Abstract
Background
Intestinal schistosomiasis caused by Schistosoma mansoni is a wide spread disease in most parts of Ethiopian highlands. Snail control is one major strategy in schistosomiasis control. The use of molluscicidal plant products is becoming interesting due to their environmental friendliness, accessibility and easy application. This research is aimed to evaluate the molluscicidal effect of Achyranthes aspera on Biomphalaria pfeifferi and Lymnaea natalensis snails, which are of great medical and veterinary importance in Ethiopia.
Methods
Adult B. pfeifferi snails were exposed to the various concentrations of A. aspera aqueous leaf extract for 24, 48 and 72 h. Similarly, adult L. natalensis snails were exposed to the extract for 24 h. Mortality data were analyzed using probit regression model. Phytochemical content of the plant was analyzed using standard screening methods.
Results
The plant’s molluscicidal effect on the two snail species was demonstrated. The 24 h LC50 and LC90 values against L. natalensis were 69.5 and 93.9 ppm respectively. In the 24, 48 and 72 h exposure of B. pfeifferi, the LC50 values were 72.4, 69.9, 64.7 ppm and the LC90 were 96.5, 93.8, 92.8 ppm, respectively. The phytochemical screening tests indicated presence of saponins.
Conclusion
From the findings of this study, A. aspera has a molluscicidal potential. The result provides a useful foundation for further in-depth studies to ensure its wider applicability in different water bodies and evaluate its toxic effects on non-target species.
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-017-0349-4) contains supplementary material, which is available to authorized users.
Keywords: Achyrantes aspera, Ethiopia, Snail control, Plant molluscicides
Multilingual abstract
Please see Additional file 1 for traslations of the abstract into the five official working languages of the United Nations.
Background
Schistosomiasis is a widespread parasitic disease endemic to over 74 countries infecting more than 200 million people in Africa, South America and Asia [1–3]. About 85% of the infection is found in Sub-Saharan Africa [4]. In Ethiopia, the snail Biomphalaria pfeifferi is the principal intermediate host of Schistosoma mansoni [1, 5]. The other species, Lymnaea natalensis is responsible for Fasciola, which is of great veterinary importance. Intestinal schistosomiasis is prevalent in the country, especially in the range of 1300 to 2000 m altitudes [6].
In addition to chemotherapy, integrated snail control strategies are important measures in schistosomiasis control especially in low endemic areas [7]. Currently, synthetic molluscicides are disregarded due to their high cost and environmental issues [8]. Plant products are becoming more and more interesting alternatives for their wide range of ideal properties including biodegradability and target specificity. As a result, molluscicidal plant researches are becoming so popular that many plant species have been screened [8, 9]. According to recent studies, Glinus lotoides [7] and Jatropha gossypiifolia [10] were found to be effective molluscicidal plants. In spite of a high botanical biodiversity potential and a variety of traditionally claimed medicinal plants, such studies in Ethiopia are limited [11, 12].
Achyranthes aspera (Family Amaranthaceae) is locally known as “Telenge” or “Ambulale” in many parts of Ethiopia. It is an esteemed medicinal plant in Asia, South America and Africa [13]. In Ethiopian folklore, it is used to control fertility, placental retention, postpartum bleeding, for eruption treatments and wound dressing [14, 15]. Yet, the action of A. aspera on those medically important snails is not known so far. The objective of this study is, therefore, to investigate the molluscicidal property of this plant.
Methods
This is an experimental study designed to investigate the molluscicidal effect of A. aspera aqueous leaf extract on B. pfeifferi and L. natalensis snails in the laboratory condition.
Plant material collection and processing
The plant A. aspera is selected based on the result from a preliminary screening test carried out earlier by the same researchers. Mature green leaves were collected from a place located at 9°43′45.59″ N, 39°73′2.71″ E nearby Debre Berhan Town, central Ethiopia. An expert called Mr. Melaku Wondafrash carried out plant species identification at Addis Ababa University herbarium. Voucher specimen was kept in the herbarium with specimen number: M.B.1. Some 400 g of leaves were dried in the shade to stable weight and ground to fine powder of mesh size about 200 μm according to Ndamukong et al. [4]. This was kept in tight plastic bag labeled and stored in dry and cool place in the laboratory of Environmental Health Science and Technology, Jimma University, Ethiopia.
