Abstract
The traditional view of nicotinic acetylcholine receptors (nAChRs) is that they strictly exert their functions via their well-known ion channel activity. With the identification of the cholinergic anti-inflammatory pathway and the critical involvement of the α7 nAChR, an alternate modality of function has emerged for the receptor involving metabotropic-like activity. The new emerging pharmacology for the receptor includes ligands termed silent agonists, which exert little or no ionotropic activity, yet are capable of modulating cellular inflammatory responses.
The nicotinic acetylcholine receptor (nAChR) family is well-known as archetypical representatives of the Cys-loop pentameric ligand gated ion channel (LGIC) class of signaling proteins.1 Each monomer of the pentameric receptor consists of three domains: a large extracellular domain (ECD) involved in binding the agonist acetylcholine (ACh, 1; Figure 1) at subunit interfaces, a four-helix transmembrane (TM) region that contributes one helix per monomer to create the ion pore, and an intrinsically disordered intracellular domain (ICD), located between the third and fourth TM helices, whose functions are variable and largely unknown.2 The structural biology of the receptor remains a major challenge, with only one human nAChR (α4β2) structure reported3 (ECD and TM regions) and no structures reported for an entire pentameric receptor consisting of all three domains. The receptors are distributed widely in higher eukaryotes including invertebrates such as insects, mollusks, and worms. More distantly related bacterial homologues that are pH gated are also known. The stereotypical view of nAChR function is that it is associated with fast synaptic transmission, though this is often not the case as it is found in nonsynaptic locations within the brain, as well as in non-neuronal cells. The basis for receptor signaling has been considered to be the permeability of the receptor to cations (primarily Na+ and K+, but in some cases also Ca2+) when the receptor is gated open by binding activating ligands, such as ACh or in some cases choline. The receptor is found in the brain, peripheral nervous system, neuromuscular junctions, and, as noted above, in tissues and cells that are not associated with synaptic transmission. Work that first emerged nearly two decades ago points to a distinctly different mode of action and pharmacology for the homomeric α7 subtype of the nAChR in which it exhibits ligand mediated metabotropic-like signal transduction behavior, which appears to be independent of detectable ion-currents, and is involved in the mediation of anti-inflammatory signaling and neuropathic pain. In this Viewpoint, we consider aspects of the medicinal chemistry and pharmacology of this rapidly growing area of nAChR research, and do so in the light of possible new therapeutic avenues.
Figure 1.
Acetylcholine receptor ligands discussed in this Viewpoint.
The cholinergic anti-inflammatory pathway4 (CAP) involves communication between the brain and the immune system via the vagal nerve. This pathway involves, in part, release of ACh from the vagal nerve, which is then able to modulate production of pro-inflammatory cytokines in macrophages.5 While the overall interplay between the nervous and immune systems is complex, several works have provided compelling evidence that the α7 nAChR is the mediator of this reduction in immune response and that there are viable pharmacological strategies to selectively target α7 with small molecules other than ACh. In cell culture, macrophages challenged with ACh and nicotine 2 showed strong dose-dependent reduction of TNF production. Interestingly, muscarine 3 appeared to show a weak effect, but it was not blocked by atropine, suggesting that G-protein coupled muscarinic AChR was not involved. Vagal nerve stimulation reduces the inflammatory response to endotoxin in rodents, and in α7 knock out mice, the anti-inflammatory activity of vagal nerve stimulation was lost. In 2012, Thomsen and Mikkelson showed that, whereas α7 agonists and positive allosteric modulators (PAMs) that increase α7 ion channel currents failed to reduce TNFα produced in cultured microglial cells, the very weak partial agonist NS6740, 4, the antagonist methyllycaconitine, 5, and partial agonist GTS-21, 6, were able to lower LPS-induced TNFα.6 This led to the suggestion that ionotropic action was not required for this effect, but rather, the binding of nonactivating ligands to the α7 nAChR produced metabotropic-like activity. Additionally, further support for a metabotropic mode of activity for the α7 nAChR was reported in 2016 by King et al. in a study that showed a critical region of the receptor’s intracellular domain was required for G-protein binding and mediation of release of intracellular calcium stores. Other works have suggested models for downstream signaling via the α7 nAChR interacting in JAK/STAT pathways.5,7 Indeed, for a number of different ligand-gated ion channels, the possible significant duality of function, both ionotropic and metabotropic, has been an emerging and exciting area of work.8 One challenge in considering this line of inquiry for therapeutics is to develop agents that are selective for the metabotropic activity, while not activating ionotropic activity. For example, it has been shown that the silent agonist NS6740, demonstrated to be an effective activator of the cholinergic anti-inflammatory pathway (CAP), is inactive in cognitive tests compared to agents with good ionotropic activity. This supports the hypothesis that the CAP activity is a special and unique indication that is targetable relative to ionotropic activity.
