Abstract
The central nervous system (CNS) impairment is a consequence seen in SIV infection of rhesus macaques of Indian-origin, which is more common in infected macaques with rapid disease progression than in those with conventional disease progression. Here, we investigated the CNS damages in SIVmac239-infected Chinese rhesus macaques. We demonstrated that SIV infection of Chinese macaques could cause neuropathological impairments, which was evidenced by appearance of SIV-RNA positive cells, the infiltration of activated macrophages and abundant multinucleated giant cells (MNGCs) in the different regions of the brains. The animals with high viremia and short survival time (average of 16 weeks, rapid progression, RP) had severer neuropathological changes than those with conventional progression (CP). As compared with the RP animals, CP macaques had lower viremia and much longer survival time (average of 154 weeks). These findings indicate that SIVmac239 infection of Chinese rhesus macaque can be used as a suitable animal model and alternative resource for nueroAIDS research.
Keywords: SIVmac239, Chinese rhesus macaque, Neuropathological lesion, Rapid progression, NeuroAIDS
Introduction
Neurological disease is a well-documented complication of HIV-1 infection, especially in the terminal stages of AIDS (Budka 1991). Although the incidence of severe dementia in HIV-infected individuals (HAD) has declined due to highly active antiretroviral therapy (HAART), the cumulative prevalence of HIV-associated neurocognitive disorders (HAND) has continued to increase (Clifford and Ances 2013; Maschke et al. 2000; McArthur et al. 2003; Sacktor et al. 2002). Mild neurocognitive impairment is currently the most prevalent form of HIV-1 associated central nervous system (CNS) disease (Heaton et al. 2010; Liner et al. 2008; McArthur et al. 2010; Simpson et al. 2006; Smyth et al. 2007). Similar to HIV, Simian immunodeficiency viruses (SIV) and Simian-human immunodeficiency virus (SHIV) (Barber et al. 2006; Chakrabarti et al. 1991; Gonzalez et al. 2000; Horn et al. 1998; Lackner et al. 1991; Sharma et al. 1992) were found to invade the CNS of pig-tailed or rhesus macaques (RMs) of Indian-origin, causing neuropathological lesions that recapitulate the characteristics of HIV neuropathogenesis (Harbison et al. 2014). Moreover, investigators have demonstrated that rapid disease progression is the best correlate of the development of SIVencephalitis in Indian RMs (Westmoreland et al. 1998). Given that AIDS is a disease that affects humans of diverse origins, it is important to examine HIV animal models with different geographical background, including evaluation of the neuropathogenesis of SIV infection in Chinese RMs.
In comparison to Indian macaques, little has been devoted to research on the neuropathogenesis in SIVor SHIV-infected Chinese RMs. We previously reported the severe lesions in the CNS of two SHIVKU-1 infected-Chinese RMs with conventional progression (Xiao et al. 2015), demonstrating that the infected animals had the neuropathological damages, which are similar to the features of HIVencephalitis or SIV- induced encephalitis (SIVE) in Indian macaques. In the present study, we further investigate the neurological damages in the brains of SIV-infected Chinese RMs with rapid and conventional progression of disease.
Materials and Methods
Experimental Animals and SIV Challenge
All animals were housed at the Animal BioSafety Level III (ABSL-III) laboratory of the Wuhan University, in accordance with the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care International. Protocols used in this study were approved by the Institutional Animal Care and Use Committee of Wuhan University School of Medicine. Eleven female RMs (M. mulatta) of Chinese origin were enrolled in this study. At the beginning of the study, the animals were screened and found negative for infections of simian retrovirus D, SIV, simian T leukemia virus type 1 (STLV-1) and Herpes virus B. The animals were inoculated with 103–104 TCID50 of SIVmac239 by either intravenous or intravaginal route. Whole blood from the inoculated macaques was collected for monitoring SIV viral load and CD4/CD8 Tcell ratio. Animals were euthanized if they lost >20 % of their body weight or developed intractable diarrhea that was unresponsive to supportive or antibiotic treatment, respiratory signs with radiographic evidence of pneumonia, persistent anorexia and lethargy, or neurologic signs. Brain tissues were collected at the time of necropsy.
Quantification of Virus in Plasma
Plasma samples were collected during the course of study. Expression level of SIV RNA in plasma was measured by real-time RT-PCR assay. Total RNA was extracted from cell-free plasma by using TRI Reagent (Molecular Research Center, Inc) as the manufacturer’s instructions. Viral RNA was then reverse transcribed to generate cDNA for real-time quantitative RT-PCR. Real-time PCR was performed using following primers: 5′-GCAGAGGAGGAAATTACCCAGTAC-3′ (forward) and 5′-CAATTTTACCCAGGCATTTAATGTT-3′ (reverse). A serial of 10-fold dilutions of standard RNA template was used to generate a standard curve for each assay. All amplifications were performed in duplicate. Assay results were normalized to the volume of plasma extracted and expressed as numbers of SIV RNA copy equivalents per milliliter of plasma.