Snail collection
Adult snails of B. pfeifferi and L. natalensis were collected from stream habitats located at 7°41′15.39″ N, 36°50′52.32″ E in Jimma prison agricultural field, southwestern Ethiopia. They were taken to the same laboratory in a clean bucket with some water from the stream and were acclimatized for 3 days at room temperature, 12 h light and 12 h dark photoperiod in aged tap water. They feed on par-boiled dried shred of lettuce (Lactuca sativa) leaf. In the course of the experiment, the snails were ethically handled in accordance with the principles of animal welfare in scientific experiments.
Aqueous extract and stock solution preparation
Aqueous leaf extract of A. aspera was prepared based on Ndamukong et al. [4]. 1 g plant leaf powder was soaked in 800 ml aged tap water in a flat-bottomed airtight flask and shook for 24 h on an orbital shaker at 110 rpm. It was then reconstituted to 1000 ml and directly used as a stock solution of 1000 ppm as mentioned in Kiros et al. [7].
Test on adult L. natalensis snails
Exactly 60 ml serial dilutions of 25, 50, 75, 100, 200, and 400 ppm were prepared from the stock solution, each in a clean petri dish. Ten adult snails, 9-11 mm shell height, were exposed to each dilution for 24 h at room temperature. The test was performed in three replicates together with negative controls consisting of only aged tap water. Positive control was prepared from a 0.28 ppm niclosamide (Bayer and Pro-Serv. Inc. Germany). This concentration was considered as the LC50 value from the researcher’s previous experiment on the same snail species.
Test on adult B. pfeifferi snails
This experiment was arranged in to three sets of exposure time: 24, 48, and 72 h. Exactly 60 ml test solutions containing 25, 50, 75, 100, 200, and 400 ppm were prepared to each of the three sets. Ten adult snails, 8.5-11 mm shell diameter, were exposed to each dilution. Each set was prepared in three replicates together with controls. Negative control was made from aged water only and positive control was made from 0.28 ppm niclosamide (for 50% mortality).
Snail death recording
Once snails are exposed to the treatments in relation to the respective exposure times, each group of snails were removed, washed with tap water, and kept in aged water with food for another 24 h. Finally, dead snails in each group were counted with thorough inspection. Snails were considered dead if they remain inactive when pocked with a needle or remain retracted in to the shell or else the color of the shell and foot changes to foggy white [4, 7].
Phytochemical analysis
Phytochemical screening tests were done using standard procedures as described in Akinyemi et al. [16] as well as Aiyegoro and Okoh [17]. Reducing sugars were identified by Fehling’s test in which the filtrate was heated with Fehling’s reagent for 5-10 min in water bath. For protein identification, Biuret test was performed by adding 4% sodium hydroxide and 1% copper sulfate solution. In addition, Ninhydrin test was done by heating the extract with 5% Ninhydrin (in butanol) solution in water bath for 10 min. Saponins were identified by formation of persistent foam after vigorous shaking of the aqueous extract. Total phenolics and tannins were tested by 5% and 10% ferric chloride solutions, respectively. Test for flavonoids was by treating the extract with concentrated hydrochloric acid and few pellets of magnesium turning for appearance of tomato red color. On top of that, Wagner’s test was applied for alkaloids using solution of iodine in potassium iodide for a reddish-brown precipitate.
Data analysis
Probit regression analysis in IBM SPSS software version 20 was used to determine the LC50 and LC90 values, which denote effective doses for 50% and 90% mortality respectively. These are the commonly used endpoints in modern dose-response experiments [18–20]. The associated Chi-square (χ 2) values were used to assess the Pearson’s goodness-of-fit for the appropriateness of probit model to this particular data [19].
Results
When B. pfeifferi were exposed to the concentrations of 200 and 400 ppm, they stopped crawling and defecating within the first two or three hours. In the beginning, they profusely secrete mucus and later start bleeding. L. natalensis snails produce less mucus than B. pfeifferi and remain active for longer time up to the eighth hour. Both species were not be able to attach firmly to the surface of petri dishes. Lymnaed snails seemed more irritated that they tried to crawl out of the solution. Snail mortalities in different concentrations and exposure times are presented in Tables 1 and 2.
Table 1.
