Abstract
Regulation of mitochondrial biogenesis and respiration is a complex process that involves several signaling pathways and transcription factors as well as communication between the nuclear and mitochondrial genomes. Under aerobic conditions, the budding yeast Saccharomyces cerevisiae metabolizes glucose predominantly by glycolysis and fermentation. We have recently shown that altered chromatin structure in yeast induces respiration by a mechanism that requires transport and metabolism of pyruvate in mitochondria. However, how pyruvate controls the transcriptional responses underlying the metabolic switch from fermentation to respiration is unknown. Here, we report that this pyruvate effect involves heme. We found that heme induces transcription of HAP4, the transcriptional activation subunit of the Hap2/3/4/5p complex, required for growth on nonfermentable carbon sources, in a Hap1p- and Hap2/3/4/5p-dependent manner. Increasing cellular heme levels by inactivating ROX1, which encodes a repressor of many hypoxic genes, or by overexpressing HEM3 or HEM12 induced respiration and elevated ATP levels. Increased heme synthesis, even under conditions of glucose repression, activated Hap1p and the Hap2/3/4/5p complex and induced transcription of HAP4 and genes required for the tricarboxylic acid (TCA) cycle, electron transport chain, and oxidative phosphorylation, leading to a switch from fermentation to respiration. Conversely, inhibiting metabolic flux into the TCA cycle reduced cellular heme levels and HAP4 transcription. Together, our results indicate that the glucose-mediated repression of respiration in budding yeast is at least partly due to the low cellular heme level.
Keywords: heme, metabolic regulation, metabolism, respiration, transcription, tricarboxylic acid cycle (TCA cycle) (Krebs cycle), HAP complex, Hap1, metabolic reprogramming, oxidative phosphorylation
Introduction
The yeast Saccharomyces cerevisiae, even under aerobic conditions, metabolizes glucose predominantly by glycolysis and fermentation, producing ethanol and carbon dioxide (1–4). This metabolic specialty of the budding yeast is partly due to the high activity of pyruvate decarboxylase Pdc1p (5, 6), which leads to cytoplasmic conversion of pyruvate to acetaldehyde and subsequently to ethanol. Because the majority of the glycolytically produced pyruvate in glucose-grown cells is converted to acetaldehyde, only a small fraction of pyruvate is translocated into mitochondria and used for the tricarboxylic acid (TCA)2 cycle (7–9) (see Fig. 1). Under these conditions of low metabolic flux into the TCA cycle, the expression of genes encoding enzymes of the TCA cycle, electron transport chain (ETC), and oxidative phosphorylation (OXPHOS) is low. After glucose is exhausted during diauxic shift, yeast cells activate TCA cycle, ETC, and OXPHOS genes and switch metabolism from fermentation to respiration, utilizing the produced ethanol as a carbon source (3, 10–12).
Figure 1.
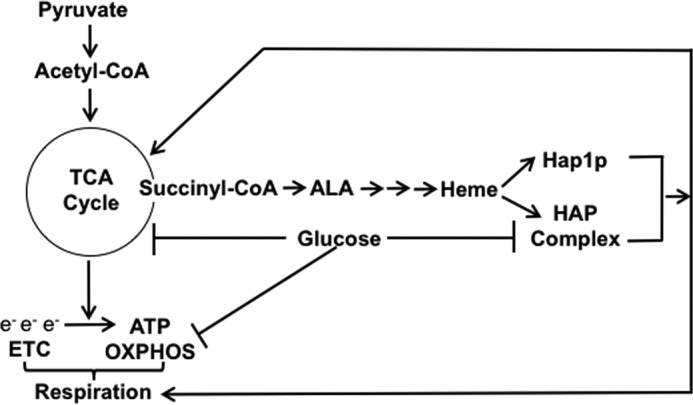
A model depicting the role of heme and glucose in regulation of respiration. Synthesis of heme requires succinyl-CoA, an intermediate of the TCA cycle. Heme activates Hap1p and the HAP complex, both of which activate genes of the TCA cycle, ETC, and OXPHOS. Glucose represses transcription of genes encoding enzymes of the TCA cycle, ETC, and OXPHOS. Glucose also down-regulates the HAP complex by repressing transcription of HAP4. When cells are grown on glucose, only a small fraction of pyruvate is transported into mitochondria and converted to acetyl-CoA.
The transition from fermentation to respiration is controlled by PKA, target of rapamycin (TOR), Sch9p, Snf1p, and Mec1p/Rad53p signaling pathways and requires several transcription factors, including Hap1p, Hap2/3/4/5p, and Rtg1/3p (10, 13–19). However, despite the central position of glycolysis, the TCA cycle, ETC, and OXPHOS in cell metabolism and physiology, the signaling mechanisms and transcriptional regulation by which these pathways are coordinated and aligned with nutritional conditions are not well understood.
The expression of TCA cycle, ETC, and OXPHOS genes is regulated by the heterotetrameric transcription complex Hap2/3/4/5p (heme-activated protein (HAP) complex) independently of PKA and SNF1, suggesting that the HAP complex provides an additional, separate mechanism of transcriptional regulation of mitochondrial respiration (4, 20) (Fig. 1). The HAP complex binds to DNA through the Hap2, -3, and -5 subunits, which are constitutively expressed (21). The activation domain of the complex is contained within the Hap4p subunit (22). HAP4 expression increases upon glucose depletion, and overexpression of HAP4 induces respiration even in a glucose-repressed state, resulting in an extension of replicative life span (23–26). The HAP complex is activated by heme and is required for growth on non-fermentable carbon sources (27, 28). The human homolog of the HAP complex is the heterotrimeric NF-Y complex (29). Interestingly, the NF-Y complex is also regulated by heme (30).
Heme stimulates the transcriptional activity of Hap1p, and as such, Hap1p acts as the key sensor of heme (20, 31). Heme synthesis in yeast is regulated by the availability of oxygen, and heme serves as an intermediate in the signaling mechanism for sensing oxygen levels (32, 33). Thus, under hypoxic conditions, when heme is not synthesized, Hap1p functions as a repressor (34). In addition, Hap1p can repress its own transcription independently of the heme level (35). The Hap1p-regulated genes include genes required for respiration, such as CYC1, CYC7, and CYT1 as well as ROX1, which encodes repressor of many hypoxic genes (20, 32).
Our previous work has shown that altered chromatin structure in S. cerevisiae induces transcription of HAP4 and respiration (36). On the basis of nucleosomal chromatin architecture, yeast genes can be classified into two broad groups: growth genes and stress genes (37). Growth genes are typically expressed at high levels and feature a nucleosome-free region upstream of the coding region where transcription factors can bind in an unobstructed way. The stress genes are generally expressed at lower levels, and their promoters are dominated by delocalized nucleosomes rather than by a nucleosome-free region. Consequently, stress genes are regulated by factors that affect the structure of chromatin, including histone level. The respiratory genes in S. cerevisiae belong to the stress gene category unlike respiratory genes in higher eukaryotes (38, 39). Altered chromatin structure in S. cerevisiae makes promotors of respiratory genes more accessible to transcription factors and allows increased expression of respiratory genes and transition from fermentation to respiration (36). The increased transcription of respiratory genes and induction of respiration require a product of mitochondrial carbon metabolism (36).
In this study, we show that this metabolite is heme. We found that the effect of heme does not require an altered chromatin structure; in cells with normal chromatin architecture, heme regulates transcription of HAP4 in a Hap1p- and HAP complex-dependent manner. Perhaps more importantly, increasing synthesis of heme induces a switch of metabolic mode from fermentation to respiration even under conditions of glucose repression. Together, our results indicate that the glucose-mediated repression of respiration in budding yeast is at least partly due to the low metabolic flux into the TCA cycle and low cellular heme level.
Results
Heme regulates HAP4 transcription
We have previously shown that altered chromatin structure activates transcription of HAP4 and induces respiration by a mechanism that requires transport and metabolism of pyruvate in mitochondria (36). Because many of the TCA cycle and OXPHOS genes are regulated by the heme-activated Hap1p and the HAP complex (27, 28), we speculated that a defect in transport and metabolism of pyruvate in mitochondria would result in diminished synthesis of heme and decreased activities of Hap1p, HAP complex, TCA cycle, and OXPHOS.
