Abstract
Cell transplantation is a promising therapeutic strategy for the treatment of traumatic muscle injury in humans. Typically, previous investigations have focused on the identification of potent cell and growth factor treatments and optimization of spatial control over delivery. However, the optimal time point for cell transplantation remains unclear. Here we study how myoblast and morphogen delivery timed to coincide with specific phases of the inflammatory response affects donor cell engraftment and the functional repair of severely injured muscle. Delivery of a biomaterial-based therapy timed with the peak of injury-induced inflammation leads to potent early and long-term regenerative benefits. Diminished inflammation and fibrosis, enhanced angiogenesis, and increased cell engraftment are seen during the acute stage following optimally timed treatment. Over the long-term, treatment during peak inflammation leads to enhanced functional regeneration, as indicated by reduced chronic inflammation and fibrosis and increased tissue perfusion and muscle contractile force. Treatments initiated immediately after injury or after inflammation had largely resolved provided more limited benefits. These results demonstrate the importance of appropriately timing the delivery of biologic therapy in the context of muscle regeneration. Biomaterial-based timed delivery can likely be applied to other tissues and is of potential wide utility in regenerative medicine.
Keywords: ferrogel scaffolds, magnetic biomaterials, inflammation kinetics, cell therapy, controlled delivery
ToC figure

Externally actuated ferrogels are used to demonstrate the importance of timing of biologic therapies with respect to injury-induced inflammation. Delaying treatment until the peak of inflammation leads to both early and long-term regenerative benefits surpassing those seen with treatment initiated at the time of injury. This strategy can likely be applied broadly to both new and existing cell transplantation therapies.
1. Introduction
Early clinical trials in myoblast transplantation demonstrated the safety of intramuscular cell injections and the ability of transplanted myoblasts to participate in muscle regeneration.[1, 2] Unfortunately, no functional benefit was observed following these treatments, likely due to poor donor cell engraftment resulting from rapid death, poor migration, and immune rejection of the injected cells.[2–4] To address limitations highlighted by these early clinical trials, many subsequent studies have focused on optimizing therapeutic cell populations and transplantation strategies. For example, pretreatment regimens involving irradiation-induced injury have been developed to enhance donor cell engraftment in murine models of muscular dystrophy.[5] More generally, as culturing satellite cells on tissue culture plastic results in cell populations with greatly reduced regenerative capacity, new minimal isolation and sorting procedures have been developed.[6, 7] Freshly isolated, minimally manipulated myogenic precursors,[8–10] and satellite cells transplanted with their parent myofiber[11–13] have been shown to efficiently engraft and contribute to muscle repair in mouse models, suggesting cell transplantation remains a promising strategy for the clinical treatment of traumatic skeletal muscle injury.
Biomaterial systems that provide cell-instructive cues and a protective microenvironment have shown great promise as controlled cell delivery vehicles that can promote in vivo regeneration by enhancing transplanted cell survival and engraftment.[14–18] Myoblast death following bolus injection into injured muscle results in part from a lack of available extracellular matrix contacts. Material systems that incorporate adhesive cues prevent anoikis of anchorage-dependent cells by allowing them to recognize and interact with their environment.[19] Polymeric biomaterials functionalized with cell adhesion ligands such as RGD, the cell binding domain of fibronectin, have also been shown to regulate myoblast proliferation and differentiation.[20–22] In addition, immediate exposure of intramuscularly injected cells to the harsh wound environment likely plays a role in their rapid death and poor long-term viability.[4, 23] Material systems can be designed to provide temporary protection from post-injury inflammation while allowing interactions with host cells when necessary. Due to their ability to improve survival, engraftment, and fate control of the delivered cells, material-based cell transplantation approaches will likely be of great clinical utility for muscle repair.
Further improvements in biomaterial-based cell therapy strategies may result from the appropriate timing of cell delivery to coincide with specific phases of the innate inflammatory response following injury. Macrophages, for example, are known to play key roles in muscle regeneration and interact with satellite cells in dual and beneficial ways.[24–26] The early influx of proinflammatory (M1) macrophages generally coincides with satellite cell recruitment and activation in vivo. M1 macrophages and their secreted factors have been shown to enhance the proliferation and motility of myogenic progenitors, as well as inhibit myogenic differentiation and cell fusion in vitro.[27–31] In contrast, a later rise in the anti-inflammatory (M2) macrophage population generally coincides with satellite cell differentiation and termination of inflammation. M2 macrophages and their secreted factors have been shown to promote myogenic differentiation and mature myotube formation in vitro.[27–29] Interestingly, improved myoblast engraftment has been observed following coinjection of myogenic progenitors with anti-inflammatory macrophages in an mdx mouse model[32] and also with proinflammatory macrophages in a cryoinjury model, with the latter leading to increased donor cell proliferation following injection.[31] These findings suggest that biomaterial-based cell therapy strategies temporally controlled to coincide with specific phases of the inflammatory response following acute injury may lead to improved outcomes.