The α7 nAChR and Silent Agonists
The α7 nAChR is remarkable for its low probability to gate into the open state and its ability to readily enter desensitized, nonconducting states. Whereas many agonists and partial agonists are capable of desensitizing the receptor, those that are extremely weak partial agonists are of particular interest, as exemplified by NS6740, which is able to produce significant and remarkably prolonged desensitization.9 One key tool in the arsenal of characterizing these desensitized states is to utilize type II positive allosteric modulators such as PNU-120596, 7, in conjunction with two-electrode voltage clamping electrophysiological measurements. The type II PAM is capable of rendering nonconducting state(s) conductive and thus provides a quantifiable metric for desensitization of the receptor (Figure 2). We have termed compounds like NS6740 that only weakly activate the receptor but show substantial desensitization (as evidenced by PAM potentiated currents) as silent agonists. The “agonism” part of this name refers to the idea of metabotropic signaling of the α7 nAChR. In other words, silent agonists are relatively silent in the ionotropic sense but can be agonists in the metabotropic sense. The temporal- and concentration-dependent nature of evolving α7 state distributions renders the complete description of these states a long-term goal, but in the conjunction of a silent agonist and a PAM, the latter is a tool that has been used to identify at least two types of desensitization states: one, able to be rendered conductive with the PAM, and others, insensitive to the PAM. Indeed, in the case of NS6740,10 the relevant desensitized state associated with analgesia of neuropathic pain may be the one insensitive to the PAM.
Figure 2.
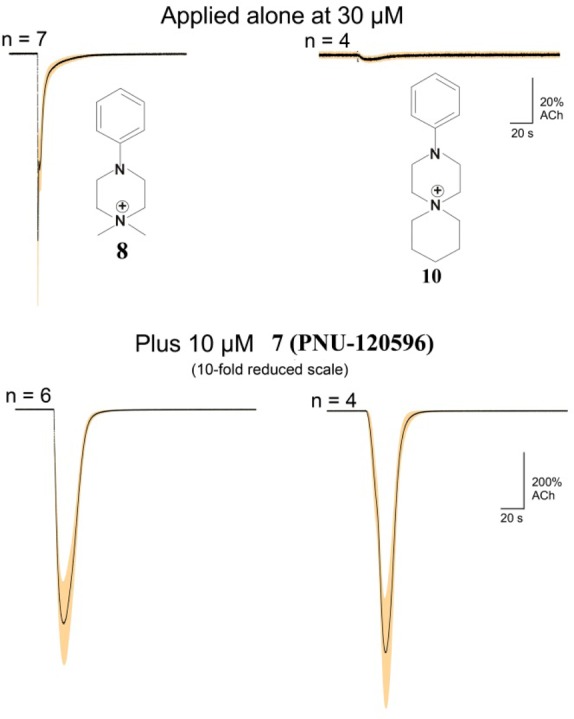
Two-electrode voltage clamp electrophysiology illustrating the behavior of a strong partial agonist 8 (left) versus a silent agonist 10 (right). The top two traces represent response of the α7 nAChR to applied compound, and the bottom two traces represent the receptor response when compounds were coapplied with compound 7, PNU-120596. Note that 10 shows barely detectable ion current, yet is able to effectively desensitize the receptor.
The idea that some α7 nAChR ligands are not conventional agonists or partial agonists, but rather work in a metabotropic way from a nonconductive (desensitized) state, represents an alternative pharmacology for the receptor specifically targeting CAP. Pragmatically, one would like to define the rules for this new pharmacology and be able to identify compounds that are completely lacking in ionotropic activity but capable of metabotropic signaling. Several structural classes of ligand appear to be capable of facile receptor desensitization in lieu of receptor activation, and some emergent principles have been noted. One common feature of all silent agonists noted thus far is that they either bear a fixed positive charge or are sufficiently basic at a central nitrogen atom that they may be predominantly protonated at physiologic pH. The simplest agonist for any nAChR is tetramethylammonium cation, and it has been shown that, as the alkyl substituents are made more bulky, initially selectivity for α7 as a partial agonist is enhanced over other receptor subtypes, but with sufficient bulk, e.g., tetraethylammonium cation (TEA), partial agonism is extremely weak, but TEA will induce a desensitized state of the receptor.11 This observation was generalized into a number of series of alkylammonium ligands, which led to the idea that steric bulk around the positively charged nitrogen (or sulfur, see below) will diminish agonism but preserve the ability to desensitize. While simple ammonium compounds are not drug-like, this work led to the idea of taking N,N-dimethyl N′-phenyl piperazine (DMPP, 8) and replacing the two methyls with ethyl groups to produce silent agonists (DEPP, 9). This experiment was successful and provided further validation of the “steric bulk” aspect of the silent agonist pharmacophore. The spirocyclic version of DEPP, 10, was found to have enhanced desensitization properties relative to DEPP (Figure 2). Work within this series revealed a profound substituent effect at the meta and para positions of the phenyl ring, producing a wide range of α7 activities ranging from partial agonism to silent agonists with enhanced PNU-120596 sensitive desensitization. More recently, we synthesized the sulfonium analogue of diethyl phenyl piperazine in which we replaced the ammonium nitrogen atom with a sulfonium sulfur, 11, and we found that the sulfur substitution was effective for enhanced desensitization of the receptor and diminished partial agonism, relative to the nitrogen analogue.11a Another aspect of a silent agonism pharmacophore is based on the structure of NS6740, which has a bicyclo [3.2.2] ring system bearing the requisite positive charge, a central heterocycle, and a terminal meta-trifluoromethylbenzene substituent. We considered that the right-hand portion of this molecule was similar to the known partial agonist anabaseine, 12, and that if we derivatized the pyridyl ring with a phenyl ring (KC1, 13)12 we could mimic the overall spatial and charge features of the much more complex NS6740. KC1 indeed proved to be a silent agonist, and it is noteworthy that, whereas the original inspiration, NS6740, had a somewhat bulky, bicyclic structure surrounding the charged nitrogen, KC1 is planar at nitrogen, suggesting the silent agonism for this compound does not rely on the aforementioned steric factor for its ability to promote desensitization and diminish partial agonism.
