Abstract
Here, we report a method to specifically bind liposomal radiopharmaceuticals to a CoCrMo alloy, which can be used in arterial stents, via an irreversible inverse electron‐demand Diels–Alder reaction. Inspired by recent accomplishments in pre‐targeted imaging using tetrazine‐trans‐cyclooctene click chemistry, we synthesized 89Zr‐labeled trans‐cyclooctene‐functionalized liposomal nanoparticles, which were validated on a tetrazine‐appended polydopamine‐coated CoCrMo surface. In efforts to ultimately translate this new material to biomedical applications, we compared the ability of 89Zr‐TCO–liposomal nanoparticles (89Zr‐TCO‐LNP) to be immobilized on the tetrazine surface to the control suspensions of non‐TCO functionalized 89Zr‐liposomal nanoparticles. Ultimately, this platform technology could result in a systemic decrease of the radiotherapeutic dose deposited in non‐targeted tissues by specific removal of long‐circulating liposomal radiopharmaceuticals from the blood pool.
Keywords: 89Zr, click chemistry, CoCrMo alloys, liposomes, tetrazine, trans-cyclooctene
Liposomal radiopharmaceuticals have drawn much attention as clinical diagnostics and/or therapeutics in recent years.1, 2, 3, 4, 5 They have been employed as low excretion blood‐pool imaging agents, labeled with 18F,2 as nanoreporters to predict therapeutic outcome during Doxil® treatment,3 and as bone marrow imaging agents, tagged with 64Cu.5 Nanoparticles that are not decorated with a molecularly targeted small molecule, peptide, or antibody can still passively accumulate in tumors, exploiting the enhanced permeability and retention (EPR) effect.6 This increased accumulation of the liposomal radiopharmaceutical in tumor regions allows delineation of tumor margins with positron emission tomography (PET), generally at late time points between 24 and 48 h. However, long‐circulating liposomal radiopharmaceuticals tagged with long‐lived radionuclides such as 89Zr and 64Cu can deposit a significant dose of radiation to non‐targeted organs, such as the liver and spleen of patients.7
Thus, it would be beneficial to utilize early time‐point imaging (ideally between 4–6 h post‐injection) of long‐circulating liposomal nanoparticles so that sufficient tumor signal‐to‐background ratios can be realized without unnecessary circulation of potentially deleterious radioisotope nanocarriers. We, therefore, designed a liposome‐based tetrazine (Tz)/trans‐cyclooctene (TCO) targeting system by using the inverse electron‐demand Diels–Alder (IEDDA) click reaction (Figure 1). Over the last six years, the potential of this reaction has been exploited as a tool for the in vitro and in vivo labeling of biologically relevant probes.8 This extremely fast (k> 30 000 m −1 s−1), selective, and bioorthogonal click reaction9 has been successfully introduced as a pretargeting system for in vivo PET imaging10 as well as a radioimmunotherapeutic.11
Figure 1.
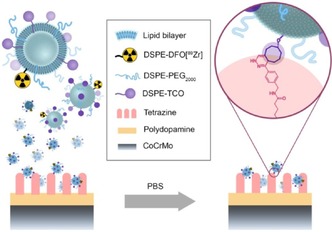
Schematic overview of 89Zr‐TCO‐liposome uptake via bioorthogonal inverse electron‐demand Diels–Alder click reaction.
In this Communication, we present our recent results towards the development of a platform technology for in vivo removal of a liposomal radiopharmaceutical through the IEDDA reaction. Bioorthogonally reactive liposomes can potentially result in a systemic decrease of the radiotherapeutic dose deposited in healthy tissues, yield a larger therapeutic window, better treatment outcomes, and ultimately more satisfactory prognoses.