Here we study how myoblast and morphogen delivery timed to coincide with specific phases of muscle injury-induced inflammation affects regeneration. Time frames representing various phases of the inflammatory response were established in a murine model of severe muscle injury that does not spontaneously heal, unlike other commonly used injury models.[33] Myogenic cells and growth factors previously shown to support engraftment[18] were then delivered to injured muscles from ferrogel scaffolds before, during, and after peak inflammation. Magnetically-responsive ferrogels, capable of on-demand release of cells and growth factors,[34, 35] were utilized here to enhance cell survival and allow externally triggered release during distinct phases of the injury response. We hypothesized that avoiding the early inflammatory stage would enhance donor cell engraftment and the regeneration of severely injured skeletal muscle.
2. Results
2.1 Inflammation Kinetics and Experimental Design
The inflammation kinetics of the tibialis anterior muscles of C57BL6/J mice subjected to a severe muscle injury were first analyzed. Tibialis anterior muscles were injected intramuscularly with notexin, and hindlimb ischemia was induced six days later upon resolution of notexin-induced inflammation (Figure S1A–D). Following combined injury with notexin and ischemia, loss of locomotion of the injured hindlimb was observed. IVIS imaging indicated a peak in inflammation 7 days post-injury that largely resolved by day 10 (Figure 1A, 1C). Similarly, quantification of the inflammatory infiltrate from hematoxylin and eosin stained sections demonstrated a peak in inflammation 3 to 7 days post-injury and resolution by day 10 (Figure 1B, 1D). CCR7+ M1 macrophage presence peaked relatively early at 3 days, while CD206+ M2 macrophage presence peaked later at day 7 post-injury (Figure S2A–D). To assess the optimal time point for treatment with regards to inflammation following combined injury, muscles were treated 0, 6, or 10 days after ischemic surgery, corresponding to points before, during, and after peak inflammation, respectively (Figure 1E). At these inflammation-directed time points, tibialis anterior muscles were treated with a subcutaneously implanted biphasic ferrogel scaffold containing a previously optimized combination of myogenic cells and growth factors known to induce myogenesis (IGF) and angiogenesis (VEGF).[18] Minimal culture of freshly isolated muscle-derived precursors provided a population of primary myoblasts expressing muscle-specific markers shared with certain populations of satellite cells (Figure S3A–B). External magnets were used to noninvasively actuate the ferrogels each day, for four consecutive days, to trigger release of the cells and factors from the ferrogels over this time frame. Muscles were then harvested for histological and functional assessment 4 days following the initiation of treatment and 6 weeks following induction of ischemia.
Figure 1.
Severe muscle injury model results in acute inflammation that largely resolves by day 10. (A) and (C) Representative images and quantification of inflammation probe dye (DYE) accumulating at injury site at varying time points following induction of ischemia, as measured by IVIS. Regions of interest used for quantification are marked with blue ovals. Ratios of dye accumulation in injured hindlimbs to uninjured contralateral hindlimbs were calculated to account for variability associated with dye leakage from and around the sinus following retro-orbital injections.[54] Footpads of injured hindlimbs were not used for quantification due to minimal blood flow and dye accumulation downstream of ischemic injuries. (B) and (D) Representative images and quantification of inflammatory infiltrate (INFLAM) in histological cross-sections of tibialis anterior muscles stained with hematoxylin and eosin at varying time points following ischemia injury. Scale bar represents 200 µm. (E) Experimental design showing injury, scaffold implantation, and sacrifice time points. The time points at which scaffolds were stimulated to induce release of cells and factors are also indicated. Values represent the mean and standard deviation (n = 5–7). Data in C and D were compared using ANOVA with Bonferroni's post-hoc test (*p < 0.05).
2.2 Impact of Timing of Therapy on Early Muscle Regeneration
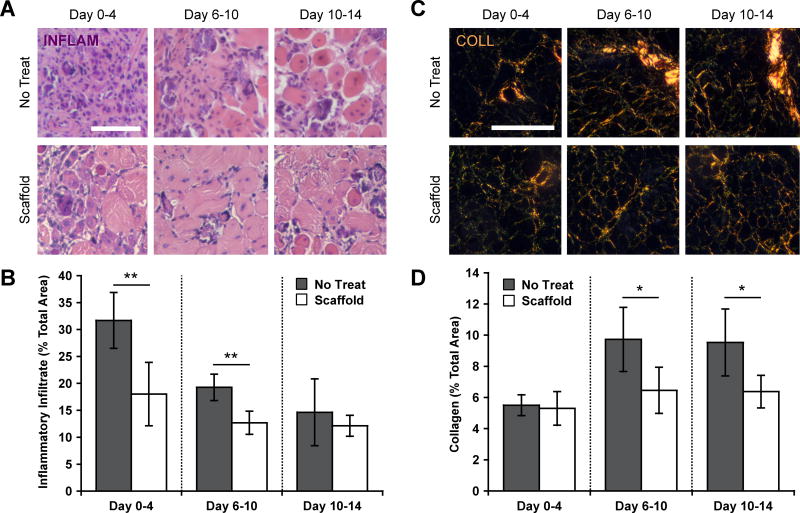
The influence of timed cell and growth factor delivery on acute inflammation and fibrosis was first determined 4 days after the start of each treatment time using histological analysis. Quantification of the inflammatory infiltrate revealed significant decreases, as compared to no treatment controls, with both immediate scaffold treatment starting at day 0 and delayed scaffold treatment starting at day 6 (Figure 2A–B). In contrast, no significant difference in the number of infiltrating inflammatory cells was observed following delayed treatment starting at day 10. The extent of muscle fibrosis was assessed by visualizing picrosirius red stained collagen I and III under polarized light. Large quantities of fibrotic tissue were present in all conditions following muscle injury with notexin and induction of ischemia (Figure 2C–D). Delayed scaffold treatments, starting at day 6 and day 10, resulted in significant decreases in interstitial collagen when compared to no treatment controls. In contrast, treatment initiated immediately after injury on day 0 led to no significant difference in collagen deposition.
