Abstract
Objective
To compare all-cause and liver-related hospital resource use in the 6 and 12 months pre-rifaximin-α and post-rifaximin-α initiation in UK patients with hepatic encephalopathy (HE).
Design
A UK multicentre, retrospective, observational study. Patients' medical records were reviewed for demographics, clinical outcomes and adverse events (AEs) to rifaximin-α. Details of hospital admissions/attendances in the 6 and 12 months pre-rifaximin-α and post-rifaximin-α initiation were extracted from hospital electronic databases.
Setting
13 National Health Service centres.
Patients
207 patients with HE who initiated rifaximin-α between July 2008 and May 2014. Hospital resource use data were available for 145/207 patients.
Main outcome measure
Change in mean number of liver-related hospital bed days/patient (total and critical care) between the 6 months pre-rifaximin-α and post-rifaximin-α initiation.
Results
Comparing the 6 months pre-rifaximin-α and post-rifaximin-α initiation in alive patients at the end of the observation period (N=114): there were significant reductions in the mean number of hospitalisations/patient (liver-related 1.3 to 0.5, p<0.001; all-cause 1.9 to 0.9, p<0.001), hospital bed days/patient (liver-related 17.8 to 6.8, p<0.001; all-cause 25.4 to 10.6, p<0.001), 30-day hospital readmissions/patient (liver-related 0.5 to 0.2, p=0.039; all-cause 0.8 to 0.4, p=0.024) and emergency department (ED) attendances/patient (all-cause, 1.0 to 0.5, p<0.001). The mean critical care bed days/patient reduced significantly for all-cause admissions (1.3 to 0.3, p=0.049); non-significant reduction for liver-related admissions. 4% of patients (9/207) developed AEs.
Conclusions
In UK clinical practice, treatment with rifaximin-α for HE is well-tolerated and associated with significant reductions in hospitalisations, bed days (including critical care), ED attendances and 30-day readmissions.
Keywords: LIVER CIRRHOSIS, HEPATIC ENCEPHALOPATHY, HEALTH ECONOMICS, ANTIBIOTIC THERAPY
Introduction
Hepatic encephalopathy (HE) is a well-recognised complication of cirrhosis, which is associated with considerable morbidity and mortality. Subtle signs of HE are observed in approximately 60%1 and overt HE (OHE) in about 30–45% of patients with cirrhosis.2 After a first OHE episode, the 1-year cumulative probability of recurrence is 40%,3 and the 1-year and 3-year survival probabilities are 42% and 23%, respectively.4 The mortality risk associated with HE is higher than that associated with other major hepatic decompensation events.5 HE can adversely affect patients' quality of life (QoL) and cognitive function.6 7 Furthermore, because OHE requires hospital management, it places a considerable burden on healthcare resources.8
Treatment for HE aims to resolve OHE episodes and prevent recurrences.9 Rifaximin-α (TARGAXAN, Norgine) is a minimally absorbed, oral antibiotic which has been shown, in clinical trials, to reduce the risk of OHE episodes, HE-related hospitalisations and improve health-related QoL, compared with placebo.10–12 It is licensed for the reduction in recurrence of OHE episodes and, in March 2015, the National Institute for Health and Care Excellence (NICE) approved Technology Appraisal 337 (TA337) for rifaximin-α use in the UK National Health Service (NHS) within its marketing authorisation.13
Currently, there is limited information describing the impact of rifaximin-α on healthcare resource use in a real-world setting. This is critical to enable clinical decision makers to evaluate the cost-effectiveness of rifaximin-α in routine clinical practice. A number of UK audits have shown reductions in all-cause hospitalisations and bed occupancy with rifaximin-α treatment,14–18 but these were mainly single-centre studies and the results need to be confirmed in a range of centres using a standardised data collection methodology. To date, no European studies have been published on the impact of rifaximin-α on critical care admissions, emergency department (ED) attendances and 30-day emergency readmissions.
IMPRESS was a retrospective, observational study conducted in 13 UK centres ranging from District General Hospitals to liver transplant units to evaluate the real-world impact of rifaximin-α on all-cause and liver-related NHS hospital resource use.
Materials and methods
Patient population and data collection
The study was conducted between August 2014 and June 2015 in 13 geographically dispersed UK NHS hospitals.
Patients with a documented clinical diagnosis of HE, who were initiated on rifaximin-α ≥12 months before data collection, were included in the study. Exclusion criteria included initiation of rifaximin-α at other hospitals or in primary care and unavailability of medical records. Patients with new prescriptions for rifaximin-α were identified from pharmacy records and selected in reverse chronological order until the centre-specific recruitment target was reached (or until all eligible patients were identified, if below target).
Patients' medical records were reviewed for baseline demographic and disease characteristics, rifaximin-α prescribing, concomitant medications, clinical outcomes and adverse events (AEs) during treatment. Resource use data were extracted from hospitals' central management information systems; parameters included hospitalisations, critical care admissions, non-elective admissions, 30-day emergency readmissions, ED attendances and reasons for hospitalisations (using International Classification of Diseases (ICD)-10 codes). The maximum observation period for each patient was 12 months before and after the date of rifaximin-α initiation, irrespective of treatment duration.