Flow Cytometry
CD4/CD8 T cell ratio was monitored in 50 μl of EDTA anti-coagulated whole blood stained with anti-human CD3-FITC, anti-human CD4-PerCP-Cy5.5 and anti-human CD8-APC. All antibodies were obtained from BD Biosciences (San Diego, CA) and used for cell labeling according to the manufacturer’s instructions. The cells were fixed with 0.5 % paraformaldehyde and analyzed on a flow cytometer (BD FACSVerse). Results were analyzed using FlowJo software (TreeStar, v7.6.1).
SIV-Specific In Situ Hybridization
Formalin-fixed, paraffin-embedded brain sections were assayed for SIV viral RNA expression by in situ hybridization (ISH). Briefly, deparaffinized and rehydrated sections were first incubated with pre-hybridization buffer at 50 °C for 30 min to avoid background staining. Tissue sections were hybridized overnight at 50 °C with either sense or antisense digoxigenin-UTP-labeled SIVmac239 probe that spanned the entire genome. The hybridized sections were then blocked with 2 % normal horse serum (Gibco) and 1 % bovine serum albumin (BSA) in 0.1 M Tris/HCl (pH 7.4) for 1 h and incubated with sheep anti-digoxigenin-alkaline phosphatase (Roche Molecular Biochemicals) at 37 °C for 30 min, followed by staining with BCIP/NBT (Sigma) and counter staining with methyl green. ISH-stained sections were visualized and photographed with a camera-assisted microscope (OLYMPUS, BX53).
Immunohistochemistry and Immunofluorescence
Formalin-fixed, paraffin-embedded brain tissues obtained from all macaques at necropsy were consecutively cut into 5 μm sections, and mounted on glass slides for immunohistochemistry (IHC) staining. Sections were deparaffinized and rehydrated before heat-mediated epitope retrieval was performed. To avoid non-specific staining in tissue sections, hydrogen peroxide blocking and serum blocking were applied at room temperature. Primary antibodies were diluted in PBS and incubated with the sections at 4 °C overnight. After primary antibody incubation, sections were washed and stained with goat anti-mouse/rabbit IgG secondary antibody (HRP Polymer) for 30 min at room temperature. The color reaction product was developed using 3, 3′-diaminobenzidine tetrahydrochloride (DAB). Sections were counterstained with hematoxylin followed by dehydration and mounting. The primary antibodies used were: the pan-macrophage marker CD68 (1/50; Boster), the macrophage scavenger receptor CD163 (1/400; Boster), the microglia activation marker Iba-1(1/1000; Wako) and SIVmac251 p28 antibody (1/400; MyBioSource). Sections were visualized, and imaged with a camera-assisted microscope (OLYMPUS, BX53). For immunofluorescence staining, primary antibody labeled brain sections were incubated with either goat-anti mouse IgG-Cy3 secondary antibody (1/100; Boster) or goat-anti rabbit IgG-FITC secondary antibody (1/100; Boster) for 30 min at room temperature. 4′, 6-diamidine-2′-pheynylindole dihydrochloride (DAPI) staining was used for all fluorescence staining conditions to identify nuclear in cells. Slides were examined and photographed using a camera-assisted fluorescent microscope (OLYMPUS, IX71).
Results
Outcomes of SIVmac239 Infection in Chinese Rhesus Macaques
The animals were inoculated with 103–104 TCID50 of SIVmac239 by either intravenous (n = 9) or intravaginal route (n = 2) (Table 1). The infected animals were classified as RP and CP macaques based on the viremia and the rate of disease progression (Mellors et al. 1996). As shown in Table 1, three macaques identified as RP macaques were euthanized at 9, 15 and 24 weeks post infection, with the average survival time was only 16 weeks, which is similar to the rapid disease progression reported among Indian RMs (Brown et al. 2007; Williams et al. 2008). In contrast, 7 out of 8 CP macaques survived more than 100 weeks post infection, with an average survival time of 154 weeks. Macaque WSP12 did not have any signs of advanced HIV disease and euthanized at 215 weeks post infection. Statistically, there is a significant difference in survival time between CP and RP macaques (Table 1, P < 0.05; log rank test). In addition, disease phenotypes also differed distinctly between RP and CP macaques. Most CP macaques (7/8) commonly succumbed to the gastrointestinal dysfunctions, such as severe diarrhea at the end-stage. In contrast, clinical signs in RP macaques appeared to be quite diverse. The macaque WSL02 gradually showed HAD-like syndrome including ataxia and dyspraxia, while WSL06 exhibited diarrhea and severe wasting and then euthanized at week 15. The RP macaque (WSL01) unexpectedly died at week 9 post infection due to acute heart failure caused by heart thrombus. We classified WSL01 to the RP group because this animal had sustained high level of viremia over the course of infection.
Table 1.
