Competing interest statement
Conflict of interest: the authors declare no potential conflict of interest.
Abstract
The bidirectional communication between the central nervous system and gut microbiota, referred to as the gut-brain-axis, has been of significant interest in recent years. Increasing evidence has associated gut microbiota to both gastrointestinal and extragastrointestinal diseases. Dysbiosis and inflammation of the gut have been linked to causing several mental illnesses including anxiety and depression, which are prevalent in society today. Probiotics have the ability to restore normal microbial balance, and therefore have a potential role in the treatment and prevention of anxiety and depression. This review aims to discuss the development of the gut microbiota, the linkage of dysbiosis to anxiety and depression, and possible applications of probiotics to reduce symptoms.
Introduction
Healthy gut function has been linked to normal central nervous system (CNS) function.1-4 Hormones, neurotransmitters and immunological factors released from the gut are known to send signals to the brain either directly or via autonomic neurons. The existence of the gut-brain axis was proposed in the landmark study by Sudo and colleagues that discovered the impaired stress response in germ-free mice. Other studies using germ-free mice not only supported this existence, but also the idea that the gut-brain-axis (GBA) extends even beyond these two systems into the endocrine, neural, and immune pathways.2,5
Recently, studies have emerged focusing on variations in the microbiome and the effect on various CNS disorders, including, but not limited to anxiety, depressive disorders, schizophrenia, and autism.2,8,9 This review focuses on the GBA in the context of anxiety and depressive disorders. Therapeutic interventions to treat dysbiosis, or disturbance in the gut, and mitigate its effects on the GBA are only recently coming to the forefront as more is known about this unique relationship. As a result, research has been done on the use of probiotics in treatment of anxiety and depression both as standalone therapy and as adjunct to commonly prescribed medications. These findings as well as their potential impact on treatment are discussed in this paper.4,9 An overview of the role of the gut microbiome, from its development, to its relationship with the emotional and cognitive centers of the brain, while also providing ideas for future research, are included in this review.
The microbiome is defined as all microorganisms in the human body and their respective genetic material. The microbiota is defined as all microorganisms in a particular location, such as the GI tract or skin.10,11 This distinction is relevant as this review will focus on the microbiota of the gut in the context of the gut-brain axis, though there will be discussion of the human microbiome where appropriate.
Materials and Methods
This literature review is based on English-language articles sourced from PubMed. Keywords searched included: microbiome development, neonatal microbiome, negative aspects of probiotic use, anxiety and depressive disorders, gut brain axis, anxiety, depression, hypothalamic-pituitary axis (HPA), stress and the microbiome, microbiome composition, intestinal bowel disease, cytokines, TNF-a, interleukins, leaky gut, anxiety, depression, and prostaglandins. Antibiotics were not included in the search as the authors felt it was beyond the scope of the discussion regarding the existing microbiome, stress responses, and their relationship with depression and anxiety disorders. No geographical limitations were included in the search. Publications were initially excluded if they were published before 2010. However, in order to include an in depth understanding of the research, articles published before 2010 were included if they were cited in research published after 2010. This review contains articles published through July of 2017.
