Abstract
Background
Gastric electric stimulation (GES) is a treatment approach to refractory gastroparesis, possibly acting centrally via afferent vagus nerve stimulation (VNS). Non-invasive VNS (nVNS) is a potential alternative to GES that could eliminate the safety risks of or identify likely responders to implantable neurostimulators.
Objective
This open-label proof-of-concept study assessed the effects of nVNS in patients with severe drug-refractory gastroparesis.
Methods
Patients used the Gastroparesis Cardinal Symptom Index (GCSI) to grade symptoms in diaries daily for 2 weeks before treatment (baseline) and during ≥3 weeks of nVNS therapy. Adverse events (AEs) were also diarised. Treatment was self-administered using an nVNS device (gammaCore, electroCore) and consisted of 120 s stimulations to the vagus nerve in the neck (two stimulations to each side three times daily during weeks 1 and 2; three stimulations to each side three times daily during week 3 and beyond). Response was defined as a ≥1 point decrease from baseline in GCSI score.
Results
Thirty-five patients enrolled; 23 were compliant with study procedures and were included in the analysis; 7 continued treatment beyond 3 weeks. Response rates were 35% (8/23) at 3 weeks and 43% (10/23) for the duration of therapy (3–6 weeks). For the entire cohort and the 10 responders, improvements from baseline were noted for mean total GCSI and GCSI subscale scores (nausea/vomiting, postprandial fullness/early satiety, bloating). No serious AEs were reported.
Conclusions
These preliminary results provide a signal that nVNS may be useful for treating refractory gastroparesis. Larger controlled studies are warranted.
Keywords: GASTROPARESIS, AUTONOMIC NERVOUS SYSTEM, NERVE - GUT INTERACTIONS
Introduction
Gastroparesis is a chronic motility disorder characterised by disruption in the timing or strength of normal gastric contractility, leading to profound delay in gastric emptying in the absence of mechanical obstruction.1 2 Symptoms include persistent nausea, vomiting, bloating, early satiety and abdominal pain.1 Its prevalence has been estimated at 9.6 per 100 000 men and 37.8 per 100 000 women,3 and data on gastroparesis-related hospitalisations suggest that its prevalence is increasing.4 The condition is poorly understood, often difficult to treat and associated with substantial economic impacts.4
Several aetiological processes have been hypothesised for this disorder. In many cases of idiopathic gastroparesis, viral infection, which results in damage to nerves and/or other components that affect gastric contractility, is believed to be an underlying cause.5 6 Gastroparesis also is often associated with diabetes mellitus or surgery involving the upper gastrointestinal tract.1 Autonomic neuropathy of the parasympathetic nervous system appears to be an important factor in diabetic gastroparesis,7–9 whereas postsurgical gastroparesis is often related to vagus nerve injury.1 Chronic intestinal pseudo-obstruction, Parkinson disease, scleroderma and systemic lupus erythematosus are also associated with gastroparesis, albeit less commonly.1
Pharmacological treatment for gastroparesis usually involves the combination of prokinetic and antiemetic medications including metoclopramide, domperidone, cyclizine and ondansetron.10–12 Symptomatic improvement has been reported after endoscopic injection of botulinum toxin in the pyloric muscle, but randomised trials have failed to confirm such benefit.13 In treatment-refractory gastroparesis, patients may require enteral nutrition via a jejunostomy feeding tube, an approach that is often complicated by obstruction, displacement or aspiration pneumonia.14
Implantation of a gastric electric stimulation (GES) device (Enterra, Medtronic, Minneapolis, Minnesota, USA) is a therapeutic option for patients with gastroparesis who do not respond to pharmacological treatment.15 This device is CE marked in Europe and is available in the USA for compassionate use in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to gastroparesis. Results from a double-blind crossover study and meta-analyses suggest that GES is associated with significant reductions in total symptom severity as well as in nausea and vomiting severity;16 17 however, the clinical effect may become evident only after many months of GES therapy.18 Additionally, as with any surgical implantation procedure, inherent risks of complications such as infection and pain at the implantation site are associated with GES.16 17