To this end, we synthesized 89Zr‐radiolabeled TCO‐functionalized liposomes (Figure 2). The preparation of TCO‐functionalized liposomal nanoparticles required the synthesis of a derivative of the phospholipid 1,2‐distearoyl‐sn‐glycero‐3‐ phosphoethanolamine (DSPE‐NH2, Figure S1). DSPE‐NH2 was treated with a TCO‐functionalized N‐hydroxysuccinimide (TCO‐NHS) ester in the presence of triethylamine to obtain the desired TCO‐functionalized phospholipid in moderate yields. After purification, DSPE‐TCO was characterized by using mass spectrometry with electrospray ionization (ESI‐MS) (Figure S1B). The TCO‐functionalized liposomal nanoparticles can then be assembled by using a previously established protocol.4 Together with 1,2‐dipalmitoyl‐sn‐glycero‐3‐phosphocholine (DPPC), cholesterol, DSPE‐PEG2000, DSPE‐TCO, and DSPE‐desferrioxamine (DSPE‐DFO), a high‐pressure tip‐sonication method yielded the TCO‐functionalized liposomal nanoparticle (TCO‐LNP) suspension of narrow size distributions (mean diameter=109.4±8.8 nm, PDI=0.153±0.027). The overall charge of TCO‐LNP was measured to be slightly negative, with a zeta potential of −6.8±4.4 mV. Subsequent radiolabeling of the TCO‐LNP surface chelate moieties was achieved through treatment with radioactive 89Zr(IV)‐oxalate (Figure 2 A). The desired 89Zr‐radiolabeled TCO‐functionalized liposome (89Zr‐TCO‐LNP) was isolated by centrifugal filtration in good radiochemical yield (49±5 %). Size exclusion chromatography (SEC) exhibited high radiochemical purity (>97 %) of the radiolabeled, or “hot”, liposome population (Figure 2 B) with no statistically significant difference in particle size and distributions compared to its “cold” precursor (Figure 2 C). Biodistribution studies in 4T1‐tumor‐bearing nude mice showed typical pharmacokinetics of PEGylated liposomal nanoparticles with increasing ratios of tumor to blood over 24 h of up to 3.12±0.57 (Figure 2 D and Figure S2). It is important to note that while tumor/blood ratios increase between 6 and 24 h, sufficient tumoral uptake of 89Zr‐TCO‐LNPs was observed as early as the 6 h time point. In fact, the mean percent injected dose per gram (%ID g−1) of tumoral tissue actually decreases between 6 and 24 h (17.0±4.2 and 14.0±1.2 %ID g−1 for 6 and 24 h, respectively). Enhanced contrast should not only be achieved by increased particle accumulation, but rather clearance of 89Zr‐TCO‐LNPs from the blood pool. A rapid route for selective tracer removal from the blood pool would, therefore, decrease the radiation dose as well as allow for earlier imaging time points.
Figure 2.
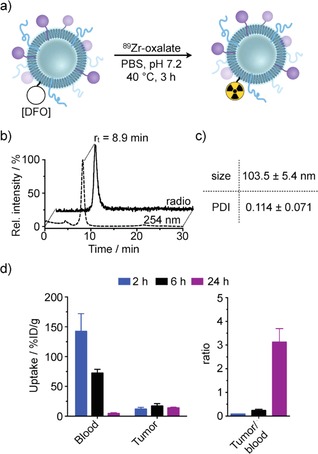
Synthesis, chemical characterization, and in vivo biodistribution of 89Zr‐labeled TCO‐functionalized liposomal nanoparticles. a) Formulation and radiolabeling. b) UV (254 nm) and radio‐chromatogram by size exclusion chromatography. c) Analysis of size and polydispersity of 89Zr‐labeled liposomal nanoparticle. d) In vivo biodistribution of 89Zr‐TCO liposomal nanoparticles in 4T1‐tumor‐bearing nude mice.