Conclusion
The α7 nAChR is an important target for noncanonical metabotropic signaling, but it is also clear that silent agonism and metabotropic-like pathways is not limited to the α7 subtype. It has been reported13 that phosphocholine is capable of modulating interleukin 1β levels in cultured monocytes in an α9- and α10-dependent fashion; phosphocholine is not a partial agonist of the human α9 receptor when heterologously expressed in Xenopus oocytes. It may be a general trend that nicotinic receptors, with their highly variable intracellular domains,2 have evolved to include a metabotropic function in addition to the canonical ion channel function they are well-known for. The further elucidation of what constitutes the pharmacophore(s) for silent agonists will enable the design of new compounds that are able to selectively target inflammatory responses derived from the receptor’s metabotropic-like function, while minimizing ionotropic activity.
Acknowledgments
We wish to thank Marta Quadri for the synthesis of compound 10 and Clare Stokes for electrophysiological measurements.
Author Contributions
The authors have contributed equally to the writing of this manuscript.
This work was supported under NIH grant GM57481
Views expressed in this editorial are those of the authors and not necessarily the views of the ACS.
The authors declare no competing financial interest.
References
- Changeux J. P. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem. 2012, 287 (48), 40207–15. 10.1074/jbc.R112.407668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C.; Treinin M.; Papke R. L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2015, 36 (8), 514–23. 10.1016/j.tips.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Perez C. L.; Noviello C. M.; Hibbs R. E. X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 2016, 538 (7625), 411–415. 10.1038/nature19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007, 117 (2), 289–96. 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge W. J.; van der Zanden E. P.; The F. O.; Bijlsma M. F.; van Westerloo D. J.; Bennink R. J.; Berthoud H. R.; Uematsu S.; Akira S.; van den Wijngaard R. M.; Boeckxstaens G. E. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005, 6 (8), 844–51. 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- Thomsen M. S.; Mikkelsen J. D. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J. Neuroimmunol. 2012, 251 (1–2), 65–72. 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Egea J.; Buendia I.; Parada E.; Navarro E.; Leon R.; Lopez M. G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015, 97 (4), 463–72. 10.1016/j.bcp.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Valbuena S.; Lerma J. Non-canonical Signaling, the Hidden Life of Ligand-Gated Ion Channels. Neuron 2016, 92 (2), 316–329. 10.1016/j.neuron.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Papke R. L.; Stokes C.; Damaj M. I.; Thakur G. A.; Manther K.; Treinin M.; Bagdas D.; Kulkarni A. R.; Horenstein N. A. Persistent activation of alpha7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br. J. Pharmacol. 2017, 10.1111/bph.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R. L.; Bagdas D.; Kulkarni A. R.; Gould T.; AlSharari S.; Thakur G. A.; Damaj I. M. The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with nonconducting conformations of the receptor. Neuropharmacology 2015, 91, 34–42. 10.1016/j.neuropharm.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R. L.; Chojnacka K.; Horenstein N. A. The minimal pharmacophore for silent agonism of alpha7 nAChR. J. Pharmacol. Exp. Ther. 2014, 350 (3), 665–680. 10.1124/jpet.114.215236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M.; Stokes C.; Gulsevin A.; Felts A.; Abboud K. A.; Papke R. L.; Horenstein N. A.. Sulfonium as a surrogate for ammonium: A new α7 nicotinic acetylcholine receptor partial agonist with desensitizing activity. J. Med. Chem. 2017, Articles ASAP, DOI: 10.1021/acs.jmedchem.7b00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka K.; Papke R. L.; Horenstein N. A. Synthesis and evaluation of a conditionally-silent agonist for the alpha7 nicotinic acetylcholine receptor. Bioorg. Med. Chem. Lett. 2013, 23 (14), 4145–9. 10.1016/j.bmcl.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K.; Mathes V.; Fronius M.; Althaus M.; Hecker A.; Krasteva-Christ G.; Padberg W.; Hone A. J.; McIntosh J. M.; Zakrzewicz A.; Grau V. Phosphocholine - an agonist of metabotropic but not of ionotropic functions of alpha9-containing nicotinic acetylcholine receptors. Sci. Rep. 2016, 6, 28660. 10.1038/srep28660. [DOI] [PMC free article] [PubMed] [Google Scholar]