Characteristics of SIVmac239-infected Chinese RMs
| Disease progression | Animal # | Infection route | Infection dose (TICD50) | Time to autopsy (week) | Peripheral viremia (copies/ml)
|
|
|---|---|---|---|---|---|---|
| Peak | Autopsy | |||||
| Rapid | WSL01 | IV | 10,000 | 9 | 2.8 × 107 | 5.8 × 107 |
| WSL02 | IV | 10,000 | 24 | 9.6 × 107 | 4.4 × 107 | |
| WSL06 | IV | 10,000 | 15 | 4.4 × 107 | 1.7 × 109 | |
| Conventional | WSL03 | IV | 10,000 | 151 | 3.8 × 107 | 9.6 × 107 |
| WSL04 | IV | 10,000 | 184 | 8.9 × 106 | 2.1 × 106 | |
| WSL05 | IV | 10,000 | 181 | 1.5 × 106 | 7.3 × 107 | |
| WSP08 | IV | 1,500 | 125 | 1.6 × 107 | 8.3 × 108 | |
| WSP09 | IV | 1,500 | 128 | 2.9 × 106 | 4.6 × 107 | |
| WSP10 | IV | 1,500 | 96 | 2.0 × 108 | 3.5 × 106 | |
| WSP11 | I.Vag | 1,500 | 150 | 5.1 × 107 | 3.4 × 106 | |
| WSP12 | I.Vag | 1,500 | 216 | 5.3 × 106 | 1.0 × 107 | |
IV, intravenous; I.Vag, intravaginal
All 11 animals became infected as evidenced by increased levels of plasma SIV RNA (Fig. 1a and c). Peak viremia of 6–8 log10 RNA copies per milliliter plasma was detected in both RP and CP macaques, with no significant difference in the magnitude between the two groups. However, the set point viral loads were significantly higher in the RP macaques than those of the CP macaques (Fig. 1b, p < 0.001). As shown in Fig. 1c, all three RP macaques exhibited higher persistent plasma viremia than CP animals over the course of SIV infection, with a slight decline after peak viremia. All the infected animals showed the transient decline of peripheral CD4/CD8 ratio (Fig. 1d). In general, CD4/CD8 ratio in RP macaques is comparable to those in CP macaques over the course of infection, with a median ratio value of 0.5 in both groups at the time of necropsy.
Fig. 1.
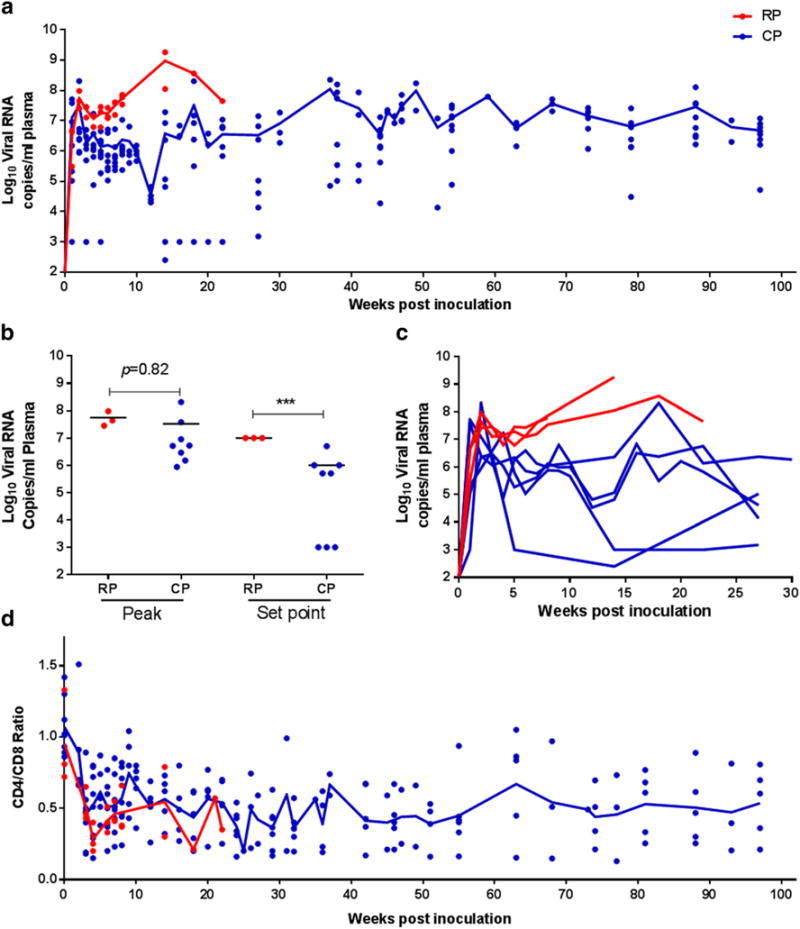
Virologic and immunologic parameters in SIVmac239-infected macaques a. Comparison of the changes in viral load in RP and CP macaques over the course of infection (100 weeks). b. Comparison of peak and set point between RP and CP macaques. Asterisks (***) indicates statistical significance (p < 0.001). c. Kinetics of virus dissemination within the first 30 weeks of infection in RP and CP macaques. d. Comparison of changes in CD4/CD8 ratios in RP and CP macaques over the course of infection (100 weeks). Trend lines in a and d were created using median viral load or CD4/CD8 ratio of RP (n = 3) and CP (n = 8) macaques at each time point
Pathologic Changes in Brains of Infected Chinese Rhesus Macaques
The immunohistological examination was completed in 3 RP macaques (WSL01, WSL02 and WSL06) and 5 CP macaques (WSP08, WSP09, WSP10, WSP11 and WSL03) (Table 2). IHC staining and ISH for SIV-RNA expression in brain tissues obtained at the necropsy of 8 animals revealed distinct neuropathologic characteristics in SIV-induced brain lesions between RP and CP macaques.