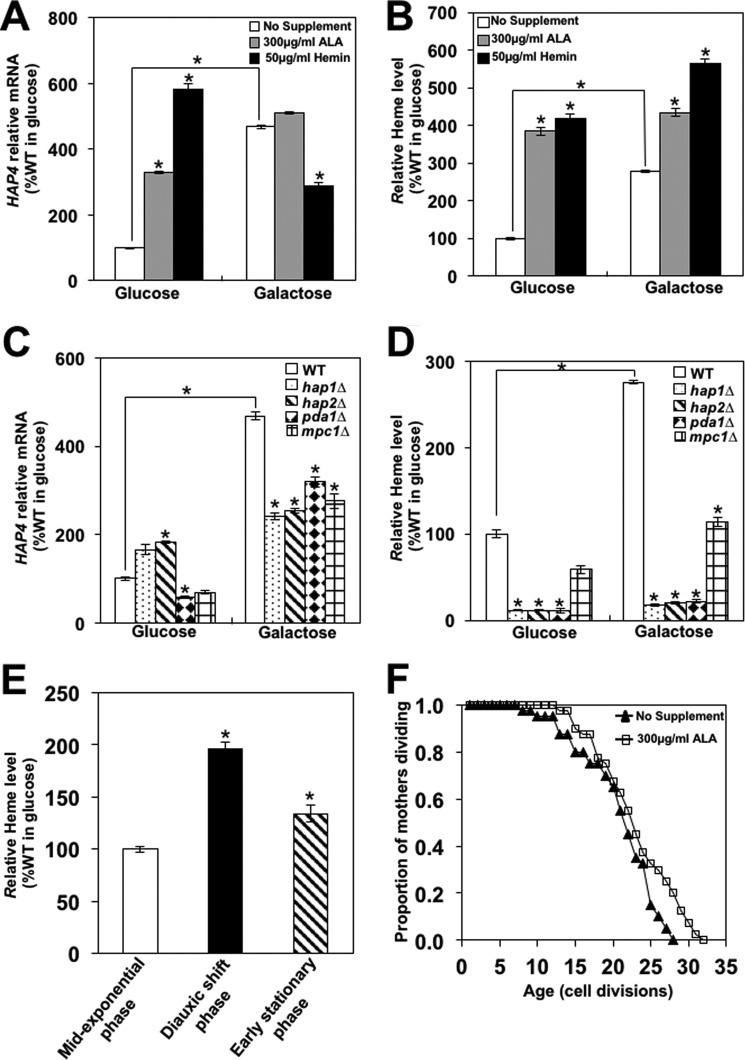
Heme biosynthesis starts in mitochondria and requires succinyl-CoA, an intermediate of the TCA cycle, and glycine to produce a key heme intermediate, 5-aminolevulinic acid (ALA) (20, 31). To determine whether the heme level is limiting for HAP4 transcription in wild-type cells, we added ALA or hemin (a heme derivative used to treat porphyrias) to cells grown in rich media containing glucose or galactose. We found that in cells grown on glucose, addition of ALA or hemin increased HAP4 expression 3.4- and 5.9-fold, respectively (Fig. 2A). In cells grown on galactose, HAP4 expression was elevated 4.7-fold in comparison with glucose-grown cells; however, addition of ALA did not significantly increase HAP4 expression, and addition of hemin decreased HAP4 expression. In glucose-grown cells, addition of ALA and hemin increased the heme level 3.8- and 4.2-fold, respectively (Fig. 2B). In cells grown on galactose, the heme level was elevated 2.7-fold in comparison with glucose-grown cells. Addition of ALA or hemin further increased the heme level 4.3- and 5.5-fold in comparison with glucose-grown cells (Fig. 2B). The effect of ALA or hemin addition on HAP4 transcription appears to be more significant in cells grown on glucose where the effect of ALA or hemin addition on the cellular heme level is more pronounced than in cells grown on galactose. These results suggest that HAP4 transcription is regulated by heme and that the heme synthesis is limiting for HAP4 expression only in cells grown on glucose.
Figure 2.
HAP4 transcription is regulated by heme in Hap1p- and HAP complex-dependent manner. A and B, WT (W303-1a) cells were grown in YEP medium containing 2% glucose or 2% galactose with or without addition of ALA (300 μg/ml) or hemin (50 μg/ml). Relative HAP4 mRNA levels (A) and cellular heme levels (B) were determined. The heme level in WT cells grown on glucose was 17.3 pmol/1010 cells and was set as 100%. C and D, WT (W303-1a), hap1Δ (TZ766), hap2Δ (TZ742), pda1Δ (TZ354), and mpc1Δ (TZ341) cells were grown in YEP medium containing 2% glucose or 2% galactose. Relative HAP4 mRNA levels (C) and cellular heme levels (D) were determined. E, WT (W303-1a) cells were grown in YEP medium containing 2% glucose to midexponential phase (A600 nm of 0.8), diauxic shift (A600 nm of 5.0), and early stationary phase (A600 nm of 9.0), and cellular heme levels were determined. F, replicative life span was determined for wild-type (W303-1a) cells grown on YEP medium containing 2% glucose (no supplement) or the same medium supplemented with 300 μg/ml ALA. Forty cells were analyzed for each condition. A–E, the experiments were repeated three times, and the results are shown as means ± S.D. (error bars). Values that are statistically significantly different (p < 0.05) from the WT cells grown on the corresponding carbon source are indicated by an asterisk. Values that are statistically significantly different (p < 0.05) from each other are indicated by a bracket and an asterisk. The results are expressed relative to the value for the wild-type strain grown in 2% glucose.
Heme synthesis and HAP4 transcription are regulated by metabolic flux into the TCA cycle
The lower heme level in glucose-grown cells suggests that heme synthesis is limited by metabolic flux into the TCA cycle and by availability of succinyl-CoA. To test this possibility, we determined the heme level and HAP4 expression in pda1Δ and mpc1Δ cells. Pda1p is a subunit of pyruvate dehydrogenase complex that catalyzes the conversion of pyruvate to acetyl-CoA in mitochondria. Mpc1p is a pyruvate transporter localized in the inner mitochondrial membrane; mitochondria isolated from mpc1Δ cells have decreased pyruvate uptake (40, 41). Both HAP4 expression and heme levels in pda1Δ and mpc1Δ cells were significantly decreased in comparison with wild-type cells both in glucose and galactose media (Fig. 2, C and D). To extend these findings, we compared heme levels in cells grown to midexponential phase, diauxic shift, and early stationary phase (Fig. 2E). The heme level in cells undergoing diauxic shift was elevated almost 2-fold in comparison with midexponential phase cells, indicating that heme synthesis is up-regulated when cells transition from utilizing glucose to ethanol. We interpret these results to mean that metabolic flux into the TCA cycle regulates the cellular heme level, most likely by regulating succinyl-CoA synthesis. These results are also consistent with the role of heme in regulation of HAP4 expression (Fig. 2, A and B) and with the observed increase of HAP4 transcription during diauxic shift (23). Overexpression of HAP4 induces respiration and extends yeast replicative life span (23–26). Because addition of ALA increased HAP4 expression (Fig. 2A), we wanted to determine whether ALA also increases life span. Indeed, we found that addition of ALA to glucose medium increases the replicative life span of wild-type cells (Fig. 2F).
HAP4 transcription is regulated by Hap1p and HAP complex
Because HAP4 transcription is affected by heme level and Hap1p and the HAP complex are the only known yeast transcription factors regulated by heme, we next determined HAP4 transcription in wild-type, hap1Δ, and hap2Δ cells grown on glucose or galactose (Fig. 2C). As noted above, HAP4 expression was elevated 4.7-fold in cells grown on galactose in comparison with glucose-grown cells, but this induction was largely abolished in hap1Δ and hap2Δ cells (Fig. 2C). These results indicate that HAP4 transcription is regulated by Hap1p and the HAP complex. Alternatively, the HAP4 transcription might be regulated by another, previously uncharacterized heme-responsive factor. In that case, the roles of Hap1p and the HAP complex would be only indirect, mediated by the cellular heme level. This possibility is supported by the decreased levels of heme in both hap1Δ and hap2Δ cells (Fig. 2D).
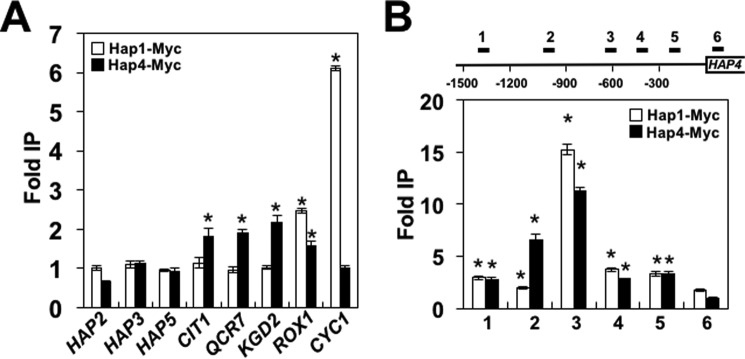
To determine whether Hap1p and the HAP complex regulate HAP4 transcription directly or indirectly, we performed a chromatin immunoprecipitation (ChIP) experiment with myc-tagged Hap1p and Hap4p. As expected, we found that Hap1p was recruited to promoters of ROX1 and CYC1 genes, which are known targets of Hap1p (Fig. 3A and Ref. 31). Hap4p was recruited to promoters of CIT1, QCR7, and KGD2, known targets of the HAP complex (42). In addition, we detected occupancy of Hap4p at the ROX1 promoter. Neither Hap1p nor Hap4p was recruited to the constitutively expressed HAP2, HAP3, or HAP5 genes. To determine whether Hap1p and the HAP complex directly regulate transcription of HAP4, we assayed occupancy of Hap1p and Hap4p within 1.5 kb upstream of the HAP4 coding region (Fig. 3B). Both Hap1p and Hap4p bound to the HAP4 promoter; the maximum occupancy was found at around 600 bp upstream of the HAP4 transcriptional start site. Together, these results suggest that HAP4 transcription is directly regulated by both Hap1p and the HAP complex.