Figure 2.
Timing of therapy modulates acute inflammation and fibrosis. (A) and (B) Representative images and quantification of inflammatory infiltrate (INFLAM) in histological cross-sections of tibialis anterior muscles stained with hematoxylin and eosin. Muscles were treated at each time point via scaffold delivery (Scaffold) or left untreated (No Treat). Treatments included placement of the scaffold immediately after induction of ischemia (Day 0–4), 6 days after induction of ischemia (Day 6–10), or 10 days after induction of ischemia (Day 10–14) followed by stimulation for four consecutive days. Analysis was always performed 4 days after initiation of each treatment. (C) and (D) Representative images and quantification of collagen deposition, as assessed from picrosirius red stained cross-sections, with the same treatments and time of analysis. All scale bars represent 200 µm. Values represent the mean and standard deviation (n = 5). Data in B and Figure S4B, as well as data in D and Figure S4D were compared using ANOVA with Bonferroni's post-hoc test (*p < 0.05, **p < 0.01).
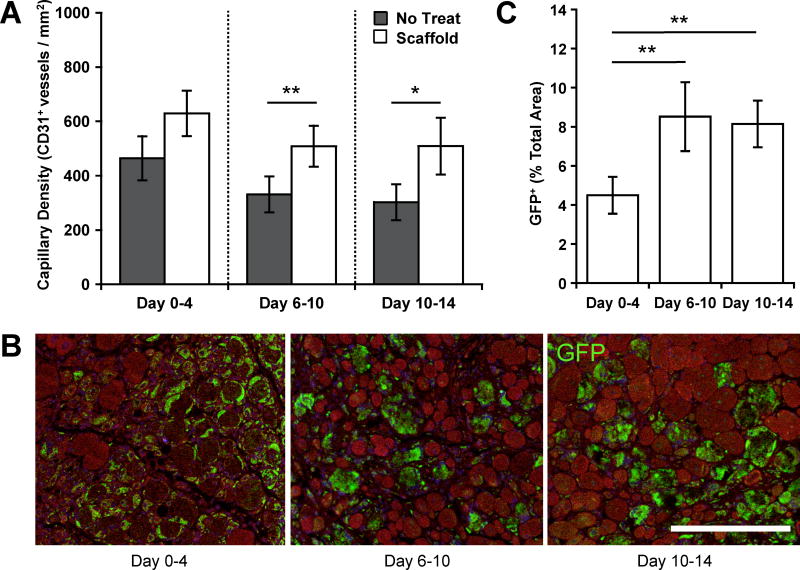
The effects of temporally controlled delivery of myogenic cells and morphogens on angiogenesis and muscle regeneration at 4 days after the start of each treatment time were also determined. At this early time point, statistically significant increases in the average muscle capillary density compared to the no treatment control, as measured by immunostaining for the endothelial cell marker CD31, were only observed for delayed treatments initiated at day 6 and day 10 post-injury (Figure 3A). The persistence of the transplanted cells was also measured but differed significantly between immediate and delayed treatment conditions. Following immediate scaffold treatment, abundant GFP positive cells were found surrounding damaged myofibers, while few GFP positive fibers were noted (Figure 3B–C). In contrast, scaffold treatments starting at day 6 and day 10 resulted in abundant GFP positive fibers with fewer single myoblasts (Figure 3B–C).
Figure 3.
Timing of therapy modulates early angiogenesis and transplanted cell engraftment efficiency. (A) Muscle capillary densities, as assessed by CD31+ staining of tissue samples 4 days after initiation of scaffold treatment. (B) and (C) Representative images and quantification of the percentage of donor GFP myoblasts and newly formed GFP myofibers 4 days after initiation of scaffold treatment in histological cross-sections of tibialis anterior muscles. GFP donor cells remaining as single cells and GFP myofibers were quantified together. Scale bar represents 100 µm. Values represent the mean and standard deviation (n = 5). Data in A and Figure S5A, as well as data in C and Figure S5C were compared using ANOVA with Bonferroni's post-hoc test (*p < 0.05, **p < 0.01).
Timed delivery of therapy was investigated further using cell injections timed with injury-induced inflammation. In general, results from injections mirrored those obtained with scaffold-based cell delivery, but benefits were reduced and at times not statistically significant when compared to no treatment controls (Figure S4–S5). As a result, long-term studies were completed using the scaffold-based delivery system.
