Abstract
The aquatic bacterium and human intestinal pathogen, Vibrio cholerae, senses and responds to a variety of environment-specific cues to regulate biofilm formation. Specifically, the polyamines norspermidine and spermidine enhance and repress V. cholerae biofilm formation, respectively. These effects are relevant for understanding V. cholerae pathogenicity and are mediated through the periplasmic binding protein NspS and the transmembrane bis-(3′-5′) cyclic diguanosine monophosphate (c-di-GMP) phosphodiesterase MbaA. However, the levels of spermidine required to inhibit biofilm formation through this pathway are unlikely to be encountered by V. cholerae in aquatic reservoirs or within the human host during infection. We therefore hypothesized that other polyamines in the gastrointestinal tract may control V. cholerae biofilm formation at physiological levels. The tetramine spermine has been reported to be present at nearly 50 μm concentrations in the intestinal lumen. Here, we report that spermine acts as an exogenous cue that inhibits V. cholerae biofilm formation through the NspS–MbaA signaling system. We found that this effect probably occurs through a direct interaction of spermine with NspS, as purified NspS protein could bind spermine in vitro. Spermine also inhibited biofilm formation by altering the transcription of the vps genes involved in biofilm matrix production. Global c-di-GMP levels were unaffected by spermine supplementation, suggesting that biofilm formation may be regulated by variations in local rather than global c-di-GMP pools. Finally, we propose a model illustrating how the NspS–MbaA signaling system may communicate exogenous polyamine content to the cell to control biofilm formation in the aquatic environment and within the human intestine.
Keywords: bacterial signal transduction, biofilm, cyclic di-GMP (c-di-GMP), polyamine, Vibrio cholerae
Introduction
Vibrio cholerae is an aquatic organism that can be highly motile by use of a single polar flagellum in the planktonic state or sessile through production of biofilms, aggregates of bacteria often found attached to surfaces (1). V. cholerae biofilms are primarily composed of microbes encased in a self-secreted matrix of Vibrio polysaccharide (VPS),2 DNA, and protein and are thought to be the primary form in which these bacteria persist in aquatic environments (2). In support of this, V. cholerae has been found in association with various aquatic organisms, including aquatic plants, zooplankton, bivalves, and arthropods, and forms biofilms on chitinous structures, including the exoskeleton of several of these aquatic organisms (3–8). Formation of biofilms is advantageous for bacteria, as they permit enhanced resistance to a variety of environmental stressors, including osmotic stress, UV radiation, predation, and antimicrobials (2, 9).
V. cholerae is responsible for the severe diarrheal disease cholera, which is typically contracted following consumption of contaminated drinking water. In addition to enhancing environmental persistence, biofilm formation has been implicated as a virulence factor for this organism. V. cholerae in a biofilm are about 1000-fold more resistant to acidic pH compared with their free-swimming counterparts. Therefore, biofilms are thought to permit survival in the acidic environment of the stomach (10). Following passage into the small intestine, biofilm-associated V. cholerae must disseminate from the biofilm and revert to the planktonic state to induce production of the virulence factors, toxin-coregulated pilus and cholera toxin (8, 11, 12). Toxin-coregulated pilus is required for efficient colonization of the intestinal crypts, and cholera toxin causes ionic dysregulation of intestinal cells, resulting in the massive diarrhea and vomiting associated with the disease (13, 14).
The key regulator controlling the transition to and from the biofilm-associated state in most bacteria is the intracellular second messenger bis-(3′–5′) cyclic diguanosine monophosphate (c-di-GMP) (9, 15). In V. cholerae, high levels of c-di-GMP result in increased biofilm formation, whereas low levels lead to enhanced motility and virulence factor production (2). c-di-GMP primarily influences V. cholerae biofilm formation through activation of the transcriptional regulator VpsT, which enhances vps gene transcription in its c-di-GMP–bound form (16). VpsT also activates transcription of vpsR, which encodes the primary transcriptional activator of the vps genes, VpsR (17). c-di-GMP is synthesized from two molecules of GTP by diguanylate cyclases (DGCs) with conserved GGDEF catalytic domains and is degraded by phosphodiesterases (PDEs) with EAL or HD-GYP domains to 5′-pGpG or GMP, respectively (18). DGC and PDE enzymatic activity is modulated through detection of extracellular and intracellular signals via sensory domains that are often directly linked to and control activity of the catalytic domains (19). Although the V. cholerae genome encodes 62 c-di-GMP turnover enzymes, the precise signals governing activity of many of these proteins have yet to be identified.
We have previously identified a two-protein signaling system controlling V. cholerae biofilm formation, probably through modulation of c-di-GMP levels (20). This system is composed of the transmembrane PDE, MbaA, and the periplasmic solute-binding protein, NspS. MbaA is composed of a large N-terminal periplasmic domain flanked by two transmembrane regions and tandem cytoplasmic GGDEF and EAL domains at the C terminus (20, 21). Deletion of mbaA results in robust biofilm formation compared with wild-type cells, and the C-terminal domain of MbaA has been shown to degrade c-di-GMP in vitro (15, 21). In contrast, nspS mutants produce very low biofilms, suggesting that NspS inhibits PDE activity of MbaA. Furthermore, NspS- and MbaA-dependent biofilm phenotypes occur in a vps-dependent manner (20).
The NspS–MbaA signaling system controls V. cholerae biofilm formation in response to a class of molecules known as polyamines. Polyamines are short hydrocarbon chains with two or more amine groups that are positively charged at physiological pH. These molecules are ubiquitous in living organisms and have been shown to control a variety of cellular functions, including biofilm formation, toxin activity, oxidative and acid stress tolerance, protein synthesis, and DNA replication in bacteria (22, 23). Norspermidine, a triamine (structure shown in Fig. 1A) produced by various aquatic organisms as well as members of Vibrionaceae, enhances biofilm formation at as low as 10 μm (20).
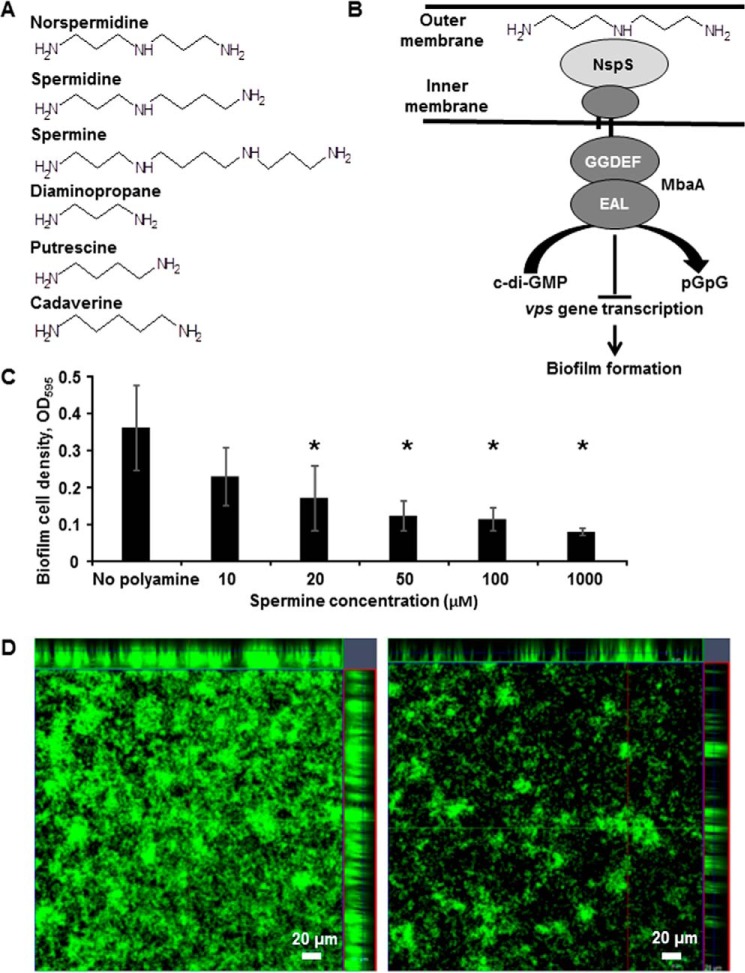
Figure 1.
