B., Oruganti S. Restoring mitochondrial function: a small molecule-mediated approach to enhance glucose stimulated insulin secretion in cholesterol accumulated pancreatic beta cells. Scientific Reports. 2016;6:27513. doi: 10.1038/srep27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogan Jonathan S., Xu Yingke, Hao Mingming. Cholesterol accumulation increases insulin granule size andimpairs membrane trafficking. Traffic. 2012;13(11):1466–1480. doi: 10.1111/j.1600-0854.2012.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian X., Kang D.S., Benovic J.L. Beta-arrestins and G protein-coupled receptor trafficking. Handbook of Experimental Pharmacology. 2014;219:173–186. doi: 10.1007/978-3-642-41199-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonoda N., Imamura T., Yoshizaki T., Babendure J.L., Lu J.C., Olefsky J.M. Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(18):6614–6619. doi: 10.1073/pnas.0710402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel J.A., Malladi C.S., Kettle E., McCluskey A., Robinson P.J. Analysis of synaptic vesicle endocytosis in synaptosomes by high-content screening. Nature Protocols. 2012;7(8):1439–1455. doi: 10.1038/nprot.2012.070. [DOI] [PubMed] [Google Scholar]
- 33.Williams D.R., Kim G.H., Lee M.R., Shin I. Fluorescent high-throughput screening of chemical inducers of neuronal differentiation in skeletal muscle cells. Nature Protocols. 2008;3(5):835–839. doi: 10.1038/nprot.2008.47. [DOI] [PubMed] [Google Scholar]
- 34.Culhane K.J., Liu Y., Cai Y., Yan E.C. Transmembrane signal transduction by peptide hormones via family B G protein-coupled receptors. Frontiers in Pharmacology. 2015;6:264. doi: 10.3389/fphar.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Reviews Molecular Cell Biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 36.Seachrist J.L., Anborgh P.H., Ferguson S.S. Beta 2 adrenergic receptor internalization, endosomal sorting and plasma membrane recycling are regulated by rab GTPases. Journal of Biological Chemistry. 2000;275(35):27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- 37.Amaral E., Guatimosim S., Guatimosim C. Using the fluorescent styryl dye FM1-43 to visualize synaptic vesicles exocytosis and endocytosis in motor nerve terminals. Methods in Molecular Biology. 2011;689:137–148. doi: 10.1007/978-1-60761-950-5_8. [DOI] [PubMed] [Google Scholar]