2.3 Impact of Timing of Therapy on Long-term Muscle Regeneration
In order to determine the long-term impact of therapy timing on muscle regeneration, animals were analyzed 6 weeks post-injury. Similar to results of the analysis 4 days post-treatment, quantification of the inflammatory infiltrate revealed significantly reduced inflammation in muscles treated with scaffolds starting at day 0 and day 6, when compared to no treatment controls and scaffold treatment starting at day 10 (Figure 4A, C). In addition, muscle fibrosis was significantly reduced following scaffold treatment starting at day 6, as compared to no treatment or other treatment times (Figure 4B, D). Differences in tissue perfusion were also observed, as treatments starting at day 0 and day 6 led to significantly greater recovery following ischemic injury than achieved with no treatment or scaffold treatment at day 10 (Figure 4E). Strikingly, scaffold treatment starting at day 6 during peak inflammation resulted in a significantly enhanced muscle contractile force (2800 µN/mg) when compared to conditions involving no treatment, immediate treatment starting at day 0, and scaffold treatment starting at day 10 (Figure 4F).
Figure 4.
Long-term functional muscle regeneration results from appropriate timing of therapy. (A) and (C) Representative images and quantification of inflammatory infiltrate in histological cross-sections of tibialis anterior muscles stained with hematoxylin and eosin 6 weeks post-injury. (B) and (D) Representative images and quantification of collagen deposition from picrosirius red stained cross-sections 6 weeks post-injury. (E) Perfusion of injured hindlimbs, normalized to uninjured contralateral controls, as measured by Laser doppler perfusion imaging (LDPI). (F) Maximum contractile force following tetanic stimulation, 6 weeks post-injury of intact muscles receiving no treatment or scaffold treatment at day 0, 6, or 10. All scale bars represent 500 µm. Values represent the mean and standard deviation (n = 6). Data in C–F were compared using ANOVA with Bonferroni's post-hoc test (*p < 0.05, **p < 0.01, ***p < 0.001).
3. Discussion
This study investigates the timing of cell therapy with respect to specific phases of injury-induced inflammation and its affect on muscle regeneration using a scaffold-based delivery system. In order to identify time points for introduction of cell therapy before, during, and after peak inflammation, the kinetics of inflammation arising from the combined muscle injury model used in this study were determined. Following the subsidence of notexin-induced inflammation, induction of ischemia led to a wave of inflammation that peaked over the following 3–7 days. Previous reports have demonstrated similar results with severe tissue hypoxia and hypoxia-induced inflammation following femoral artery ligation.[36] Specifically, dramatic increases in intramuscular immune cell presence from day 2 to day 9[37] and in blood vessel permeability in hypoxic tissues from day 1 to day 7 were observed following the procedure.[38] These results are consistent with the kinetics of inflammation reported in this study and used to generate time points for introduction of therapy. In order to allow externally triggered release during distinct phases of the injury response, a ferrogel system capable of on-demand release of cells and growth factors was used.[34, 35] While it is expected that a majority of cells were released during ferrogel stimulation based on previous reports, it is possible that migration of non-expelled cells to the injury site occurred randomly or in response to cytokine release by the injured muscle or invading inflammatory cells.
During the acute phase of regeneration, delivery of therapy initiated at the peak of the inflammatory response proved most beneficial, as evidenced by a combination of increased acute engraftment of transplanted cells, reduced inflammation and fibrosis, and enhanced angiogenesis. Treatment initiated immediately after injury (day 0 initiation) or after inflammation had subsided (day 10 initiation) provided benefit, but not as broadly. Delaying scaffold treatment until the peak of inflammation (day 6 initiation) likely diminished donor cell contact with invading neutrophils and the highly inflammatory microenvironment present early in muscle repair. It is widely known that neutrophils induce muscle membrane damage through the release of myeloperoxidase (MPO) and target tissue debris for phagocytosis through the production of free radicals.[39] These substances are deleterious to the health of donor cells and likely reduce the regenerative benefit of cells delivered immediately upon injury. In addition to avoiding early neutrophil infiltration, scaffold treatment timed to peak inflammation may have allowed donor cells maximal contact with specific populations of invading macrophages known to enhance the regenerative capacity of myogenic cells.[31] Cell delivery initiated at day 6 overlaps with peak M2 macrophage presence, and their secretion of anti-inflammatory cytokines has been shown to dramatically enhance satellite cell differentiation.[27–29] M1 macrophages which have been shown to secrete pro-inflammatory cytokines that enhance satellite cell proliferation and motility also remain present at this time, but at a greatly reduced level when compared to peak presence at day 3.[27–30] In this study, the most potent and preferable regenerative response correlated most strongly with M2 macrophage presence. In contrast, delaying scaffold treatment to day 10 diminishes cell therapy overlap with M1 macrophages and decreases overlap with M2 macrophages, potentially leading to minimal donor cell interactions with these inflammatory cell types. Cells transplanted at this time may successfully engraft and contribute to myofiber formation but perhaps without significant proliferation.