Results and Discussion
Development of the microbiome
The microbiome is initially developed via vertical transmission through the placenta, amniotic fluid, and meconium.12-14 Two animal study suggested that fetuses exposed to prenatal stress in the form of maternal stress develop a gut microbiota with decreased Bifidobacterium.14,15 Two separate studies concluded that the mode of delivery affects the initial microbiome and gut microbiota. Infants delivered vaginally had higher amounts of bacteria in their gut compared to infants delivered by Cesarean section.12,13 Beginning with the first week of life, gastric colonization is highly dynamic. This critical period during birth and GI development is essential for newborn health and immunity.16 Microbiota underdevelopment during this period has been correlated with numerous stress states including lateonset sepsis,17 cardiovascular disease, and atopic disease.18
Early nutrition also appears to play a role in shaping the developing gut microbiota. Studies found that breastfeeding was directly correlated with both IgA levels and the number of organisms in the Bifidobacterium genus present in the gut and indirectly correlated with IL-6 levels.13,15 IgA is predominantly a secretory immunoglobulin that provides immunity within the intestines and other mucosal membranes. In contrast, IL-6 is a proinflammatory cytokine that normally presents in acute and chronic inflammation. Bifidobacterium is an important part of the infant microbiome,19 and together with species in the Lactobacillus genus, is key in producing gamma-Aminobutyric acid (GABA), an inhibitory regulator of various neural pathways.20 Breastmilk’s ability to increase IgA and Bifidobacterium species and to decrease IL-6 levels, and subsequently inflammation, reduces the risk of age-related gastroenteritis.13
In comparison, infants fed formula during their first four weeks of life demonstrated a decrease in total number of bacterial species.15 Breast milk oligosaccharides includes lactose as well as over 1000 distinct non-digestible molecules.21 Researchers suggest the non-digestible sugars of breast milk provide a prime nutritional source for bacterial fermentation.12 Breast milk had similar effects in preterm infants who were shown to have a different bacterial makeup, with a predominance of proteobacteria rather than Bifidobacterium and Lactobacillus. Preterm infants fed breast milk showed an increase only in the number of Bifidobacterium, supporting the concept that breast milk’s non-digestible sugars create an environment better suited for of specific species.20
Cessation of breastfeeding is the primary diet change that leads to an adult-like microbiome.22 Children who were weaned from breast milk up to age four showed similar patterns of microbiota development as children weaned at an earlier age, indicating that the length of time to transition from breast milk to solid foods was not as important as the transition itself.22
The key relationship between the gut microbiota and diet continues throughout life. Diet alterations can have significant impact on the gut bacterial composition in as little as 24 hours.20 However, the bacterial composition is restored if the change in diet is only temporary. Regardless of the species inhabiting the gut, as long as their symbiotic role is the same, the human host will be able to function as normal.20 Symbiotic bacteria assist with immune tolerance, intestinal homeostasis, amino acid and vitamin synthesis of the host, leading to a healthy metabolism.13
The adult microbiome
As infants consume increasing amounts of solid food, the microbiome is exposed to diverse energy substrates, developing its carbon metabolism.22,23 The adult microbiome becomes dominated by the Bacteroidetes and Firmicutes phyla, rather than the Lactobacillus and Bifidobacterium genera.24 Relatively smaller quantities of the Proteobacteria, Verrucomicrobia, Actinobacteria, and Cyanobactera phyla, and Fusobacteria genus can also be found.13 However, due to many factors including diet, environment, season, health status, it is almost impossible to define a “normal” microbiome for the average human population. It is important to note that although microbiomes differ between every individual due to genetic diversity, researchers have found that every microbiome falls into one of three enterotypes. These enterotypes differ by which species dominates one’s bacterial composition, and include Bacteroides, Prevotella, or Ruminococcus species. The dominant species and therefore enterotype results from the composition of a person’s diet. Prevotella species enterotype is associated with diets high in carbohydrates versus people eating high amounts of protein are more likely to possess a Bacteriodes species enterotype.25 Interestingly, these enterotypes are independent of environmental components such as age, body-mass index, gender and geographic location and seem to only be dependent on diet and genetics.26
A Danish study of the gut microbiome created the concept of high gene count (HGC) and low gene count (LGC), both of which are implicated in digestive health.27 Due to a functionally more prosperous microbiome, the HGC group had a decreased risk of both metabolic disease and obesity. Important microbiome functions of the HGC group included an increased proportion of butyrate producing organisms, increased propensity for hydrogen production, and reduced production of hydrogen sulfide. It has also been shown that short chain fatty acids offer relevant benefits in terms of regulatory T cell induction as well as blood-brain barrier integrity.28,29 In contrast, the LGC group had a larger proportion of pro-inflammatory bacteria which predisposed them to IBD and related disorders.30,31 The Human Microbiome Project confirms this notion with studies of stool specimens demonstrating that humans with a less diverse microbiome were more likely to be diagnosed with IBD.25
When the human microbiome is challenged with changes in diet, stress, or antibiotics, the physiology of the normal microbiome undergoes change. A dysbiotic state leads to increased intestinal permeability and allows contents such as bacterial metabolites and molecules as well as bacteria themselves to leak through the submucosa and into the systemic circulation, a phenomenon aptly named leaky gut syndrome. A study by Zoppi et al. demonstrated that the gut microbiota uses the intestinal endocannabinoid system to control the degree of intestinal permeability.32 A separate study was able to reduce translocation of bacterial antigens such as LPS by using antagonists to the intestinal cannabinoid type 1 receptor in mice. Specifically, the CB1R antagonists cannabidiol and tetrahydrocannabidiol were protective against intestinal permeability, suggesting that cannabinoids could play an important role in treating inflammatory gastrointestinal diseases such as IBD.33 Increased intestinal permeability leads to detrimental effects on the host immune system, which have been demonstrated in diseases such as inflammatory bowel disease (IBD), diabetes, asthma, and psychiatric disorders including depression, anxiety, and autism.2,4,10,34,35
Although most of these studies have focused on bacterial species in the gut microbiome, other studies have elucidated the importance of other microorganisms, such as yeast. A study done by Burrus and colleagues suggested that colonization with Candida species may contribute to Autism spectrum disorders.36 By preventing absorption of carbohydrates and minerals and allowing excessive build-up of toxins, colonization with Candida albicans was shown to increase autistic behaviors in children with autistic spectrum disorder. A similar study suggested that it is the interaction between propionic acid and ammonia released by Candida albicans that results in increased autistic behaviors.37 This interaction produces an excessive amount of betaalanine, which is similar in structure to GABA and has been proposed to be an important contributor to autism spectrum disorders.