Rationally, a tetrazine‐functionalized polydopamine‐coated CoCrMo stent alloy was prepared so that bioorthogonally selective removal of 89Zr‐TCO‐LNPs can be realized (Figure 3). The inherent chemical robustness of these particular alloys allows them to be suitable as core materials for frameworking arterial stents. The noble metal surface character of these alloys also suggests that a thorough oxidation treatment can allow for a subsequent treatment with a functionalized organic substrate through the resulting strong Van der Waals forces and covalent interactions between the substrate and the metal. Recent preparations of polydopamine (PDA)‐coated CoCr alloys utilized such strategies to install functional amines on the metal surface primarily through the chelating catechol moieties of the dopamine monomer.12 These functional handles were ultimately employed as linkers to slow‐releasing crosslinked cyclodextrins for drug delivery, although PDA and its polymeric analogues can be utilized in many other applications.13 In this report, a PDA‐coated CoCrMo alloy was prepared by peroxide‐mediated surface oxidation, rendering the metal surface reactive to the catechol groups on dopamine molecules (Figure 3 A). Subsequent thermal treatment cured the surface coating through polymerization of the adjacently grafted dopamine molecules. It should be noted that the microstructure of polydopamine can vary from monomer to monomer unit, where quinone, indole, and quinhydrone‐derived moieties may exist, although their favorable intermolecular interactions contribute to not only the robustness of the bio‐inspired resin,14 but can even contribute to enhanced hemocompatibility.15 The grafted amines were converted in the second step to tetrazine‐appended amides by using N‐hydroxysuccinimide‐displacement chemistry. The amide‐bound tetrazines can selectively “click” with trans‐cyclooctene groups on substrates such as liposomes (Figure 3 B), immobilizing the macromolecular carriers and potentially removing them from circulation. Different concentrations of Tz‐NHS ester solutions (0.2 mm, 2.0 mm, and 20 mm) were evaluated for the functionalization of PDA@CoCrMo‐alloy disks to achieve maximum deposition of Tz functionalities on the polydopamine‐coated metal surface. After tetrazine functionalization, Tz‐PDA@CoCrMo disks (n=3) were incubated with 89Zr‐TCO‐liposomes at room temperature for 5 min and the bound activity was measured through γ‐counting. In all cases, we observed a statistically significant increase in uptake of 89Zr‐TCO‐liposomal nanoparticles on the Tz‐functionalized CoCrMo alloy disks (Tz‐PDA@CoCrMo) in comparison to PDA@CrCoMo metal disks, demonstrating the successful deposition of tetrazine functionalities on the polydopamine‐coated metal disks. However, no statistical difference between the different concentrations of Tz‐NHS ester was observed.
Figure 3.
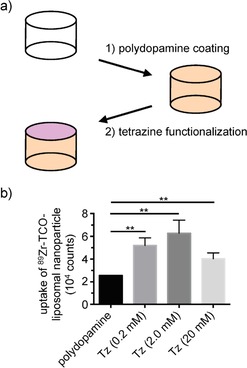
Synthesis and optimization of a tetrazine‐functionalized polydopamine‐coated CoCrMo stent alloy (Tz‐PDA@CoCrMo). a) A schematic illustration of thin‐film deposition of polydopamine and tetrazine functionalization through amide‐bond formation. b) Retention of 89Zr‐TCO liposomal nanoparticles at different concentrations of Tz NHS ester on tetrazine functionalized of polydopamine‐coated metal disks.
The viability of a conceptual liposome “filter” that could be suitable as a stent material was evaluated through radiolabeling efficiencies of CoCrMo alloy disks of various coatings (Figure 4). The tetrazine‐appended polydopamine‐coated CoCrMo surface (Tz‐PDA@CoCrMo) was treated with a buffered radiolabeled TCO‐functionalized liposome formulation as well as non‐TCO functionalized 89Zr‐labeled liposomes (Figure 4 A), and was left to stand for 5 min prior to a rinse to remove residual “unbound” liposomes (Figure 4 B). The amount of radioactivity retained was compared to a bare CoCrMo alloy and a non‐tetrazine‐functionalized PDA@CoCrMo, both of which were treated with the same radiolabeled liposome solutions. Figure 4 C depicts phosphor autoradiography films displaying the radioactivity that had remained on the disk surfaces. Four times less radioactivity was retained on the bare CoCrMo alloy compared to the Tz‐functionalized disks with the highest activity, suggesting that the TCO‐tetrazine click reaction is responsible for the enhanced uptake of the liposomes. Although considerably more radioactivity was retained on the tetrazine‐functionalized PDA@CoCrMo disk, a two‐fold decrease in the amount was still observed on the disk that was not treated with Tz NHS‐ester. This could be because of side reactions of the strained Z‐isomer of the cyclooctene, which can include Michael addition to the polydopamine amine or even Diels–Alder couplings with catechol‐derived quinone dienes.14 Owing to the bioorthogonal click reaction, the majority of PDA–liposome binding originates from the positively charged polydopamine surface itself, which may have an inherent electrostatic affinity for the negatively charged liposomes.
Figure 4.
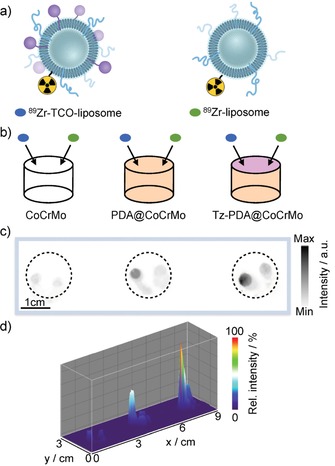
Comparison in uptake of 89Zr‐TCO‐liposomal nanoparticles versus 89Zr‐liposomal nanoparticles on CoCrMo alloy stent surfaces. a) Synthesis of TCO‐functionalized and non‐TCO‐functionalized 89Zr‐labeled liposomal nanoparticles. b) Schematic illustration of the experimental setup. c, d) Analysis of bound activity of 89Zr‐labeled nanoparticles by phosphor autoradiography.
