Competing interest statement
Conflict of interest: the authors declare no potential conflict of interest.
Abstract
Leukemia-initiating cells of core binding factor (CBF) acute myeloid leukemia (AML) likely derive from early committed hematopoietic precursors expressing CD33. As such, targeting CD33 could ameliorate the chance of cure of CBF AML patients. We compared 12 CBF AML patients treated with Fludarabine, Cytarabine, Idarubicin and Gemtuzumab Ozogamicin (FLAI-GO regimen) with 25 CBF AML patients treated with the same schedule, but without GO. With the limit of small numbers, we observed a consistent trend toward better overall survival, disease free survival and event free survival in the FLAI-GO group. We also demonstrated the ability of GO to induce the disappearance in vitro of the AML1-ETO molecular transcript in a polymerase chain reaction-positive graft without decreasing the clonogenic potential of CD34+/CD38- cells. This represent the proof of principle for using GO in a purging strategy before autologous stem cell transplantation. Therefore, our data argue in favor of the reinstitution of GO in the therapy of CBF AML.
Brief Report
Core Binding Factor (CBF) Acute Myeloid Leukemia (AML) patients experience treatment failure in the order of 40-50%.1As such, efforts have been made to increase the dose intensity of first line treatment over the standard Daunorubicin/Cytarabine 3+7 induction, under the assumption that better survival could be achieved by obtaining a deeper early clearance of blasts before clonal evolution of the disease. Among other attempts, the addition of a third chemotherapeutic drug, such as Fludarabine, has been tested in various clinical trials,2,3 with conflicting results regarding Overall Survival (OS), but, in most cases, with an increase in the rate of complete remission (CR) and Disease-Free Survival (DFS).2,3 Moreover, novel agents with compatible safety profile have been tested in addition to chemotherapy; among these, the immunoconjugate Gemtuzumab Ozogamicin (GO), which combines in a single agent a monoclonal antibody targeting CD33 with the DNA damaging toxin calicheamicin. Following initial studies, GO has been combined to chemotherapy to improve efficacy, particularly in the case of CBF AML, in which blasts highly express the target antigen.1,3 Three-drug Fludarabinebased regimens combined with GO proved successful mainly in the setting of cytogenetically favorable, such as CBF AML, or intermediate-risk AML,3,4 whereas results have somehow been disappointing in adverse risk patients.3,4 Despite this, the clinical development of GO has suffered from concerns raised by an increased incidence of hepatotoxicity and venoocclusive disease when GO was used at the dose of 9 mg/m2 twice during induction,5 and from the early results of a randomized phase-3 trial that showed no advantage in OS and a significant increase of Treatment- Related Mortality (TRM) (5% vs 1%) in the GO-treated group.6 This ultimately led to the withdrawal of the drug from the market in 2010. Later studies3,7,8 showed, on the opposite, an unequivocal survival benefit by GO, even if mainly restricted to CBF AML, at the lower schedule of 3 to 6 mg/m2, without neither increased hepatotoxicity nor higher TRM.3,7,8 This led to the revaluation of GO, which, unfortunately, has not yet resulted in the reinstitution of the drug to clinical practice.5
In order to address the role of GO in the treatment of CBF AML, we retrospectively reviewed 12 CBF AML patients [t(8;21) n=8; inv(16) n=4] treated from 2006 to 2009 with the FLAI-GO regimen (Fludarabine 30 mg/m2 on days1-5; Cytarabine 2 gr/m2 on days1-5; Idarubicin 10 mg/m2 on days 1,3,5; GO 3 mg/m2 on day 6),4 and consolidated with two high-dose cytarabine (HiDAC)- based cycles (overall dose 24 gr/m2/cycle). We applied a regimen that was previously described in an independent series of AML patients.4 Patients with c-KIT tyrosine kinase domain mutation at codon 816 (TKD816) at diagnosis (n=2) or with the persistence of molecular transcript, assessed by sequential polymerase chain reaction (PCR), at the end of consolidation (n=5), i.e. Minimal Residual Disease (MRD)- positivity, were then consolidated with either allogeneic or autologous hematopoietic stem cell transplantation (HSCT) based on the availability of a donor. We decided to further intensify the treatment of KIT mutated patients early on, based on initial studies showing an adverse prognosis of these patients as compared to KIT wild-type CBF AML.9 Conversely, the persistence of the molecular transcript at the end of consolidation was considered a predictor of adverse prognosis based on previous experiences,10,11 as well as our own unpublished data.
We compared this group with 25 CBF AML patients [t(8;21) n=13; inv(16) n=12] treated according to the same criteria and with the same schedule, but without GO, in the years 2003-2006 and 2010-2013. The two groups were comparable in all clinical and laboratory features (Table 1). In the latter group, autologous HSCT was performed in 5 patients because of MRDpositivity, and allogeneic HSCT was performed in 2 patients because of the lack of cytogenetic response after induction therapy.