Spermine inhibits V. cholerae O139 biofilm formation. A, structures of polyamines used or mentioned in this study. B, schematic diagram of the NspS–MbaA polyamine signaling pathway. Exogenous polyamines (norspermidine shown) bind NspS in the periplasm. Polyamine-specific alterations at the NspS–MbaA interface consequently modulate the c-di-GMP phosphodiesterase activity of MbaA in the cytoplasm to regulate vps gene transcription and biofilm formation. C, spermine inhibits V. cholerae biofilm formation in a concentration-dependent manner. Static cultures were incubated for 20 h at 27 °C in LB only or with varying spermine concentrations. Biofilms were washed, dispersed, and measured at A595. Shown are averages and S.D. (error bars) of three separate experiments with three technical replicates each. Asterisks, statistically significant difference from the mean of the untreated samples as determined by one-way ANOVA using SigmaPlot version 12.5 (Systat Software, Inc., San Jose, CA) (p ≤ 0.05). D, laser-scanning confocal microscopy images of wild-type V. cholerae grown in LB only (left) or LB supplemented with 100 μm spermine (right). Static cultures were incubated for 20 h at 27 °C in the indicated medium conditions. Biofilms were washed with PBS and incubated with 2.5 μm Syto9 for 30 min before imaging with a Zeiss LSM 510 microscope. Images show transverse and vertical cross-sections through the biofilms.
The current working model for norspermidine-mediated effects on V. cholerae biofilm formation is as follows: NspS interacts with exogenous norspermidine in the periplasm, causing modifications at the NspS–MbaA interface (Fig. 1B). The resulting conformational changes in MbaA are transmitted to the cytoplasmic domain to inhibit its phosphodiesterase activity. Subsequent accumulation of c-di-GMP indirectly enhances vps gene transcription and ultimately biofilm formation. In contrast, the triamine spermidine, which differs from norspermidine by just one methylene group (Fig. 1A), causes a reduction in biofilm formation at concentrations 500 μm and above (20). Spermidine is produced by the human host and resident bacteria of the intestine, where V. cholerae is thought to favor the planktonic lifestyle; however, the concentration of spermidine required to inhibit biofilm formation through the NspS—MbaA pathway far exceeds reported levels of this polyamine in the intestines (24–26). We therefore sought to identify other major polyamines encountered in the human gastrointestinal tract that may alter biofilm formation by V. cholerae. Here, we report that spermine (Fig. 1A), another abundant intestinal polyamine, inhibits V. cholerae biofilm formation at physiologically relevant concentrations (27). In addition, we provide evidence that spermine-mediated biofilm inhibition requires the periplasmic sensor protein, NspS, and transmembrane PDE, MbaA. Furthermore, polyamine-mediated influences on biofilm formation through NspS and MbaA are conserved in the clinically relevant V. cholerae serogroups O139 and O1 El Tor. Finally, we use these findings to suggest potential roles of this pathway in the V. cholerae life cycle.
Results
Spermine inhibits V. cholerae biofilm formation
We have previously shown that norspermidine enhances V. cholerae biofilm formation through the NspS–MbaA signaling system, whereas spermidine reverses this process (20, 28). V. cholerae is able to synthesize norspermidine, and this polyamine is also produced by a variety of organisms in the aquatic environment, where biofilm formation is thought to be the dominant phenotype (29); however, norspermidine is not produced by the human host or the intestinal microbiota (30). In contrast, the major polyamines present in the human gastrointestinal tract are putrescine, cadaverine, spermidine, and spermine (31). Spermidine inhibits V. cholerae biofilm formation at 500 μm (28); however, human intestinal spermidine levels have only been reported as high as 95 μm (24–26). Additionally, we reported that putrescine and cadaverine have no effect on biofilm formation at levels as high as 1000 μm (15). Spermine is produced by the human host and is present at high levels in a variety of foods, including meat and vegetables (32). Additionally, spermine levels in the human intestine have been reported at as high as 46 μm (24–26). We therefore hypothesized that spermine may alter biofilm formation in the human intestine. Using quantitative biofilm assays, we found that spermine inhibits biofilm formation in a concentration-dependent manner, and these effects were seen at levels as low as 20 μm in V. cholerae O139 (Fig. 1C). Importantly, growth curves of shaking cultures revealed no growth defect with spermine addition up to 1000 μm levels, indicating that the inhibition of biofilm formation is not due to growth inhibition or cellular toxicity (supplemental Fig. S1).
Laser-scanning confocal microscopy was used to visualize biofilms formed by wild-type V. cholerae grown in LB alone or LB supplemented with 100 μm spermine (Fig. 1D). Wild-type V. cholerae grown in LB alone produced biofilms with many large pillars and extensive surface coverage. In contrast, the spermine-treated culture produced biofilms with fewer and thinner pillars and much reduced surface coverage, confirming that spermine inhibits development of wild-type biofilms.
Spermine does not disperse preformed biofilms
Upon passage through the stomach and into the intestine, V. cholerae is believed to encounter various intestinal cues that promote biofilm dispersal, increased motility, and up-regulation of virulence factor production (11, 12, 33, 34). We therefore hypothesized that spermine may act as a host-specific cue for biofilm dispersal. To test this hypothesis, we quantified biofilm cell densities following treatment of mature biofilms with spermine over the course of 8 h (supplemental Fig. S2). We found that biofilms treated with 100 μm spermine remained unchanged as compared with those without spermine addition, indicating that spermine does not serve as a biofilm dispersal signal under these conditions. Similar results have been reported for another Gram-negative pathogen, Neisseria gonorrhoeae, where spermine inhibited biofilm formation but did not disperse preformed biofilms (35).
Spermine competes with norspermidine to regulate biofilms
As norspermidine enhances and spermine diminishes biofilm formation, and the opposing effects of these polyamines are observed at just 10–20 μm, we sought to determine whether these polyamines could compete to regulate the biofilm phenotype. In competition biofilm assays, we found that norspermidine is the dominant signal, as equal concentrations (10 μm) of these polyamines led to an increase in biofilm formation by ∼50% over that of cultures without treatment, and increasing norspermidine levels further increased biofilm formation (Fig. 2). However, increasing spermine concentrations could override the pro-biofilm effects of 10 μm norspermidine when supplied at 5-fold greater concentrations (Fig. 2). These results suggest that whereas norspermidine is the more potent signal, spermine can compete with norspermidine to inhibit biofilm formation. This is in contrast to previously reported spermidine and norspermidine competition experiments, where it was determined that a 100-fold excess of spermidine was required to reverse the effects of norspermidine on biofilm formation (28).
Figure 2.
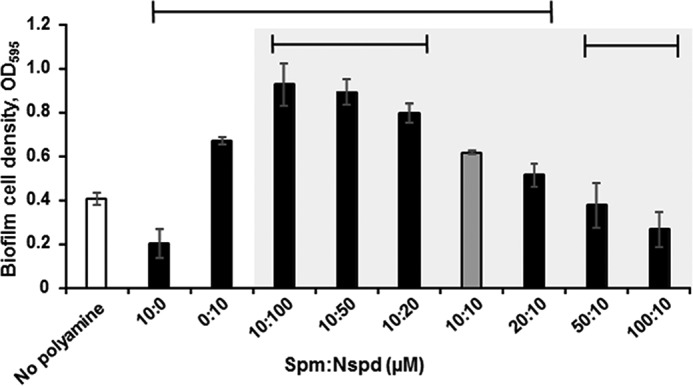
Spermine can override the pro-biofilm effects of norspermidine. Biofilms were formed in LB broth for 20 h at 27 °C with the indicated concentration of spermine (Spm) and norspermidine (Nspd). Biofilms were washed, dispersed, and measured at A595. Shown are averages and S.D. (error bars) of three separate experiments with three technical replicates each. Sample means under the top horizontal line are statistically different from the mean of the untreated samples (white bar). Sample means under the bottom horizontal lines are statistically different from the mean of the samples treated with 10 μm norspermidine and spermine (gray bar); for this set of comparisons, only samples containing both polyamines (within the shaded area) were used. Statistical analyses were performed by one-way ANOVA using SigmaPlot version 12.5 (p ≤ 0.05).
Spermine inhibits biofilm formation through the NspS–MbaA polyamine signaling system
Norspermidine enhances biofilm formation in an NspS- and MbaA-dependent manner. As spermine can compete with norspermidine to control biofilm formation, we hypothesized that spermine also signals through NspS and MbaA. To address this possibility, we compared the effects of spermine addition in ΔmbaA and ΔnspS mutants with that of the wild-type strain (Fig. 3). As shown above, 100 μm or greater spermine treatment significantly inhibited biofilm formation in wild-type V. cholerae. Biofilm cell densities of ΔmbaA mutants reached about triple that of wild-type cultures and were not reduced by spermine treatment. Deletion of nspS resulted in heavily attenuated biofilm phenotypes that were unaffected by spermine addition. Similar to the ΔmbaA single mutant, the ΔnspSΔmbaA double mutant formed robust biofilms regardless of spermine addition (Fig. 3). To determine whether the roles of NspS and MbaA were conserved under different environmental conditions, we repeated the biofilm assays using polypropylene tubes instead of glass tubes and trypticase soy broth instead of LB. Similar trends were observed for most of these conditions (supplemental Fig. S3). In polypropylene tubes, 100 but not 1000 μm exogenous spermine led to a very small but statistically significant (p = 0.019) decrease in biofilm levels for the ΔnspS mutant. Although surprising given the lack of responsiveness observed for ΔnspS under all other conditions tested, it is possible that a narrow range of spermine concentration might lead to further suppression of biofilms in the ΔnspS mutant through an additional intracellular effect when grown on polypropylene. Nevertheless, these data show that NspS and MbaA are necessary for responsiveness to spermine under different environmental conditions.