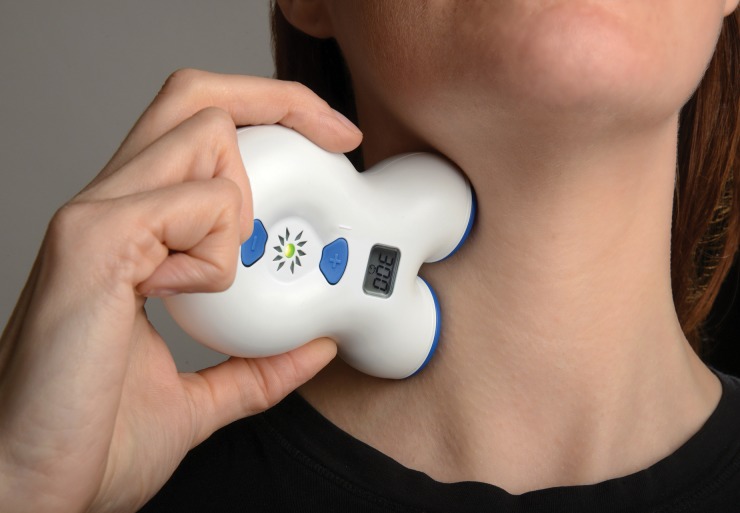
The vagus nerve plays a key role in regulation of nausea and vomiting,19 and there is evidence of vagus afferent effects on nociception,20 which suggests that electrical neurostimulation modulates nausea and vomiting. The current proof-of-concept study explored the possibility that non-invasive vagus nerve stimulation (nVNS) could have clinical effects similar to those of an implanted GES device in patients with gastroparesis. nVNS was delivered by a battery-powered neurostimulator (gammaCore, electroCore; Basking Ridge, New Jersey, USA) designed primarily to stimulate myelinated sensory afferent vagus nerve fibres as they ascend through the neck in the carotid sheath (figure 1). The device has been approved and is being prescribed in several countries mainly for the treatment of primary headache and is CE marked in the European Union for the treatment of primary headache, epilepsy, bronchoconstriction, anxiety, depression and gastric motility disorders.21 When studied in patients with primary headache, the device demonstrated a favourable safety profile and was not associated with significant adverse events (AEs).22 This short-term pilot study assessed whether nVNS therapy might have a positive effect on symptoms in patients with treatment-refractory gastroparesis. We proposed that if implanted GES devices act centrally through vagus afferents, nVNS applied to the vagus nerve traversing the neck might have a similar therapeutic effect.
Figure 1.
Positioning of the non-invasive vagus nerve stimulation device. Image provided courtesy of electroCore.
Materials and methods
This open-label proof-of-concept study was conducted at the Royal Free Hospital in London. It was estimated that between 30 and 40 patients would be sufficient to identify a signal of any therapeutic effects of nVNS, but this sample was not statistically powered to demonstrate the efficacy of this therapy. All participants had been referred to a tertiary referral centre because of severe gastroparesis with persistent symptoms that were refractory to antiemetic and prokinetic medication (thus fulfilling the criteria for consideration of non-pharmacological treatments such as GES) and agreed to participate in this trial of nVNS while awaiting funding for implantable gastric neurostimulation.23 The use of nVNS in this study was endorsed by the New Devices Committee at the Royal Free London NHS Foundation Trust. All examinations, data collection and follow-up were conducted between January 2014 and September 2014.
An explanatory video regarding nVNS in the treatment of gastrointestinal disorders was produced and made available on YouTube (https://www.youtube.com/watch?v=tH_Lw_vDo3U) for patients who wished to consider nVNS for gastroparesis. All participants provided written informed consent and were asked to complete a baseline 2-week pretreatment daily symptom diary and to continue this diary throughout the treatment period, which lasted at least 3 weeks. In daily entries, patients graded their symptoms on a nine-item Gastroparesis Cardinal Symptom Index (GCSI) using a six-point Likert scale (0=no symptoms; 5=very severe symptoms).24 The GCSI includes three subscales: nausea/vomiting, postprandial fullness/early satiety and bloating. Patients also recorded AEs in diaries.
The nVNS device delivered a low-voltage electrical signal consisting of a 5 kHz sine wave series occurring for 1 ms and repeated every 40 ms (25 Hz). Stimulation was administered via two stainless steel contact surfaces that were coated with conductive gel before each treatment, and the device was positioned in parallel with the carotid pulse in the neck (figure 1). A single stimulation of nVNS was programmed as a 2 min period, and the patient could adjust the stimulation intensity using the + and − buttons to achieve a comfortable tingling sensation in tissues beneath the stimulation plates.