In summary, 89Zr‐radiolabeled TCO‐functionalized liposomal nanoparticles (89Zr‐TCO‐LNP) with good in vivo pharmacokinetics were prepared for selective binding to a rationally engineered tetrazine‐appended polydopamine‐coated CoCrMo alloy disk. We have shown specific binding between these two counterparts through the rapid bioorthogonal inverse electron‐demand Diels–Alder click reaction between tetrazines and trans‐cyclooctenes. This report represents the first steps taken towards a new technology of on‐demand removal of circulating liposomal nanoparticles in vivo.
Experimental Section
Materials
Unless otherwise stated, all chemicals and solvents were used without further purification. Water used for this study was ultrapure (>18.2 MΩ cm−1 at 25 °C), and acetonitrile (CH3CN) as well as ethanol were of HPLC grade purity. l‐Dopamine hydrochloride, Trizma® base, p‐benzylamino tetrazine N‐hydroxysuccinimidyl ester (Tz NHS ester), triethylamine, and dimethylformamide were purchased from Sigma–Aldrich (USA) and used as received. Phospholipids were purchased from Avanti polar Lipids. Cobalt chromium molybdenum alloy disks (CoCrMo, 63:30:7 wt %) were purchased from American Elements (CA, USA). TCO‐NHS ester was purchased from KeraFast (MA, USA). Chelexed water was prepared with BT Chelex 100 Resin (Bio‐Rad) and phosphate‐buffered saline (PBS) was purchased from the media preparation facility at Memorial Sloan Kettering Cancer Center. 89Zr‐oxalate was purchased from the radiochemistry core at Memorial Sloan Kettering Cancer Center. HPLC reactions and quality controls (1.0 mL min−1, phosphate‐buffered saline) were performed on a Shimadzu UFLC HPLC system equipped with a DGU‐20A degasser, a SPD‐M20A UV detector, a LC‐20AB pump system, a CBM‐20A communication BUS module, and a Scan‐RAM radio‐TLC/HPLC‐detector form Lablogic using a size exclusion column (GE SuperdexTM 200, 10/300 GL). Electrospray ionization mass spectrometry (ESI‐MS) spectra were recorded with a Waters Acquity UPLC (Milford, CA) with electrospray ionization SQ detector. Size and zeta potential measurements were performed on a Malvern Zetasizer NanoSeries.
Formulation of TCO‐Functionalized Liposomal Nanoparticles
The formulation of TCO‐functionalized liposomal nanoparticles is based on a previously established protocol.4 In detail, the liposomal nanoparticle suspension consisting of 1,2‐dipalmitoyl‐sn‐glycero‐3‐phosphocholine (DPPC), cholesterol, pegylated DSPE (DSPE‐PEG2000), DSPE‐TCO, and DSPE‐DFO in a 1.85:1:0.15:0.03:0.01 molar ratio was prepared by adding the individual lipids to a reaction vessel in a solution of chloroform. After removing the organic solvent under reduced pressure, the resulting lipid film was dried under high vacuum for 2 h. Phosphate‐buffered saline (pH 7.4, 4.0 mL) was added to the reaction vessel and stirred at 40 °C for 1 h. Followed by tip sonication and centrifugation, TCO‐functionalized liposomal nanoparticles were isolated and stored as a suspension in PBS. Particle diameters were measured to be 109.4±8.8 nm (n=3) with a polydispersity index [PDI] of 0.153±0.027 (n=3) and a zeta potential of −6.8±4.4 mV (n=3). Non‐functionalized liposomal nanoparticle suspensions without the DSPE‐TCO constituent were prepared as control formulations and exhibited analogous properties to those reported by Perez et al.4
Radiolabeling of 89Zr‐TCO‐Liposomes
89Zr‐oxalate (7.3 μL, 98 μCi) was added to phosphate‐buffered saline (100 μL) and the pH was adjusted to 7.5 by additing Na2CO3 (1 m, 7.0 μL). After the addition of a solution of TCO‐liposomal nanoparticles (100 μL), the reaction mixture was incubated at 40 °C for 1 h. After purification by sequential spin filtration (5×) using Amicon® filters (100 kDa, EMD Millipore), 89Zr‐TCO‐liposomal nanoparticles were isolated (49±5 % radiochemical yield, >97 % radiochemical purity, n=5) with similar characteristics (size: 103.5±5.4 nm and PDI: 0.114±0.071, n=3) as their precursors.