Figure 3.
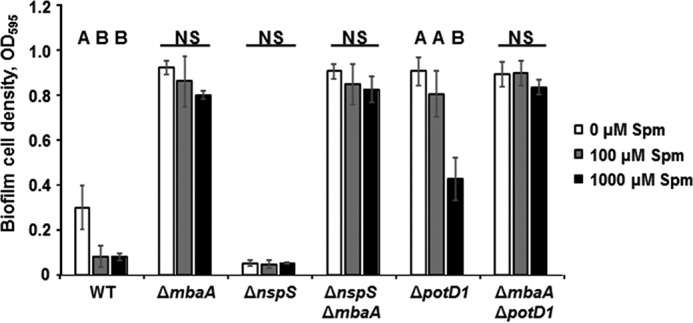
Spermine inhibits V. cholerae biofilms in an NspS/MbaA-dependent manner. Static cultures were incubated for 20 h at 27 °C in the LB only or with varying spermine concentrations. Biofilms were washed, disrupted, and measured at A595 (Spm, spermine). Shown are averages and S.D. (error bars) of three separate experiments with three technical replicates each. Means not followed by the same letter are statistically different at p ≤ 0.05 from the untreated samples within a given strain, as determined by one-way ANOVA using SigmaPlot version 12.5 (NS, not significant).
We have previously shown that nspS and mbaA are conserved in nearly all sequenced V. cholerae isolates, including O1 El Tor, another serotype responsible for epidemic cholera (15). To determine whether spermine could also inhibit V. cholerae O1 El Tor biofilm formation, we conducted biofilm assays using the O1 El Tor strain C6706 (supplemental Fig. S4). We found that, although the IC50 for spermine is >100 μm, this effect does require NspS and MbaA (supplemental Fig. S4). Importantly, O1 El Tor strains required longer incubation times (48 h versus 20 h) to accumulate in sufficient biofilm levels to be measured by our assay. Additionally and in contrast to observations made in the O139 strain, the O1 El Tor ΔmbaA mutant did not produce biofilms greater than that of wild-type.
As ΔmbaA deletion mutants in the O139 background exhibit strong induction of biofilm formation, we wanted to determine whether the decreased responsiveness of this mutant to spermine addition may result from obstruction of spermine entry into the periplasm due to increased exopolysaccharide production or electrostatic interactions between the polyamine and other components of the biofilm matrix. Therefore, we performed biofilm assays with a ΔpotD1 mutant. PotD1 is the periplasmic substrate binding component of an ABC-type transporter responsible for import of both norspermidine and spermidine (28). We have shown previously that the ΔpotD1 mutant produces robust biofilms comparable with the ΔmbaA mutant, although the mechanism behind this effect is not understood (28). The ΔpotD1 mutant still produced robust biofilms with the addition of up to 100 μm spermine, but these biofilm densities were reduced by ∼50% when culture medium was supplemented with 1000 μm spermine (Fig. 3). We hypothesize that the inability of 100 μm spermine to inhibit biofilm formation in the ΔpotD1 mutant may be a result of ionic interactions between spermine and some component(s) of the biofilm exopolymeric matrix that is heavily produced by this mutant even in shaking culture (supplemental Fig. S5), hindering entry of spermine into the periplasm. Similar to the observations made with the ΔmbaA single mutant, a ΔmbaAΔpotD1 double mutant was unresponsive to spermine at all concentrations tested (Fig. 3). Together, these data suggest that spermine represses biofilm formation through modulation of MbaA and that even other robust biofilm-forming mutants respond to spermine in an NspS–MbaA–dependent manner.
As PotD1 is involved in polyamine metabolism, we tested the effects of spermine on another robust biofilm former, ΔcytR. In V. cholerae, CytR is a transcriptional regulator involved in nucleoside scavenging and natural competence; however, it is unlikely to influence polyamine levels in the cell (36, 37). We observed a dose-dependent decrease in biofilm levels in response to spermine treatment in this mutant as well, further corroborating that robust biofilm formers are able adjust their biofilm levels in response to spermine (supplemental Fig. S6).
Spermine-mediated repression of biofilm formation is dependent on vps gene transcription
We have previously reported that the NspS–MbaA signaling system influences biofilm formation through modulation of vps gene transcription (20). Therefore, we hypothesized that inhibition of biofilm formation by spermine is also a direct result of altered vps expression. To investigate this possibility, we used a colorimetric reporter assay by making use of a chromosomal vpsLp-lacZ fusion in cell cultures containing varying amounts of spermine. The vpsL promoter drives transcription of the vpsII operon encoding a set of proteins involved in biofilm matrix production (38). To maintain consistency between the biofilm experimental setup and that of vps assays, biofilms were first permitted to form and then homogenized along with planktonic cells before cell lysis and β-galactosidase activity measurements. As predicted, wild-type vpsL gene transcription decreased with increasing concentrations of spermine (Fig. 4). In contrast, ΔmbaA single mutants and ΔnspSΔmbaA double mutants exhibited much greater and ΔnspS mutants exhibited much lower vpsL gene transcription than wild-type cells regardless of spermine addition. Further corroborating biofilm data, the ΔpotD1 mutant showed initially high vpsL gene transcription, but a decrease of 60–80% was observed in cultures supplemented with 1000 μm spermine. Additionally, the ΔmbaAΔpotD1 double mutant yielded high vpsL gene transcription compared with wild type, and this phenotype was not influenced by spermine at any concentration tested. Altogether, these data indicate that spermine influences biofilm formation through the NspS–MbaA signaling pathway at the level of vps gene transcription.
Figure 4.
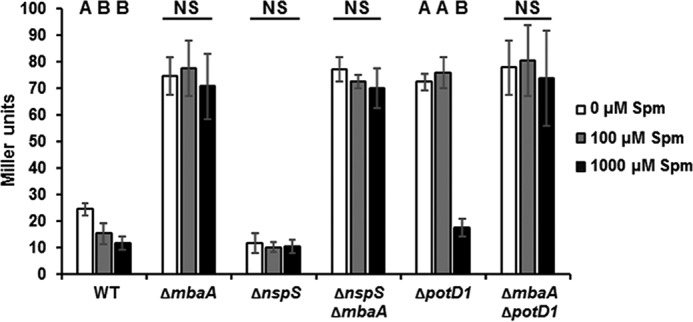
Spermine inhibits vpsL gene transcription in a NspS—MbaA-dependent manner. Static cultures were grown for 20 h with the indicated concentration of spermine (Spm) at 27 °C. Planktonic cells and biofilms were homogenized by disruption with glass beads and vortexing. Cells were pelleted and lysed in Z-buffer. Lysates were incubated with the colorimetric β-galactosidase substrate ortho-nitrophenyl-β-galactoside, and Miller units were calculated using A415 of reaction mixtures. Shown are the averages and S.D. (error bars) of three experiments with three technical replicates each. Means not followed by the same letter are statistically different at p ≤ 0.05 from the untreated samples within a given strain, as determined by one-way ANOVA using SigmaPlot version 12.5 (NS, not significant).
Ectopic expression of nspS restores the spermine phenotype in a ΔnspS mutant
In an attempt to restore the loss of spermine phenotype in the ΔnspS and ΔmbaA deletion mutants, we expressed each of these genes from a plasmid in the respective mutant backgrounds and performed biofilm assays in the presence of spermine. The ΔmbaA mutant carrying an empty pEVS143 vector formed robust biofilms and did not respond to spermine addition, as expected. Complementation of mbaA from an expression plasmid resulted in minimal biofilm, and spermine had no additional impact on biofilm levels in this strain (supplemental Fig. S7A). We hypothesize that overexpression of mbaA resulted in an increased MbaA/NspS ratio, and as MbaA is downstream of NspS in this signaling pathway, the native NspS levels were unable to hinder PDE activity of a considerable portion of the MbaA protein. This would result in increased c-di-GMP degradation and very low biofilm formation even in the absence of an external inhibitory signal. In contrast, a ΔnspS mutant carrying an empty plasmid formed very low biofilms, whereas complementation with a plasmid carrying the wild-type nspS gene formed robust biofilms that were diminished with spermine addition (supplemental Fig. S7B). Notably, 100 μm spermine only reduced biofilm formation by ∼25% in the complemented strain as compared with wild type, which showed a reduction of 66% (Fig. 1C and supplemental Fig. S7B). However, complementation of nspS from the overexpression plasmid would lead to increased NspS levels in the periplasm and would therefore require increased spermine concentrations to occupy enough NspS for a wild-type response through MbaA. Altogether, these results support the observations that the inhibitory effects of spermine on biofilm formation occur in an NspS- and MbaA-dependent manner.