Two gastroenterologists in the hospital outpatient department provided training on the correct use of the nVNS device. Throughout the treatment period, patients self-administered nVNS at home. For the first two weeks of the treatment phase, patients administered two stimulations sequentially to each of the left and right vagus nerves three times daily (12 stimulations per day); during the third week, they delivered three stimulations to each side three times daily (18 stimulations per day). A subgroup of patients continued this regimen beyond 3 weeks and recorded symptoms for up to 6 weeks total. At the end of the treatment period, patients were reassessed by a gastroenterologist. Compliance was assessed through patient interviews and diary completion.
For each compliant patient (defined as a patient who returned a diary containing at least three consecutive weeks of data), a mean GCSI aggregate score was calculated for the 2 week pretreatment phase (ie, baseline) and at the end of each treatment week. Building upon previous reports,24 25 we defined a responder for this study as a patient who experienced a ≥1 point decrease from baseline in GCSI aggregate score. In addition, percentage changes in GCSI aggregate scores were calculated by comparing the mean baseline score with the mean score at the end of each treatment week. The proportions of patients with >30% and >50% decreases from baseline in GCSI aggregate score after 3 weeks of treatment were calculated. Scores for the nausea/vomiting, postprandial fullness/early satiety and bloating GCSI subscales were assessed independently via similar methods.
Results
Thirty-five patients (28 women, 7 men) enrolled in the study. Of these, 12 (34%) enrolled patients discontinued from the study or were non-compliant with study procedures for various reasons: 6 (50%) did not attend follow-up appointments, 3 (25%) stopped because of lack of response, 2 (17%) had intercurrent hospital admissions and 1 (8%) lost the diaries. Twenty-three patients (66%) completed the baseline assessment, at least 3 weeks of treatment, and the study diaries (completing patients). Of these, seven patients (30%) elected to continue treatment for up to 6 weeks total. Patient characteristics at baseline are presented in table 1.
Table 1.
Baseline characteristics
| Characteristic | Total n=23 |
Responders n=10 |
Non-responders n=13 |
|---|---|---|---|
| Age (mean±SD), years | 39±14 | 37±10 | 41±17 |
| Sex, n (%) | |||
| Male | 6 (26) | 2 (20) | 4 (31) |
| Aetiology, n (%) | |||
| Idiopathic | 15 (65) | 6 (60) | 9 (69) |
| Diabetic | 6 (26) | 3 (30) | 3 (23) |
| Postsurgical | 2 (9) | 1 (10) | 1 (8) |
| Duration of symptoms (mean±SD), years | 4.1±3.7 | 4.1±3.1 | 4.0±4.2 |
| Patients on jejunal feed, n (%) | 7 (30) | 2 (20) | 5 (38) |
| Severity of symptoms before treatment (mean±SD), Gastroparesis Cardinal Symptom Index score | 2.9±0.9 | 3.2±0.7 | 2.6±1.0 |
After 3 weeks of treatment, 8 of the 23 completing patients (35%) were classified as responders to nVNS therapy. An additional 2 of 7 patients who continued nVNS therapy for up to 6 weeks (total) achieved response during that time, bringing the total number of responders to 10 (43%) among patients who completed ≥3 weeks of nVNS therapy. All responders experienced symptom relapse within 1 week of stopping treatment.