The inflammatory response
Inflammation of the GI tract places stress on the microbiome through the release of cytokines and neurotransmitters. Coupled with the increase in intestinal permeability, these molecules then travel systemically. Elevated blood levels of cytokines TNF-a and MCP (monocyte chemoattractant protein) increase the permeability of the blood-brain barrier, enhancing the effects of rogue molecules from the permeable gut.38,39 Their release influences brain function, leading to anxiety, depression, and memory loss.39-41
Depressive disorders are characterized by both neuroplastic, organizational changes, and neurochemical dysfunction.42 Illness is thought to begin when there is deregulation of these systems and can largely be attributed to cytokine release secondary to an exaggerated systemic response to stressors.39,41 Endotoxin infusions to healthy subjects with no history of depressive disorders triggered cytokine release and subsequent emergence of classical depressive symptoms. The study established a direct correlation between increased levels of IL-6 and TNF-a with symptoms of depression and anxiety,43 indicating that pro-inflammatory cytokines play a role in the development of anxiety and depression. These effects correlated with a state of chronic inflammation and altered immune cells in the peripheral blood. However, TNF-a administered to healthy subjects resulted in no depressive symptoms,38 suggesting that toxin induced inflammation caused the mood disturbance.
Pro-inflammatory cytokines are also important stimulators of the hypothalamic-pituitary-adrenal (HPA) axis (Figure 1). The hypothalamus releases corticotropin releasing factor from the hypothalamus, stimulating the adenohypophysis to release adreno-corticotropic hormone (ACTH). In turn, ACTH stimulates the adrenal release of cortisol, a known stress hormone that acts as a negative feedback signal in the pro-inflammatory signal transduction machinery.3,41
Figure 1.
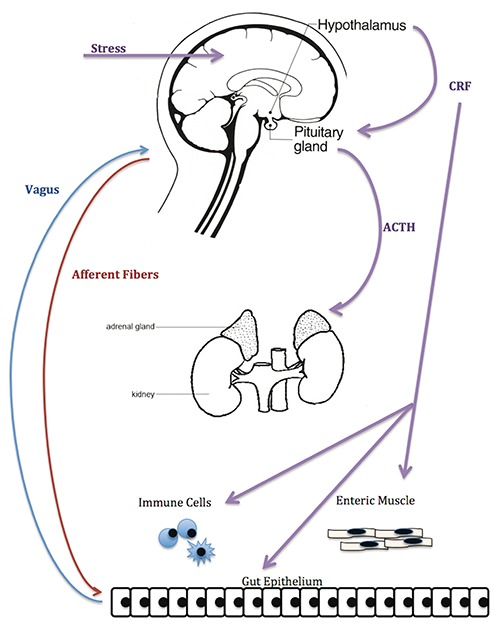
The gut-brain axis pathway. Image created by Megan Clapp and Emily Wilen.