Table 2.
Summary of IHC and ISH results
| Disease progression | Animal # | Tissue | CD68 | CD163 | SIV (ISH) |
|---|---|---|---|---|---|
| Rapid | WSL01 | CC | − | − | − |
| CB | + | − | − | ||
| WSL02 | CC | + | + | NP | |
| CB-1 | + | + | + | ||
| CB-2 | + | + | + | ||
| WSL06 | CC | + | + | + | |
| CB | + | + | + | ||
| Conventional | WSL03 | TC | − | − | + |
| OC | + | − | + | ||
| CB | + | + | NP | ||
| WSP08 | TC | − | − | + | |
| OC | + | − | + | ||
| CB | + | NP | − | ||
| WSP09 | TC | + | − | − | |
| OC | + | + | − | ||
| CB | + | + | − | ||
| WSP10 | TC | + | − | + | |
| OC | + | + | + | ||
| CB | + | + | + | ||
| WSP11 | TC | − | − | + | |
| OC | − | − | + | ||
| CB | + | + | + |
+, IHC or ISH positive signals were found in tissues slides; −, no positive signal in tissues slides
CC, cerebral cortex; CB, cerebellum; TC, temporal cortex; OC, occipital cortex; NP, not performed
Among three RP macaques, two (WSL02 and WSL06) were demonstrated to have the neuropathologic damages in the cerebral cortex and the cerebellum (Table 2, Fig. 2). IHC staining for CD68 and CD163 revealed the robust macrophage activation (Fig. 2b–e), together with a number of MNGCs formation, throughout the brain sections of WSL02. In addition, many of SIV-infected cells were identified as macrophages (Fig. 2a–c, e–f) in these sections. Furthermore, the immunofluorescence demonstrated double staining of SIVp28 antigen (red) and CD68-positive cells (green) in the brain section of macaque WSL02 (Fig. 2g). Compared with WSL02, macaque WSL06 presented milder neuropathologic lesions in the brain, despite the fact that activated CD68+/CD163+ macrophages and MNGCs as well as SIV-infected cells could be observed in the most regions of the brain (Fig. 2h–k). Moreover, similar to SIV/SHIV-infected Indian macaques and our earlier report (Xiao et al. 2015), the brain lesions in SIVmac239-infected RP macaques could be divided into two main histopathological patterns. The first one represents classical perivascular lesions comprised of CD68+/CD163+ SIV-infected macrophages and MNGCs (Fig. 2a–g); and the second pattern is characterized by scattered SIV infected cells, with extensive macrophage activation.
Fig. 2.