Intracellular c-di-GMP levels are unaffected by polyamines or removal of NspS or MbaA
As MbaA is a confirmed PDE and is thought to influence vps gene transcription and biofilm formation through degradation of c-di-GMP, we quantified cellular c-di-GMP levels of wild type, ΔnspS, and ΔmbaA single mutants grown to early log phase in the presence of 100 μm norspermidine or spermine (Fig. 5). Interestingly, despite drastic influences on biofilm formation, cellular c-di-GMP levels were unaffected, regardless of strain or treatment. These results suggest that the influence of MbaA on cellular c-di-GMP is a localized phenomenon and that global c-di-GMP levels remain unaffected by the NspS–MbaA signaling system regardless of exogenous polyamine content.
Figure 5.
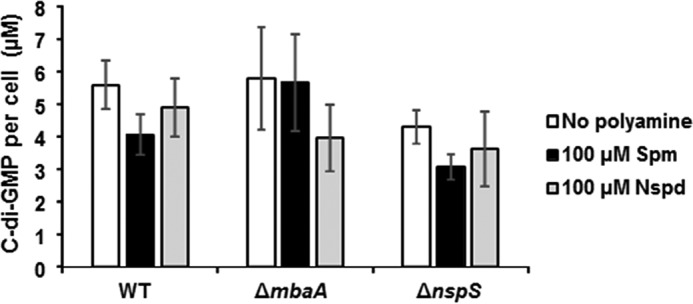
Cellular c-di-GMP levels are not altered as a result of polyamine treatment or deletion of nspS or mbaA genes. Cultures were grown to mid-log phase in LB only or supplemented with 100 μm spermine (Spm) or norspermidine (Nspd). Cells were lysed, and nucleotides were extracted for quantification by LC-MS/MS. Shown are averages and S.D. (error bars) of three biological replicates. Comparisons between treatments for a given strain and treatment duration were made by a two-way ANOVA followed by Tukey's post hoc test, using SigmaPlot version 12.5. No significant differences were found at p ≤ 0.05 between treatments or for various treatment durations (not indicated for clarity).
NspS binds spermine in vitro
We have previously shown that NspS mediates the effect of norspermidine and spermidine by directly binding these polyamines (15); therefore, we hypothesized that NspS can also interact with spermine. We investigated the capacity of purified NspS protein to bind spermine in vitro using a thermal shift assay (TSA). This assay relies on the inherently increased thermal stability of ligand-bound proteins as compared with unbound counterparts (39). As shown previously (15), purified NspS had a melting temperature (Tm) of ∼47 °C (Fig. 6). The addition of norspermidine resulted in an increase in thermal stability of about 4 °C, whereas spermidine caused a shift of 2 °C, indicating a binding event, as reported previously (Fig. 6). Additionally, cadaverine and putrescine had no effect on thermal stability of NspS, confirming that these polyamines are unable to bind NspS, as shown previously. However, the addition of spermine to the reaction mixture resulted in a thermal shift of ∼5 °C, indicating that purified NspS protein can bind spermine under these conditions (Fig. 6).
Figure 6.
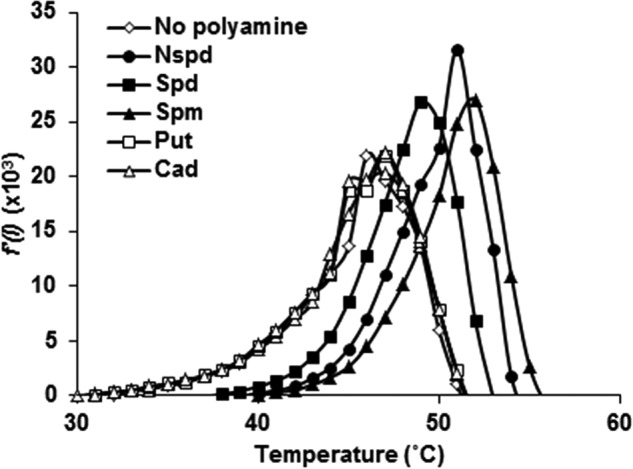
NspS binds spermine in vitro. In optical 96-well plates, 10 μm purified NspS protein alone or with polyamines (10 μm) was combined with 5× SYPRO® Orange (Nspd, norspermidine; Spd, spermidine; Spm, spermine; Put, putrescine; Cad, cadaverine). The temperature was raised in 1 °C increments from 25 to 95 °C, and fluorescence was recorded in an Applied Biosystems 7300 real-time PCR system. Shown are representative first derivative curves of the raw fluorescence data. Peaks indicate Tm.
Spermine is imported independently of PotD1 and NspS
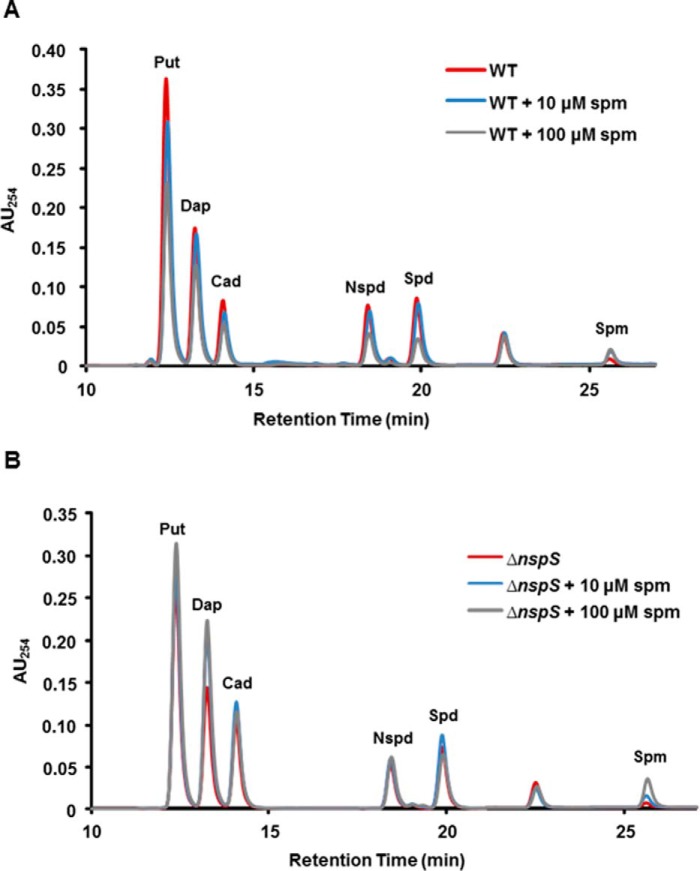
It was possible that, in addition to acting as an external signal through NspS and MbaA, spermine could impact biofilm formation through an intracellular mechanism. To investigate whether spermine may act as an internal signal, we quantified internal polyamine levels of V. cholerae grown in the presence of spermine. As shown previously (28, 29), V. cholerae can synthesize putrescine, diaminopropane, cadaverine, and norspermidine (Fig. 7A). V. cholerae cannot synthesize spermidine under most conditions but can import this polyamine from the LB medium used in these experiments (28, 29). We found that spermine can also be imported at as low as 10 μm levels by wild-type V. cholerae.
Figure 7.
Spermine is imported by wild-type V. cholerae and the ΔnspS mutant. Cells were grown to mid-log phase in LB only or LB supplemented with spermine. Cells were lysed, and polyamines were extracted and benzoylated. Benzoylated polyamines were identified using HPLC (Put, putrescine; Dap, diaminopropane; Cad, cadaverine; Spd, spermidine; Nspd, norspermidine; Spm, spermine). A, wild type. B, ΔnspS mutant. The identity of the peak between spermidine and spermine is unknown. Shown are representative HPLC traces of four independent experiments.
As the periplasmic ligand-binding protein, PotD1, mediates transport of spermidine and norspermidine, we hypothesized that it may also mediate transport of spermine (15, 28). However, the ΔpotD1 mutant was also capable of importing spermine at as low as 10 μm, similar to wild type (Fig. 7A and supplemental Fig. S8), indicating that spermine is imported by a mechanism independent of PotD1. Polyamines have been reported to associate with the outer membranes of Gram-negative bacteria; therefore, it was possible that spermine potentially associated with the membrane might be released during processing (40). To ensure that our polyamine extraction approach allowed for detection of cytoplasmic polyamine levels without contribution from polyamines potentially associated with the outer membrane, we repeated this experiment after removing membranes by ultracentrifugation following cell lysis (41). We were able to detect spermine in cytoplasmic extracts in both the wild type and the ΔpotD1 mutant, indicating that spermine is indeed imported into the cell (supplemental Fig. S9).