Hyperactivity or dysregulation of the HPA axis is one of the most reliable biological readouts in major depression and anxiety.39 Rats with activated stress circuits demonstrated anxiety and depressive-like behaviors. Removal of the stimulus normalized HPA hyper-reactivity, as measured by their endogenous corticosterone levels, and in turn reversed or mitigated their abnormal behaviors.10
The interconnection of the endocrine, neural, and immune pathways is demonstrated in the relationship between brain derived neurotrophic factor (BDNF) mRNA in the dentate gyrus of the hippocampus and the stress response in germ free mice. BDNF supports the development of neurons and synapses involved in regulation of emotions and cognition; male germ free mice have an increased stress response associated with decreased hippocampus BDNF, which could be reversed by recolonization with Bifidobacteria species. Futhermore, the Bifidobacteria was shown to alter mRNA expression of GABA receptors and decrease serum cortisol. This change was not seen after the mice underwent vagatomies, suggesting that the parasympathetic nervous system was imperative for the bacteria’s effects on their stress response.24
Probiotics, inflammation, and the HPA axis
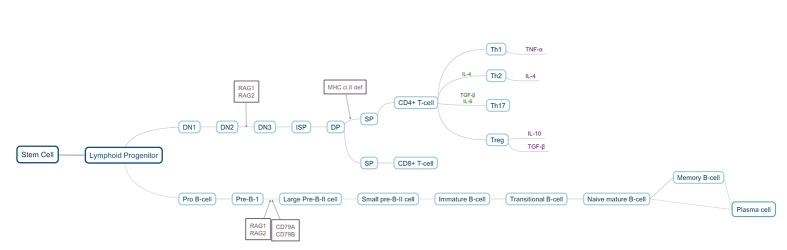
Probiotics are living microorganisms, typically yeasts and bacteria, that have been utilized as supplements to other medications or as alternative treatments for anxiety and depression.44 Probiotics have also been studied in the context of suppression of inflammatory cytokines. Some studies have found that human patients suffering from chronic inflammation responded positively to the ingestion of probiotics, as they decreased production of TNF-a.45.46 In patients with inflammatory bowel disease, probiotics correlated with suppressed levels of pro-inflammatory cytokines, and improved intestinal barrier integrity. This led to a decrease in differentiation of CD4+ T cells into Th2 cells, and inhibition of nuclear factor kappa B, both of which are highly involved in inflammation47 (Figure 2).
Figure 2.
B and T cell development. Image created by Megan Clapp and Emily Wilen.
Mothers who consumed probiotics compared to controls were found to have an altered gene expression associated with improved inflammatory responsiveness in the placenta and neonatal gut.20 Probiotic usage in late pregnancy led to a decrease in IL-4, IL-10, and Atopbium, a species of the Actinobacteria phylum, with a concurrent increase in Bifidobacterium species.48 Mothers who consumed probiotics two weeks prior to delivery had babies with altered expression of TLR-related genes in the placenta and neonatal gut; the TLR gene expression varied based on the type of probiotics the mother consumed.49 These infants were found to directly respond and modify their inflammatory responses to pathogenic bacteria compared to controls.49 Thus, providing mothers with specific probiotic formulas may protect the infant from persistent metabolic and immunologic disease processes.
Though human symptomatology is the primary interest, animal studies have elucidated the mechanisms underlying the relationship between probiotics and the immune response. Mice with B and T lymphocytes deficient in Rag1, a gene responsible for B and T cell maturation, had increased colonic ion transport, resulting in a state of dysbiosis and altered HPA axis status. These mice were treated with probiotics containing Lactobacillus species and demonstrated reduced intestinal permeability and restored microbiome and HPA-axis functionality.50 A separate study used mice with a stress-induced reduction of HPA axis function and neuronal firing. Probiotic therapy maintained neurogenesis and synaptic plasticity in the hippocampus, allowing the survival and differentiation of cells into neurons. These mice also produced lower amounts of stress hormones, and preserved intestinal permeability. The Lactobacillus strain in the administered probiotic upregulated BDNF and resulted in increased glucocorticoid regulation of the HPA axis.