Biodistribution of 89Zr‐TCO‐Liposomes
Evaluating the in vivo performance of 89Zr‐TCO‐liposomes, 4T1‐tumor‐bearing (150–200 mm3) mice (female, nude, 6–8 weeks, 20–25 g) were intravenously injected with a solution of 89Zr‐TCO‐liposomes (13.3–18.2 μCi, 0.5 μmol of lipid) in PBS (150 μL). At predetermined time points (2, 6, and 24 h post‐injection), the mice were euthanized by asphyxiation with CO2 and blood was collected via cardiac puncture. Selected organs were harvested, weighed, and counted by using a Wizard2 automatic γ‐counter from PerkinElmer. The percentage of tracer uptake stated as percentage injected dose per gram of tissue (%ID g−1) was calculated as the activity bound to tissue per organ weight per actual injected dose, decay‐corrected to the start time of counting. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Memorial Sloan Kettering Cancer Center.
Preparation of Tetrazine‐Functionalized Stent Alloy
Functionalization of commercial CoCrMo alloy disks was achieved by coating the surface with a polydopamine coating using a modified literature protocol.12 Amidization of surface amines through N‐hydroxysuccinimide (NHS) displacement yields tetrazine covalencies. The alloy disk was first surface‐treated with piranha solution (3:1 w/w H2SO4 [conc.]/H2O2 [30 % w/w]) for 16 h. After rinsing with water, the disk was submerged in a freshly prepared aqueous solution of Trizma® base (1.2 mg mL−1) and dopamine (2 mg mL−1) (pH 8.5) and agitated at 20 °C for 16 h. After rinsing with water, the disks were cured at 150 °C for 1 h. The resulting polydopamine‐coated disks (PDA@CoCrMo) were then incubated in the dark at 20 °C with layer of pipette‐dropped solutions of Tz NHS ester (0.2 mm, 2.0 mm, or 20 mm) and triethylamine (0.15 mm) for 1 h prior to a water rinse to afford the tetrazine‐appended surface‐coated alloy (Tz‐PDA@CoCrMo). Then, the reaction solution was removed by washing with water (3×1.0 mL).
Optimization of Tetrazine Functionalization of Polydopamine‐Coated CoCrMo Alloy Disks
After the dilution of 89Zr‐TCO‐liposomal nanoparticles (100 μL, 62 μCi) with PBS (1.0 mL), Tz‐PDA@CoCrMo disks prepared under various Tz NHS ester conditions (0.2 mm, 2.0 mm, and 20 mm; n=3) and non‐functionalized PDA@CoCrMo disks (n=3) were incubated with a solution of 89Zr‐TCO‐liposomal nanoparticles (100 μL) for 5 min at 20 °C. The disks were then carefully washed with PBS (10 mL) and residual radioactivity on the disks was measured by using a Wizard2 automatic γ‐counter from PerkinElmer.
Comparison in Uptake of 89Zr‐TCO‐Liposomal Nanoparticles and 89Zr‐Liposomal Nanoparticles
For digital autoradiography experiments, bare CoCrMo, PDA@CoCrMo, and Tz‐PDA@CoCrMo disks were incubated with pipette‐delivered drops of 89Zr‐TCO‐liposomal nanoparticles (2 μL, 2.0 μCi) or 89Zr‐liposomal nanoparticles (2 μL, 2.0 μCi) for 5 min at 20 °C (Figure 4 A and Figure 4 B). After washing with PBS (10 mL), bound radioactivity on the metal disks was measured by exposing them to a phosphor‐imaging plate (Fujifilm, BAS‐MS2325, Fuji Photo, Japan) for 24 h at 20 °C. After exposure, the imaging plate was analyzed by using a Typhoon FLA 7000 laser scanner (GE Healthcare, Port Washington, NY) with 25 μm pixel resolution.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by a Center for Molecular Imaging & Nanotechnology grant of Memorial Sloan Kettering Cancer Center (to T.R.); NIH R01 EB017748 and K08 CA16396 (to M.F.K.); M.F.K. is a Damon Runyon‐Rachleff Innovator supported (in part) by the Damon Runyon Cancer Research Foundation (DRR‐29‐14). The authors thank the Small Animal Imaging Core and the Radiochemistry and Molecular Imaging Probes Core for support (P30 CA008748).