NspS is a homolog of PotD1; however, we have shown previously that NspS does not play a role in norspermidine or spermidine uptake (15). Nevertheless, it remained possible that NspS might be involved in spermine import. We therefore investigated the polyamine profiles of the ΔnspS mutant cultured with spermine supplementation. This mutant was still able to take up spermine, indicating that NspS is not involved in spermine import (Fig. 7B). Curiously, the presence of 100 μm spermine in the culture medium of both the wild-type bacteria and the ΔnspS mutant led to increased import of spermine but also resulted in a reduction of spermidine in the cell.
Discussion
Bacterial pathogens are often exposed to very diverse and dynamic environments throughout their life cycle. As such, these organisms must detect and respond to a variety of environment-specific signals to exhibit the most beneficial gene expression profile and phenotypes. In the case of V. cholerae, survival in aquatic environments is thought to be enhanced through formation of a biofilm, and a variety of aquatic signals, including low temperature (<20 °C) (42), high salinity (43), and chitin (44), induce this phenotype. In contrast, dispersal from the biofilm and reversion to the planktonic state is necessary for virulence factor production in the intestinal environment. Indeed, several host factors, including elevated temperature (37 °C) (42), the bile salt taurocholate (11), and bicarbonate (45), have been shown to inhibit biofilm formation. Additionally, taurocholate was reported to disperse preformed biofilms (11). Here, we show that one of the major intestinal polyamines, spermine, is capable of inhibiting vps-dependent biofilms in a dose-dependent manner and that this inhibition requires a polyamine signaling pathway composed of the substrate-binding protein NspS and the transmembrane phosphodiesterase MbaA. Spermine appears to act as an external signal to control biofilm formation as it is imported into the cell regardless of the presence of NspS, whereas the effects on biofilm formation are abolished in the absence of this pathway. Our results strengthen the notion that NspS signals exogenous polyamine content through MbaA rather than playing a role in polyamine transport.
We also show that although the polyamine-binding protein PotD1 is responsible for both norspermidine and spermidine import, it does not appear to play a role in spermine import. However, there may be an additional transporter that is responsible for both spermidine and spermine import, as uptake of large amounts of spermine resulted in a reduction of spermidine in the cell. Alternatively, spermidine may be degraded or exported to maintain polyamine homeostasis in the cell in the presence of large amounts of spermine. Reduction of spermidine levels in the cell is not expected to contribute to the inhibitory effect of spermine on biofilms, as ΔpotD1 mutants, which have minimal to no spermidine in the cell, are robust biofilm formers. V. cholerae contains two additional potD1 paralogs in its genome. One of these, potD2, is located on the large chromosome and is adjacent to and co-transcribed with potD1 (28). The other (NCBI accession number WP_000846694) is located on the small chromosome. Ligands for these have not yet been identified. It is possible that periplasmic binding proteins encoded by one of these genes are responsible for spermine uptake. We are currently testing this hypothesis.
We did observe differences in polyamine responsiveness through the NspS—MbaA signaling pathway between the O139 and O1 El Tor strains used in this study. Mainly, the concentration of spermine required to inhibit O1 El Tor biofilms was higher than that required to inhibit O139 biofilms. In addition, deletion of mbaA did not enhance O1 El Tor biofilms. Notably, the enhanced biofilm phenotype of the ΔmbaA mutant was originally observed in the O1 El Tor strain N16961 (21); however, these observations were made using crystal violet staining for quantification of the biofilms as opposed to the optical density measurements used here. Also notable is the observation that V. cholerae is capable of spermine uptake from the medium, which may alter the effective concentration acting through the NspS–MbaA signaling pathway during longer incubation times. Nevertheless, the observation that spermine inhibits biofilm formation through NspS and MbaA is consistent between both strains. It is possible that variations in assay conditions and/or the increased incubation times necessary to develop O1 El Tor biofilms as compared with that of O139 may account for the differences between these results. Alternatively, these findings may exemplify physiological differences between the clinically important serogroups, O139 and O1 El Tor, responsible for recent cholera epidemics.
It is intriguing to note that all polyamines shown to control biofilm formation through NspS and MbaA contain the diaminopropane moiety (structure shown in Fig. 1A). However, it appears that the 3 + 3 structure of norspermidine (where numbers indicate the number of methylene groups between flanking amine groups) stabilizes NspS in a conformation that may promote an interaction with MbaA that hinders its PDE activity, thereby enhancing biofilm formation. In contrast, the 3 + 4 spacing of amine groups within spermidine and spermine may result in an NspS conformation that either relieves inhibition of the MbaA PDE activity or altogether inhibits the NspS—MbaA interaction. Notably, the diaminobutyl moiety alone is insufficient to bind NspS and therefore cannot affect biofilm formation through this pathway (15). It is interesting that whereas both spermidine and spermine can bind NspS, only spermine can inhibit NspS-dependent activation of biofilm formation at low concentrations. Clearly, the additional diaminopropane moiety present in spermine enhances the inhibitory effect through this pathway. We hope to elucidate the mechanism behind this effect in more detailed structural and biochemical analyses currently under way.
Cellular c-di-GMP levels were unaffected by the addition of polyamines or deletion of nspS or mbaA despite very robust effects on c-di-GMP–dependent phenotypes, including vps gene transcription and biofilm formation. This was surprising because c-di-GMP phosphodiesterase activity of MbaA has been confirmed in vitro, indicating that repression of biofilm formation by this protein should be mediated by its effect on c-di-GMP levels (15). However, it has been suggested that c-di-GMP signaling is a high specificity phenomenon (46). In other words, signals communicated through distinct DGCs or PDEs bring about c-di-GMP–mediated responses through alterations in subcellular pools of c-di-GMP as opposed to global levels (46). Importantly, we have previously reported that at least 15 different genes related to biofilm matrix production are differentially regulated in an ΔmbaA mutant (20). One of these, vpsT, encodes a transcriptional regulator of vps gene expression that is active when bound to c-di-GMP (47). Specifically, VpsT up-regulates the neighboring gene clusters vps (composed of vpsA–K), followed by the rbmA–F gene cluster, and vpsII (composed of vpsL–Q). The rbm genes encode proteins involved in biofilm matrix production and stabilization (48). In addition, VpsT regulates transcription of vpsR, encoding the master regulator of biofilm formation, VpsR, which also enhances transcription of the biofilm gene clusters mentioned above (47). VpsR and c-di-GMP–bound VpsT also activate their own transcription in a positive feedback loop (17). Thus, increases in vpsT transcripts in an ΔmbaA mutant indicate that the cell perceives an increase in the c-di-GMP levels under these conditions. Therefore, we believe that c-di-GMP data reported here further support the notion of high specificity signaling by this second messenger. Future studies will investigate the effects of a catalytically inactive form of MbaA on biofilm formation and vps gene transcription to ensure that it is indeed c-di-GMP turnover and not a secondary effect resulting from loss of MbaA that directs these phenotypes.
Norspermidine is a relatively uncommon polyamine in nature; however, this polyamine has been detected at nanomolar amounts in coastal waters, in various aquatic eukaryotic organisms, and is produced by Vibrionaceae (29, 49). It is possible that norspermidine released into the environment (actively or by cell lysis) may be sensed by V. cholerae to signal an environment that favors biofilm formation. We have shown that norspermidine is not exported by V. cholerae; however, other members of the Vibrionaceae may actively export this polyamine (20, 50). Additionally, norspermidine-producing eukaryotes could present a surface for V. cholerae to form biofilms on as well as provide the necessary signal to promote this lifestyle. In support of this hypothesis, V. cholerae has been found to associate with Volvox spp. and algae, some of which have been shown to produce norspermidine (6, 51, 52). Interestingly, one study reporting cellular concentrations of polyamines in eukaryotic algae revealed that of 42 algae tested, 32 contained intracellular norspermidine (52). In addition, of the 17 organisms in which spermine was detected, 13 also contained norspermidine, which was typically at greater levels than spermine. As the biofilm lifestyle has been implicated as an important physiological adaptation necessary for interepidemic persistence by V. cholerae (53), we propose that norspermidine may act as an environment-specific cue for enhancing survival in aquatic environments (Fig. 8). Specifically, we hypothesize that norspermidine interacts with NspS to inhibit the phosphodiesterase activity of MbaA, allowing for increases in localized pools of c-di-GMP. Increases in c-di-GMP pools result in activation of VpsT, leading to increased transcription of vps and rbm genes to enhance production of biofilm matrix components (Fig. 8, left). In contrast, we show here that the tetramine spermine is an inhibitor of biofilm formation. Spermine is present at up to several hundred micromolar levels in a variety of foodstuffs typically consumed by humans, including meats, dairy products, vegetables, fruits, cereals, and legumes (32, 54). In addition, spermine is produced by the human host and has been detected at up to 46 μm levels in fluids within the intestinal lumen (25, 26, 31, 54). Although these studies are sparse, they suggest that spermine may be present well above the concentrations necessary to promote the planktonic lifestyle necessary for virulence factor production within the human intestine (34). We propose that, following passage of ingested V. cholerae into the human intestine, host-specific cues may disperse preformed biofilms, and spermine may then act as a host-derived signal to maintain the planktonic lifestyle by decreasing local c-di-GMP pools (Fig. 8, right). Importantly, although spermine could not disperse preformed biofilms in vitro, it remains possible that this signal could have dispersal effects in the gastrointestinal tract during infection. Finally, the NspS–MbaA signaling system appears to be the primary mediator of exogenous polyamine-based influences on biofilm formation in V. cholerae and permits the physiological adaptations necessary to achieve maximum fitness in the drastically disparate aquatic and human intestinal environments through recognition of this ubiquitous class of molecules.