Probiotics provide a neuroprotective role by preventing stress-induced synaptic dysfunction between neurons. Treatment for as little as two weeks created an appreciable decrease in ACTH and corticosterone levels in rats, illustrating the suppressive effects of probiotics on HPA axis.1,4 Probiotics have the potential to diminish the HPA axis response to chronic stressors, and prevent or reverse physiologic damage.4
Human and animal studies of probiotics show similar reductions in anxiety and depressive symptoms. Human patients suffering from chronic stress were given a three-week probiotic treatment containing Bifidobacteria species. Subjects in the bottom third of the elated/depressed scale demonstrated the most improvement with treatment. These patients rated an overall happier mood on daily analogue scales using six dimensions of mood including energetic/tired, composed/anxious, elated/depressed, clearheaded/muddled, confident/unsure, and agreeable/angry.51
In a 30-day study, healthy volunteers with no previous depressive symptoms were given either probiotics or antidepressants. Those given probiotics showed reduced cortisol levels and improved self-reported psychological effects to a similar degree as participants administered Diazepam, a commonly used anti-anxiety medication.52 Analogous studies found that probiotic therapy reduced depressive symptoms and improved HPA-axis functionality as well as Citalopram and Diazepam.53,54
Comparing probiotics to the antidepressant escitalopram in mice, the probiotics were discovered to have similar effects. They were equally successful in anxiety reduction and were more effective than the escitalopram in maintaining healthy metabolism and body weight.55 Though researchers have not determined the mechanism of action in humans, those who studied probiotics in rats found that oral ingestion of Bifidobacterium infantis resulted in increased tryptophan, a serotonin precursor,9 and GABA.56
Despite treatment with multiple antidepressants, each with different methods of action, roughly 20% of patients do not show improvement in reduction of anxiety or depressive symptoms.57 The human and mouse studies cited above indicated that probiotics normalize cortisol levels, regulate the HPA axis and reduce circulating pro-inflammatory cytokines. These mechanisms suggest probiotic therapies may confer certain benefits over therapeutic drugs. Advantages include ease of availability, lower cost, less dependence, and fewer side effects compared to pharmaceutics. Regulation of the microflora composition offers the possibility to improve immune function, homeostasis, and gut inflammation.58 Despite numerous studies citing the benefits of probiotic treatment, their specific mechanisms of action are often unknown and understudied, unlike prescription drugs.59 Thus, dosage becomes an issue, as the mechanisms and long-term effects have yet to be studied in a human population.60 Probiotics enhance resistance to infectious diseases via excretion of antimicrobial components and increase the concentration of anaerobic gram positive bacteria. However, in some studies, subjects administered probiotics reported fever, headaches, and nausea with increased frequency after a bacterial challenge.61,62 One study indicated that the probiotics administered in mouse subjects were not sufficient to prevent maleffects from a second immune challenge. This suggests that while probiotics may be helpful in the acute phase, they are not a cure-all in the long term.50
Prebiotics such as fructo-oligosaccharides and galacto-oligosaccharides are soluble fibers used to stimulate the preexisting gut microbiota. Additional studies in recent years have shown that prebiotics confer similar anxiolytic and antidepressant effects as probiotics as they also diminish stress-induced changes to the colonic microbiota and create stabilized levels of Bifidobacteria and Lactobacilli populations.63
Conclusions
The bidirectional link between the brain, gut, and microbiome has come to the forefront of the medical research community in the past few years. The growing amount of evidence substantiating this link indicates it will be a valuable area for future medical and nutritional practice, and research. This review demonstrates the importance of a healthy microbiome, particularly the gut microbiota, for patients suffering from anxiety and depression, as dysbiosis and inflammation in the CNS have been linked as potential causes of mental illness. Of note, studies have shown that probiotics effectively mitigated anxiety and depressive symptoms similar to conventional prescription medications.7,51,53,54,56
However, several weaknesses are identified in the course of this selected review. First, research linking TNF, cytokines, and other stressors to the pathogenesis of mental health disorders, particularly anxiety and depression, is lacking, and thus provides an area for future research, particularly regarding levels of intestinal bacteria and their correlation with levels of circulating cytokines.
The utility of probiotics is questionable as no form is currently regulated by the FDA, including natural sources such as yogurt, kefir, or sauerkraut. Patients may be more likely to use these natural sources of probiotics both due to increased accessibility as well as the resurgence in food trends of a return to more ancient food preparation techniques. Recent research has shown that the use of fermented foods in diets did confer gastrointestinal and cognitive benefits.64,65 However, until more evidence behind the use of probiotics as therapy for anxiety and depressive disorders is available, probiotics in any form cannot be considered a reliable therapy to anxiety and depressive disorders as compared to psychiatric medications. Furthermore, gender differences as well as comorbidities such as obesity, lifestyle, and tobacco and alcohol use may impact the overall benefit of probiotics.
Despite the lack of regulations, patients prescribed mood-altering drugs may benefit from concomitant use of probiotics. The dysbiosis created by the prescribed medications, or resulting from the neurological disturbance itself, may be mitigated by the introduction of beneficial gut flora in a probiotic form. Ultimately, the question that needs to be addressed is can probiotics alone fix the problem, or do they need to be used with mood stabilizers?