A ‘liver-related’ hospitalisation was defined as any hospitalisation with a primary ICD-10 diagnosis code of K70-77/C22.0/I85-86 (see online supplementary table S1). For hospitalisations with the primary ICD-10 codes R18/R41.0/R41/G93.4/F10.3/F05.9/I98.2/I98.3 (see online supplementary table S2), anonymised records were reviewed on a case-by-case basis by a medical representative of the study sponsor to determine whether the admission was liver-related.
flgastro-2016-100792supp001.pdf (548KB, pdf)
Primary and secondary outcomes
The primary outcome was the change in the mean number of liver-related hospital bed days per patient (total and critical care) between the 6 months pre-rifaximin-α and post-rifaximin-α initiation. Secondary outcomes included changes between the 6 or 12 months pre-rifaximin-α and post-rifaximin-α initiation in the number of all-cause and liver-related hospitalisations (overnight stay), hospital bed days, critical care admissions and bed days, non-elective admissions, 30-day emergency readmissions (defined as a hospital admission occurring within 30 days of a previous discharge) and ED attendances (without admission); also clinical outcomes and AEs.
Statistical analysis
The target sample size of 250–300 patients was based on two previous UK evaluations,15 17 in which reductions of ∼1.5 hospital bed days/patient/month were observed in the 6 months post-rifaximin-α initiation. To describe a similar reduction at p<0.05, a sample of 25–30 patients was required. It was important for the sample size to give sufficient reliability for analysis of both the overall study population and subgroups of patients with different Model for End-Stage Liver Disease (MELD) scores. A MELD score of 19–24 was expected to occur at a frequency of ∼10%;10 a 10% subgroup of 25–30 thus required an overall sample of 250–300. Analyses were conducted using Microsoft Excel and Stata using the available data, with no imputation of missing values.
Resource use endpoints were analysed for the intention-to-treat (ITT) population and for the patients who were alive (‘surviving patients’) at the end of the period investigated (6 or 12 months post-rifaximin-α initiation). The latter analysis was conducted to reduce non-survivor confounding, as resource use, calculated including deceased patients, may overestimate the benefit of rifaximin-α. The statistical significance of the mean change for each parameter was calculated using a paired t-test.
Results
Patient and treatment characteristics
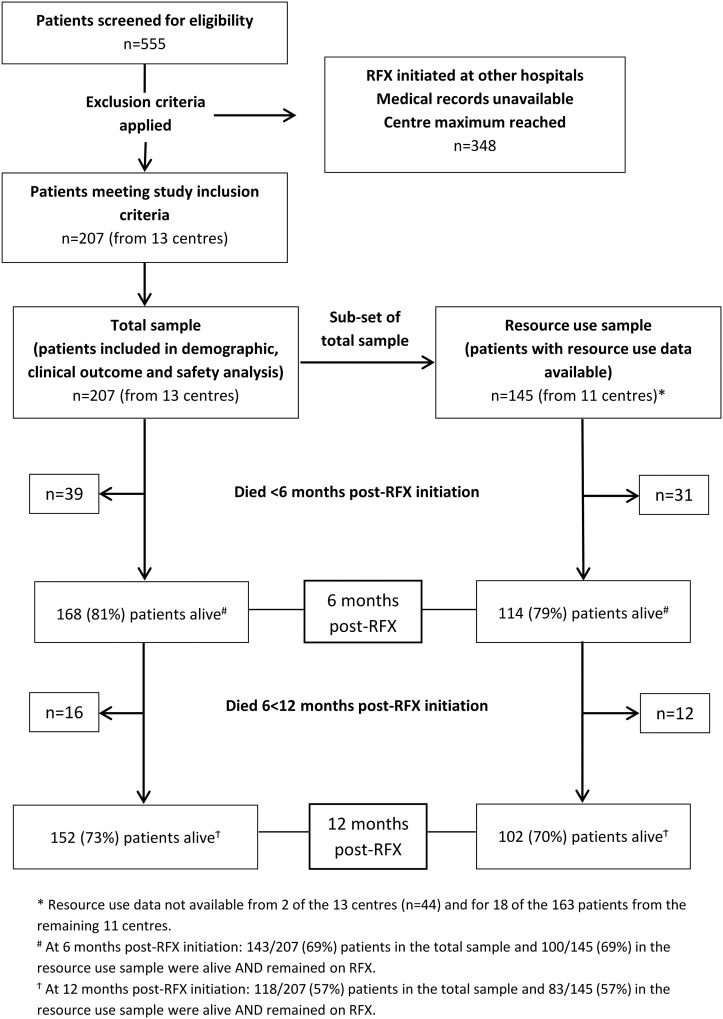
The study included 207 patients (range 5–25 per centre), who initiated rifaximin-α between 3 July 2008 and 8 May 2014. Resource use data were available for 145 patients (70%), from 11 of the 13 centres. The flow of patients through the study is shown in figure 1. Table 1 summarises patient baseline characteristics, which did not differ significantly between the total sample and the resource use cohort. The mean age of patients was 59 years (SD±11.4) at diagnosis of HE, 61% were male and 64% had alcohol-related liver disease (ARLD) (table 1).