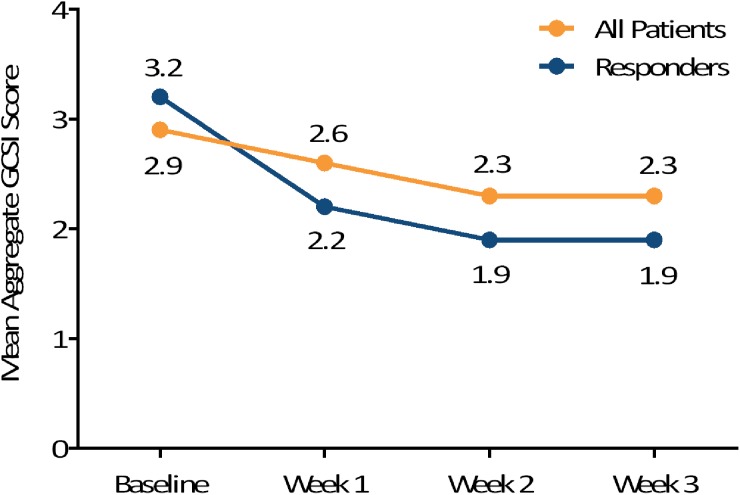
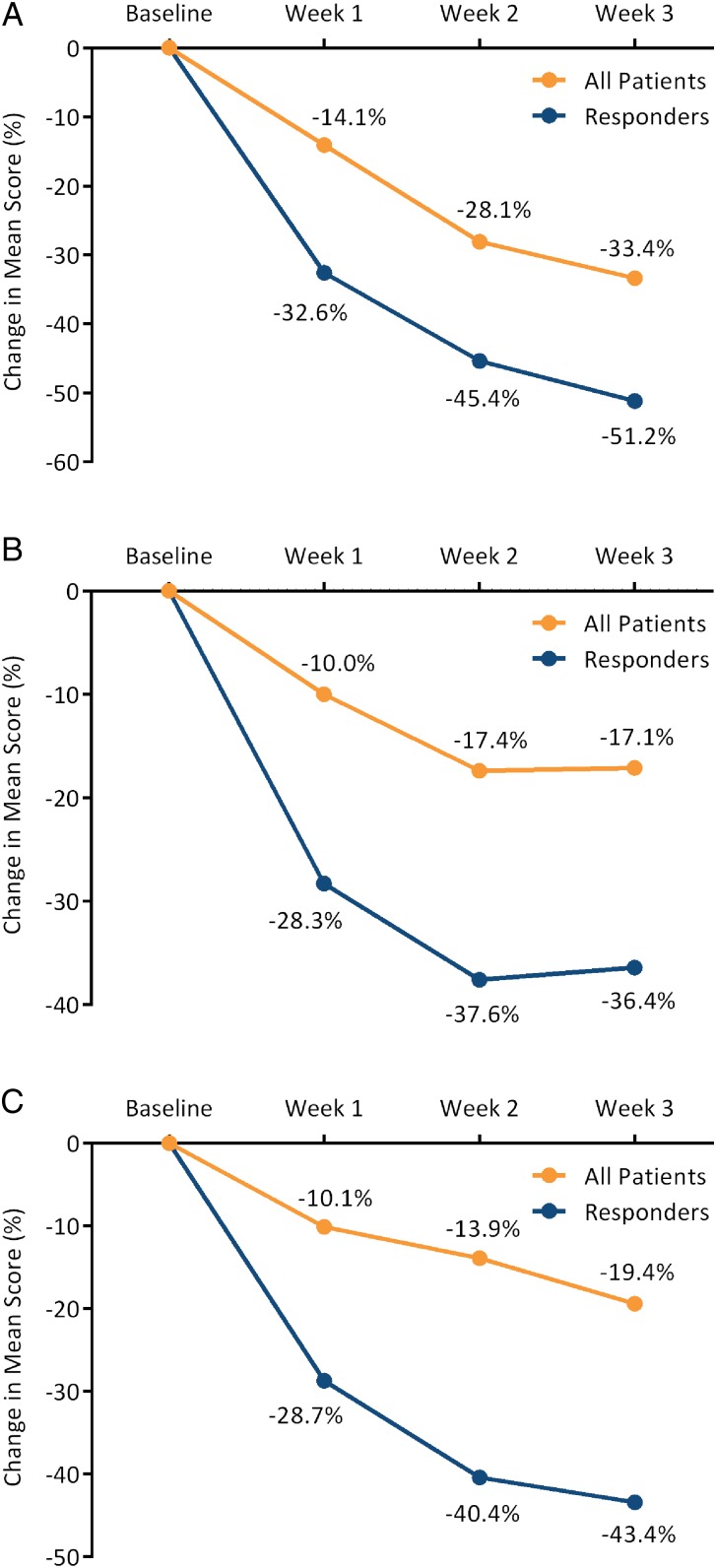
Figure 2 illustrates the mean weekly GCSI aggregate scores at baseline and during the first three weeks of treatment, indicating decreases (ie, improvements) in these scores for the completing patients (n=23) and for the subgroup classified as responders (n=10). When GCSI aggregate scores at baseline and after 3 weeks of treatment were compared, 12 of 23 patients (52%) had a >30% decrease from baseline and 6 (26%) had a >50% decrease from baseline. Mean GCSI subscale scores also improved during the treatment phase (figure 3), with nVNS having a particularly dramatic effect on the individual symptoms of nausea and stomach fullness. Only skin irritation (one case) and neck discomfort (one case) were reported as AEs. No serious device-related AEs were reported.