Figure 3.
Hap1p and HAP complex directly regulate HAP4 transcription. A, Hap1p and Hap4p are not recruited to HAP2, HAP3, and HAP5 promoters. B, Hap1p and Hap4p are recruited to HAP4 promoter. A and B, ChIP was performed using chromatin from cells expressing myc-Hap1p (FY2612) and Hap4p-myc (TZ909). Each immunoprecipitation (IP) was performed at least three times using different chromatin samples, and the occupancy at the indicated genes was calculated using the POL1 coding sequence as a negative control. The data are presented as -fold occupancy over the POL1 coding sequence control and represent means ± S.D. (error bars). Values that are statistically different (p < 0.05) from recruitment of Hap1p or Hap4p to the POL1 locus are indicated by an asterisk.
HAP4 transcription correlates with cellular heme level
To test the heme responsiveness of HAP4 transcription, we grew hem1Δ, hem1Δhap1Δ, hem1Δhap2Δ, and hem1Δhap1Δhap2Δ cells in YPD medium containing different concentrations of ALA. HEM1 encodes ALA synthase, the first enzyme in the heme biosynthetic pathway (43). Yeast with the hem1Δ mutation require the addition of ALA to the growth medium, and the cellular heme level can be regulated by the concentration of ALA in the medium (27).
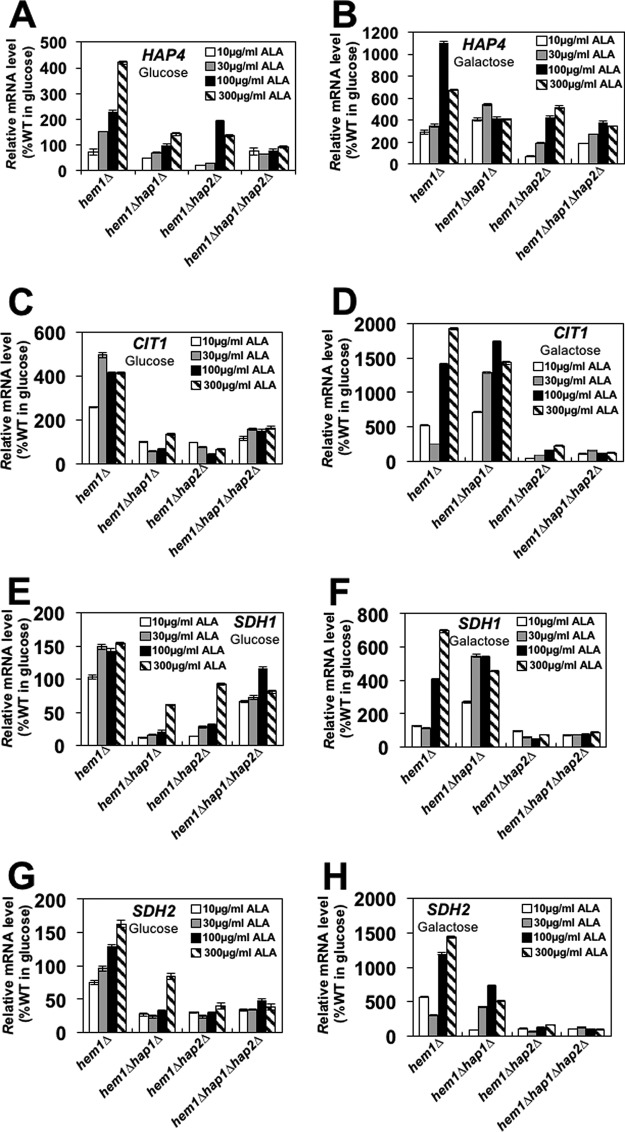
In hem1Δ cells grown on glucose, HAP4 expression increased with increasing concentration of ALA (Fig. 4A). In hem1Δhap1Δ and hem1Δhap2Δ cells, the expression of HAP4 was decreased in comparison with hem1Δ cells but still correlated with the ALA concentration in the medium. The heme responsiveness was completely lost in hem1Δhap1Δhap2Δ cells. These results indicate that in cells grown on glucose both Hap1p and the HAP complex mediate the heme responsiveness of HAP4 expression.
Figure 4.
Transcription of HAP4 and TCA cycle genes correlates with cellular heme level. Strains hem1Δ (TZ779), hem1Δhap1Δ (TZ864), hem1Δhap2Δ (TZ881), and hem1Δhap1Δ hap2Δ (TZ884) were grown in YEP medium containing 2% glucose (A, C, E, and G) or 2% galactose (B, D, F, and H) and 10, 30, 100, or 300 μg/ml ALA. Shown are relative mRNA levels for HAP4 (A and B), CIT1 (C and D), SDH1 (E and F), and SDH2 (G and H). The experiments were repeated three times, and the results are shown as means ± S.D. (error bars). The results are expressed relative to the value for the wild-type strain grown in 2% glucose.
In hem1Δ and hem1Δhap2Δ cells grown on galactose, HAP4 expression correlated with the ALA concentration in the medium. However, at high concentrations of ALA, HAP4 expression was higher in hem1Δ than in hem1Δhap2Δ or hem1Δhap1Δ cells (Fig. 4B). The heme responsiveness was lost in hem1Δhap1Δ cells and significantly reduced in hem1Δhap1Δhap2Δ cells. These results indicate that in cells grown on galactose the heme responsiveness of HAP4 transcription is mediated primarily by Hap1p. The lack of heme responsiveness in hem1Δhap1Δ cells is surprising because it would be expected that the heme activation of the HAP complex would still result in increased HAP4 transcription, similarly to hem1Δhap1Δ cells grown on glucose (Fig. 4A). In addition, transcription of CIT1, SDH1, and SDH2 in hem1Δhap1Δ cells grown on glucose or galactose correlates with the concentration of ALA in the medium, indicating the activation of the HAP complex by heme (Fig. 4, C–H). It is possible that full activation of HAP4 transcription by heme requires a synergistic action of both Hap1p and the HAP complex. Similar synergistic regulation was described for genes regulated by repressors Rox1p and Mot3p (44–46). Alternatively, heme-mediated activation of the HAP complex may induce expression of a repressor that, directly or indirectly, counteracts the activation of the HAP complex and down-regulates transcription of HAP4. A similar situation occurs at the HEM1 promoter where positive and negative regulators cancel each other's effects (47). The logical candidate for this repressor in the case of HAP4 transcription would be Rox1p, which is transcriptionally regulated by heme in a Hap1p-dependent manner (48). However, HAP4 has not been identified as a target of Rox1p yet (32, 49).
Transcription of TCA cycle genes is regulated by heme
To determine whether the transcription of HAP complex-regulated genes encoding enzymes of the TCA cycle is also regulated by heme, we measured mRNA levels of CIT1, SDH1, and SDH2 in hem1Δ, hem1Δhap1Δ, hem1Δhap2Δ, and hem1Δhap1Δhap2Δ cells in YEP medium (1% yeast extract, 2% Bacto peptone) containing glucose or galactose and different concentrations of ALA. As expected, the mRNA levels were significantly lower in cells grown on glucose in comparison with galactose-grown cells. In hem1Δ cells grown on glucose or galactose, the mRNA levels of the three genes correlated with the ALA concentration (Fig. 4, C–H). Both Hap1p and the HAP complex make comparable contributions to transcription of CIT1, SDH1, and SDH2 in glucose-grown cells (Fig. 4, C, E, and G). However, the HAP complex appears to play a more dominant role than Hap1p in cells grown on galactose (Fig. 4, D, F, and H). This is likely due to the higher activity of the HAP complex due to the higher cellular heme level in cells grown on galactose (Figs. 2B and 5, A and B).
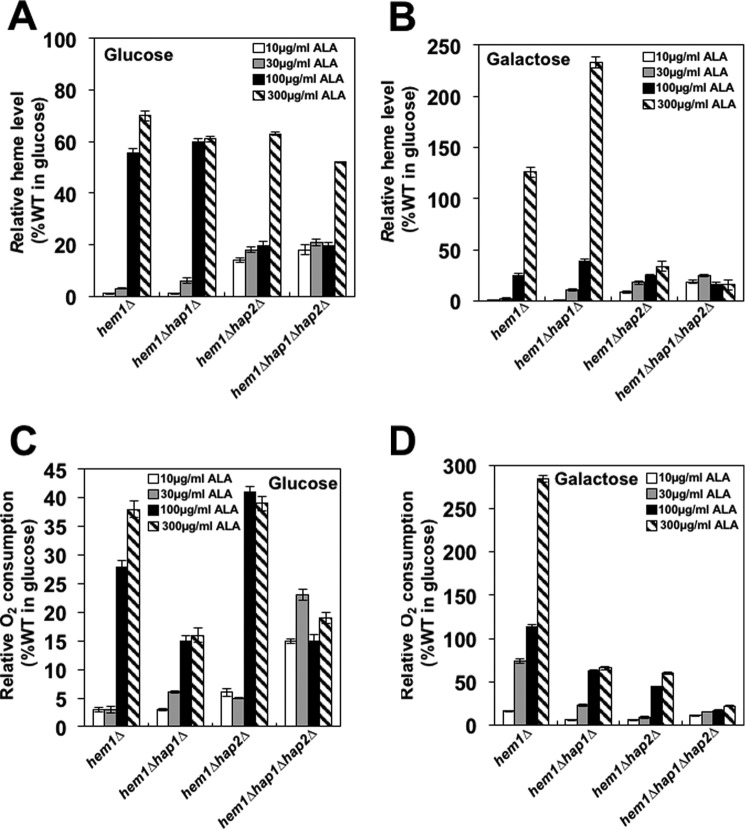
Figure 5.