Figure 1.
Study flow. RFX, rifaximin-α.
Table 1.
Baseline demographic, disease and treatment characteristics
| Characteristic | Total sample (N=207) | Resource use cohort (N=145) |
|---|---|---|
| Gender (n, %) | ||
| Male | 127 (61%) | 89 (61%) |
| Female | 80 (39%) | 56 (39%) |
| Age (years) — mean (±SD) | ||
| At diagnosis of cirrhosis | 57.3 (±11.9) N=191 | 58.7 (±11.7) N=132 |
| At diagnosis of HE | 59.3 (±11.4) | 60.3 (±11.5) |
| At initiation of rifaximin-α | 60.0 (±11.4) | 60.9 (±11.5) |
| Baseline Child-Pugh score (n, %) | ||
| A | 12 (6%) | 7 (5%) |
| B | 58 (28%) | 36 (25%) |
| C | 44 (21%) | 24 (17%) |
| Not recorded | 93 (45%) | 78 (54%) |
| Baseline MELD score (n, %) | ||
| ≤10 | 29 (14%) | 19 (13%) |
| 11–18 | 76 (37%) | 43 (30%) |
| 19–24 | 25 (12%) | 14 (10%) |
| ≥25 | 29 (14%) | 19 (13%) |
| Not recorded | 48 (23%) | 50 (34%) |
| Time from cirrhosis diagnosis to initiation of rifaximin-α, months | ||
| Mean (SD) | 38.8 (49.2) N=191 | 33.9 (±46.6) N=132 |
| Median (IQR) | 22.4 (6.8–54.3) | 17.9 (4.7–47.3) |
| Time from HE diagnosis to initiation of rifaximin-α, months | ||
| Mean (SD) | 8.3 (17.5) | 7.5 (±15.1) |
| Median (IQR) | 1.6 (0.1–9.0) | 1.9 (0.1–9.2) |
| Underlying liver disease aetiology (not mutually exclusive) | ||
| Alcohol-related liver disease | 133 (64%) | 98 (68%) |
| Non-alcoholic steatohepatitis | 50 (24%) | 32 (22%) |
| Hepatitis B or C | 22 (11%) | 13 (9%) |
| Autoimmune hepatitis | 5 (2%) | 2 (1%) |
| Primary biliary cirrhosis | 1 (0.5%) | 0 (0%) |
| Haematochromatosis | 1 (0.5%) | 0 (0%) |
| Non-alcoholic fatty liver disease | 2 (1%) | 2 (1%) |
| Cryptogenic | 6 (3%) | 5 (3%) |
| Other | 7 (3%) | 6 (4%) |
| Not recorded | 2 (1%) | 2 (1%) |
| Rifaximin-α dose (n, %) | ||
| 1100 mg/day | 70 (34%) | 43 (30%) |
| 1200 mg/day | 126 (61%) | 93 (64%) |
| Other doses | 11 (5%) | 9 (6%) |
| Rifaximin-α initiated during an overt HE episode | 152 (73%) | 100 (69%) |
| Patients drinking alcohol at rifaximin-α initiation (n, %) | ||
| Yes | 35 (17%) | 26 (18%) |
| No | 143 (69%) | 102 (70%) |
| Unknown | 29 (14%) | 17 (12%) |
| Concomitant lactulose use (n, %) | 174 (84%) | 119 (82%) |
| Listed for liver transplantation (n, %) | 19 (9%) | 7 (5%) |
There were no significant differences between the total sample and the resource use cohort in gender distribution (χ2 test, p=0.996), age (t-test, p=0.2961 (at diagnosis of cirrhosis), p=0.402 (at diagnosis of HE), p=0.4681 (at initiation of rifaximin-α)) or baseline MELD score (χ2 test, p=0.221). Remaining characteristics not compared.
HE, hepatic encephalopathy; MELD, Model for End-Stage Liver Disease.
Rifaximin-α and lactulose treatment are presented in table 1. Rifaximin-α was discontinued within 12 months in 43% (88/207) of patients, most commonly due to death (52%, 46/88), transplant (14%, 12/88) or HE resolution (7%, 6/88). Of the 88 patients who discontinued treatment, 47% (41/88) stopped <12 weeks, 22% (19/88) 12<26 weeks and 28% (25/88) 26<52 weeks after rifaximin-α initiation (3% (3/88), time unknown).