Furthermore, scaffold delivery initiated at the peak of inflammation demonstrates a striking combination of reduced inflammation and fibrosis with enhanced angiogenesis, possibly resulting from timing of growth factor delivery consistent with the kinetics of endogenous growth factor expression following muscle injury. It has been previously reported that IGF expression is transiently induced during regeneration following hindlimb ischemia with substantial induction from day 1 to day 10.[40] Interestingly, expression of supplemental IGF in transgenic mice has been previously shown to accelerate muscle regeneration by modulating the inflammatory response and reducing fibrosis, consistent with results obtained following treatment initiation at day 6 in this study.[41] Similarly, previous work detailing the kinetics of VEGF shows significant protein detection following induction of ischemia in activated satellite cells at 3 days and in regenerating fibers at 7 days, with a significant reduction by day 14, suggesting angiogenic benefits exhibited following delayed therapy delivery may be associated with appropriately timed VEGF delivery.[42] Overall, these results demonstrate the importance of temporally controlled delivery on the acute effects of delivered cells and factors.
Over the long-term, treatment delayed to coincide with the peak of inflammation substantially enhanced muscle regeneration, as indicated by all metrics analyzed. In addition to reduced inflammation and fibrosis, enhancements in the functional metrics of muscle contractile force and tissue perfusion were observed. Treatment initiated immediately after injury provided some benefit, although reduced, while treatment delayed until 10 days had no long-term impact on muscle regeneration. Surprisingly, relatively few studies have investigated the optimal time-point for cell transplantation by either injection or scaffold delivery to acutely injured muscle or other tissues. Despite infrequent investigations, the data from this study is consistent with past reports that concluded treatment initiated at an intermediate time point between early and late stage inflammation provides the most potent regenerative benefit. For example, one study examined the impact at 4 weeks of timed delivery of muscle-derived stem cells (MDSCs) to contusion-injured muscle and found that MDSC transplantation during the acute inflammatory response (4 days post-injury) led to reduced fibrosis, enhanced angiogenesis, and greater functional recovery as compared to immediate (1 day post-injury) and late (7 days post-injury) delivery.[43] Similar results have also been obtained following inflammation-timed cell delivery in the cardiac and neural regeneration fields. Cardiomyocyte transplantation to cryoinjured cardiac tissue provided the most functional benefit following delivery via intracardial injection 2 weeks post-injury rather than immediately or 4 weeks post-injury.[44] The optimal time point of 2 weeks was identified as subsequent to early inflammation but prior to fibrous tissue formation and ventricular remodeling. In addition, the importance of timing of neural stem cell (NSC) delivery following peripheral nerve injury was examined in the context of preventing skeletal muscle atrophy.[45] Consistent with results reported here, the optimal time for NSC transplantation was found to be 1 week following nerve injury, as cells transplanted immediately after injury suffered massive death attributed to the hostile microenvironment associated with acute inflammation, and cells transplanted later did not efficiently engraft. In general, these results suggest that the suitability of the wound microenvironment for cell engraftment and tissue repair may be optimal between early inflammation and extensive fibrous tissue formation, regardless of cell type and tissue injury.
4. Conclusions
This study demonstrates the importance of temporal control over biologic delivery in the context of skeletal muscle regeneration. Delaying the delivery of therapy so that it coincided with the peak of injury-induced inflammation led to potent early and long-term regenerative benefits. In contrast, treatments initiated before the start of the acute inflammatory stage or after its decline had a more limited impact on muscle repair. Due to their ability to noninvasively trigger drug and cell release,[34, 35] ferrogels and other stimuli-responsive scaffolds are likely to be highly useful for future investigations and clinical studies exploring the importance of the timing of therapeutic strategies. Although the optimal timing is likely to vary with cell type and injury, the concept of inflammation-timed delivery using biomaterial scaffolds is of potential wide utility for tissue engineering and regenerative medicine.
5. Experimental Section
Materials
Medical grade, high molecular weight (~250 kDa) sodium alginate with high guluronate content (Protanal LF 20/40) was purchased from FMC Biopolymers (Oslo, Norway). Alginates were used following covalent RGD modification and dialysis purification, as previously described.[46] All other chemicals including adipic acid dihydrazide (AAD), 1-ethyl-3-(dimethylaminopropyl) carbodiimide (EDC), MES, 1-hydroxybenzotriazole (HOBT), and Iron(II,III) oxide powder (<5 µm, 310069) were purchased from Sigma–Aldrich (St. Louis, MO).