Figure 8.
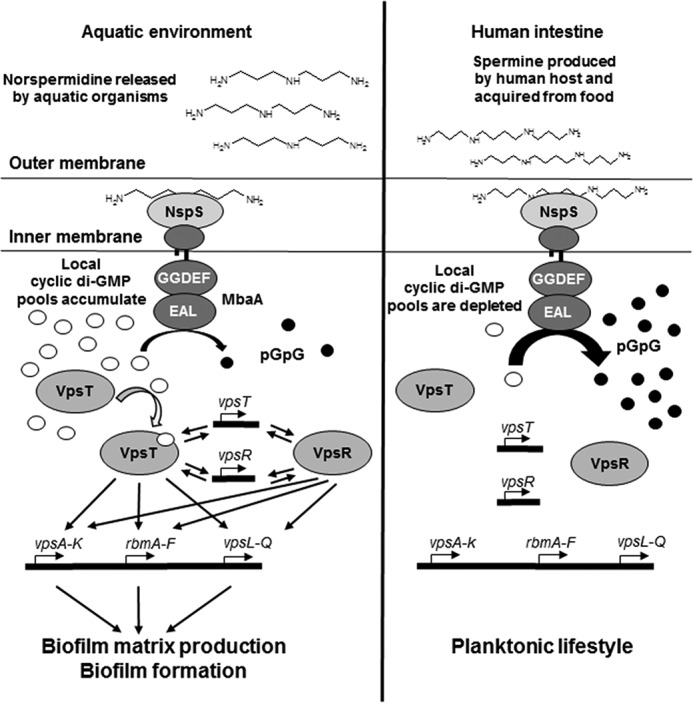
Proposed environmental roles of polyamine sensing by the NspS–MbaA signaling system in the V. cholerae life cycle. Left, in aquatic environments, V. cholerae NspS senses norspermidine that is released actively or following cell lysis by aquatic organisms. NspS in turn inhibits MbaA phosphodiesterase activity, allowing for increases in localized cytoplasmic c-di-GMP pools and VpsT binding to c-di-GMP. Activation of VpsT by c-di-GMP results in increased transcription of vps-I and vps-II operons and rbm gene clusters either by direct binding to the promoters or indirectly by affecting VpsR levels. VpsR and c-di-GMP–bound VpsT additionally enhance their own gene transcription as well as that of each other. These processes culminate in increased production of biofilm matrix components and formation of V. cholerae biofilms. Right, following passage into the human intestine, NspS senses spermine pools derived from ingested food as well as that produced by the human host, which relieves repression of phosphodiesterase activity by MbaA. Local c-di-GMP pools are degraded, thus leaving VpsT unbound and unable to activate biofilm matrix-related gene clusters.
Experimental procedures
Growth conditions
All bacterial strains and plasmids used in this study are listed in Table 1. The V. cholerae strains used in this study were all derived from O139 MO10 or O1 El Tor C6706. All V. cholerae cultures were grown in Luria broth (LB) supplemented with streptomycin (100 μg/ml) at 27 °C. pACYC184 and pEVS143 derivatives were maintained in V. cholerae with 2.5 μg/ml tetracycline and 100 μg/ml kanamycin, respectively. All polyamines were purchased from Sigma-Aldrich. Escherichia coli strains were grown on LB agar plates and LB supplemented with 50 μg/ml kanamycin at 37 °C.
Table 1.
Bacterial strains and plasmids
| Strain/plasmid | Genotype | Reference/source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F−Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK−, mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | Invitrogen |
| Shuffle T7 Express | fhuA2 lacZ::T7 gene1 [lon] ompT ahpC gal λatt::pNEB3-r1-cDsbC (SpecR, lacIq) ΔtrxB sulA11 R(mcr-73::miniTn10–TetS)2 [dcm] R(zgb-210::Tn10–TetS) endA1 Δgor Δ(mcrC-mrr)114::IS10 | New England Biolabs |
| AK356 | Shuffle T7 Express, pNP37 | Ref. 15 |
| V. cholerae | ||
| MO10 | O139 clinical isolate from India, SmR | Ref. 56 |
| PW357 | MO10 lacZ::vpsLp→lacZ, SmR | Ref. 37 |
| PW444 | MO10 lacZ::vpsLp→lacZ, ΔmbaA, SmR | Ref. 20 |
| PW514 | MO10 lacZ::vpsLp→lacZ, ΔnspS, SmR | Ref. 20 |
| PW522 | MO10 lacZ::vpsLp→lacZ, ΔnspSΔmbaA, SmR | Ref. 20 |
| AK160 | MO10 lacZ::vpsLp→lacZ, ΔpotD1, SmR | Ref. 15 |
| PW358 | MO10 lacZ::vpsLp→lacZ, ΔcytR, SmR | Ref. 37 |
| AK474 | MO10 lacZ::vpsLp→lacZ, ΔmbaAΔpotD1, SmR | This study |
| AK007 | MO10 lacZ::vpsLp→lacZ, ΔnspS, pACYC184, SmR, TetR | This study |
| AK192 | MO10 lacZ::vpsLp→lacZ, ΔnspS, pACYC184::nspS, SmR, TetR | This study |
| AK555 | MO10 lacZ::vpsLp→lacZ, ΔmbaA, pmbaA, SmR, KanR | This study |
| AK564 | MO10 lacZ::vpsLp→lacZ, ΔmbaA, pEVS143, SmR, KanR | This study |
| C6706 str. 2 | O1 El Tor clinical isolate from Peru, spontaneous SmR | Ref. 14 |
| AK635 | C6706 str. 2, ΔmbaA, SmR | This study |
| AK638 | C6706 str. 2, ΔnspS, SmRr | This study |
| Plasmids | ||
| pCR2.1-TOPO | Plasmid for TOPO cloning, ApR | Invitrogen |
| pET28b | KanR | Novagen |
| pACYC184 | TetRr CmR | Ref. 57 |
| pNP1 | pACYC184::nspS-V5 | This study |
| pEVS143 | KanR | Ref. 58 |
| pmbaA | pEVS143::mbaA | Ref. 46 |
Complementation of ΔnspS and ΔmbaA mutants
For nspS complementation, the nspS open reading frame was amplified from V. cholerae chromosomal DNA with an additional 99 base pairs upstream of the coding sequence using the forward primer P215 (5′-GCGACCAAATTATACATAGAG-3′) and reverse primer P382 (5′GCTGCCATGGCTACGTAGAATCGAGACCGAGGAGAGGGTTAGGGATAGGCTTACCGCCGCTGCCGCTGCCTGGTTTAGCTTCATATTGGTAAG-3′), and cloned into pCR2.1-TOPO® (Invitrogen). The reverse primer encodes a V5 tag (indicated in boldface type). The plasmid was transformed into E. coli DH5α, and proper construction of the recombinant molecule was confirmed by PCR and sequencing (Eurofins MWG Operon, Louisville, KY). The nspS gene was excised from the recombinant plasmid by sequential restriction digest with EcoRI followed by NcoI and cloned into these sites in similarly digested pACYC184. The resulting plasmid (pNP1, herein referred to as pnspS) or empty vector was transformed into V. cholerae ΔnspS. The pEVS143 and pEVS143::mbaA (herein referred to as pmbaA) plasmids were transformed into V. cholerae O139 ΔmbaA deletion mutants.
Biofilm and competition assays
Media used for biofilm assays were LB except for one set of experiments, where trypticase soy broth was used. Overnight cultures were diluted 1:50 into 2 ml of fresh medium with antibiotic and grown to mid-log phase at 27 °C with shaking. One ml of cells was pelleted, medium was removed, and pellets were resuspended in 100 μl of fresh medium. Three hundred μl of medium with or without polyamines were inoculated at an A595 of 0.04 in borosilicate tubes unless otherwise noted. Static cultures were grown at 27 °C for ∼20 h for O139 unless otherwise noted and 48 h for O1 El Tor. Strains with pACYC184 or pnspS were incubated statically for 24 h. Experiments with the cytR mutant were scored after 16 h, as longer incubation times resulted in the spermine effect being lost. We hypothesize that as CytR is a global transcriptional regulator, the spermine signal might be overridden by other pathways regulated by CytR as biofilm cell density increases. Planktonic and biofilm cell densities were measured as described previously (15). All experiments were performed in triplicate and repeated several times.