Clinical outcomes
A total of 81% (168/207) of patients were alive at 6 months and 73% (152/207) at 12 months post-rifaximin-α initiation. There were 55 deaths; of these, 55% (30/55) were known to be related to ARLD or end-stage liver disease, although no specific cause of death was documented. Causes of death in the remaining patients included hepatocellular carcinoma (11%, 6/55), pneumonia (7%, 4/55), multiorgan failure (5%, 3/55), gastrointestinal bleed (5%, 3/55) and other (5%, 3/55); cause not documented in 11% (6/55) of patients. The mean baseline MELD score was 15.8 (SD±8.1) in surviving and 20.7 (SD±6.4) in deceased patients.
Overall, OHE episodes were experienced by 57% (117/207) of patients in the 12 months pre-rifaximin-α versus 38% (79/207) in the 12 months post-rifaximin-α initiation; the mean number of episodes per patient (N=207) decreased from 1.01 (SD±1.5) in the 12 months pre-rifaximin-α to 0.77 (SD±1.3) in the 12 months post-rifaximin-α initiation (p=0.047). In the subgroup of 152 patients who were alive at 12 months post-rifaximin-α initiation, OHE episodes were experienced by 55% (84/152) of patients in the 12 months pre-rifaximin-α versus 36% (55/152) in the 12 months post-rifaximin-α initiation; the mean number of episodes per patient (N=152) decreased from 1.02 (SD±1.3) in the 12 months pre-rifaximin-α to 0.74 (SD±1.3) in the 12 months post-rifaximin-α initiation (p=0.068).
During the study, 6% (13/207) of patients received a liver transplant and 9% (16/173 with data available) were placed on a palliative care pathway. At 12 months post-rifaximin-α initiation, 9% (11/118) of the patients who were alive and on rifaximin-α were not abstinent.
Safety data
Nine patients (4%) had documented AEs: Clostridium difficile infection (n=4), rash (n=2), abdominal pain, vomiting and discolouration of teeth (one patient each). No serious AEs were reported. Of the four patients who developed C difficile infection, none had a history of C difficile and none was on concomitant antibiotics. All patients continued rifaximin-α therapy.
Resource use: ITT population
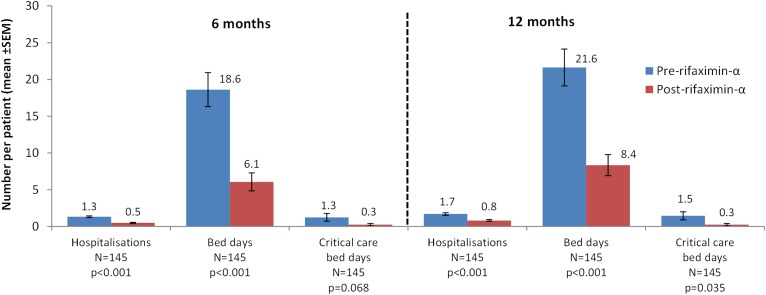
The number of liver-related hospital bed days (primary outcome), critical care bed days and hospitalisations per patient in the pre-rifaximin-α and post-rifaximin-α initiation periods is shown in figure 2 for the ITT population; this analysis included deceased patients.
Figure 2.
Liver-related resource use in the 6 and 12 months pre-rifaximin-α and post-rifaximin-α initiation — intention-to-treat population.
In the 6 months post-rifaximin-α initiation, there were reductions in the mean number of liver-related hospitalisations (p<0.001), hospital bed days (p<0.001) and critical care bed days (p=0.068) per patient (figure 2). Similar results were observed for the 12-month rifaximin-α period, however, with a significant reduction in critical care bed days per patient (p=0.035) (figure 2).
Resource use: surviving patients
The analysis in ‘surviving patients’ included 114 patients alive at 6 months and 102 patients alive at 12 months post-rifaximin-α initiation.
The proportion of hospitalised patients decreased from 69% (79/114) in the 6 months pre-rifaximin-α to 32% (37/114) in the 6 months post-rifaximin-α initiation for liver-related hospitalisations and from 80% (91/114) to 50% (57/114) for all-cause hospitalisations. For the 12 months pre-rifaximin-α and post-rifaximin-α initiation, the proportion of hospitalised patients decreased from 72% (73/102) to 44% (45/102) for liver-related hospitalisations and from 85% (87/102) to 65% (66/102) for all-cause; 3% (3/102) of patients had no hospitalisations. Overall, 63% (170/271) of all hospitalisations in the 12 months pre-rifaximin-α and 49% (83/168) in the 12 months post-rifaximin-α initiation were liver-related.
Hospital resource use in surviving patients is shown in table 2. In the 6 months post-rifaximin-α initiation, there were significant reductions in the mean number of hospitalisations per patient (p<0.001 liver-related, p<0.001 all-cause), hospital bed days per patient (p<0.001 liver-related, p<0.001 all-cause) and hospital bed days per admission (p=0.001 liver-related, p=0.001 all-cause). Similar reductions were observed for the 12-month observation period.
Table 2.