Mortalities of B. pfeifferi snails exposed to different concentrations of A. aspera aqueous leaf extract for 24, 48 and 72 h
| Concentration (ppm) | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|
| Exposed | Dead | Exposed | Dead | Exposed | Dead | |
| 0 | 30 | 0 | 30 | 0 | 30 | 0 |
| 25 | 30 | 0 | 30 | 0 | 30 | 0 |
| 50 | 30 | 3 | 30 | 4 | 30 | 7 |
| 75 | 30 | 12 | 30 | 15 | 30 | 17 |
| 100 | 30 | 30 | 30 | 30 | 30 | 30 |
| 200 | 30 | 30 | 30 | 30 | 30 | 30 |
| 400 | 30 | 30 | 30 | 30 | 30 | 30 |
Table 2.
Mortalities of L. natalensis snails exposed to different concentrations of A. aspera aqueous leaf extract for 24 h
| Concentration (ppm) | Exposed snails | Dead snails | Mortality rate |
|---|---|---|---|
| 0 | 30 | 0 | 0% |
| 25 | 30 | 0 | 0% |
| 50 | 30 | 3 | 10% |
| 75 | 30 | 17 | 56.7% |
| 100 | 30 | 29 | 93.3% |
| 200 | 30 | 30 | 100% |
| 400 | 30 | 30 | 100% |
In addition to the negative controls made of only aged water, 0.28 ppm niclosamide solution was used as a positive control where, about 50% mortality rates were expected. The resulting mortalities were 56.7% (17 out of 30) and 53.3% (16 out of 30) for B. pfeifferi and L. natalensis, respectively, asserting to the normal experimental condition.
The data in Tables 1 and 2 showed that the plant has molluscicidal property. The noticeable concentration dependent mortalities of the two snail species ranged 25-100 ppm. Snail mortality in mid concentrations (50-75 ppm) also increased with prolonged exposure time. Analysis of effective doses, specifically LC50 and LC90 values that are useful to evaluate the plant’s molluscicidal efficacy are presented in Table 3.
Table 3.
Molluscicidal effect of A. aspera aqueous leaf extract against B. pfeifferi and L. natalensis adult snails in terms of LC50 and LC90 values with 95% confidence limits (CL)
| Snail species | Time | LC50 with CL | LC90 with CL | χ 2 | Slope |
|---|---|---|---|---|---|
| B. pfeifferi | 24 h | 72.4 (66.5-78.1) | 96.5 (88.0-112.6) | 7.264 | 10.247 |
| 48 h | 69.9 (63.5-74.9) | 93.8 (84.9-110.9) | 5.063 | 9.650 | |
| 72 h | 64.7 (58.5-70.7) | 92.8 (82.9-112.7) | 5.092 | 8.184 | |
| L. natalensis | 24 h | 69.5 (63.7-75.2) | 93.9 (85.4-109.8) | 1.021 | 9.824 |
The calculated chi-square (χ 2) values in the analysis are less than the tabular values within the given degree of freedom indicating the mortality counts are not significantly heterogeneous and the goodness-of-fit of the probit model is acceptable. Moreover, the LC50 and LC90 values lie within the 95% confidence limits. As it can be seen from Table 3, for 24 h of exposure, the potency difference of the plant against the two snail species was relatively small. The graph below (Fig. 1) clarifies the trend along the entire LC levels. Yet, B. pfeifferi is somehow less sensitive than L. natalensis especially in concentrations below the LC90 level.
Fig. 1.
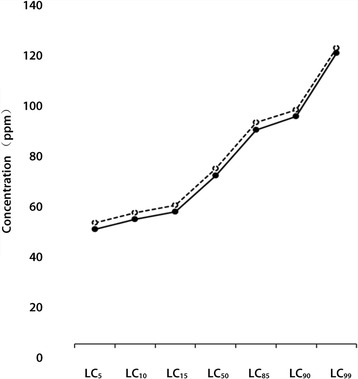
Relative sensitivities of B. pfeifferi and L. natalensis snails to various concentrations of A. aspera aqueous leaf extract. Broken line (-----) = B. pfeifferi, Solid line (_____) = L. natalensis
The phytochemical analysis of the aqueous leaf extract indicated presence of saponins and carbohydrates. However, tannins and other phenolics as well as flavonoids and alkaloids were absent. High saponin content is presumed from the vigorous emergence of persistent foam.