Respiration correlates with cellular heme level. Strains hem1Δ (TZ779), hem1Δhap1Δ (TZ864), hem1Δhap2Δ (TZ881), and hem1Δhap1Δ hap2Δ (TZ884) were grown in YEP medium containing 2% glucose (A and C) or 2% galactose (B and D) and 10, 30, 100, or 300 μg/ml ALA. Shown are cellular heme levels (A and B) and oxygen consumption rates (C and D). The oxygen consumption rate in WT cells grown on glucose (YPD medium) was 5.08 pmol/106 cells/s and was set as 100%. The experiments were repeated three times, and the results are shown as means ± S.D. (error bars). The results are expressed relative to the value for the wild-type strain grown in 2% glucose.
Mitochondrial respiration correlates with cellular heme level
To confirm that the heme synthesis correlates with the concentration of ALA in the medium, we determined the cellular heme levels in hem1Δ, hem1Δhap1Δ, hem1Δhap2Δ, and hem1Δhap1Δhap2Δ cells grown in media containing glucose or galactose and different concentrations of ALA (Fig. 5, A and B). In both glucose- and galactose-grown cells, the cellular heme levels correlated with the ALA concentration in the medium. However, even at the highest concentration of ALA (300 μg/ml), the heme levels in hem1Δ cells grown on glucose or galactose did not reach the levels in wild-type cells grown on the corresponding carbon source (compare Figs. 2B and 5, A and B). In cells grown in glucose with the highest concentration of ALA, the heme levels in hem1Δhap1Δ, hem1Δhap2Δ, and hem1Δhap1Δhap2Δ cells did not significantly differ from hem1Δ cells. However, the heme levels in galactose-grown hem1Δhap1Δ cells were elevated about 2-fold in comparison with the hem1Δ cells, whereas the heme levels in hem1Δhap2Δ and hem1Δhap1Δhap2Δ cells were significantly decreased (Fig. 5B).
HAP4 transcription in hem1Δ cells grown on either glucose or galactose media supplemented with high concentrations of ALA was significantly increased in comparison with HAP4 transcription in wild-type cells grown on the corresponding carbon source (compare Figs. 2A and 4, A and B) despite the fact that the cellular heme levels in hem1Δ cells were decreased (compare Figs. 2B and 5, A and B). This result suggests that one or several intermediates of the heme biosynthetic pathway, perhaps ALA itself, accumulates in hem1Δ cells supplemented with high concentrations of ALA and activates Hap1p and the HAP complex, resulting in increased HAP4 transcription.
To determine whether respiration is affected by cellular heme levels, we measured oxygen consumption in hem1Δ, hem1Δhap1Δ, hem1Δhap2Δ, and hem1Δhap1Δhap2Δ cells grown in media containing glucose or galactose and different concentrations of ALA (Fig. 5, C and D). In glucose-grown cells, the oxygen consumption correlated with the ALA concentration in the medium. However, even at the highest concentration of ALA (300 μg/ml), the oxygen consumption in hem1Δ cells did not reach the oxygen consumption of wild-type cells. As expected, the highest oxygen consumption in hem1Δhap1Δ, hem1Δhap2Δ, and hem1Δhap1Δhap2Δ cells was reached in medium containing the highest concentration of ALA. Still, the values were reduced in comparison with hem1Δ cells (Fig. 5, C and D). We observed similar trends in hem1Δ, hem1Δhap1Δ, hem1Δhap2Δ, and hem1Δhap1Δhap2Δ cells grown on galactose, but the oxygen consumption reached at the highest concentrations of ALA were higher than in glucose-containing medium. This was expected because we reported previously that oxygen consumption in wild-type cells grown on galactose was about 2.5-fold higher than in wild-type cells grown on glucose (36).
Inactivation of ROX1 induces heme synthesis and mitochondrial respiration
The addition of excess ALA to hem1Δ cells did not result in cellular heme levels above the levels found in the wild-type cells grown on the corresponding carbon source (compare Figs. 2B and 5, A and B). The reason for this is currently not clear. It is probable that the exogenous ALA is not completely taken up by the cells or efficiently transported to the appropriate cellular compartment. It is also possible that the excess of exogenous ALA results in accumulation of intermediates of the heme biosynthetic pathway. These intermediates might not be effectively converted to heme and might affect activity of enzymes in the heme biosynthetic pathway (50).
In an effort to find a model with which we could test the effect of elevated cellular heme level on transcription of HAP4 and mitochondrial respiration, we characterized the rox1Δ mutant. Rox1p is a repressor of anaerobic genes, such as ANB1. The heme-dependent repression by Rox1p is due to Hap1p-mediated activation of ROX1 transcription. When the cellular level of heme increases, Hap1p is activated and stimulates ROX1 transcription. Under hypoxic conditions when heme is absent, ROX1 is not transcribed, and hypoxic genes are activated (32, 49, 51, 52). In addition, Rox1p represses transcription of its own gene (53, 54).
One of the genes regulated by Rox1p is HEM13, which encodes coproporphyrinogen III oxidase, one of the enzymes of the heme biosynthetic pathway (48). We speculated that derepression of HEM13 upon inactivation of ROX1 would result in an elevated cellular heme level. This hypothesis was correct; the heme level in glucose-grown cells was about 2.6-fold higher in rox1Δ cells than in the wild-type cells (Fig. 6A). In addition, the increased cellular level of heme correlated with increased oxygen consumption, which increased about 2.8-times in rox1Δ cells in comparison with wild-type cells (Fig. 6B). These results show that the oxygen consumption in rox1Δ cells grown on glucose is almost identical to the oxygen consumption of wild-type cells grown on galactose (36), suggesting that respiration is not repressed by glucose in rox1Δ cells. The increased cellular heme level and oxygen consumption in rox1Δ cells are associated with elevated ATP level and increased transcription of HAP4, CIT1, SDH1, and SDH2 (Fig. 6, C and D). The increased heme and ATP levels, oxygen consumption, and transcription of the TCA cycle genes in rox1Δ cells require Hap1p and the HAP complex (Fig. 6). This finding is consistent with the notion that Hap1p and the HAP complex function as heme receptors and mediate the effect of heme on respiration.
Figure 6.
Inactivation of ROX1 induces heme synthesis and mitochondrial respiration. Cellular heme levels (A), oxygen consumption (B), cellular ATP levels (C), and relative mRNA levels of the indicated genes (D) were determined in WT (W303-1a), rox1Δ (TZ917), hap1Δ (TZ766), rox1Δhap1Δ (TZ992), hap4Δ (LG579), and rox1Δhap4Δ (TZ974) strains. The ATP level in WT cells grown on glucose (YPD medium) was 0.58 μmol/1010 cells and was set as 100%. The cells were grown in YEP medium containing 2% glucose. The experiments were repeated three times, and the results are shown as means ± S.D. (error bars). Values that are statistically different (p < 0.05) from the WT cells are indicated by an asterisk.
Interestingly, the cellular ATP level was also significantly up-regulated in hap4Δ cells (Fig. 6C). Because HAP4 inactivation decreases oxygen consumption (Fig. 6B) and expression of TCA cycle genes (Fig. 6D), the increased ATP level in hap4Δ cells is likely not caused by increased activity of the TCA cycle, ETC, and OXPHOS. It seems more likely that activation of glycolysis is responsible for the increased ATP level, but the mechanism of this activation is not known.