Resource use in surviving patients
| 6 months (N=114) |
12 months (N=102) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Resource use parameter* | n† | Pre-RFX initiation‡ | Post-RFX initiation‡ | p Value§ | n† | Pre-RFX initiation‡ | Post-RFX initiation‡ | p Value§ |
| Liver-related resource use | ||||||||
| Total hospital bed days | 89 | 2034 | 769 | – | 85 | 2135 | 910 | – |
| Hospitalisations per patient | 89 | 1.3 (0.1) | 0.5 (0.1) | <0.001 | 85 | 1.7 (0.2) | 0.8 (0.1) | <0.001 |
| Hospital bed days per patient | 89 | 17.8 (2.6) | 6.8 (1.5) | <0.001 | 85 | 20.9 (2.9) | 8.9 (1.9) | <0.001 |
| Hospital bed days per admission | 89 | 10.9 (1.5) | 4.8 (1.1) | 0.001 | 85 | 10.9 (1.4) | 5.2 (1.2) | 0.002 |
| Critical care admissions per patient | 16 | 0.2 (0.04) | 0.1 (0.02) | 0.027 | 15 | 0.2 (0.04) | 0.1 (0.03) | 0.01 |
| Critical care bed days per patient | 16 | 1.1 (0.4) | 0.3 (0.2) | 0.109 | 15 | 1.4 (0.5) | 0.3 (0.2) | 0.059 |
| Non-elective admissions per patient | 74 | 1.0 (0.1) | 0.4 (0.1) | <0.001 | 71 | 1.3 (0.2) | 0.6 (0.1) | <0.001 |
| 30-Day emergency readmissions per patient | 37 | 0.5 (0.1) | 0.2 (0.1) | 0.039 | 34 | 0.6 (0.2) | 0.3 (0.1) | 0.084 |
| All-cause resource use | ||||||||
| Total hospital bed days | 101 | 2890 | 1206 | – | 99 | 3138 | 1621 | – |
| Hospitalisations per patient | 101 | 1.9 (0.2) | 0.9 (0.1) | <0.001 | 99 | 2.7 (0.3) | 1.7 (0.2) | 0.002 |
| Hospital bed days per patient | 101 | 25.4 (2.9) | 10.6 (2.1) | <0.001 | 99 | 30.8 (3.5) | 15.9 (2.9) | <0.001 |
| Hospital bed days per admission | 101 | 12.5 (1.5) | 6.4 (1.3) | 0.001 | 99 | 12.6 (1.5) | 5.8 (0.9) | <0.001 |
| Critical care admissions per patient | 19 | 0.2 (0.04) | 0.1 (0.02) | 0.008 | 18 | 0.2 (0.05) | 0.1 (0.03) | 0.002 |
| Critical care bed days per patient | 19 | 1.3 (0.5) | 0.3 (0.2) | 0.049 | 18 | 2.0 (0.6) | 0.4 (0.3) | 0.021 |
| Non-elective admissions per patient | 88 | 1.6 (0.2) | 0.8 (0.1) | <0.001 | 87 | 2.2 (0.3) | 1.3 (0.2) | 0.006 |
| 30-Day emergency readmissions per patient | 50 | 0.8 (0.2) | 0.4 (0.1) | 0.024 | 52 | 0.9 (0.2) | 0.6 (0.1) | 0.16 |
| ED attendances per patient¶ | 61 | 1.0 (0.2) | 0.5 (0.1) | <0.001 | 62 | 1.5 (0.3) | 1.1 (0.2) | 0.116 |
Definitions: ‘Hospitalisation’ includes overnight stay admissions only (ie, excluding day case); it includes both non-elective (unplanned) admissions (via ED or GP direct referral route) and elective (planned) admissions. ‘ED attendance’ includes only attendances which did not result in hospital admission.
*Data are presented for all surviving patients at the end of 6 months (N=114) or 12 months (N=102).
†Number of patients with ≥1 admission/attendance in the observed periods (pre-RFX, post-RFX or both).
‡Shown as the mean (SEM) per patient.
§Paired t-test.
¶Data only available for all-cause (not liver-related).
ED, emergency department; GP, general practitioner; RFX, rifaximin-α.
There were also significant reductions in liver-related and all-cause critical care admissions per patient at both 6 (p=0.027 liver-related, p=0.008 all-cause) and 12 months (p=0.01 liver-related, p=0.002 all-cause) post-rifaximin-α initiation. The reduction in all-cause critical care bed days per patient was significant at both 6 (p=0.049) and 12 months (p=0.021), but the reduction in liver-related critical care bed days was not significant at either time point (table 2, see online supplementary figure S1).
There were significant reductions in liver-related and all-cause non-elective admissions at both 6 (p<0.001 liver-related, p<0.001 all-cause) and 12 months (p<0.001 liver-related, p=0.006 all-cause).