Laser-scanning confocal microscopy
Overnight cultures were diluted 1:50 in 2 ml of fresh medium with antibiotic and grown to mid-log phase at 27 °C with shaking. Cells were subcultured at A595 of 0.005 in 10 ml of LB only or LB supplemented with 100 μm spermine. Biofilms were formed on a number 1 borosilicate glass coverslip (VWR) inserted into the culture tube by incubation at 27 °C for ∼20 h. After washing with 1× PBS, biofilms were stained with 2.5 μm Syto9 (Invitrogen). Images were collected at room temperature with Zeiss ZEN 2009 acquisition software using a Zeiss LSM 510 microscope equipped with a Zeiss LD Plan-Neofluar 40×/0.6 numerical aperture Korr objective lens.
β-Galactosidase measurements
vps gene transcription was measured using a chromosomal lacZp-vpsL fusion and a modified protocol described by Haugo and Watnick (37). Briefly, mid-log phase cells were diluted to A595 of 0.04 in 2 ml of fresh LB with or without spermine and incubated statically at 27 °C for ∼20 h. Biofilms were homogenized along with planktonic cells by vortexing with glass beads for 30 s, and 1 ml of the homogenized cells was pelleted and frozen for at least 30 min at −20 °C before further processing. A595 of 150 μl of the remaining homogenate was measured. Cell pellets were thawed and resuspended in 200 μl of cold Z-buffer (100 mm NaH2PO4, 10 mm KCl, 1 mm MgSO4, pH 7) supplemented with 20 μl/ml protease inhibitors (Sigma-Aldrich) and 39 mm β-mercaptoethanol. Cells were disrupted by sonication using a Branson SFX150 Sonifier® (Swedesboro, NJ). Forty μl of a 4 mg/ml solution of o-nitrophenyl-β-galactopyranoside (Acros Organics, Thermo Fisher Scientific) were added, and the reaction mixture was incubated at 37 °C until sufficient color change was observed. Cell debris was pelleted, and β-galactosidase activity was determined by measuring the A415. Miller units were calculated using the equation,
| (Eq. 1) |
where A415 is the absorbance of the reaction mixture, t is the duration of the reaction in minutes, V is the volume in microliters, and A595 is the optical density of the culture (55). Experiments were performed in triplicate and repeated multiple times to ensure reproducibility.
Extraction and quantification of c-di-GMP
Nucleotides were extracted and quantified essentially as described previously (46). Briefly, overnight cultures were diluted 1:100 in 2 ml of fresh medium with the indicated concentration of polyamine and grown to mid-log phase at 27 °C with shaking at 200 rpm. Cells were lysed in cold extraction buffer (40% methanol, 40% acetonitrile, 20% water, 0.1 n formic acid), followed by incubation at 65 °C for 10 min. Cell debris was pelleted, and supernatants were used for quantification of cellular nucleotides by LC-MS/MS as described previously (46).
NspS purification
The V. cholerae nspS gene lacking the periplasmic signal sequence was expressed from the isopropyl 1-thio-β-d-galactopyranoside-inducible promoter in pET28b in Shuffle E. coli cells and purified as described previously, except the pH of the buffers was adjusted to 8.0 (15). Purified NspS protein was eluted in three 1.5-ml fractions of elution buffer (50 mm Na2HPO4, 300 mm NaCl, 300 mm imidazole, pH 8.0), and all fractions were analyzed by SDS-PAGE before further processing. Eluates were dialyzed in a 3,500 molecular weight cut-off dialysis cassette (Thermo Fisher Scientific) in HEPES buffer (100 mm HEPES, 150 mm NaCl, pH 8.0) at 4 °C with stirring overnight and a second time in fresh HEPES buffer for at least 4 h.
Thermal shift assays
TSAs were performed as described previously (15). TSA reaction mixtures contained 10 μm NspS only or NspS with a 10 μm concentration of the indicated polyamine in TSA reaction buffer (100 mm HEPES, 150 mm NaCl, pH 7.5) with 5× SYPRO Orange (Invitrogen). Samples were loaded into an Optical 96-well reaction plate with adhesive cover and monitored in an Applied Biosystems 7300 real-time PCR system using a TAMRA dye detection filter. Temperature was increased in 1 °C min−1 increments from 25 to 95 °C. Results shown are representative of three independent experiments. To ensure that no reaction was occurring between polyamines and SYPRO Orange, negative controls included polyamines and SYPRO Orange dye in reaction buffer.
Extraction, benzoylation, and detection of polyamines
Bacteria were grown at 27 °C to mid-exponential phase, pelleted, washed twice with 1× PBS, and resuspended in 10 μl of water/mg wet cell weight. The cell suspension was lysed using sonication, and the cell debris was removed by centrifugation. Where indicated, membranes were removed by ultracentrifugation at 100,000 × g as described previously (41). Cellular proteins were precipitated with 50% (w/v) trichloroacetic acid and centrifuged, leaving the supernatant with polyamines. This supernatant was removed and benzoylated as described previously (28). Benzoylated polyamine samples were separated using a Phenomenex Spherclone ODS column (5 μm, 250 × 4.6 mm) that was fitted with a 4.0 × 3.0-mm guard cartridge with a Waters 1525 Binary HPLC pump and analyzed using a Waters 2487 Dual λ absorbance detector. The separation was performed using a gradient of 45–80% methanol in water for 40 min with a 10-min isocratic equilibration of 45% methanol in water. A set of polyamine standards was also benzoylated and analyzed with each experiment.
Author contributions
R. C. S. performed experiments along with C. K. W., W. G. B., N. F., and E. L. B. R. C. S. and E. K. conceived and designed experiments. R. C. S. and H. S. N. performed the statistical analyses. R. C. S., C. K. W., H. S. N., C. M. W., and E. K. interpreted data. J. P. Z. and M. A. B. developed new strains used in this study. R. C. S. and E. K. wrote the manuscript, and all authors approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We acknowledge Dr. Anthony Michael for helpful discussions and Dr. Guichuan Hou for help with microscopy.
This work was supported in part by National Institutes of Health Grants AI096358 (to E. K.) and GM109259 (to C. M. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Figs. S1–S9.
- VPS
- Vibrio polysaccharide
- c-di-GMP
- bis-(3′-5′) cyclic diguanosine monophosphate
- DGC
- diguanylate cyclase
- PDE
- phosphodiesterase
- TSA
- thermal shift assay
- ANOVA
- analysis of variance.