Overexpression of HEM3 or HEM12 increases cellular heme level and induces mitochondrial respiration
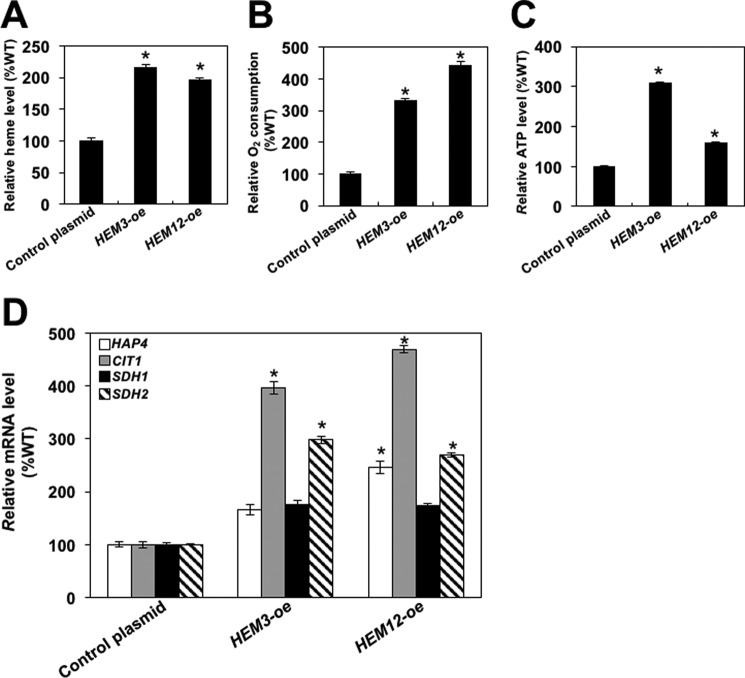
Because Rox1p is a transcriptional repressor, it may directly or indirectly affect transcription of HAP4 and genes required for the TCA cycle and OXPHOS, independently of its role in regulating the cellular heme level. To eliminate this possibility, we considered other approaches to increase cellular heme level. Heme synthesis can be increased by overexpressing HEM3, which encodes porphobilinogen deaminase, or HEM12, which encodes uroporphyrinogen decarboxylase (50, 55). To conclusively prove that it is the heme level that is responsible for the induction of mitochondrial respiration, we individually overexpressed HEM3 and HEM12 in wild-type cells.
Overexpression of HEM3 or HEM12 in wild-type cells increased the cellular heme level 2.2- and 2-fold, respectively (Fig. 7A). Importantly, overexpression of HEM3 or HEM12 increased oxygen consumption more than 3- and 4-fold, respectively (Fig. 7B). The oxygen consumption in glucose-grown cells overexpressing HEM3 or HEM12 is even higher than the oxygen consumption of wild-type cells grown on galactose or non-fermentable carbon sources (36). As expected, overexpression of HEM3 or HEM12 also increased the cellular ATP levels and transcription of HAP4, CIT1, SDH1, and SDH2 (Fig. 7, C and D). Because Hem3p and Hem12p do not play any role in transcriptional regulation, the effect of overexpressing the corresponding genes on the induction of respiration and transcription of HAP4 and genes encoding enzymes of the TCA cycle can be solely attributed to the increased cellular level of heme.
Figure 7.
Overexpression of HEM3 or HEM12 increases cellular heme level and induces mitochondrial respiration. Cellular heme levels (A), oxygen consumption (B), cellular ATP levels (C), and relative mRNA levels of the indicated genes (D) were determined in wild-type cells containing either the control plasmid (TZ1099) or high-copy-number plasmid expressing HEM3 (HEM3-oe; strain TZ1100) or HEM12 (HEM12-oe; strain TZ1101). The cells were pregrown under selection in synthetic complete medium and inoculated to an A600 nm of 0.2 into YEP medium containing 2% glucose and grown for two generations. The experiments were repeated three times, and the results are shown as means ± S.D. (error bars). Values that are statistically different (p < 0.05) from the wild-type cells containing the control plasmid are indicated by an asterisk.
Discussion
The key finding of this study is that heme induces metabolic transition from fermentation to mitochondrial respiration even under glucose-repressing conditions (Fig. 8). Glucose represses many genes involved in the TCA cycle, ETC, and OXPHOS by a mechanism that is independent of PKA and Mig1p/Snf1p pathways. A number of these genes are regulated by the HAP complex (4, 11). Our results indicate that the mechanism of glucose repression of these genes involves low cellular heme level due to the low metabolic flux into the TCA cycle and insufficient availability of succinyl-CoA for ALA and heme synthesis. Low cellular heme level in glucose-grown cells results in low activity of the HAP complex and low transcription of HAP complex-regulated genes. Increasing heme synthesis activates the HAP complex and induces transcription of HAP complex-regulated genes and transition from fermentation to respiration.
Figure 8.
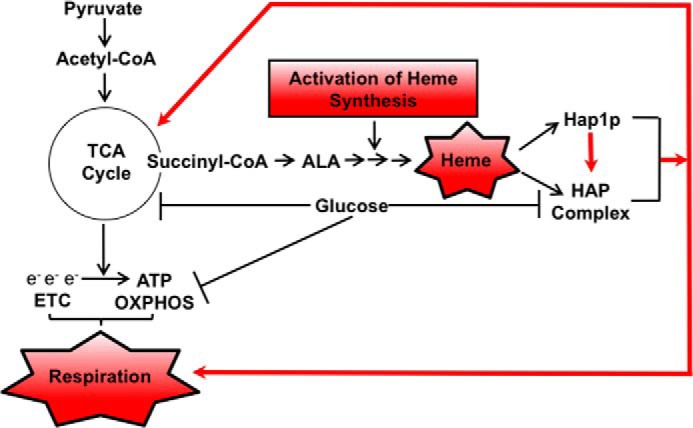
Increased synthesis of heme overcomes glucose repression of mitochondrial respiration. Glucose represses TCA cycle genes and synthesis of succinyl-CoA, resulting in a low level of ALA and heme. Activation of heme synthesis by overexpressing HEM3 or HEM12 or by derepressing HEM13 (in rox1Δ cells) results in elevated levels of heme and activation of Hap1p and the HAP complex, leading to activation of genes encoding enzymes of the TCA cycle, ETC, and OXPHOS.
In aerobic organisms, heme serves as a prosthetic group in proteins and enzymes that sense, transport, or use oxygen, such as hemoglobin, myoglobin, cytochrome complexes, catalases, and cyclooxygenases (31). Heme is also required for ATP production by the mitochondrial ETC and OXPHOS. To accomplish these diverse roles, heme controls activities of a number of proteins involved in signal transduction and transcription (31). In yeast, the major players involved in the heme transcriptional regulatory network are Hap1p and the HAP complex as well as repressors Rox1p and Mot3p.
Heme synthesis in yeast is regulated by the availability of oxygen, and heme serves as an intermediate in the signaling mechanism for sensing oxygen levels (32, 33). Our results suggest that the cellular heme level is also regulated by a carbon source; compared with glucose-grown cells, the heme levels in cells grown on galactose are 2.7 times higher (Fig. 2B). The increased heme level caused by addition of ALA in cells grown on glucose medium (Fig. 2B) indicates that the limiting step of heme synthesis in glucose-grown cells is the synthesis of ALA. ALA is produced from succinyl-CoA and glycine by 5-aminolevulinate synthase, encoded by the HEM1 gene. Because the metabolic flux into the TCA cycle is low in glucose-grown cells (7, 8), it is likely that the synthesis of succinyl-CoA, an intermediate of the TCA cycle, is limiting for the production of ALA and heme (Figs. 1 and 8). Derepression of HEM13 in rox1Δ cells or overexpression of HEM3 or HEM12 leads to increased conversion of ALA to heme (Figs. 6 and 7), which in turn induces transcription of HAP4 and activates Hap1p and the HAP complex. Activation of Hap1p and the HAP complex leads to increased expression of genes encoding the TCA cycle enzymes and increased synthesis of succinyl-CoA, ALA, and heme. The negative regulator in this mechanism is Rox1p. Transcription of ROX1 is activated by heme in a Hap1p-dependent manner, and Rox1p controls heme synthesis by repressing HEM13 and HEM14 (48, 49). This model is also consistent with the fact that the addition of ALA to wild-type cells results in about a 4-fold increase of cellular heme level (Fig. 2B) and that the same addition of ALA to hem1Δ cells results in cellular heme levels that correspond to only about 70% of the wild-type level (Fig. 5A). We interpret this result to mean that the addition of ALA to wild-type cells increases heme synthesis, resulting in increased activation of Hap1p and the HAP complex, increased transcription of HAP4 and genes encoding TCA cycle enzymes, and increased synthesis of succinyl-CoA and, therefore, ALA. Introducing the hem1Δ mutation prevents de novo synthesis of ALA and does not allow the cellular heme to reach the wild-type levels.
In hem1Δ cells grown on glucose, high concentrations of ALA do not increase heme levels (Fig. 5A), but they do lead to about a 4-fold increase in HAP4 transcription (Fig. 4A). However, this increased HAP4 transcription is not accompanied by induction of respiration (Fig. 5C). Thus, increased transcription of HAP4 in the absence of elevated heme level is not able to induce respiration. In contrast, increased heme synthesis in rox1Δ cells or in cells overexpressing HEM3 or HEM12 results in the induction of respiration despite the fact that it induces only a relatively modest increase in HAP4 transcription (Figs. 6 and 7). These findings indicate that the cellular level of heme is the primary signal that triggers the metabolic transition from fermentation to respiration (Fig. 8). The heme-induced activation of HAP4 transcription is part of the cellular response to increased heme level, but when HAP4 transcription is dissociated from heme synthesis, it does not induce respiration (compare Figs. 4 and 6). This conclusion underscores the importance of Hap1p and HAP complex activation by heme.