There were significant reductions in both liver-related and all-cause 30-day emergency readmissions at 6 months (p=0.039 liver-related, p=0.024 all-cause); however, the reductions at 12 months were not significant. Similarly, for ED attendances (all-cause only) there was a significant reduction at 6 (p<0.001) but not 12 months (table 2).
In the subgroup of alive patients prescribed rifaximin-α 1100 mg/day (n=31, 6 months; n=30, 12 months), significant reductions in all-cause hospitalisations and bed days per patient were observed at 6 months, and were maintained at 12 months (see online supplementary table S3).
All-cause and liver-related resource use calculated in surviving patients with ≥1 admissions/attendances in the observed periods is shown in online supplementary table S4.
Discussion
IMPRESS included 207 UK patients with HE treated with rifaximin-α in routine clinical practice. Treatment with rifaximin-α was well-tolerated and associated with significant reductions in all-cause hospitalisations and bed days, affirming the findings of previous randomised controlled trials (RCTs) and UK audits.14–18 Also, importantly, there were significant reductions in critical care admissions and bed days, non-elective admissions, 30-day emergency readmissions and ED attendances, which have not been demonstrated previously. Statistically significant reductions in liver-related resource use were observed within 6 months of treatment initiation and largely sustained at 12 months.
Clinical outcomes
The proportion of patients experiencing OHE episodes decreased in the 12 months post-rifaximin-α initiation (absolute reduction 18% in the overall group) and the number of episodes per patient also decreased significantly. Although anticipated, this finding confirms the improvements in HE outcomes observed in rifaximin-α clinical trials in routine clinical practice.10
The mortality rate in this study (19% at 6 months, 27% at 12 months) is similar to the recent seven-centre UK audit (12-month mortality 21%)18 but higher than the pivotal trial (6-month mortality 6.4% in rifaximin-α-treated patients).10 This difference is likely to be because patients with MELD scores ≥25 were excluded from the RCT.10
The low number of AEs and C difficile cases in our study demonstrates good tolerability and is consistent with both the clinical trials of rifaximin-α and previous UK audits.10 12 18 All patients experiencing C difficile had several other risk factors; rifaximin-α therapy was maintained.
Resource use
Overall, this study indicates a large burden of inpatient hospital care for HE patients, with 97% of the surviving patients hospitalised during the 24-month observation period and 51% having at least one 30-day emergency readmission. There are limited UK studies quantifying the resource use burden associated with HE with which to compare these results.18 19 However, the pattern of repeat hospitalisations in HE patients has been widely reported and is associated with negative consequences including increased in-hospital mortality.6 20 In the present study, fewer patients were hospitalised in the post-rifaximin-α initiation period, and the number of all-cause hospitalisations and bed days, as well as critical care admissions and bed days, reduced significantly. The reduction in all-cause hospital bed days per patient from 30.8 in the 12 months pre-rifaximin-α to 15.9 in the 12 months post-rifaximin-α initiation, in the ‘surviving patient’ group, is similar to the recent seven-centre audit.18 There were also significant reductions in all-cause non-elective hospitalisations, ED attendances and 30-day emergency readmissions, suggesting that rifaximin-α is associated with a considerable reduction in the burden of unplanned hospital care for patients with HE.
In addition to all-cause resource use, the present study demonstrated significant reductions in liver-related hospitalisations and bed days after rifaximin-α initiation, not evaluated in the earlier UK audits.
As the estimated cost of a general liver-related hospitalisation is £400/day21 and a critical care admission for patients with cirrhosis £1000/day,22 these findings suggest potential for significant NHS cost reductions and will be useful to payers and clinical decision makers when evaluating the cost-effectiveness of rifaximin-α.
Strengths and limitations
IMPRESS was conducted in a real-world setting in a range of geographically dispersed UK centres. Baseline patient characteristics were similar to previous studies,10 18 and broadly representative of UK patients with advanced liver disease.23 Consequently, the data should be generalisable to wider patient populations. Additionally, we evaluated a broader range of resource use descriptors than previous UK audits; the impact of rifaximin-α on critical care resources, 30-day emergency readmissions, ED attendances and liver-related admissions specifically, has not been studied previously.
There are a number of limitations. This was not an RCT, rather, patients acted as their own controls in a pre-study versus post-study design. This is a well-established method in observational research, which was employed in previous UK audits of rifaximin-α effectiveness; nevertheless, the results are open to confounding by the effects of unidentified changes in management over time, such as referral for liver transplantation, placement on the palliative care pathway, increased contact with specialist hepatologists or reduced alcohol consumption. However, overall, the comparison between the pre-rifaximin-α and post-rifaximin-α periods is probably biased against rifaximin-α since the patients' condition will have deteriorated over time.
The study quantifies resource use in the hospital setting only, not including primary care, hospices or nursing homes, but this limitation applies equally in both observation periods.
Although we planned to include 250 patients in the study, resource use data were only available for analysis from 145 patients; this may have affected the power of the study and its ability to detect differences between the pre-rifaximin-α and post-rifaximin-α initiation periods. Nevertheless, significant changes were observed in most resource use parameters.