Muscle Injury Model
All animal work was performed in compliance with NIH and institutional guidelines. Six-week-old female wild-type C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were anesthetized with an intraperitoneal injection of ketamine (80 mg kg-1) and xylazine (5 mg kg-1). For myotoxin injuries, the tibialis anterior muscles of the right legs of anesthetized mice were injected with 10 µl of 10 µg/ml Notexin Np myotoxin from Notechis scutatus snake venom (Latoxan) using a 25 µl Hamilton syringe. Notexin is a potent phospholipase A2 toxin known to cause complete myofiber breakdown and loss of muscle functional capacity.[47] Six days after notexin injection, hindlimb ischemia was induced by unilateral external iliac and femoral artery and vein ligation, as previously described.[48, 49]
Inflammation Probe
Following notexin and ischemia injury, the kinetics of the inflammatory response were assessed using a near-IR probe (760536, Caliper Life Sciences) and an in vivo imaging system (IVIS Spectrum, Perkin Elmer, Waltham, MA). Briefly, mice were anesthetized and injected with the probe by retro-orbital injection. Periodically following injury, whole body images were acquired for a duration of 25 seconds using an ex/em of 745/800 nm and analyzed using Living Image software (Perkin Elmer). The extent of inflammation is related to the amount of dye accumulating at the ischemic site, which is increased due to the enhanced permeability of blood vessels in hypoxic tissues.[38, 50, 51] Values were expressed as the ratio of signals attained from the injured to the uninjured contralateral limb of the same animal. Inflammation probe results were then verified through histological assessment of the inflammatory infiltrate.
Primary myoblast isolation
Primary myoblasts were isolated from 6 week old, female, transgenic mice with a C57BL/6J background (C57BL/6-Tg(ACTb-EGFP)1Osb/J, Jackson Laboratories, Bar Harbor, ME) constitutively expressing GFP in all cells. Cells were isolated from cardiotoxin (C9759, Sigma) pre-injured hindlimbs three days post-injury rather than from resting muscle to maximize cell yield, as previously described.[52, 53] Briefly, tibialis anterior and gastrocnemius muscles were harvested, incubated in a collagenase solution (250 U/ml in DMEM), and triturated with a Pasteur pipet to disrupt muscle fibers. Following centrifugation at 300g, muscle fibers were incubated in a collagenase-dispase solution (250 U/ml-10 U/ml in F10 media) and vortexed to release muscle-derived precursors. The cells were then filtered through a 40 µm filter, collected via centrifugation at 500g, and plated on collagen-coated culture dishes (BioCoat Collagen I, BD Biosciences). Primary myoblasts were cultured for 3–4 days prior to collection for characterization and animal studies.
Ferrogel Scaffold Implantation and Stimulation
Biphasic ferrogel scaffolds (7 wt% iron oxide) were fabricated as previously described using RGD-modified alginate to ensure adequate retention of cells before initiation of triggered release.[34, 35] Prior to implantation, ferrogels were rehydrated with 50 µl of DMEM containing 4 × 105 GFP primary myoblasts, 3 µg IGF, and 3 µg VEGF. The scaffolds were placed subcutaneously on the tibialis anterior muscle 0, 6, or 10 days following induction of ischemia. Scaffold placement was expected to minimally impact the significant inflammation induced by the injury as it was performed without muscle tissue manipulation. Biphasic ferrogels were then stimulated for 2 min at 1 Hz every 24 hours for 4 days to ensure more complete release of cells and growth factors from the scaffolds. Stimulation was carried out by approaching and retracting a stack of permanent magnets with a surface field of ~6600 Gauss (K&J Magnetics, D78). The magnet and ferrogel were oriented so that stimulation resulted in ferrogel compression against the injured muscle during application of the magnetic field (~1700 Gauss at the implant site). For future use in large animals and humans, it may be necessary to change the orientation of the magnet and ferrogel so that stimulation results in ferrogel compression against the skin. Due to the rapid decay of field strength with distance from the magnet source, a stronger magnet (e.g. MRI scanner) may also be needed.
Ischemia and Perfusion Analysis
Blood perfusion measurements of ischemic and normal limbs were performed on anesthetized animals (n = 5–6 per condition) using a Laser Doppler Perfusion Imaging (LDPI) analyzer (PeriScan PIM II, Perimed Instruments, Ardmore, PA). Entire hindlimbs were scanned at 2 d and every week following surgery. Perfusion was calculated as the ratio of ischemic to non-ischemic limb perfusion for each animal.
Muscle Function Testing
Intact tibialis anterior muscles were dissected (n = 5–6 per condition) and mounted vertically midway between two cylindrical parallel steel wire electrodes (1.6 mm diameter, 21 mm long), attached by their tendons to microclips connected to a force transducer (FORT 25, WPII). The muscles were bathed in a physiologic saline solution in a chamber with continuously bubbled oxygen at 37°C, and muscle length was adjusted to a physiological relevant length. A wave pulse was then initiated using a custom-written LabVIEW program and delivered to the stimulation electrodes via a purpose-built power amplifier (QSC USA 1310). Contractions were evoked every 5 min. Tetani was evoked at 250–300 Hz and 25–30 V, with a constant pulse width of 2 ms and a train duration of 1 s. Peak tetanic force was determined as the difference between the maximum force during contraction and the baseline level. Forces were then normalized to muscle wet weight.