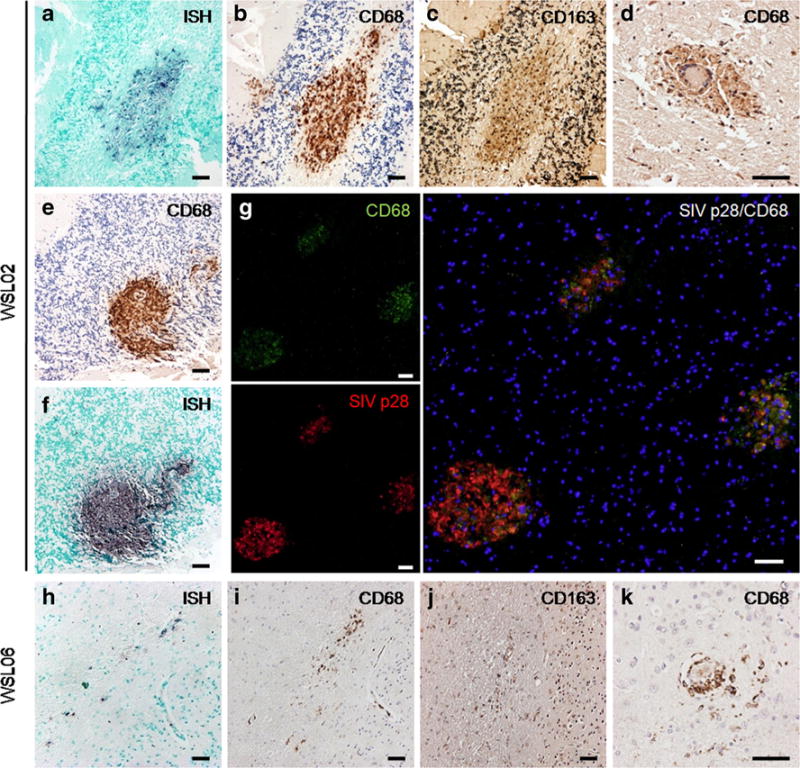
Neuropathologic lesions of SIVE in brains of SIVmac239-infected Chinese RMs with rapid disease progression a–c. Representative regions of SIVE lesions in the brain of WSL02. There was a high expression of SIV-RNA positive signals (a, BCIP/NBT; indigo) within CD68+ (b, DAB; brown) and CD163+ (c, DAB; brown) perivascular macrophages. d. Classic MNGCs with CD68+ macrophages surrounded in the brain of WSL02. e–f. Massive CD68+ macrophages accumulated (e) with SIV-RNA positive cells (f) in the cerebellum of WSL02. g. dual-color immunofluorescence staining of MNGCs formation in the cerebellum of WSL02, with CD68+ macrophages (green) and SIVp28 (red) overlapping. Nuclear staining is DAPI (blue). h–j. Typical SIV induced brain lesion in WSL06 comprised of SIV-RNA positive cells (h) are CD68+ (i) and CD163+ (j) macrophages. k. MNGCs with CD68+ macrophages in the brain of WSL06. a–c, e–f are from the cerebellum of WSL02; d and g are from the cerebral cortex of WSL02; h–k are from the cerebral cortex of WSL06. Scale bar: 50 μm
As compared with RP macaques, the SIV infection and pathological changes in the brains of CP macaques were significantly less severe (Table 2, Fig. 3). SIV-RNA positive cells detected by ISH scattered over the cerebrum of WSP10 (Fig. 3a), but the majority of SIV-RNA positive cells were rarely identified as macrophages by IHC staining with CD68 and CD163 antibodies (Fig. 3b–d). IHC staining for SIV gag p28 was positive in the brain of WSP08 (Fig. 3e) without CD68-positive macrophages (Fig. 3f), but with activated microglia marked as Iba-1 positive (Fig. 3g). Future research is necessary to determine the target cells responsible for SIV infection in the CNS. Interestingly, scattered but widely distributed CD68+ macrophages were seen throughout the cerebellum of WSP09 (Fig. 3h), but SIV-RNA (ISH) was not.
Fig. 3.
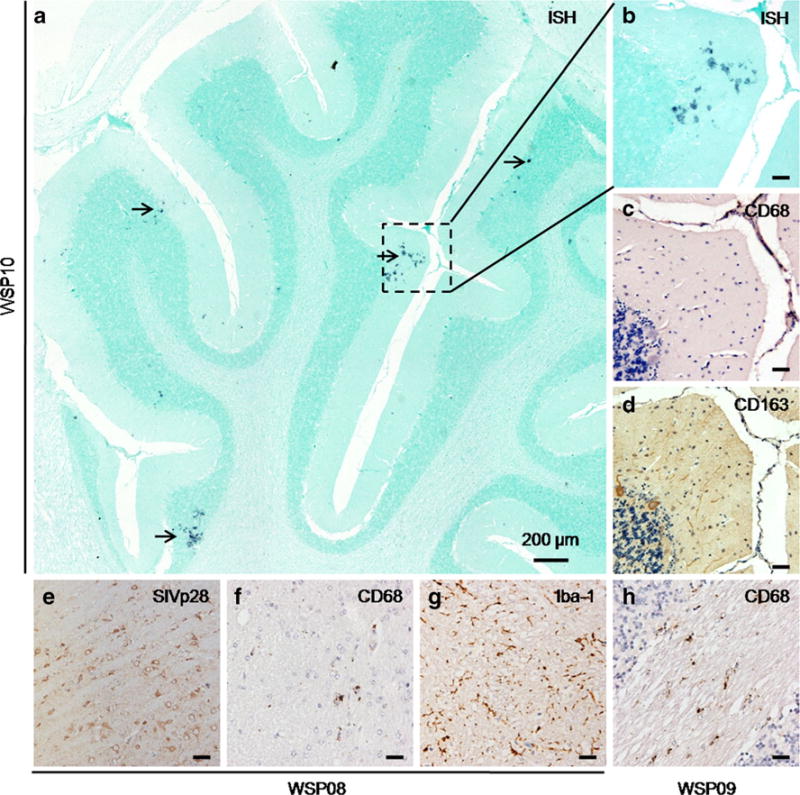
Neuropathological changes in brains of SIVmac239-infected Chinese RMs with conventional disease progression a. In situ hybridization for SIV viral RNA in the cerebellum of WSP10 (magnification, ×10). Cells with SIV-RNA positive signals (BCIP/NBT; indigo) are shown with arrows. b–d. Enlargements of the area indicated by the rectangle in a, that SIV-RNA positive cells (b) are CD68− (c) and CD163− cells. (d). e–g. IHC staining for SIVp28 (DAB; brown) was positive in the brain of WSP08 (e), with less CD68+ macrophages (DAB; brown) recruitment (f), but obvious activated microglia expression marked by Iba-1 (g, DAB; brown). h. Scattered CD68+ macrophages in the cerebellum of WSP09. Scale bar: 50 μm (except for a)
CD4/CD8 Ratio in Peripheral Blood is Associated with SIV Infection of the CNS in CP Macaques