The classification of liver-related and all-cause resource use involved a degree of subjective interpretation and the validity of categorisation could have been affected by factors including clinical coding accuracy and inconsistencies between centres. Additionally, the involvement of the study sponsor (rather than an independent source) in this process is a potential source of commercial bias.
Conclusions
This study provides strong evidence for the positive impact of rifaximin-α on NHS hospital resource use in the real-world setting. The findings confirm the reductions in hospitalisations and bed days observed in previous studies and, additionally, show significant reductions in critical care resources, non-elective admissions, 30-day emergency readmissions and ED attendances. Overall, the results support the use of rifaximin-α for HE management to improve care and outcomes for patients with advanced liver disease. A prospective real-world study investigating the resource use, clinical effectiveness and safety of rifaximin-α in HE (NCT02488993) is underway and will address some of the limitations of this study.
Significance of this study.
What is already known on this topic
A number of single-centre UK audits have shown reductions in all-cause hospitalisation frequency and bed occupancy with rifaximin-α treatment, but these results need to be confirmed in a range of centres.
What this study adds
This is the first UK study that provides clear evidence of the positive real-world impact of rifaximin-α on National Health Service hospital resource use. There were significant reductions in all-cause hospitalisations and bed days, and in critical care admissions and bed days. Reductions were also seen in non-elective admissions, 30-day emergency readmissions and ED attendances, which were significant within 6 months of treatment initiation and largely sustained at 12 months. In addition to all-cause resource use, the present study demonstrated significant reductions in liver-related hospital bed days and hospitalisations after rifaximin-α initiation, not evaluated in the earlier UK audits.
How might it impact on clinical practice in the foreseeable future
This study supports the use of rifaximin-α for the management of hepatic encephalopathy in patients with advanced liver disease and will help clinical decision makers to evaluate the cost-effectiveness of rifaximin-α to advocate its use in routine clinical care.
Acknowledgments
The authors thank the following for their contribution to study data collection: Sister Lorna Brownlee, Research Nurse at The Freeman Hospital, Newcastle Upon Tyne. Dr William Bernal (Co-investigator), Dr Vishal C Patel (Clinical Research Fellow), Ms Ane Zamalloa (Liver Research Nurse) and Mr Andrew Ayers at King's College Hospital, London. Dr Rosario Spinella at Royal Free Hospital, London. The Research and Development Unit at Royal Free Hospital, London. Dr Anne Robins at Addenbrookes Hospital, Cambridge. Ms Amber Kane and Ms Beverley Longhurst at Portsmouth Hospitals NHS Trust. Ms Melanie Kent, Hepatology Research Nurse at University Hospital of North Durham. The Hepatology Research Team at Royal Liverpool Hospital. The authors also thank Laura Baldock of pH Associates, Marlow, who provided medical writing support.
Footnotes
Contributors: MH and RJA contributed to the design of the study and the collection, analysis and interpretation of the study data. AR contributed to the design of the study and the analysis and interpretation of the study data. PDM contributed to the analysis and interpretation of the study data, to the publication planning and management. RC analysed the data. SDR, JFD, RTP, RJ, PR and EF contributed to the collection of the study data. WJC, DLS and MA contributed to the design of the study and the collection of the study data. MW contributed to the collection and interpretation of the study data. SS and PR contributed to the design of the study and the collection and interpretation of the study data. All authors critically reviewed the manuscript and approved the final version for publication.
Funding: This study was sponsored and funded by Norgine. Norgine personnel were involved in the design of the study, analysis and interpretation of the study data, review of the draft manuscript and approval of the final version. pH Associates, an independent research consultancy, was commissioned by Norgine to provide support with the design and conduct of the study, data analysis and medical writing.
Competing interests: AR was an employee of Norgine at the time this work was undertaken. PDM is an employee of Norgine. RC is an employee of pH Associates, which was commissioned by Norgine to provide support with the design and conduct of the study, data analysis and medical writing. JFD has received honoraria for lectures from Norgine. DS and MH have served as a speaker, consultant and an advisory board member for Norgine. RJ: Inventor (Ornithine phenyl acetate for the treatment of hepatic encephalopathy (licensed to Ocera Therapeutics)); Consultancy and Speaker Fees (Ocera Therapeutics, Norgine); Research Collaboration (Ocera Therapeutics, Takeda); Chief Investigator (Sequana medical sponsored study of alfapump); Founder (UCL spin-out company, Yaqrit (Yaq-001, TLR4 antagonist, DIALIVE: Albumin exchange and Endotoxin removal)). MA and RJA have attended UK Advisory Boards for Norgine. EF has served as consultant and speaker for Norgine. SS has received travel support from Norgine, AbbVie, Merck and Roche and attended, in advisor capacity, two meetings hosted by Norgine related to research in HE. SDR, WJC, RTP, MW and PR have no competing interests.