Table 1.
Baseline patient characteristics.
| FLAI5 | My-FLAI5 | P | |
|---|---|---|---|
| Median age | 41.3 (18-66) | 46.3 (29-67) | 0.2602 |
| Sex | 12 M + 13 F | 6 M + 6 F | 0.909 |
| Secondary acute myeloid leukemia | 0 | 0 | NA |
| Hepatomegaly | 4 | 2 | 1 |
| Splenomegaly | 3 | 2 | 1 |
| Sarcoma | 0 | 1 | 1 |
| Hemoglobin gr/dL | 8.4 (4.2-11) | 8.5 (5-13.6) | 0.91 |
| White blood cells ×103/ L | 19.0 (1.6-95) | 18.7 (4.5-45.5) | 0.9706 |
| N ×103/ L | 1.86 (0.33-6.35) | 2.58 (0.45-11.36) | 0.368 |
| Mo ×103/ L | 0.95 (0.01-2.56) | 0.58 (0.01-2.68) | 0.133 |
| Ly ×103/ L | 2.67 (0.50-6.80) | 2.86 (0.60-7.73) | 0.8016 |
| Blasts ×103/ L | 10.9 (0.01-65.55) | 11.6 (0.22-32.0) | 0.9074 |
| Platelets ×103/ L | 60.66 (8-255) | 73.72 (6-531) | 0.715 |
| Elevated LDH | 18 | 10 | 0.638 |
| DIC | 2 | 2 | 0.305 |
| Acute renal failure | 0 | 1 | 0.314 |
| t(8;21)/inv(16) | 13/12 | 4-Aug | 0.491 |
| FLT3-ITD | 3 | 2 | 1 |
| NPM1 mutated | 0 | 0 | NA |
| KIT TKD 816 mutated | 1 | 1 | 1 |
| Packed BM (>80%) | 12 | 5 | 0.717 |
| Additional cytogenetic abnormalities | None: 14 pts; 1: 7 pts; 2: 3 pts; 3: 1 pts | None: 4; 1: 5 pts; 2: 1 pts; 3: 2 pts | 0.387 |
Patients in the two groups reached a comparable rate of CR after induction (n=12/12 vs 22/25, P=0.540); no death in induction was observed, and all patients completed their therapeutic schedule. At median follow-up of 69.2 months, 3 patients in the FLAI-GO group relapsed at 8, 14 and 42 months after the achievement of CR; of these, two out of three achieved second CR following rescue therapy and underwent allogeneic HSCT. Conversely, at median DFS 14.7 months, 11 patients of the FLAI group relapsed; among these, 7 out of 10 achieved second CR and were consolidated with allogeneic HSCT.
As shown in Figure 1, we observed a consistent, yet non-statistical trend towards better OS, DFS and, most importantly in evaluating the efficacy of first line therapy, Event Free Survival (EFS) in the FLAI-GO group (5-yrs OS 69.4% vs 48.6%, P=0.202; 5-yrs DFS 54.7% vs 42.4%, P=0.327; 5-yrs EFS 54.7% vs 36.9%, P=0.136; Figure 1). Besides, patients tended to relapse later when treated with FLAI-GO (median DFS: unreached vs 14.7 months; Figure 1). We believe these differences did not reach the statistical significance mainly due to small numbers. The achievement of MRDnegativity (FLAI-GO=100% of the 4 patients analyzed; FLAI=63% of the 13 patient analyzed), assessed by PCR, was of pivotal prognostic importance (P<0.001 for either OS, DFS and EFS).
Figure 1.
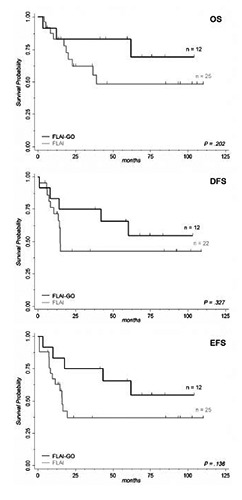
Survival by treatment with Gemtuzumab Ozogamicin (GO). Patients receiving FLAI-GO induction therapy showed a consistent, yet nonstatistical, trend towards better Overall Survival (OS), Disease-Free Survival (DFS) and, most importantly in determining the effect of first line treatment, Event-Free Survival (EFS), as compared to a group of patients treated with FLAI. Numbers of the two groups are showed as n; only patients achieving complete remission after induction therapy were considered in determining DFS. Inclusion criteria and treatment schedule was the same in the two groups apart from the addition of GO at 3 mg/m2 to induction therapy at day 6 of the FLAIGO regimen.