SIV infection in the brain was demonstrated in 4 out of 5 CP macaques with little SIV-induced neuropathological changes. The numbers of SIV-infected cells and activated macrophage in the brains of these CP macaques varied, which was ranked in the following order: WSP10, WSL03 > WSP08, WSP11 > WSP09. The animal WSL03 (Fig. 4a and e) and WSP10 (Fig. 4b and f) had more SIV-RNA positive cells and activated CD68+ macrophages than the animals WSP08 (Fig. 4c and g) and WSP11 (Fig. 4d and h). Corresponding to the neuropathologic changes in CP macaques, the early decline of CD4/CD8 ratios was seen in macaque WSP10 and WSL03, with the value of ratio dropping from 0.9 at day 0 to 0.25 at week 25 and week 35, respectively. These animals maintained the low CD4/CD8 ratio (<0.2) until the time of euthanasia (Fig. 4i).
Fig. 4.
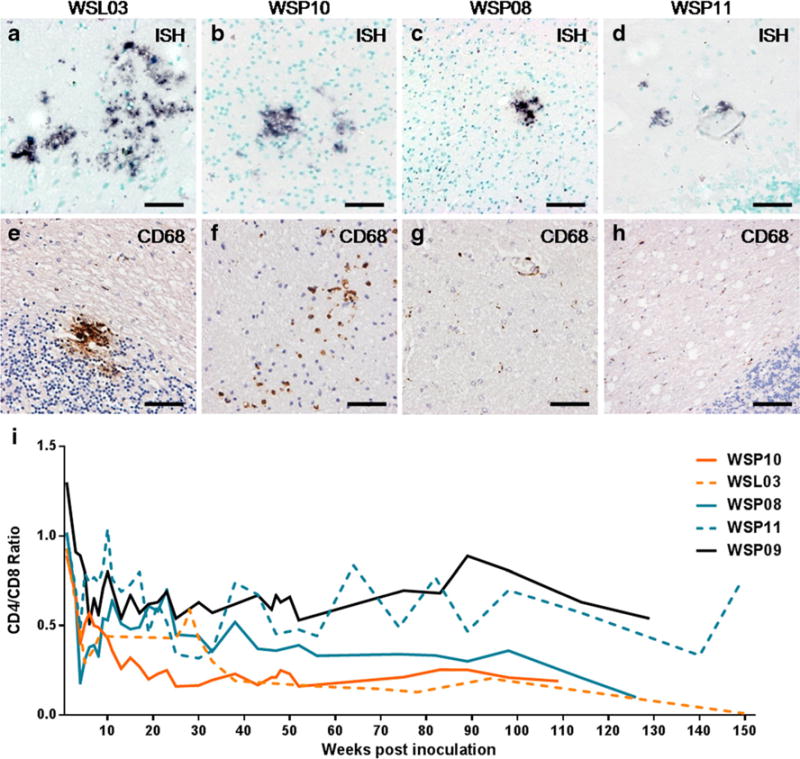
Variant neuropathological changes and CD4/CD8 ratio over time in CP macaques a–d. ISH for SIV RNA (BCIP/NBT; indigo) in brains of CP macaques. More SIV-expressing cells presented in the brain of WSL03 (a, temporal cortex) and WSP10 (b, occipital cortex) comparing to WSP08 (c, occipital cortex) and WSP11 (d, cerebellum). e–h. Distribution of CD68+ macrophages (DAB; brown) in brains of CP macaques with SIVmac239 infection. e. CD68+ macrophage accumulation was seen in the cerebellum of WSL03. f. CD68+ macrophages scattered in the brain of WSP10. g–h. IHC staining of CD68 was not significant in the brain of WSP08 (g) and WSP11 (h), while ISH for SIV-RNA was positive (c and d). i. CD4/CD8 ratio in 5 CP macaques with a neuropathological diagnosis. Scale bars: 50 μm
Discussion
Central nervous system disease is a major feature of SIV infection of macaques of Indian origin (Matsuda et al. 2013; Orandle et al. 2001). SIVE was strongly associated with a rapid disease course (Nowlin et al. 2015; O’Neil et al. 2004; Orandle et al. 2001; Westmoreland et al. 1998). However, there is little information about neuropathology in the brains of SIV-infected Chinese RMs. In the present study, we observed that the neuropathological features in RP Chinese RMs were similar to those in RP macaques of Indian origin, including MNGCs formation and SIV expression in macrophages (Baskin et al. 1991; Brown et al. 2007; Zhuang et al. 2014). In addition, we showed that the majority of SIVmac239-infected Chinese RMs at end stage disease had the brain impairments in various degrees. The RP macaques (WSL02 and WSL06) had severe neuropathological damages as evidenced by the positive staining of activated CD68+/CD163+ macrophages, abundant MNGCs and many SIV-RNA+ cells in the brains (Fig. 2). In contrast, the pathological examination of the brains of CP macaques showed few of activated macrophages and only scattered SIV-RNA+ cells (Figs. 3 and 4). Although the CP and RP macaques had similar peak viremia, RP macaques maintained higher levels of viral loads than CP macaques during the course of infection.