Ethics approval: Proportionate Review Sub-Committee of the West of Scotland 3 Research Ethics Committee (reference 14/WS/1017).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Das A, Dhiman RK, Saraswat VA, et al. . Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol 2001;16:531–5. doi:10.1046/j.1440-1746.2001.02487.x [DOI] [PubMed] [Google Scholar]
- 2.Amodio P, Del Piccolo F, Pettenò E, et al. . Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol 2001;35:37–45. doi:10.1016/S0168-8278(01)00129-5 [DOI] [PubMed] [Google Scholar]
- 3.Sharma BC, Sharma P, Agrawal A, et al. . Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology 2009;137:885–91, 891.e1 doi:10.1053/j.gastro.2009.05.056 [DOI] [PubMed] [Google Scholar]
- 4.Bustamante J, Rimola A, Ventura PJ, et al. . Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999;30:890–5. doi:10.1016/S0168-8278(99)80144-5 [DOI] [PubMed] [Google Scholar]
- 5.Jepsen P, Ott P, Andersen PK, et al. . Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1675–82. doi:10.1002/hep.23500 [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Schubert CM, Heuman DM, et al. . Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010;138:2332–40. doi:10.1053/j.gastro.2010.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj JS, Wade JB, Gibson DP, et al. . The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011;106:1646–53. doi:10.1038/ajg.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007;25(Suppl 1):3–9. doi:10.1111/j.1746-6342.2006.03215.x [DOI] [PubMed] [Google Scholar]
- 9.Vilstrup H, Amodio P, Bajaj JS, et al. . Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by AASLD and EASL. The American Association for the Study of Liver Diseases 2014 http://www.aasld.org/sites/default/files/guideline_documents/hepaticencephenhanced.pdf. [DOI] [PubMed]
- 10.Bass NM, Mullen KD, Sanyal A, et al. . Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071–81. doi:10.1056/NEJMoa0907893 [DOI] [PubMed] [Google Scholar]
- 11.Sanyal A, Younossi ZM, Bass NM, et al. . Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy—a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011;34:853–61. doi:10.1111/j.1365-2036.2011.04808.x [DOI] [PubMed] [Google Scholar]
- 12.Mullen KD, Sanyal AJ, Bass NM, et al. . Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 2014;12:1390–7.e2. doi:10.1016/j.cgh.2013.12.021 [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Rifaximin for preventing episodes of overt hepatic encephalopathy: NICE technology appraisal guidance [TA337] 2015. http://www.nice.org.uk/guidance/ta337.
- 14.Goel A, Patel N, Blackwell R, et al. . PWE-125 rifaximin treatment in hepatic encephalopathy (HE)—marked reduction in hospital admissions and hospital bed day occupancy in a District General Hospital. Gut 2014;63:A179–80. doi:10.1136/gutjnl-2013-305112 [Google Scholar]
- 15.Orr J, Perez F, Mitchison H, et al. . PTU-112 rifaximin for hepatic encephalopathy is cost effective at reducing emergency hospital admission. Gut 2013;62:A91–2. doi:10.1136/gutjnl-2013-304907.202 [Google Scholar]
- 16.Roach J, Heron T, Henry E, et al. . The Real World Experience of Rifaximin in Hepatic Encephalopathy. Poster presentation at Falk Symposium 191. Liver Diseases in 2013: Advances in Pathogenesis and Treatment; 2013 October 4–5, London, UK.
- 17.Valliani T, Sumner M, Gordon F. Rifaximin treatment in hepatic encephalopathy—A single centre experience. Poster presentation at Falk Symposium 186. Challenges of Liver Cirrhosis and Tumors: Prevent it, Treat it, Manage Consequences; 2012, October 5–6, Mainz, Germany.
- 18.Orr JG, Currie CJ, Berni E, et al. . The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-α. Liver Int Off J Int Assoc Study Liver 2016;36:1295–303. doi:10.1111/liv.13111 [DOI] [PubMed] [Google Scholar]
- 19.Orr J, Morgan CL, Hudson M, et al. . PTU-127 resource use associated with hepatic encephalopathy in patients with severe liver disease. Gut 2014;63:A94–5. doi:10.1136/gutjnl-2014-307263.201 [Google Scholar]
- 20.Saab S. Evaluation of the impact of rehospitalization in the management of hepatic encephalopathy. Int J Gen Med 2015;8:165–73. doi:10.2147/IJGM.S81878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department of Health. NHS reference costs 2014 to 2015 2015. http://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015.
- 22.Shawcross DL, Austin MJ, Abeles RD, et al. . The impact of organ dysfunction in cirrhosis: survival at a cost? J Hepatol 2012;56:1054–62. doi:10.1016/j.jhep.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 23.National End of Life Care Intelligence Network. Deaths from liver disease: Implications for end of life care in England. 2012. file:///C:/Users/lbaldock/Downloads/Deaths_fr_liver_disease_report_FINAL_Report%20(1).pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2016-100792supp001.pdf (548KB, pdf)