The potential of GO in the treatment of CBF AML is based on strong biological bases: first of all, most t(8;21) AML blasts do not express the transporter P-glycoprotein (Pgp),12 i.e. the Multi-Drug Resistance 1 (MDR1) gene product, and this seems related to a selective repression of the promoter of MDR1 by AML1-ETO.13 Several studies have pointed out how GO extrusion by Pgp affects clinical response.14 Moreover, CBF fusion transcripts promote leukemogenesis by inducing the initial expansion of a preleukemic myeloid cell compartment predisposed to secondary mutations and characterized by CD33+ early committed myeloid precursors incorporating the AML1-ETO or CBFB-MYH11 transcripts.15 According to this model, founding Leukemia-Initiating Cells of CBF AML, differently from other types of AML, would arise from these early committed myeloid precursors rather than the more immature Hematopoietic Stem Cells,15 and therefore be more sensitive to GO therapy because of their markedly higher expression of CD33. The distinctive chemosensitivity shown by CBF AML1 could also be explained by these biological differences.15
As such, autologous HSCT could be used to increase the dose intensity of first line therapy in selected patients, improving OS, as shown by some studies.16 Results are best when MRD-negativity in the bone marrow and in the products of leukapheresis is obtained before transplantation.17 Recently, a 5-year EFS of 93% was reported in a small series of CBF AML patients undergoing autologous HSCT with a PCRnegative graft, with the disappearance of CBF transcripts after HSCT in 8 out of 10 previously MRD-positive patients.18 Nonetheless, in a previous series we observed that clinical outcome of patients could be improved also when autologous HSCT had been performed with CD34+ grafts with persistent molecular transcripts.16 We explain this finding by the higher overall dose-intensity achieved by first line treatment by including autologous HSCT.
The achievement of MRD-negativity has been related to a better prognosis by many trials.19 Autologous HSCT, performed at the end of first line treatment, might be beneficial for selected CBF AML patients to achieve the disappearance of MRD, especially when performed using MRDnegative Peripheral Blood Stem Cells (PBSC).17 Monoclonal antibodies are thus being tested to provide in vivo purging of PBSC. This approach resembles the use of Rituximab in the therapy of patients affected by CD20+ lymphomas and undergoing autologous HSCT. Concerns are there, though, that treatment with GO might affect hematopoietic reconstitution by reducing the number of hematopoietic long-term repopulating cells collected by leukapheresis.
We therefore evaluated the clonogenic growth of PBSC collected at the end of consolidation therapy from five patients with CBF AML. Briefly, mononuclear cells were cultured in RPMI medium in the presence or absence of GO at a concentration of 5 μg/mL for two hours, a previously in vitro dose proved able to induce the near-complete saturation of the CD33 antigen sites.20 Cells were then collected, GO removed by centrifugation, and cells reseeded in Petri dishes containing semisolid MethoCult GFH4434 medium. The number of Colony-Forming Units (CFU) was determined after 14 days incubation.
Using unsorted cells, we observed a significant decrease in the number of CFUGEMM (clonal efficiency 0.000697 vs 0.01037, P=0.016), CFU-GM (0.002606 vs 0.004071, P=0.038) and BFU-E colonies (0.003213 vs 0.004697, P=0.031) in the cells exposed to GO. We then performed the same experiments on samples from the same PBSC units after the enrichment in CD34+/CD38- cells by immunomagnetic sorting. The efficacy of this sorting was proved by either immunophenotypic analysis and functional assays, which resulted in significantly more numerous CFU-GEMM, CFU-GM, BFU-E (data not shown). This time, though, we did not observe a significant decrease in the clonogenic potential of CD34+/CD38- cells by the exposure to GO (0.01518 vs 0.02631, P=0.351), thus confirming the preservation of more immature hematopoietic precursors. Moreover, in one MRD-positive patient we could observe the disappearance of the AML1-ETO molecular transcript following in vitro incubation with GO at a concentration of 5 μg/mL for two hours (Figure 2).
Figure 2.
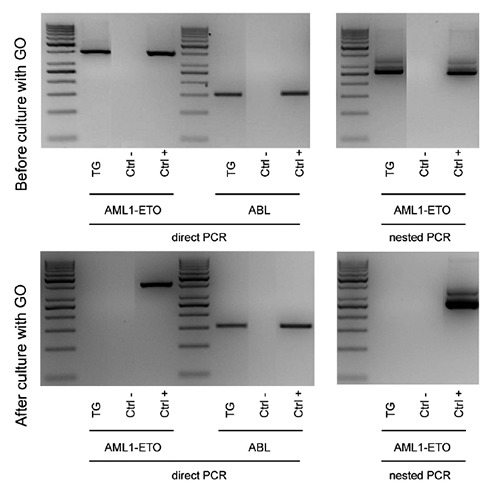
Disappearance of AML1-ETO molecular transcript following in vitro purging of PBSC with Gemtuzumab Ozogamicin. Samples from PBSC collected from 5 patients affected by CBF AML at the end of consolidation were cultured in the presence or absence of Gemtuzumab Ozogamicin at a concentration of 5 μg/mL for two hours. In one patient (i.e. TG) affected by t(8;21) AML with persistent AML1-ETO transcript at the end of consolidation and in the PBSC, incubation with GO obtained the disappearance of cells expressing AML1-ETO, as tested by either direct and nested PCR. Abelson (ABL) amplification was used as internal control. TG: initials of the patient’s name; Ctrl- /Ctrl+: negative and positive controls.