Although Chinese RM and Indian RM belong to the same species, they demonstrate differences in physiological and immunological responses (Champoux et al. 1997; Clarke and O’Neil 1999; Doxiadis et al. 2003), as well as genetic background (Doxiadis et al. 2003; Otting et al. 2007; Penedo et al. 2005; Viray et al. 2001). Ling et al. showed that compared with Indian RM, SIV pathogenesis in Chinese RM was closer to HIV infection in humans, as the infected Chinese RMs had significantly lower setpoint viral load than Indian RMs (Ling et al. 2002). Such difference in viral load was also observed in SIVmac251-infected Chinese RMs (Reimann et al. 2005; Marthas et al. 2001; Marcondes et al. 2010). Consistent with these reports, we demonstrated that the majority of SIVmac239-infected Chinese RMs had low viremia and long survival time (average 154 weeks). Similar to HIV infection in humans (Forbi and Agwale 2009), inverted CD4/CD8 ratio is associated with the onset of disease in CP macaques. We found that CD4/CD8 ratio in eight CP macaques gradually declined over the course of infection. The CD4/CD8 ratio of macaques (WSP10 and WSL03) dropped significantly (<0.25) at the early stage of infection (at 25 and 35 weeks post infection, respectively), and maintained such lower ratios until the time of euthanasia (week 109 and week 150, respectively). These two animals also had severe SIV infection in the CNS, as demonstrated by SIV-RNA positive cells and infiltrated macrophages in the brains. Interestingly, RP macaques (WSL02 and WSL06) with severer neuropathological damages did not have significant decrease in CD4/CD8 ratio. Thus, it is likely that high and sustained levels of viral loads would be responsible for the SIVmac239-induced brain lesions.
In summary, the present study demonstrated that SIVmac239 infection could induce neurological impairments in the brain of the infected Chinese rhesus macaques. The CNS injury in the infected animals was positively associated with the disease progression. These data indicate that Chinese rhesus macaque is a suitable and alternative non-human primate model for neuroAIDS studies.
Acknowledgments
We are grateful to Dr. Vanessa M Hirsch for the SIVmac239 ISH probes templates and Dr. Kenta Matsuda for the technique support of ISH. This work was supported by the National Natural Science Foundation of China (81471943 to K.Z.; 81301428 to L.Z.; 81271334 to W.Z.H. and 81201261 to J.Z.) and by the Fundamental Research Funds for the Central Universities (2042016kf0186 to K.Z. and 2042015kf0188 to L.Z.).
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflicts of interest.
References
- Barber SA, Gama L, Dudaronek JM, et al. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infectious Diseases. 2006;193:963–970. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- Baskin G, Murphey-Corb M, Martin L, et al. Lentivirus-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Veterinary Pathol Online. 1991;28:506–513. doi: 10.1177/030098589102800607. [DOI] [PubMed] [Google Scholar]
- Brown CR, Czapiga M, Kabat J, et al. Unique pathology in simian immunodeficiency virus-infected rapid progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J Virol. 2007;81:5594–5606. doi: 10.1128/JVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Hurtrel M, Maire MA, et al. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Champoux M, Higley JD, Suomi SJ, et al. Behavioral and physiological characteristics of Indian and Chinese-Indian hybrid rhesus macaque infants. Dev Psychobiol. 1997;31:49–63. [PubMed] [Google Scholar]
- Clarke MR, O’Neil JAS. Morphometric comparison of Chinese–origin and Indian–derived rhesus monkeys (Macaca mulatta) Am J Primatol. 1999;47:335–346. doi: 10.1002/(SICI)1098-2345(1999)47:4<335::AID-AJP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxiadis GG, Otting N, de Groot NG, et al. Evolutionary stability of MHC class II haplotypes in diverse rhesus macaque populations. Immunogenetics. 2003;55:540–551. doi: 10.1007/s00251-003-0590-9. [DOI] [PubMed] [Google Scholar]
- Forbi J, Agwale S. Inverted CD4+/CD8+ ratio associated with AIDS event and death in HIV-1 infected individuals in Nasarawa State, Nigeria. Tanzania journal of health research. 2009;11(3) doi: 10.4314/thrb.v11i3.47701. [DOI] [PubMed] [Google Scholar]