How does heme orchestrate the transition of the metabolic mode from fermentation to respiration? Our results show that the transition requires both Hap1p and the HAP complex. The increased respiration due to the increased synthesis of heme in rox1Δ cells is attenuated by introducing hap1Δ or hap4Δ mutation (Fig. 6). The requirement for HAP4 is not surprising because HAP4 and the integrity of the HAP complex are required for induction of respiration and viability when cells are grown on non-fermentable carbon sources (28). In addition, overexpression of HAP4 results in increased respiration even in cells grown on glucose (24–26). The requirement of Hap1p for heme-induced respiration is consistent with the role of Hap1p in the regulation of HAP4 transcription and suggests that activation of the HAP complex by heme is alone not sufficient for induction of respiration. Our results thus indicate that heme has at least two targets required for activation of respiration: Hap1p and the HAP complex. However, we cannot exclude the possibility that heme directly regulates other proteins important for respiration. For example, Mss51p, a COX1 mRNA-specific translational activator and Cox1p chaperone, is a heme-binding protein whose function in cytochrome c oxidase biogenesis is also regulated by heme (56).
The transcriptional regulation of HAP4 is not completely understood. The HAP4 promoter contains Cat8p-binding sites; however, cat8Δ mutation has no effect on HAP4 transcription (57). The HAP4 promoter also contains a canonical Mig1p-binding site; however, Mig1p binding to the HAP4 promoter has not been demonstrated, and the mig1Δ mutation does not result in activation of respiration (7, 42, 58). Our results show that both Hap1p and Hap4p are recruited to the HAP4 promoter and that HAP4 transcription is stimulated by heme (Figs. 2 and 4). Thus, heme regulates both the activity of HAP complex (27) and transcription of HAP4 (Figs. 2–4).
Altogether, this study suggests that the glucose-mediated repression of respiration in budding yeast is at least partly due to the low cellular heme level. The metabolic flux into the TCA cycle is low in glucose-grown cells (7, 8) partly due to the high activity of pyruvate decarboxylase Pdc1p (5, 6), which limits the amount of pyruvate available for conversion to acetyl-CoA in mitochondria. This results in low levels of succinyl-CoA, a precursor for ALA and heme synthesis. Increasing conversion of ALA into heme activates Hap1p and the HAP complex, which results in induction of TCA cycle genes, increased metabolic flux into the TCA cycle, and increased synthesis of succinyl-CoA. This mechanism also explains the observed activation of the HAP complex during the diauxic shift (23). As glucose is depleted and cells begin to utilize ethanol, the metabolic flux into the TCA cycle increases together with the synthesis of succinyl-CoA, ALA, and heme, leading to the activation of Hap1p and the HAP complex and induction of TCA cycle, ETC, and OXPHOS genes.
Experimental procedures
Yeast strains, plasmids, and media
All yeast strains used in this study are listed in Table 1. Standard genetic techniques were used to manipulate yeast strains and to introduce mutations from non-W303 strains into the W303 background (61). To construct HEM3 and HEM12 overexpression plasmids, HEM3 and HEM12 genomic sequences, spanning 1.0 kb upstream and 400 bp downstream of the coding region, were cloned by PCR using Phusion high-fidelity DNA polymerase (M0530S, New England Biolabs) with primers specific to HEM3 (5′-AGGGTTAGACTAGTATATCAATCCCG-3′ and 5′-TAATAACTCGAGATTTCATTTTTTATATACAAGCTA-3′) and HEM12 (5′-TGCTTCTGTCTAGAAAAACGAGC-3′ and 5′-CATTTTACTCGAGTTTTGTGAATTCATGC-3′). The HEM3 forward and reverse primers contain SpeI and XhoI sites, respectively, and the HEM12 forward and reverse primers contain NotI and XhoI sites, respectively. Following double digestion with the appropriate restriction enzymes, HEM3 and HEM12 genomic DNA fragments were ligated into the high-copy-number plasmid pRS426. Cells were grown at 28 °C in YEP medium containing 2% glucose, 2% galactose, or 2% acetate or under selection in synthetic complete medium containing 2% glucose and, when appropriate, lacking specific nutrients to select for a particular genotype.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source/Ref. |
|---|---|---|
| W303-1a | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 | R. Rothstein |
| W303-1α | MATα ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 | R. Rothstein |
| W303 | MATa/MATα ade2–1/ade2–1 his3–11,15/his3–11,15 leu2–3,112/leu2–3,112 trp1–1/ trp1–1ura3–1/ ura3–1 can1–100/ can1–100 | R. Rothstein |
| YPL101W | MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 hap1::KAN | Open Biosystems |
| TZ766 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hap1::KAN | This study |
| YGL237C | MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 hap2::KAN | Open Biosystems |
| TZ742 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hap2::KAN | This study |
| FY2867 | MATa ura3Δ0 his3Δ200 leu2Δ0 lys2–128 hap2Δ0::LEU2 | 59 |
| TZ742 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hap2::LEU | This study |
| TZ354 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 pda1::KAN | 36 |
| TZ341 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 mpc1::KAN | 36 |
| FY2612 | MATa ura3Δ0 his3Δ0 his3Δ0 lsy2–128 myc-HAP1 | 59 |
| HAP4-Myc9 | MATa ura3Δ0 his3Δ200 leu2Δ0 lys2–128 myc-HAP4::HPH | 60 |
| TZ909 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 myc-HAP4::HPH | This study |
| YDR232W | MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 hem::KAN | Open Biosystems |
| TZ779 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hem1::KAN | This study |
| TZ864 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hem1:: KAN hap1::KAN | This study |
| TZ881 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hem1:: KAN hap2::LEU | This study |
| TZ884 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hem1:: KAN hap1:: KAN hap2::LEU | This study |
| YPR065W | MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 rox1::KAN | Open Biosystems |
| TZ917 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 rox1::KAN | This study |
| TZ992 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 rox1:: KAN hap1::KAN | This study |
| YKL109W | MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 hap4::KAN | Open Biosystems |
| LG579 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 hap4::KAN | 36 |
| TZ974 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 rox1:: KAN hap4::KAN | This study |
| TZ1099 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 pRS426 [URA3, 2μ] | This study |
| TZ1100 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 pRS426-HEM3 [URA3, 2μ, HEM3] | This study |
| TZ1101 | MATa ade2–1 his3–11,15 leu2–3,112 trp1–1 ura3–1 ssd1-d2 can1–100 pRS426-HEM12 [URA3, 2μ, HEM12] | This study |
Oxygen consumption
Cells were grown to an A600 nm of 0.6 in YEP medium containing either 2% glucose or galactose, and 9 × 106 cells were harvested by centrifugation. Cells were resuspended in a buffer containing 10 mm HEPES, 25 mm K2HPO4, pH 7.0, and incubated at 30 °C in an oxygen consumption chamber (Instech Laboratories, Inc.) connected to a NeoFOX fluorescence-sensing detector using NeoFOX software (Ocean Optics, Inc.). Results were calculated as pmol of O2/106 cells/s and expressed as percentages of the wild-type value. The oxygen consumption rate in wild-type (WT) cells grown on glucose (YPD medium) was 5.08 pmol/106 cells/s and was set as 100%.
ATP and heme assays
For the ATP assay, cells were grown to an A600 nm of 0.6 in YEP medium containing 2% glucose, and 9 × 107 cells were harvested by centrifugation and lysed in 5% trichloroacetic acid with prechilled glass beads. Cell lysate was neutralized to pH 7.5 with 10 m KOH and 2 m Tris-HCl, pH 7.5. ATP levels were measured using the ENLITEN ATP assay (FF2000, Promega) according to the manufacturer's instructions and normalized by the number of cells. The ATP level in WT cells grown on glucose (YPD medium) was 0.58 μmol/1010 cells and was set as 100%. For the heme assay, cells were grown to an A600 nm of 0.8 in YEP medium containing either 2% glucose or galactose, and 3 × 108 cells were harvested by centrifugation and lysed in 200 μl of 1% NaOH and 5% Triton with prechilled glass beads. Cell lysate was neutralized to pH 7.5 with 1 m HCl and 2 m Tris-HCl, pH 7.5. Heme was assayed using a heme colorimetric assay kit (K672-100, BioVision). The heme level in WT cells grown on glucose (YPD medium) was 17.3 pmol/1010 cells and was set as 100%.
Real-time RT-PCR
Real-time RT-PCR was performed as described (62) using the following primers: ACT1, 5′-TATGTGTAAAGCCGGTTTTGC-3′ and 5′-GACAATACCGTGTTCAATTGGG-3′; HAP4, 5′-CCGCAAAGACTTTTCTACACAGG-3′ and 5′-TGTTATGATGGTTGGTATTTGGG-3′; CIT1, 5′-CAGCGATATTATCAACAACTAGCA-3′ and 5′-TAGTGGCGAGCATTCAATAGTG-3′; SDH1, 5′-CTCCAAGTTGACTTTGCTCAGAA-3′ and 5′-ACGCGGAACCGTTTACAGA-3′; and SDH2, 5′-ATTGAGAAGGAAGGCCTTTTGT-3′ and 5′-AGTTTTCAATCTGGGGGTATGC-3′.