C. Brand, P. Iacono, C. Pérez-Medina, W. J. M. Mulder, M. F. Kircher, T. Reiner, ChemistryOpen 2017, 6, 615.
References
- 1. Petersen A. L., Hansen A. E., Gabizon A., Andresen T. L., Adv. Drug Delivery Rev. 2012, 64, 1417–1435. [DOI] [PubMed] [Google Scholar]
- 2. Marik J., Tartis M. S., Zhang H., Fung J. Y., Kheirolomoom A., Sutcliffe J. L., Ferrara K. W., Nucl. Med. Biol. 2007, 34, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez-Medina C., Abdel-Atti D., Tang J., Zhao Y., Fayad Z. A., Lewis J. S., Mulder W. J. M., Reiner T., Nat. Commun. 2016, 7, 11838–11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pérez-Medina C., Abdel-Atti D., Zhang Y., Longo V. A., Irwin C. P., Binderup T., Ruiz-Cabello J., Fayad Z. A., Lewis J. S., Mulder W. J. M., Reiner T., J. Nucl. Med. 2014, 55, 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee S. G., Gangangari K., Kalidindi T. M., Punzalan B., Larson S. M., Pillarsetty N. V., Nucl. Med. Biol. 2016, 43, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsumura Y., Maeda H., Cancer Res. 1986, 46, 6387–6392. [PubMed] [Google Scholar]
- 7. van der Geest T., Laverman P., Metselaar J. M., Storm G., Boerman O. C., Expert Opin. Drug Delivery 2016, 13, 1231–1242. [DOI] [PubMed] [Google Scholar]
- 8. Patra M., Zarschler K., Pietzsch H.-J., Stephan H., Gasser G., Chem. Soc. Rev. 2016, 45, 6415–6431. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Devaraj N. K., Weissleder R., Acc. Chem. Res. 2011, 44, 816–827; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b. Karver M. R., Weissleder R., Hilderbrand S. A., Bioconjugate Chem. 2011, 22, 2263–2270; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Knall A. C., Slugovc C., Chem. Soc. Rev. 2013, 42, 5131–5142. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Zeglis B. M., Sevak K. K., Reiner T., Mohindra P., Carlin S., Zanzonico P., Weissleder R., Lewis J. S., J. Nucl. Med. 2013, 54, 1389–1396; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Zeglis B. M., Mohindra P., Weissmann G. I., Divilov V., Hilderbrand S. A., Weissleder R., Lewis J. S., Bioconjugate Chem. 2011, 22, 2048–2059; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10c. Adumeau P., Carnazza K. E., Brand C., Carlin S. D., Reiner T., Agnew B. J., Lewis J. S., Zeglis B. M., Theranostics 2016, 6, 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. Houghton J. L., Membreno R., Abdel-Atti D., Cunanan K. M., Carlin S. D., Scholz W. W., Zanzonico P. B., Lewis J. S., Zeglis B. M., Mol. Cancer Ther. 2017, 16, 124–133; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11b. Rossin R., Läppchen T., van den Bosch S. M., Laforest R., Robillard M. S., J. Nucl. Med. 2013, 54, 1989–1995. [DOI] [PubMed] [Google Scholar]
- 12. Sobocinski J., Laure W., Taha M., Courcot E., Chai F., Simon N., Addad A., Martel B., Haulon S., Woisel P., Blanchemain N., Lyskawa J., ACS Appl. Mater. Interfaces 2014, 6, 3575–3586. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Ye Q., Zhou F., Liu W., Chem. Soc. Rev. 2011, 40, 4244–4258; [DOI] [PubMed] [Google Scholar]
- 13b. Lee H., Dellatore S. M., Miller W. M., Messersmith P. B., Science 2007, 318, 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nielsen S. R., Besenbacher F., Chen M., Phys. Chem. Chem. Phys. 2013, 15, 17029–17037. [DOI] [PubMed] [Google Scholar]
- 15. Luo R., Tang L., Zhong S., Yang Z., Wang J., Weng Y., Tu Q., Jiang C., Huang N., ACS Appl. Mater. Interfaces 2013, 5, 1704–1714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