- Gonzalez RG, Cheng LL, Westmoreland SV, et al. Early brain injury in the SIV-macaque model of AIDS. AIDS. 2000;14:2841–2849. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- Harbison C, Zhuang K, Gettie A, et al. Giant cell encephalitis and microglial infection with mucosally transmitted simian-human immunodeficiency virus SHIVSF162P3N in rhesus macaques. J Neurovirology. 2014;20:62–72. doi: 10.1007/s13365-013-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R, Clifford D, Franklin D, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn TF, Huitron-Resendiz S, Weed MR, et al. Early physiological abnormalities after simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1998;95:15072–15077. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner AA, Smith MO, Munn RJ, et al. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Liner KJ, II, Hall CD, Robertson KR, et al. Effects of antiretroviral therapy on cognitive impairment. Current HIV/AIDS Reports. 2008;5:64–71. doi: 10.1007/s11904-008-0011-7. [DOI] [PubMed] [Google Scholar]
- Ling B, Veazey RS, Luckay A, et al. SIVmac pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. Aids. 2002;16:1489–1496. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- Marcondes MCG, Flynn C, Watry DD, Zandonatti M, Fox HS. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol. 2010;177:355–361. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthas ML, Lu D, Penedo MC, et al. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res Hum Retroviruses. 2001;17:1455–1466. doi: 10.1089/088922201753197123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Esser S, et al. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Brown CR, Foley B, et al. Laser capture microdissection assessment of virus compartmentalization in the central nervous systems of macaques infected with neurovirulent simian immunodeficiency virus. J Virol. 2013;87:8896–8908. doi: 10.1128/JVI.00874-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, et al. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo CR, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Nowlin BT, Burdo TH, Midkiff CC, et al. SIV encephalitis lesions are composed of CD163+ macrophages present in the central nervous system during early SIV infection and SIV-positive macrophages recruited terminally with AIDS. Am J Pathol. 2015;185:1649–1665. doi: 10.1016/j.ajpath.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil SP, Suwyn C, Anderson DC, et al. Correlation of acute humoral response with brain virus burden and survival time in pigtailed macaques infected with the neurovirulent simian immunodeficiency virus SIVsmmFGb. Am J Pathol. 2004;164:1157–1172. doi: 10.1016/S0002-9440(10)63204-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orandle MS, Williams KC, MacLean AG, et al. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J Virol. 2001;75:4448–4452. doi: 10.1128/JVI.75.9.4448-4452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N, de Vos-Rouweler AJ, Heijmans CM, et al. MHC class I A region diversity and polymorphism in macaque species. Immunogenetics. 2007;59:367–375. doi: 10.1007/s00251-007-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penedo MC, Bontrop RE, Heijmans CM, et al. Microsatellite typing of the rhesus macaque MHC region. Immunogenetics. 2005;57:198–209. doi: 10.1007/s00251-005-0787-1. [DOI] [PubMed] [Google Scholar]
- Reimann KA, Parker RA, Seaman MS, et al. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6 P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J Virol. 2005;79:8878–8885. doi: 10.1128/JVI.79.14.8878-8885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sharma DP, Zink MC, Anderson M, et al. Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J Virol. 1992;66:3550–3556. doi: 10.1128/jvi.66.6.3550-3556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DM, Kitch D, Evans SR, et al. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- Smyth K, Affandi JS, McArthur JC, et al. Prevalence of and risk factors for HIV-associated neuropathy in Melbourne, Australia 1993–2006. HIV Med. 2007;8:367–373. doi: 10.1111/j.1468-1293.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Viray J, Rolfs B, Smith DG, et al. Comparison of the frequencies of major histocompatibility (MHC) class-II DQA1 and DQB1 alleles in Indian and Chinese rhesus macaques (Macaca mulatta) Comp Med. 2001;51:555–561. [PubMed] [Google Scholar]
- Westmoreland SV, Halpern E, Lackner AA, et al. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- Williams R, Bokhari S, Silverstein P, et al. Nonhuman primate models of NeuroAIDS. J Neurovirol. 2008;14:292–300. doi: 10.1080/13550280802074539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Li J, Yu Q, et al. Distinct compartmentalization in the CNS of SHIVKU-1-infected Chinese rhesus macaque is associated with severe neuropathology. J Acquir Immune Defic Syndr. 2015;70:e168–e171. doi: 10.1097/QAI.0000000000000839. [DOI] [PubMed] [Google Scholar]
- Zhuang K, Leda AR, Tsai L, et al. Emergence of CD4 independence envelopes and astrocyte infection in R5 simian-human immunodeficiency virus model of encephalitis. J Virol. 2014;88:8407–8420. doi: 10.1128/JVI.01237-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