ChIP assays
In vivo chromatin cross-linking and immunoprecipitation were performed essentially as described (63, 64). Immunoprecipitation was performed with the anti-myc antibody (2276, Cell Signaling Technology). The primers used for real-time PCR are as follows: POL1, 5′-TCCTGACAAAGAAGGCAATAGAAG-3′ and 5′-TAAAACACCCTGATCCACCTCTG-3′; HAP2, 5′-AGACGAAACGGATGCGAAAT-3′ and 5′-AAGCTGCTGCCGTTGATGT-3′; HAP3, 5′-ATGAATACCAACGAGTCCGAACA-3′ and 5′-GAAATTTGCTGCAAACTGCC-3′; HAP4-1, 5′-GAAATAGATGCATTTTATGTGCGA-3′ and 5′-GGTTTGCATTCTATTCGTTACCC-3′; HAP4-2, 5′-CTATGAGATCACTATTTTGCCGGA-3′ and 5′-GAAATTCTCAGCAGAGGTTATCCC-3′; HAP4-3, 5′-CTTATGTTACGGATCTTGCACG-3′ and 5′-TCTGCAAAATCGATACATGACAC-3′; HAP4-4, 5′-TGGTTGTTTAGGTCCATCTCCTT-3′ and 5′-AAAACTGGGGTTTAGAGAGGTGA-3′;HAP4-5, 5′-CGCATAGGAAGAGAAAAAACACA-3′ and 5′-CAGCAACCCATTAAAATGCTC-3′; HAP4-6, 5′-CCGCAAAGACTTTTCTACACAGG-3′ and 5′-TGTTATGATGGTTGGTATTTGGG-3′; HAP5, 5′-GACTGATAGGAATTTCTCACCACA-3′ and 5′-CTCTCTGAATCATCGTACTGGGTT-3′; ROX1, 5′-CACCTAAGATTCCAAGACCCAA-3′ and 5′-GCTGTCTGAACAGAATAAATGCG-3′; CYC1, 5′-TTCTGCTAAGAAAGGTGCTACACT-3′ and 5′-ACCATGCAAGTTTGGACCAA-3′; CIT1, 5′-CAGCGATATTATCAACAACTAGCA-3′ and 5′-TAGTGGCGAGCATTCAATAGTG-3′;QCR7, 5′-ACGTCTATTGCGAGAATTGGTG-3′ and 5′-AGCCCTAACTTCTTGTAACCTGC-3′; and KGD2, 5′-TAAGGTTCGTGTCTTCGCAAAC-3′ and 5′-CCGACAATTTTGACAGTAGATGC-3′.
Replicative life span
Yeast replicative life span was determined as described (65, 66). Briefly, virgin daughter cells were allowed to grow into mother cells and produce daughter cells on YPD medium with and without ALA (300 μg/ml). The daughter cells were microdissected from mother cells and counted until the mother cells could no longer divide. Forty cells were analyzed in each experiment.
Statistical analysis
The results represent at least three independent experiments. Numerical results are presented as means ± S.D. Data were analyzed using an InStat software package (GraphPad, San Diego, CA). Statistical significance was evaluated by one-way analysis of variance, and p < 0.05 was considered significant.
Author contributions
T. Z. conceived the project, conducted most of the experiments, analyzed the results, prepared the figures, and wrote the paper. P. B. designed and conducted some of the experiments. J. Z. conducted some of the experiments. A. V. conceived the project, analyzed the data, wrote the paper, and coordinated the study.
Acknowledgments
We thank Drs. Aitchison, Barrientos, Kaplan, Muhlenhoff, and Winston for strains and plasmids and members of the Vancura and Vancurova laboratories for helpful comments.
This work was supported by National Institutes of Health Grant GM120710 (to A. V). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TCA
- tricarboxylic acid
- ETC
- electron transport chain
- OXPHOS
- oxidative phosphorylation
- HAP
- heme-activated protein
- ALA
- 5-aminolevulinic acid
- NF-Y
- nuclear transcription factor Y
- YPD
- yeast extract-peptone-dextrose.
References
- 1. Gancedo J. M. (1998) Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62, 334–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santangelo G. M. (2006) Glucose signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 253–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaman S., Lippman S. I., Zhao X., and Broach J. R. (2008) How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42, 27–81 [DOI] [PubMed] [Google Scholar]
- 4. Zaman S., Lippman S. I., Schneper L., Slonim N., and Broach J. R. (2009) Glucose regulates transcription in yeast through a network of signaling pathways. Mol. Syst. Biol. 5, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pronk J. T., Yde Steensma H., and Van Dijken J. P. (1996) Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 12, 1607–1633 [DOI] [PubMed] [Google Scholar]
- 6. Liesen T., Hollenberg C. P., and Heinisch J. J. (1996) ERA, a novel cis-acting element required for autoregulation and ethanol repression of PDC1 transcription in Saccharomyces cerevisiae. Mol. Microbiol. 21, 621–632 [DOI] [PubMed] [Google Scholar]
- 7. Gombert A. K., Moreira dos Santos M., Christensen B., and Nielsen J. (2001) Network identification and flux quantification in the central metabolism of Saccharomyces cerevisiae under different conditions of glucose repression. J. Bacteriol. 183, 1441–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heyland J., Fu J., and Blank L. M. (2009) Correlation between TCA cycle flux and glucose uptake rate during respiro-fermentative growth of Saccharomyces cerevisiae. Microbiology 155, 3827–3837 [DOI] [PubMed] [Google Scholar]
- 9. Galdieri L., Zhang T., Rogerson D., Lleshi R., and Vancura A. (2014) Protein acetylation and acetyl coenzyme a metabolism in budding yeast. Eukaryot. Cell 13, 1472–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galdieri L., Mehrotra S., Yu S., and Vancura A. (2010) Transcriptional regulation in yeast during diauxic shift and stationary phase. OMICS 14, 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broach J. R. (2012) Nutritional control of growth and development in yeast. Genetics 192, 73–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conrad M., Schothorst J., Kankipati H. N., Van Zeebroeck G., Rubio-Texeira M., and Thevelein J. M. (2014) Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 38, 254–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leadsham J. E., and Gourlay C. W. (2010) cAMP/PKA signaling balances respiratory activity with mitochondria dependent apoptosis via transcriptional regulation. BMC Cell Biol. 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor S. D., Zhang H., Eaton J. S., Rodeheffer M. S., Lebedeva M. A., O'rourke T. W., Siede W., and Shadel G. S. (2005) The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol. Biol. Cell 16, 3010–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lavoie H., and Whiteway M. (2008) Increased respiration in the sch9Δ mutant is required for increasing chronological life span but not replicative life span. Eukaryot. Cell 7, 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z., and Butow R. A. (2006) Mitochondrial retrograde signaling. Annu. Rev. Genet. 40, 159–185 [DOI] [PubMed] [Google Scholar]
- 17. Dimmer K. S., Fritz S., Fuchs F., Messerschmitt M., Weinbach N., Neupert W., and Westermann B. (2002) Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fendt S. M., and Sauer U. (2010) Transcriptional regulation of respiration in yeast metabolizing differently repressive carbon substrates. BMC Syst. Biol. 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonander N., Ferndahl C., Mostad P., Wilks M. D., Chang C., Showe L., Gustafsson L., Larsson C., and Bill R. M. (2008) Transcriptome analysis of a respiratory Saccharomyces cerevisiae strain suggests the expression of its phenotype is glucose insensitive and predominantly controlled by Hap4, Cat8 and Mig1. BMC Genomics 9, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L., and Hach A. (1999) Molecular mechanism of heme signaling in yeast: the transcriptional activator Hap1 serves as the key mediator. Cell. Mol. Life Sci. 56, 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McNabb D. S., and Pinto I. (2005) Assembly of the Hap2p/Hap3p/Hap4p/Hap5p-DNA complex in Saccharomyces cerevisiae. Eukaryot. Cell 4, 1829–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forsburg S. L., and Guarente L. (1989) Identification and characterization of HAP4: a third component of the CCAAT-bound HAP2/HAP3 heteromer. Genes Dev. 3, 1166–1178 [DOI] [PubMed] [Google Scholar]
- 23. DeRisi J. L., Iyer V. R., and Brown P. O. (1997) Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278, 680–686 [DOI] [PubMed] [Google Scholar]
- 24. Lascaris R., Bussemaker H. J., Boorsma A., Piper M., van der Spek H., Grivell L., and Blom J. (2003) Hap4p overexpression in glucose-grown Saccharomyces cerevisiae induces cells to enter a novel metabolic state. Genome Biol. 4, R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blom J., De Mattos M. J., and Grivell L. A. (2000) Redirection of the respiro-fermentative flux distribution in Saccharomyces cerevisiae by overexpression of the transcription factor Hap4p. Appl. Environ. Microbiol. 66, 1970–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin S. J., Kaeberlein M., Andalis A. A., Sturtz L. A., Defossez P. A., Culotta V. C., Fink G. R., and Guarente L. (2002) Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348 [DOI] [PubMed] [Google Scholar]
- 27. Olesen J. T., and Guarente L. (1990) The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev. 4, 1714–1729 [DOI] [PubMed] [Google Scholar]