Figure 2.
Mean aggregate Gastroparesis Cardinal Symptom Index (GCSI )scores in all patients (n=23) and responders (n=10).
Figure 3.
Percentage change in mean subscale score for nausea/vomiting (A), postprandial fullness/early satiety (B) and bloating (C) for all patients (n=23) and responders (n=10).
Discussion
Electrical neuromodulation offers a novel therapeutic approach for a number of disparate disorders in which central nervous system effects are thought to play a role.26–29 This short-term proof-of-concept study was based on evidence that implantable GES improved symptoms in patients with treatment-resistant gastroparesis.30 Several mechanisms of action have been proposed for gastrointestinal neurostimulation including central, autonomic and enteric effects.31–33 The central mechanisms of GES have been demonstrated via activation of gastric-related neurons in the paraventricular nucleus, and the effects of GES on the autonomic nervous system have been shown through direct measures of cholinergic and adrenergic effects.34 Considering the central effects of VNS, it seemed reasonable to explore this treatment in a cohort of patients with severe gastroparesis who had failed to respond to pharmacological treatment and were considered suitable candidates for implantable GES.
The vagus nerve is the longest of the cranial nerves, extending from the medulla to the colon, innervating thoracic and abdominal organs including the lungs, heart and gastrointestinal tract.35 It can be described as a continuous circuit eliciting a broad spectrum of modulatory actions in the nervous, immune, autonomic, endocrine, cardiorespiratory and gastrointestinal systems via both afferent and efferent pathways.36 Myelinated A-fibres and B-fibres of the vagus nerve have key roles in somatic sensory, motor and parasympathetic innervation.35 Emerging data further suggest that VNS may induce neuromodulatory antinociception through both peripheral and central systems, and VNS has recently demonstrated potential for use in a wide array of diseases including epilepsy, depression, headache and inflammatory disorders.36 Non-invasive VNS offers a potential alternative to implantable VNS with fewer inherent risks; no serious device-related adverse effects have been reported during trials of nVNS in patients with headache.22 27
This proof-of-concept trial represents the first prospective study of nVNS in gastroparesis. Twelve of the 35 enrolled patients discontinued or were non-compliant with the study protocol. This relatively high rate of discontinuation/non-compliance (34%) may reflect the difficulty that some chronically ill patients experience with the technique of self-administered nVNS and the discipline required for diary completion. Notably, only three patients cited lack of response as their reason for study non-compliance/discontinuation. Of the 23 patients who completed at least 3 weeks of nVNS therapy, 10 were classified as responders. For these patients, nVNS could offer an effective option that is non-invasive, safe and cost-efficient.
Gastroparesis is a severe, difficult-to-treat upper gastrointestinal functional disorder. Our preliminary evidence of symptom improvements in patients with this condition after nVNS therapy suggests that the device should be further evaluated as a therapeutic option, thus potentially allowing responders to delay or eliminate the need for surgical device implantation.
This study has several limitations, including its small sample size (which comprised a heterogeneous group of gastroparesis types), short duration, open-label design and lack of power for formal statistical analyses. The findings from the current exploratory study must be confirmed in larger controlled studies. Longer treatment durations are also needed to exclude the possibility of a placebo effect and to identify further continued benefits, as consistently seen in previous studies.
Previous literature has suggested that a meaningful response may be defined by a 0.5–0.75-point decrease from baseline in GCSI aggregate score.24 25 However, there is no clear consensus regarding the definition of response to short-term therapy for gastroparesis, and we chose a more conservative definition (≥1 point decrease from baseline in GCSI aggregate score) for the current evaluation. The validity of this definition appears to be supported by the divergence in GCSI improvements between the entire study cohort and the subgroup classified as responders (figures 2 and 3).
The mean baseline GCSI score in this study indicates the severity of gastroparesis in the patient cohort.25 Because of the small sample size, a relationship between symptom severity at baseline and likelihood of response could not be determined. The differences in outcomes between patients with diabetic gastroparesis and those with idiopathic gastroparesis and/or differences among those with comorbid depression or anxiety need to be explored. Optimal dose and duration of treatment also require further evaluation. This proof-of-concept study provides preliminary evidence that nVNS could prove to be useful in larger controlled studies of patients with drug-refractory gastroparesis.