Therefore, with the limit of small numbers, the results that we report suggest the possibility of using GO in a purging strategy that would possibly act on residual CBF AML cells without affecting the repopulating ability of PBSC. In order to avoid the limitation of in vitro purging, GO could be used in vivo before CD34+ cell collection in MRD-positive patients.
Acknowledgments
The authors are grateful to Leukemia-Lymphoma-Myeloma ONLUS Association (AIL), Section of Treviso (Italy), for the financial support.
References
- 1.Paschka P. Core binding factor acute myeloid leukemia. Semin Oncol 2008; 35:410-7. [DOI] [PubMed] [Google Scholar]
- 2.Borthakur G, Kantarjian H, Wang X, et al. Treatment of core-binding-factor acute myelogenous leukemia with fludarabine, cytarabine and granulocyte colony-stimulating factor results in improved event-free survival. Cancer 2008;113:3181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamic in: results of the MRC AML 15 trial. J Clin Oncol 2011;29:369-77. [DOI] [PubMed] [Google Scholar]
- 4.Candoni A, Martinelli G, Toffoletti E, et al. Gemtuzumab-ozogamicin in combination with fludarabine, cytarabine, idarubicin (FLAI-GO) as induction therapy in CD33-positive AML patients younger than 65 years. Leuk Res 2008;32:1800-8. [DOI] [PubMed] [Google Scholar]
- 5.Rowe JM, Lowenberg B. Gemtuzumab Ozogamycin in acute myeloid leukemia: a remarkable saga about an active drug. Blood 2013;121:4838-41. [DOI] [PubMed] [Google Scholar]
- 6.Petersdorf SH, Kopecky KJ, Slovak ML, et al. A phase III study of gemtuzumab ozogamycin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013;121:4854-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukemia (ALFA-0701): a randomised, open label, phase 3 study. Lancet 2012;379:1508-16. [DOI] [PubMed] [Google Scholar]
- 8.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012; 30:3924-31. [DOI] [PubMed] [Google Scholar]
- 9.Pashka P, Marcucci G, Ruppert AS, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol 2006;24:3904-11. [DOI] [PubMed] [Google Scholar]
- 10.Tobal K, Newton J, Macheta M, et al. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood 2000;95:815-9. [PubMed] [Google Scholar]
- 11.Martin G, Barragan E, Bolufer P, et al. Relevance of presenting white blood cell count and kinetics of molecular remission in the prognosis of acute myeloid leukemia with CBFbeta/MYH11 rearrangement. Haematologica 2000;85:699-703. [PubMed] [Google Scholar]
- 12.Del Poeta G, Venditti A, Aronica G, et al. P-glycoprotein expression in de novo acute myeloid leukemia. Leuk Lymphoma 1997;27:257-74. [DOI] [PubMed] [Google Scholar]
- 13.Lutterbach B, Sun D, Schuetz J, Hiebert SW. The MYND motif is required for repression of basal transcription from the multidrug resistance 1 promoter by the t(8;21) fusion protein. Mol Cell Biol 1998;18:3604-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linenberger ML, Hong T, Flowers D, et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood 2001;98:988-94. [DOI] [PubMed] [Google Scholar]
- 15.Mosna F, Gottardi M. Stem cell modeling of core binding factor acute myeloid leukemia. Stem Cell Int 2016;2016:7625827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosna F, Papayannidis C, Martinelli G, et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up. Am J Hematol 2015;90:515-23. [DOI] [PubMed] [Google Scholar]
- 17.Gorin NC, Giebel S, Labopin M, et al. Autologous stem cell transplantation for adult acute leukemia in 2015: time to rethink? Present status and future prospects. Bone Marrow Transplant 2015;50:1495-502. [DOI] [PubMed] [Google Scholar]
- 18.Nakasone H, Izutsu K, Wakita S, et al. Autologous stem cell transplantation with PCR-negative graft would be associated with a favourable outcome in core-binding factor acute myeloid leukemia. Biol Blood Marrow Transplant 2008;14:1262-9. [DOI] [PubMed] [Google Scholar]
- 19.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Hematol Am Soc Hematol Educ Program 2014; 2014:222-33. [DOI] [PubMed] [Google Scholar]
- 20.van Der Velden VH, te Marvelde JG, Hoogeveen PG, et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 200;97: 3197-204. [DOI] [PubMed] [Google Scholar]