- 28. Forsburg S. L., and Guarente L. (1989) Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu. Rev. Cell Biol. 5, 153–180 [DOI] [PubMed] [Google Scholar]
- 29. Dolfini D., Gatta R., and Mantovani R. (2012) NF-Y and the transcriptional activation of CCAAT promoters. Crit. Rev. Biochem. Mol. Biol. 47, 29–49 [DOI] [PubMed] [Google Scholar]
- 30. Marziali G., Perrotti E., Ilari R., Testa U., Coccia E. M., and Battistini A. (1997) Transcriptional regulation of the ferritin heavy-chain gene: the activity of the CCAAT binding factor NF-Y is modulated in heme-treated Friend leukemia cells and during monocyte-to-macrophage differentiation. Mol. Cell. Biol. 17, 1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mense S. M., and Zhang L. (2006) Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 16, 681–692 [DOI] [PubMed] [Google Scholar]
- 32. Zitomer R. S., and Lowry C. V. (1992) Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol. Rev. 56, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hon T., Dodd A., Dirmeier R., Gorman N., Sinclair P. R., Zhang L., and Poyton R. O. (2003) A mechanism of oxygen sensing in yeast. Multiple oxygen-responsive steps in the heme biosynthetic pathway affect Hap1 activity. J. Biol. Chem. 278, 50771–50780 [DOI] [PubMed] [Google Scholar]
- 34. Hickman M. J., and Winston F. (2007) Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol. Cell Biol. 27, 7414–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hon T., Lee H. C., Hu Z., Iyer V. R., and Zhang L. (2005) The heme activator protein Hap1 represses transcription by a heme-independent mechanism in Saccharomyces cerevisiae. Genetics 169, 1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galdieri L., Zhang T., Rogerson D., and Vancura A. (2016) Reduced histone expression or a defect in chromatin assembly induces respiration. Mol. Cell. Biol. 36, 1064–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rando O. J., and Winston F. (2012) Chromatin and transcription in yeast. Genetics 190, 351–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Field Y., Fondufe-Mittendorf Y., Moore I. K., Mieczkowski P., Kaplan N., Lubling Y., Lieb J. D., Widom J., and Segal E. (2009) Gene expression divergence in yeast is coupled to evolution of DNA-encoded nucleosomes organization. Nat. Genet. 41, 438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsankov A. M., Thompson D. A., Socha A., Regev A., and Rando O. J. (2010) The role of nucleosomes positioning in the evolution of gene regulation. PLoS Biol. 8, e1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Herzig S., Raemy E., Montessuit S., Veuthey J. L., Zamboni N., Westermann B., Kunji E. R., and Martinou J. C. (2012) Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96 [DOI] [PubMed] [Google Scholar]
- 41. Bricker D. K., Taylor E. B., Schell J. C., Orsak T., Boutron A., Chen Y. C., Cox J. E., Cardon C. M., Van Vranken J. G., Dephoure N., Redin C., Boudina S., Gygi S. P., Brivet M., Thummel C. S., et al. (2012) A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schüller H. J. (2003) Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr. Genet. 43, 139–160 [DOI] [PubMed] [Google Scholar]
- 43. Heinemann I. U., Jahn M., and Jahn D. (2008) The biochemistry of heme biosynthesis. Arch. Biochem. Biophys. 474, 238–251 [DOI] [PubMed] [Google Scholar]
- 44. Sertil O., Kapoor R., Cohen B. D., Abramova N., and Lowry C. V. (2003) Synergistic repression of anaerobic genes by Mot3 and Rox1 in Saccharomyces cerevisiae. Nucleic Acids Res. 31, 5831–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klinkenberg L. G., Mennella T. A., Luetkenhaus K., and Zitomer R. S. (2005) Combinatorial repression of the hypoxic genes of Saccharomyces cerevisiae by DNA binding proteins Rox1 and Mot3. Eukaryot. Cell 4, 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klinkenberg L. G., Webb T., and Zitomer R. S. (2006) Synergy among differentially regulated repressors of the ribonucleotide diphosphate reductase genes of Saccharomyces cerevisiae. Eukaryot. Cell 5, 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keng T., and Guarente L. (1987) Constitutive expression of the yeast HEM1 gene is actually a composite of activation and repression. Proc. Natl. Acad. Sci. U.S.A. 84, 9113–9117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keng T. (1992) HAP1 and ROX1 form a regulatory pathway in the repression of HEM13 transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 12, 2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ter Linde J. J., and Steensma H. Y. (2002) A microarray-assisted screen for potential Hap1 and Rox1 target genes in Saccharomyces cerevisiae. Yeast 19, 825–840 [DOI] [PubMed] [Google Scholar]
- 50. Hoffman M., Góra M., and Rytka J. (2003) Identification of rate-limiting steps in yeast heme biosynthesis. Biochem. Biophys. Res. Commun. 310, 1247–1253 [DOI] [PubMed] [Google Scholar]
- 51. Becerra M., Lombardía-Ferreira L. J., Hauser N. C., Hoheisel J. D., Tizon B., and Cerdán M. E. (2002) The yeast transcriptome in aerobic and hypoxic conditions: effects of hap1, rox1, rox3 and srb10 deletions. Mol. Microbiol. 43, 545–555 [DOI] [PubMed] [Google Scholar]
- 52. Kwast K. E., Lai L. C., Menda N., James D. T. 3rd, Aref S., and Burke P. V. (2002) Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: functional roles of Rox1 and other factors in mediating the anoxic response. J. Bacteriol. 184, 250–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Denby C. M., Im J. H., Yu R. C., Pesce C. G., and Brem R. B. (2012) Negative feedback confers mutational robustness in yeast transcription factor regulation. Proc. Natl. Acad. Sci. U.S.A. 109, 3874–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deckert J., Perini R., Balasubramanian B., and Zitomer R. S. (1995) Multiple elements and auto-repression regulate Rox1, a repressor of hypoxic genes in Saccharomyces cerevisiae. Genetics 139, 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang Z., Chen K., Xu T., Zhang J., Li Y., Li W., Agarwal A. K., Clark A. M., Phillips J. D., and Pan X. (2011) Sampangine inhibits heme biosynthesis in both yeast and human. Eukaryot. Cell 10, 1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soto I. C., Fontanesi F., Myers R. S., Hamel P., and Barrientos A. (2012) A heme-sensing mechanism in the translational regulation of mitochondrial cytochrome c oxidase biogenesis. Cell Metab. 16, 801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haurie V., Perrot M., Mini T., Jenö P., Sagliocco F., and Boucherie H. (2001) The transcriptional activator Cat8p provides a major contribution to the reprogramming of carbon metabolism during the diauxic shift in Saccharomyces cerevisiae. J. Biol. Chem. 276, 76–85 [DOI] [PubMed] [Google Scholar]
- 58. Raghevendran V., Patil K. R., Olsson L., and Nielsen J. (2006) Hap4 is not essential for activation of respiration at low specific growth rates in Saccharomyces cerevisiae. J. Biol. Chem. 281, 12308–12314 [DOI] [PubMed] [Google Scholar]
- 59. Hickman M. J., Spatt D., and Winston F. (2011) The Hog1 mitogen-activated protein kinase mediates a hypoxic response in Saccharomyces cerevisiae. Genetics 188, 325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Danziger S. A., Ratushny A. V., Smith J. J., Saleem R. A., Wan Y., Arens C. E., Armstrong A. M., Sitko K., Chen W. M., Chiang J. H., Reiss D. J., Baliga N. S., and Aitchison J. D. (2014) Molecular mechanisms of system responses to novel stimuli are predictable from public data. Nucleic Acids Res. 42, 1442–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sherman F. (1991) Getting started with yeast. Methods Enzymol. 194, 3–21 [DOI] [PubMed] [Google Scholar]
- 62. Galdieri L., and Vancura A. (2012) Acetyl-CoA carboxylase regulates global histone acetylation. J. Biol. Chem. 287, 23865–23876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang M., Galdieri L., and Vancura A. (2013) The yeast AMPK homolog SNF1 regulates acetyl coenzyme A homeostasis and histone acetylation. Mol. Cell. Biol. 33, 4701–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chang T. P., Kim M., and Vancurova I. (2014) Analysis of TGFβ1 and IL-10 transcriptional regulation in CTCL cells by chromatin immunoprecipitation. Methods Mol. Biol. 1172, 329–341 [DOI] [PubMed] [Google Scholar]
- 65. Kennedy B. K., Austriaco N. R. Jr., and Guarente L. (1994) Daughter cells of Saccharomyces cerevisiae from old mothers display a reduced life span. J. Cell Biol. 127, 1985–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Steffen K. K., Kennedy B. K., and Kaeberlein M. (2009) Measuring the replicative life span in the budding yeast. J. Vis. Exp. 1209 [DOI] [PMC free article] [PubMed] [Google Scholar]