Histological Assessment of Skeletal Muscles and Ferrogel Scaffolds
Mice were sacrificed and hindlimb muscle tissues (n = 5–6 per condition) and ferrogel scaffolds were processed separately for histological analyses at 4 days post-treatment and 6 weeks post-injury. Briefly, tibialis anterior muscles were fixed in 10% neutral buffered formalin overnight, paraffin embedded, sectioned at 7 µm thickness, and stained with hematoxylin and eosin (H&E) at the Harvard Rodent Histopathology Core. Sections stained with H&E were used for quantification of the inflammatory infiltrate, defined as hematoxylin positive cells located in the interstitial space between muscle fibers. GFP donor cells and myofibers were identified with immunostaining for mouse GFP (Abcam ab6556; Invitrogen A-11035). Vascular endothelial cells were identified by immunostaining for mouse CD31 (Abcam ab28364; Invitrogen A-11035), and capillaries were defined as CD31+ round or oval structures adjacent to myofibers. Intramuscular collagen content was assessed in picrosirius red stained sections imaged with polarized light. Quantification of inflammation, collagen, CD31, and GFP immunostaining was performed using ImageJ.
Statistical Analyses
All statistical comparisons were performed using ANOVA with Bonferroni’s post-hoc test. INSTAT 3.1a software (GraphPad Software, San Diego, CA) was used for analysis. Differences between conditions were considered significant if p < 0.05.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 DE013349), National Science Foundation Graduate Student Fellowship to CC (DMR-0820484), and the Materials Research Science and Engineering Center (MRSEC) at Harvard University. The authors would like to thank Dr. Roderick Bronson of the Harvard Rodent Histopathology Core for his pathological assessment of histology sections.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Christine A. Cezar, Harvard School of Engineering and Applied Sciences, 29 Oxford Street, Cambridge, MA, 02138, USA Wyss Institute for Biologically Inspired Engineering, 60 Oxford Street, Suite 403, Cambridge, MA, 02138, USA.
Dr. Praveen Arany, University at Buffalo Department of Oral Biology, School of Dental Medicine, 3435 Main Street, Buffalo, NY 14260, USA
Sarah A Vermillion, Harvard School of Engineering and Applied Sciences, 29 Oxford Street, Cambridge, MA, 02138, USA; Wyss Institute for Biologically Inspired Engineering, 60 Oxford Street, Suite 403, Cambridge, MA, 02138, USA.
Dr. Bo Ri Seo, Harvard School of Engineering and Applied Sciences, 29 Oxford Street, Cambridge, MA, 02138, USA Wyss Institute for Biologically Inspired Engineering, 60 Oxford Street, Suite 403, Cambridge, MA, 02138, USA.
Prof. Herman H. Vandenburgh, Brown University Department of Pathology and Lab Medicine, Providence, RI, 02912, USA
Prof. David J. Mooney, Harvard School of Engineering and Applied Sciences, 29 Oxford Street, Cambridge, MA, 02138, USA Wyss Institute for Biologically Inspired Engineering, 60 Oxford Street, Suite 403, Cambridge, MA, 02138, USA.
References
- 1.Tedesco FS, Cossu G. Curr. Opin. Neurol. 2012;25:597. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- 2.Palmieri B, Tremblay JP, Daniele L. Pediatr. Transplant. 2010;14:813. doi: 10.1111/j.1399-3046.2010.01377.x. [DOI] [PubMed] [Google Scholar]
- 3.Smythe GM, Hodgetts SI, Grounds MD. Mol. Ther. 2000;1:304. doi: 10.1006/mthe.2000.0049. [DOI] [PubMed] [Google Scholar]
- 4.Fan Y, Maley M, Beilharz M, Grounds M. Muscle Nerve. 1996;19:853. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Stem Cells. 2012;30:1971. doi: 10.1002/stem.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Science. 2005;309:2064. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 7.Cezar CA, Mooney DJ. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Cell. 2008;134:37. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Science. 2010;329:1078. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Nature. 2008;456:502. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Cell. 2005;122:289. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Parker MH, Loretz C, Tyler AE, Duddy WJ, Hall JK, Olwin BB, Bernstein ID, Storb R, Tapscott SJ. Stem Cells. 2012;30:2212. doi: 10.1002/stem.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall JK, Banks GB, Chamberlain JS, Olwin BB. Sci. Transl. Med. 2010;2:57ra83. doi: 10.1126/scitranslmed.3001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, Reggiani C, Vitiello L, Elvassore N, De Coppi P. FASEB J. 2011;25:2296. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 15.Beier JP, Stern-Straeter J, Foerster VT, Kneser U, Stark GB, Bach AD. Plast. Reconstr. Surg. 2006;118:1113. doi: 10.1097/01.prs.0000221007.97115.1d. [DOI] [PubMed] [Google Scholar]
- 16.Fuoco C, Salvatori ML, Biondo A, Shapira-Schweitzer K, Santoleri S, Antonini S, Bernardini S, Tedesco FS, Cannata S, Seliktar D, Cossu G, Gargioli C. Skeletal muscle. 2012;2:24. doi: 10.1186/2044-5040-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandyopadhyay B, Shah V, Soram M, Viswanathan C, Ghosh D. Biotechnol. Prog. 2013;29:197. doi: 10.1002/btpr.1665. [DOI] [PubMed] [Google Scholar]