Discussion
In Sub Saharan Africa, schistosomiasis is widely distributed especially in poor rural communities imposing huge socio-economic burden. Hence, community based low cost snail control measures are proved to be feasible. Furthermore, in relatively arid regions like Ethiopia where transmission is usually focal and seasonal, this self-help approach is preferable. Environmentally safe, easily applicable and locally available control agents like molluscicidal plants were recorded to be useful tools to enhance community participation [21]. The cost benefit advantage of such plant products over niclosamide is clearly stated in many literatures [22, 23]. A. aspera is an abundant wild herb in Ethiopia, easily growing on abandoned lands and roadsides. This plant is noncommercial and freely available to local communities without any cost related to transport, storage, expertise and custom. Generally, using such local materials has great economic role in import substitution, income generation to local growers and wasteland utilization.
This particular study showed that A. aspera has molluscicidal effect against B. pfeifferi and L. natalensis snails with the resulting LC50 of 72.4 and 69.5 ppm respectively in 24 h exposure. It is indeed confirmatory to the preliminary screening test we had done previously. In addition, the LC90 values are within the limit set by WHO for plant molluscicidal screening test in which the aqueous extract should be effective in less than 100 ppm [7, 21]. Some toxicological studies indicated the plant has no significant toxic effect in mice [24, 25].
The present phytochemical analysis showed presence of saponins and carbohydrates while tannins, flavonoids, and alkaloids were absent. To the contrary, phytochemical screening test in Bhosale et al. [26] has indicated presence of tannins, flavonoids and alkaloids. Such differences usually arise from variations in extraction techniques and/or parts of the plant used.
According to the present result, saponins are the principal molluscicidal compounds in the aqueous extract of this plant. The molluscicidal property of saponins is well documented in many studies. The main effect on animal cells is formation of complex with plasma and membrane cholesterols causing cell membrane damage [10].
The result shows that, in B. pfeifferi snails, as the exposure time extends from 24 to 72 h, the LC50 decreases from 72.4 to 64.7 ppm. The reason could be the active ingredient in the plant product is released slowly or it remains stable in action for longer time. Exposure to sub lethal levels may also have gradual effect on the snail survival.
The 24 h LC90 lethal dose against B. pfeifferi is 96.5 ppm. This result is nearly similar to the LC90 values (89.50 and 97.55 ppm) of mesocarp and whole fruits of Balanites aegyptiaca that was tested by Molla et al. [27]. However, A. aspera is found to be less potent than other studied plants. For instance, in the work of Kiros and colleagues [7], the aqueous extract of Glinus lotoides fruits produced LC90 of 56.96 ppm.
In the current study, the 24 h LC90 of the plant against L. natalensis was 93.9 ppm indicating higher efficacy on these snails when compared to plants such as Balanites aegyptiaca in Molla et al. [27].
This particular study also showed that the effective doses of the plant against the two snails differ only slightly. According to Utzinger and Tanner [28], the two snail species usually coexist in common habitats. Their similarity in sensitivity to this plant is a useful phenomenon that a single effective dose could be applied for the control of such mixed population altogether.
Conclusions
This is the first evaluation of the molluscicidal effect of A. aspera against two medically important snail species in Ethiopia. The study indicated the aqueous extract is effective at acceptable concentration. The plant is widely available in most parts of Ethiopia and is a well-known traditional medicine. Therefore, this plant can play a role in community based snail control activities through further studies on different snail species and life stages. Effectiveness studies in the field condition and evaluation of toxic effects on non-target organisms are the researchers’ future prospects.
Acknowledgements
Mr. Wuhib, Head, Department of Environmental Health Science and Technology, Jimma University for the provision of laboratory facilities. Mr. Yifrashewa Mengesha for his keen help in collection and maintenance of test snails.
Funding
Jimma University and Dilla University, Ethiopia, have done financial supports for the study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Additional file
Multilingual abstract in the five official working languages of the United Nations. (PDF 815 kb)
Authors’ contributions
BM: Contributed in the conception of the study, carried out the experiment, and wrote the manuscript. STM: Modified the experimental design, supervised the experiment process, and edited the manuscript. AA: Carried out the statistical analysis, organized literatures and edited the final version of the manuscript. YT: Did the phytochemical analysis section and edited the revised manuscript. All authors read and approved the final manuscript.