The findings above, coupled with the recent surge of interest of gut health in the media, underscore the importance of future research in understanding the gut flora. Anxiety and depression are rising global issues, effective and accessible treatments would benefit millions of people worldwide.
Acknowledgments
The authors extend their appreciation to John Pelley, Ph.D for guidance and Andrew Skin, Ph.D for editing.
References
- 1.Daulatzai MA. Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. CNS Neurol Disord 2015;14:110-31. [DOI] [PubMed] [Google Scholar]
- 2.Carabotti M, Scirocco A, Maselli MA, Carola S. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroent 2015;28:203-9. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Foster J. Psychobiotics and the gut-brain axis: in the pursuit of happiness. Neuropsych Dis Treat 2015;715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ait-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrino 2012;37:1885-95. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Hand T. Role of the microbiota in immunity and inflammation. Cell 2014;157:121-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitaryadrenal system for stress response in mice. J Physiol-London 2004;558:263-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroent Motil 2011;23:264, e119. [DOI] [PubMed] [Google Scholar]
- 8.Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms?. BioEssays 2014;36:933-9. [DOI] [PubMed] [Google Scholar]
- 9.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiat 2013;74:720-6. [DOI] [PubMed] [Google Scholar]
- 10.Foster JA, McVey Neufeld KA. Gutbrain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013;36:305-12. [DOI] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 2007;449:804-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev 2015;73:32-40. [DOI] [PubMed] [Google Scholar]
- 14.Gur TL, Shay L, Palkar AV, et al. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav Immun 2017;64:50-8. [DOI] [PubMed] [Google Scholar]
- 15.Patel K, Konduru K, Patra AK, et al. Trends and determinants of gastric bacterial colonization of preterm neonates in a NICU setting. PLoS One 2015;10:e0114664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Praveen V, Praveen S. Microbiome-gutbrain axis: A pathway for improving brainstem serotonin homeostasis and successful autoresuscitation in SIDS-A novel hypothesis. Front Pediatr 2016;4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mai V, Torrazza R, Ukhanova M, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One 2013;8:e52876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mai V, McCrary Q, Sinha R, Glei M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutrition J 2009;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett E, Kerr C, Murphy K, et al. The individual-specific and diverse nature of the preterm infant microbiota. Arch Dis Child-Fetal 2013;98:F334-40. [DOI] [PubMed] [Google Scholar]
- 20.Wright ML, Starkweather AR. Antenatal microbiome: potential contributor to fetal programming and establishment of the microbiome in offspring. Nurs Res 2015;64:306-19. [DOI] [PubMed] [Google Scholar]
- 21.Oozeer R, van Limpt K, Ludwig T, et al. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr 2013;98:561S-71. [DOI] [PubMed] [Google Scholar]
- 22.Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015;17:690-703. [DOI] [PubMed] [Google Scholar]
- 23.Dogra S, Sakwinska O, Soh S-E, et al. Rate of establishing the gut microbiota in infancy has consequences for future health. Gut Microbes 2015;6:321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701-12. [DOI] [PubMed] [Google Scholar]
- 25. . Structure, function and diversity of the healthy human microbiome Nature 2012;486:207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caporaso JG, Lauber CL, Costello EK, et al. Moving pictures of the human microbiome. Genome Biol 2011;12:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jandhyala SM. Role of the normal gut microbiota. World J Gastroenterol 2015;21:8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islami F, Ren J-S, Taylor PR, Kamangar F. Pickled vegetables and the risk of oesophageal cancer: a meta-analysis. Br J Cancer 2009;101:1641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiat 2007;62:55-64. [DOI] [PubMed] [Google Scholar]
- 30.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastro Hepat 2009;6:306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckburg PB. Diversity of the human intestinal microbial flora. Science 2005;308:1635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoppi S, Madrigal JLM, Pérez-Nievas BG, et al. Endogenous cannabinoid system regulates intestinal barrier function in vivo through cannabinoid type 1 receptor activation. Am J Physiol Gastr L 2012;302:565. [DOI] [PubMed] [Google Scholar]
- 33.Muccioli GG, Naslain D, Bäckhed F, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 2010;6:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho JT Chan GC Li JC.. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol 2015;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burrus CJ. A biochemical rationale for the interaction between gastrointestinal yeast and autism. Med Hypotheses 2012;79:784-5. [DOI] [PubMed] [Google Scholar]