- 18.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. Biomaterials. 2011;32:8905. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchentouf M, Benabdallah BF, Rousseau J, Schwartz LM, Tremblay JP. Am. J. Transplant. 2007;7:1491. doi: 10.1111/j.1600-6143.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 20.Boontheekul T, Kong HJ, Hsiong SX, Huang YC, Mahadevan L, Vandenburgh H, Mooney DJ. Faraday Discuss. 2008;139:53. doi: 10.1039/b719928g. [DOI] [PubMed] [Google Scholar]
- 21.Rowley JA, Mooney DJ. J. Biomed. Mater. Res. 2002;60:217. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 22.Salimath AS, Garcia AJ. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1881. [DOI] [PubMed] [Google Scholar]
- 23.Guerette B, Asselin I, Vilquin JT, Roy R, Tremblay JP. Muscle Nerve. 1995;18:39. doi: 10.1002/mus.880180107. [DOI] [PubMed] [Google Scholar]
- 24.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Exerc. Sport Sci. Rev. 2009;37:18. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 25.Kharraz Y, Guerra J, Mann CJ, Serrano AL, Munoz-Canoves P. Mediators Inflamm. 2013;2013:491497. doi: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B. FEBS J. 2013;280:4118. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- 27.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Stem Cells. 2013;31:384. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 28.Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, Viollet B, Chazaud B. Cell Metab. 2013;18:251. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. J. Exp. Med. 2007;204:1057. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak ML, Weinheimer-Haus EM, Koh TJ. J. Pathol. 2014;232:344. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bencze M, Negroni E, Vallese D, Yacoub-Youssef H, Chaouch S, Wolff A, Aamiri A, Di Santo JP, Chazaud B, Butler-Browne G, Savino W, Mouly V, Riederer I. Mol. Ther. 2012;20:2168. doi: 10.1038/mt.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesault PF, Theret M, Magnan M, Cuvellier S, Niu Y, Gherardi RK, Tremblay JP, Hittinger L, Chazaud B. PloS One. 2012;7:e46698. doi: 10.1371/journal.pone.0046698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thepenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon JM, Tajbakhsh S, Rocheteau P, Chretien F. PloS One. 2016;11:e0147198. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ. Proc. Natl. Acad. Sci. U. S. A. 2011;108:67. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cezar CA, Kennedy S, Mehta M, Weaver JC, Gu L, Vandenburgh H, Mooney D. Adv. Healthcare Mater. 2014 doi: 10.1002/adhm.201400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltzschig HK, Carmeliet P. N. Engl. J. Med. 2011;364:656. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson EM, Kwee BJ, Lewin SA, Raimondo T, Mehta M, Mooney DJ. Tissue engineering. Part A. 2015;21:1217. doi: 10.1089/ten.tea.2014.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ. Nano Lett. 2011;11:694. doi: 10.1021/nl103812a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen HX, Lusis AJ, Tidball JG. J. Physiol. 2005;565:403. doi: 10.1113/jphysiol.2005.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L. Physiol. Genomics. 2002;11:263. doi: 10.1152/physiolgenomics.00110.2002. [DOI] [PubMed] [Google Scholar]
- 41.Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musaro A. FASEB J. 2007;21:1393. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- 42.Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC. Am. J. Pathol. 2003;163:1417. doi: 10.1016/S0002-9440(10)63499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, Fu FH, Huard J. Am. J. Sports Med. 2011;39:1912. doi: 10.1177/0363546511415239. [DOI] [PubMed] [Google Scholar]
- 44.Li RK, Mickle DA, Weisel RD, Rao V, Jia ZQ. Ann. Thorac. Surg. 2001;72:1957. doi: 10.1016/s0003-4975(01)03216-7. [DOI] [PubMed] [Google Scholar]
- 45.Lin S, Xu L, Hu S, Zhang C, Wang Y, Xu J. Muscle Nerve. 2013;47:194. doi: 10.1002/mus.23447. [DOI] [PubMed] [Google Scholar]
- 46.Rowley JA, Madlambayan G, Mooney DJ. Biomaterials. 1999;20:45. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 47.Plant DR, Colarossi FE, Lynch GS. Muscle Nerve. 2006;34:577. doi: 10.1002/mus.20616. [DOI] [PubMed] [Google Scholar]
- 48.Chen RR, Silva EA, Yuen WW, Brock AA, Fischbach C, Lin AS, Guldberg RE, Mooney DJ. FASEB J. 2007;21:3896. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 49.Silva EA, Mooney DJ. J. Thromb. Haemost. 2007;5:590. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 50.Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Am. J. Physiol. 1999;277:L1057. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- 51.Detmar M, Brown LF, Berse B, Jackman RW, Elicker BM, Dvorak HF, Claffey KP. J. Invest. Dermatol. 1997;108:263. doi: 10.1111/1523-1747.ep12286453. [DOI] [PubMed] [Google Scholar]
- 52.Cousin W, Ho ML, Desai R, Tham A, Chen RY, Kung S, Elabd C, Conboy IM. PloS One. 2013;8 doi: 10.1371/journal.pone.0063528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conboy MJ, Conboy IM. Methods Mol. Biol. 2010;621:149. doi: 10.1007/978-1-60761-063-2_10. [DOI] [PubMed] [Google Scholar]
- 54.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Lab animal. 2011;40:155. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.