Significance of this study.
What is already known on this topic?
Gastric electric stimulation (GES) improves symptoms in patients with treatment-resistant gastroparesis possibly through a central mechanism of action and effects on the autonomic nervous system.
Vagus nerve stimulation (VNS) has shown promising results in treating a wide array of diseases in which central nervous system effects are thought to play a role.
What this study adds?
This is the first report of the use of non-invasive VNS (nVNS) as a new therapeutic approach to gastroparesis.
How might it impact on clinical practice in the foreseeable future?
nVNS could represent a less-invasive and less-expensive alternative to GES in patients with drug-refractory gastroparesis.
Acknowledgments
The authors acknowledge Anton Shklyayev for conducting statistical analyses. Editorial support was provided by Elizabeth Barton, MS, of MedLogix Communications, Schaumburg, Illinois.
Footnotes
Contributors: OE is the guarantor of the article and takes responsibility for the integrity of the work as a whole, from inception to publication. OE conceived the study, helped develop the protocol, provided oversight and authored the manuscript. EP was responsible for training patients to use gammaCore and record data; she also collected and collated diary data, participated in data analysis and helped write the manuscript. DN assisted with patient training, clinical follow-up and data collection. FJ was involved in patient recruitment and clinical follow-up and contributed to the manuscript. JM and EL helped develop the clinical protocol. All authors approved the final version of the article, including the authorship list.
Funding: This study was supported in part by an unrestricted grant from electroCore, Basking Ridge, New Jersey. Editorial support from MedLogix Communications was funded by electroCore and was provided under the direction of the authors in accordance with International Committee of Medical Journal Editors criteria for authorship.
Disclaimer: The authors are guarantors of this document, which expresses the opinions and conclusions of the authors and not those of their corresponding affiliations.
Competing interests: DN received a fellowship grant from electroCore. OE participated in a clinical study supported by electroCore. JM has participated in clinical trials supported by electroCore. EL is an employee of electroCore and receives stock ownership.
Ethics approval: New Devices Committee at the Royal Free London NHS Foundation Trust.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Parkman HP, Hasler WL, Fisher RS, American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004;127:1592–622. doi:10.1053/j.gastro.2004.09.055 [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Vazquez-Roque MI. Chapter 4. Gastric dysmotility at the organ level in gastroparesis. In: Parkman HP, McCallum RW. Gastroparesis: pathophysiology, presentation and treatment. New York, NY: Humana Press, 2012:37–46. [Google Scholar]
- 3.Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 2009;136:1225–33. doi:10.1053/j.gastro.2008.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol 2008;103:313–22. doi:10.1111/j.1572-0241.2007.01658.x [DOI] [PubMed] [Google Scholar]
- 5.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology 2011;140:101–15. doi:10.1053/j.gastro.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci 1998;43:2398–404. doi:10.1023/A:1026665728213 [DOI] [PubMed] [Google Scholar]
- 7.Chang J, Rayner CK, Jones KL, et al. Diabetic gastroparesis-backwards and forwards. J Gastroenterol Hepatol 2011;26(Suppl 1):46–57. doi:10.1111/j.1440-1746.2010.06573.x [DOI] [PubMed] [Google Scholar]
- 8.Moldovan C, Dumitrascu DL, Demian L, et al. Gastroparesis in diabetes mellitus: an ultrasonographic study. Rom J Gastroenterol 2005;14:19–22. [PubMed] [Google Scholar]
- 9.De Block CE, De Leeuw IH, Pelckmans PA, et al. Current concepts in gastric motility in diabetes mellitus. Curr Diabetes Rev 2006;2:113–30. doi:10.2174/157339906775473662 [DOI] [PubMed] [Google Scholar]
- 10.Hasler WL. Chapter 23: Antiemetic treatment for gastroparesis. In: Parkman HP, McCallum RW. Gastroparesis: pathophysiology, presentation and treatment. New York, NY: Humana Press, 2012:279–88. [Google Scholar]
- 11.Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol Ther 2008;27:724–40. doi:10.1111/j.1365-2036.2008.03637.x [DOI] [PubMed] [Google Scholar]