References
- 1. Liu X., Beyhan S., Lim B., Linington R. G., and Yildiz F. H. (2010) Identification and characterization of a phosphodiesterase that inversely regulates motility and biofilm formation in Vibrio cholerae. J. Bacteriol. 192, 4541–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karatan E., and Watnick P. (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73, 310–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shukla B. N., Singh D. V., and Sanyal S. C. (1995) Attachment of non-culturable toxigenic Vibrio cholerae 01 and non-01 and Aeromonas spp. to the aquatic arthropod Gerris spinolae and plants in the River Ganga, Varanasi. FEMS Immunol. Med. Microbiol. 12, 113–120 [DOI] [PubMed] [Google Scholar]
- 4. Meibom K. L., Li X. B., Nielsen A. T., Wu C.-Y., Roseman S., and Schoolnik G. K. (2004) The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. U.S.A. 101, 2524–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zampini M., Canesi L., Betti M., Ciacci C., Tarsi R., Gallo G., and Pruzzo C. (2003) Role for mannose-sensitive hemagglutinin in promoting interactions between Vibrio cholerae El Tor and mussel hemolymph. Appl. Environ. Microbiol. 69, 5711–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamplin M. L., Gauzens A. L., Huq A., Sack D. A., and Colwell R. R. (1990) Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56, 1977–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reguera G., and Kolter R. (2005) Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J. Bacteriol. 187, 3551–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamayo R., Patimalla B., and Camilli A. (2010) Growth in a biofilm induces a hyperinfectious phenotype in Vibrio cholerae. Infect. Immun. 78, 3560–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tischler A. D., and Camilli A. (2004) Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53, 857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu J., and Mekalanos J. J. (2003) Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5, 647–656 [DOI] [PubMed] [Google Scholar]
- 11. Hay A. J., and Zhu J. (2015) Host intestinal signal-promoted biofilm dispersal induces Vibrio cholerae colonization. Infect. Immun. 83, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Z., Wang Y., Liu S., Sheng Y., Rueggeberg K.-G., Wang H., Li J., Gu F. X., Zhong Z., Kan B., and Zhu J. (2015) Vibrio cholerae represses polysaccharide synthesis to promote motility in mucosa. Infect. Immun. 83, 1114–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanden Broeck D., Horvath C., and De Wolf M. J. (2007) Vibrio cholerae: cholera toxin. Int. J. Biochem. Cell Biol. 39, 1771–1775 [DOI] [PubMed] [Google Scholar]
- 14. Thelin K. H., and Taylor R. K. (1996) Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 64, 2853–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cockerell S. R., Rutkovsky A. C., Zayner J. P., Cooper R. E., Porter L. R., Pendergraft S. S., Parker Z. M., McGinnis M. W., and Karatan E. (2014) Vibrio cholerae NspS, a homologue of ABC-type periplasmic solute binding proteins, facilitates transduction of polyamine signals independent of their transport. Microbiology 160, 832–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krasteva P. V., Fong J. C., Shikuma N. J., Beyhan S., Navarro M. V., Yildiz F. H., and Sondermann H. (2010) Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327, 866–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beyhan S., Bilecen K., Salama S. R., Casper-Lindley C., and Yildiz F. H. (2007) Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J. Bacteriol. 189, 388–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jenal U., and Malone J. (2006) Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40, 385–407 [DOI] [PubMed] [Google Scholar]
- 19. Römling U., Galperin M. Y., and Gomelsky M. (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77, 1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karatan E., Duncan T. R., and Watnick P. I. (2005) NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J. Bacteriol. 187, 7434–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bomchil N., Watnick P., and Kolter R. (2003) Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 185, 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah P., and Swiatlo E. (2008) A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 68, 4–16 [DOI] [PubMed] [Google Scholar]
- 23. Tabor C. W., and Tabor H. (1985) Polyamines in microorganisms. Microbiol. Rev. 49, 81–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benamouzig R., Mahé S., Luengo C., Rautureau J., and Tomé D. (1997) Fasting and postprandial polyamine concentrations in the human digestive lumen. Am. J. Clin. Nutr. 65, 766–770 [DOI] [PubMed] [Google Scholar]
- 25. McEvoy F. A., and Hartley C. B. (1975) Polyamines in cystic fibrosis. Pediatr. Res. 9, 721–724 [DOI] [PubMed] [Google Scholar]
- 26. Osborne D. L., and Seidel E. R. (1990) Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 258, G576–G584 [DOI] [PubMed] [Google Scholar]
- 27. Bardocz S., and White A. (1999) Polyamines in Health and Nutrition, Springer Science + Business Media, Berlin [Google Scholar]
- 28. McGinnis M. W., Parker Z. M., Walter N. E., Rutkovsky A. C., Cartaya-Marin C., and Karatan E. (2009) Spermidine regulates Vibrio cholerae biofilm formation via transport and signaling pathways. FEMS Microbiol. Lett. 299, 166–174 [DOI] [PubMed] [Google Scholar]
- 29. Lee J., Sperandio V., Frantz D. E., Longgood J., Camilli A., Phillips M. A., and Michael A. J. (2009) An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae. J. Biol. Chem. 284, 9899–9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanfrey C. C., Pearson B. M., Hazeldine S., Lee J., Gaskin D. J., Woster P. M., Phillips M. A., and Michael A. J. (2011) Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota. J. Biol. Chem. 286, 43301–43312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hallak A., Rosenberg R., Gilat T., and Sömjen G. (1993) Determination of free polyamines in human bile by high-performance liquid chromatography. Clin. Sci. 85, 451–454 [DOI] [PubMed] [Google Scholar]
- 32. Atiya Ali M., Poortvliet E., Strömberg R., and Yngve A. (2011) Polyamines in foods: development of a food database. Food Nutr. Res. 10.3402/fnr.v55i0.5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Correa N. E., Lauriano C. M., McGee R., and Klose K. E. (2000) Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol. Microbiol. 35, 743–755 [DOI] [PubMed] [Google Scholar]
- 34. Holmgren J., and Svennerholm A.-M. (1977) Mechanisms of disease and immunity in cholera: a review. J. Infect. Dis. 136, S105–S112 [DOI] [PubMed] [Google Scholar]
- 35. Goytia M., Dhulipala V. L., and Shafer W. M. (2013) Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol. Lett. 343, 64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antonova E. S., Bernardy E. E., and Hammer B. K. (2015) Natural competence in Vibrio cholerae is controlled by a nucleoside scavenging response that requires CytR-dependent anti-activation. Mol. Microbiol. 97, 605. [DOI] [PubMed] [Google Scholar]
- 37. Haugo A. J., and Watnick P. I. (2002) Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 45, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fong J. C., Karplus K., Schoolnik G. K., and Yildiz F. H. (2006) Identification and characterization of RbmA, a novel protein required for the development of rugose colony morphology and biofilm structure in Vibrio cholerae. J. Bacteriol. 188, 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giuliani S. E., Frank A. M., and Collart F. R. (2008) Functional assignment of solute-binding proteins of ABC transporters using a fluorescence-based thermal shift assay. Biochemistry 47, 13974–13984 [DOI] [PubMed] [Google Scholar]
- 40. Koski P., and Vaara M. (1991) Polyamines as constituents of the outer membranes of Escherichia coli and Salmonella typhimurium. J. Bacteriol. 173, 3695–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandkvist M., Hough L. P., Bagdasarian M. M., and Bagdasarian M. (1999) Direct interaction of the EpsL and EpsM proteins of the general secretion apparatus in Vibrio cholerae. J. Bacteriol. 181, 3129–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Townsley L., and Yildiz F. H. (2015) Temperature affects c-di-GMP signalling and biofilm formation in Vibrio cholerae. Environ. Microbiol. 17, 4290–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shikuma N. J., and Yildiz F. H. (2009) Identification and characterization of OscR, a transcriptional regulator involved in osmolarity adaptation in Vibrio cholerae. J. Bacteriol. 191, 4082–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun S., Tay Q. X. M., Kjelleberg S., Rice S. A., and McDougald D. (2015) Quorum sensing-regulated chitin metabolism provides grazing resistance to Vibrio cholerae biofilms. ISME J. 9, 1812–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koestler B. J., and Waters C. M. (2014) Bile acids and bicarbonate inversely regulate intracellular cyclic di-GMP in Vibrio cholerae. Infect. Immun. 82, 3002–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Massie J. P., Reynolds E. L., Koestler B. J., Cong J.-P., Agostoni M., and Waters C. M. (2012) Quantification of high-specificity cyclic diguanylate signaling. Proc. Natl. Acad. Sci. U.S.A. 109, 12746–12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teschler J. K., Zamorano-Sánchez D., Utada A. S., Warner C. J., Wong G. C., Linington R. G., and Yildiz F. H. (2015) Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat. Rev. Microbiol. 13, 255–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fong J. C., and Yildiz F. H. (2007) The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J. Bacteriol. 189, 2319–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nishibori N., Yuasa A., Sakai M., Fujihara S., and Nishio S. (2001) Free polyamine concentrations in coastal seawater during phytoplankton bloom. Fish. Sci. 67, 79–83 [Google Scholar]
- 50. Parker Z. M., Pendergraft S. S., Sobieraj J., McGinnis M. M., and Karatan E. (2012) Elevated levels of the norspermidine synthesis enzyme NspC enhance Vibrio cholerae biofilm formation without affecting intracellular norspermidine concentrations. FEMS Microbiol. Lett. 329, 18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hamana K., Aizaki T., Arai E., Saito A., Uchikata K., and Ohnishi H. (2004) Distribution of norspermidine as a cellular polyamine within micro green algae including non-photosynthetic achlorophyllous Polytoma, Polytomella, Prototheca and Helicosporidium. J. Gen. Appl. Microbiol. 50, 289–295 [DOI] [PubMed] [Google Scholar]
- 52. Hamana K., and Matsuzaki S. (1982) Widespread occurrence of norspermidine and norspermine in eukaryotic algae. J. Biochem. 91, 1321–1328 [DOI] [PubMed] [Google Scholar]
- 53. Lutz C., Erken M., Noorian P., Sun S., and McDougald D. (2013) Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front. Microbiol. 4, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bardócz S., Grant G., Brown D. S., Ralph A., and Pusztai A. (1993) Polyamines in food: implications for growth and health. J. Nutr. Biochem. 4, 66–71 [Google Scholar]
- 55. Sexton J. A., Brown V., and Johnston M. (2007) Regulation of sugar transport and metabolism by the Candida albicans Rgt1 transcriptional repressor. Yeast 24, 847–860 [DOI] [PubMed] [Google Scholar]
- 56. Waldor M. K., and Mekalanos J. J. (1994) Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae 0139 and development of a live vaccine prototype. J. Infect. Dis. 170, 278–283 [DOI] [PubMed] [Google Scholar]
- 57. Chang A. C., and Cohen S. N. (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134, 1141–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dunn A. K., Millikan D. S., Adin D. M., Bose J. L., and Stabb E. V. (2006) New rfp-and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 72, 802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.