- 37.Kantarcioglu AS, Kiraz N, Aydin A. Microbiota-gut-brain axis: Yeast species isolated from stool samples of children with suspected or diagnosed autism spectrum disorders and in vitro susceptibility against nystatin and fluconazole. Mycopathologia 2016;181:1-7. [DOI] [PubMed] [Google Scholar]
- 38.Biesmans S, Bouwknecht JA, Ver Donck L, et al. Peripheral administration of tumor necrosis factor-alpha induces neuroinflammation and sickness but not depressive-like behavior in mice. BioMed Res Int 2015;2015:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 2013;65:1655-62. [DOI] [PubMed] [Google Scholar]
- 40.Ohland CL, Kish L, Bell H, et al. Effects of lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinol 2013;38:1738-47. [DOI] [PubMed] [Google Scholar]
- 41.Muscatello MRA. Role of negative affects in pathophysiology and clinical expression of irritable bowel syndrome. World J Gastroenterol 2014;20:7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falowski SM, Sharan A, Reyes BAS, et al. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery 2011;69:1281-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Cao S, Zhang X. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J Agr Food Chem 2015;63:7885-95. [DOI] [PubMed] [Google Scholar]
- 45.D’Mello C, Ronaghan N, Zaheer R, et al. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J Neurosci 2015;35:10821-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desbonnet L, Garrett L, Clarke G, et al. The probiotic bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 2008;43:164-74. [DOI] [PubMed] [Google Scholar]
- 47.Buckley MM, O’Mahony SM, O’Malley D. Convergence of neuro-endocrine-immune pathways in the pathophysiology of irritable bowel syndrome. World J Gastroenterol 2014;20:8846-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitali B, Cruciani F, Baldassarre ME, et al. Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiol 2012;12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rautava S, Collado MC, Salminen S, Isolauri E. Probiotics modulate hostmicrobe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology 2012;102:178-84. [DOI] [PubMed] [Google Scholar]
- 50.Smith CJ, Emge JR, Berzins K, et al. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol-Gastr L 2014;307:G793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr 2007;61:355-61. [DOI] [PubMed] [Google Scholar]
- 52.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011;105:755-64. [DOI] [PubMed] [Google Scholar]
- 53.Steenbergen L, Sellaro R, van Hemert S, et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun 2015;48:258-64. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt K, Cowen PJ, Harmer CJ, et al. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl) 2015;232:1793-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroent Motil 2014;26:1615-27. [DOI] [PubMed] [Google Scholar]
- 56.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 2011;33:574-81. [DOI] [PubMed] [Google Scholar]
- 57.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci 2011;34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marlow G, Han DY, Wickens K, et al. Differential effects of two probiotics on the risks of eczema and atopy associated with single nucleotide polymorphisms to Toll-like receptors. Pediatr Allergy Immunol 2015;26:262-71. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A Alrefai WA Borthakur A Dudeja PK.. Lactobacillus acidophilus counteracts enteropathogenic E. coliinduced inhibition of butyrate uptake in intestinal epithelial cells. Am J Physiol-Gastr L 2015;309:G602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Repa A, Thanhaeuser M, Endress D, et al. Probiotics (Lactobacillus acidophilus and Bifidobacterium bifidum) prevent NEC in VLBW infants fed breast milk but not formula. Pediatr Res 2015;77:381-8. [DOI] [PubMed] [Google Scholar]
- 61.Ouwehand AC, ten Bruggencate SJ, Schonewille AJ, et al. Lactobacillus acidophilus supplementation in human subjects and their resistance to enterotoxigenic Escherichia coli infection. Br J Nutr 2014;111:465-73. [DOI] [PubMed] [Google Scholar]
- 62.Savino F, Fornasero S, Ceratto S, et al. Probiotics and gut health in infants: a preliminary case-control observational study about early treatment with Lactobacillus reuteri DSM 17938. Clin Chim Acta 2015;451:82-7. [DOI] [PubMed] [Google Scholar]
- 63.Burokas A, Arboleya S, Moloney RD, et al. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiat 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 64.Selhub EM, Logan AC, Bested AC. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J Physiol Anthropol 2014;33:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim B, Hong VM, Yang J, et al. A review of fermented foods with beneficial effects on brain and cognitive function. Prev Nutr Food Sci 2016;21:297. [DOI] [PMC free article] [PubMed] [Google Scholar]