- 12.Acosta A, Camilleri M. Prokinetics in gastroparesis. Gastroenterol Clin North Am 2015;44:97–111. doi:10.1016/j.gtc.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 13.Clarke JO, Snape WJ Jr. Pyloric sphincter therapy: botulinum toxin, stents, and pyloromyotomy. Gastroenterol Clin North Am 2015;44:127–36. doi:10.1016/j.gtc.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 14.Enweluzo C, Aziz F. Gastroparesis: a review of current and emerging treatment options. Clin Exp Gastroenterol 2013;6:161–5. doi:10.2147/CEG.S50236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross J, Masrur M, Gonzalez-Heredia R, et al. Effectiveness of gastric neurostimulation in patients with gastroparesis. JSLS 2014;18:e2014.00400 doi:10.4293/JSLS.2014.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu H, Lin Z, Zhong L, et al. Treatment of high-frequency gastric electrical stimulation for gastroparesis. J Gastroenterol Hepatol 2012;27:1017–26. doi:10.1111/j.1440-1746.2011.06999.x [DOI] [PubMed] [Google Scholar]
- 17.O'Grady G, Egbuji JU, Du P, et al. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg 2009;33:1693–701. doi:10.1007/s00268-009-0096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 2003;125:421–8. doi:10.1016/S0016-5085(03)00878-3 [DOI] [PubMed] [Google Scholar]
- 19.Babic T, Browning KN. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol 2014;722:38–47. doi:10.1016/j.ejphar.2013.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aicher SA, Lewis SJ, Randich A. Antinociception produced by electrical stimulation of vagal afferents: independence of cervical and subdiaphragmatic branches. Brain Res 1991;542:63–70. doi:10.1016/0006-8993(91)90998-B [DOI] [PubMed] [Google Scholar]
- 21.electroCore, LLC. About Us http://www.electrocoremedical.com/about-us (accessed 14 May 2015).
- 22.Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia 2014;34:986–93. doi:10.1177/0333102414524494 [DOI] [PubMed] [Google Scholar]
- 23.Waseem S, Moshiree B, Draganov PV. Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol 2009;15:25–37. doi:10.3748/wjg.15.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res 2004;13:833–44. doi:10.1023/B:QURE.0000021689.86296.e4 [DOI] [PubMed] [Google Scholar]
- 25.Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil 2012;24:456–63. doi:10.1111/j.1365-2982.2012.01879.x [DOI] [PubMed] [Google Scholar]
- 26.Blok BF, Groen J, Bosch JL, et al. Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int 2006;98:1238–43. doi:10.1111/j.1464-410X.2006.06521.x [DOI] [PubMed] [Google Scholar]
- 27.Barbanti P, Grazzi L, Egeo G, et al. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain 2015;16:61 doi:10.1186/s10194-015-0542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krahl SE, Clark KB. Vagus nerve stimulation for epilepsy: a review of central mechanisms. Surg Neurol Int 2012;3:S255–9. doi:10.4103/2152-7806.103015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogbonnaya S, Kaliaperumal C. Vagal nerve stimulator: evolving trends. J Nat Sci Biol Med 2013;4:8–13. doi:10.4103/0976-9668.107254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JD, Yin J, McCallum RW. Chapter 29: Gastric electrical stimulation for gastroparesis. In: Parkman HP, McCallum RW. Gastroparesis: pathophysiology, presentation and treatment. New York, NY: Humana Press, 2012:353–64. [Google Scholar]
- 31.McCallum RW, Dusing RW, Sarosiek I, et al. Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterol Motil 2010;22:161–7, e50–1 doi:10.1111/j.1365-2982.2009.01389.x [DOI] [PubMed] [Google Scholar]
- 32.Monnikes H, van der Voort IR. Gastric electrical stimulation in gastroparesis: where do we stand? Dig Dis 2006;24:260–6. doi:10.1159/000092879 [DOI] [PubMed] [Google Scholar]
- 33.Yin J, Abell TD, McCallum RW, et al. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation 2012;15:224–31. doi:10.1111/j.1525-1403.2012.00429.x [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Rashed H, Eaton P, et al. Long term treatment at gastric electrical stimulation is associated with autonomic and enteric nervous system changes [abstract A6]. Dig Dis Sci 2000;46:1246. [Google Scholar]
- 35.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part I. Headache 2016;56:71–8. doi:10.1111/head.12647 [DOI] [PubMed] [Google Scholar]
- 36.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part III. Headache 2016;56:479–90. doi:10.1111/head.12649 [DOI] [PubMed] [Google Scholar]