Ethics approval
The internal review board (IRB) of college of Public Health, Jimma University, has issued ethical clearance for this study.
Consent for publication
Not applicable
Competing interests
All authors of this revised manuscript declare that they have no competing interest regarding this research.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-017-0349-4) contains supplementary material, which is available to authorized users.
Contributor Information
Belayhun Mandefro, Email: belayhunmandefro@gmail.com.
Seid Tiku Mereta, Email: seidtiku@yahoo.com, Email: seid.tiku@ju.edu.et.
Yinebeb Tariku, Email: yinebeb_tariku@yahoo.com.
Argaw Ambelu, Email: aambelu@yahoo.com.
References
- 1.Mengistu M, Shimelis T, Torben W, Terefe A, Kassa T, Hailu A. Human intestinal schistosomiasis in communities living near three rivers of Jimma town, south western Ethiopia. Ethiop J Health Sci. 2011;21(2):111–118. doi: 10.4314/ejhs.v21i2.69051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deribe K, Meribo K, Gebre T, Hailu A, Ali A, Aseffa A, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit Vectors. 2012. doi:10.1186/1756-3305-5-240. [DOI] [PMC free article] [PubMed]
- 3.Mwonga K, Waniki N, Dorcas Y, Piero N. Molluscicidal effects of aqueous extracts of selected medicinal plants from Makueni county. Kenya Pharm Anal Acta. 2015. doi:10.4172/2153-2435.1000445.
- 4.Ndamukong KJN, Ntonifor NN, Mbuh J, Atemnkeng AF, Akam MT. Molluscicidal activity of some Cameroonian plants on Bulinus species. East Afr Med J. 2006;83:102–109. doi: 10.4314/eamj.v83i3.9405. [DOI] [PubMed] [Google Scholar]
- 5.Terefe A, Shimelis T, Mengistu M, Hailu A, Erko B. Schistosomiasis mansoni and soil-transmitted helminthiasis in Bushulo village, southern Ethiopia. Ethiop J Health Dev. 2011;25(1):46–50. doi: 10.4314/ejhd.v25i1.69847. [DOI] [Google Scholar]
- 6.Alebie G, Erko B, Aemero M, Petros B. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region. Ethiopia Parasites & vectors. 2014. doi:10.1186/1756-3305-7-15. [DOI] [PMC free article] [PubMed]
- 7.Kiros G, Erko B, Giday M, Mekonnen Y. Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes. 2014. doi:10.1186/1756-0500-7-220. [DOI] [PMC free article] [PubMed]
- 8.Coelho PMZ, Caldeira RL. Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect Dis Poverty. 2016. doi:10.1186/s40249-016-0153-6. [DOI] [PMC free article] [PubMed]
- 9.Singh SK, Yadav RP, Singh A. Molluscicides from some common medicinal plants of eastern Uttar Pradesh. India J Appl Toxicol. 2010. doi:10.1002/jat.1498. [DOI] [PubMed]
- 10.Pereira Filho AA, França CRC, Oliveira DS, Mendes RJA, Gonçalves JRS, Rosa IG. Evaluation of the molluscicidal potential of hydroalcoholic extracts of Jatropha gossypiifolia Linnaeus, 1753 on Biomphalaria glabrata (say, 1818). Rev Inst Med Trop. 2014; doi:10.1590/S0036-46652014000600009. [DOI] [PMC free article] [PubMed]
- 11.Geyid A, Abebe D, Debella A, Makonnen Z, Aberra F, Teka F, et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J Ethnopharmacol. 2005. doi:10.1016/j.jep.2004.08.021. [DOI] [PubMed]
- 12.Belayneh A, Asfaw Z, Demissew S, Bussa N. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda. Eastern Ethiopia J Ethnobiol Ethnomed. 2012. doi:10.1186/1746-4269-8-42. [DOI] [PMC free article] [PubMed]
- 13.Kamalakannan S, Murugan K, Barnard DR. Toxicity of Acalypha indica (Euphorbiaceae) and Achyranthes aspera (Amaranthaceae) leaf extracts to Aedes aegypti (Diptera: Culicidae). J Asia Pac Entomol. 2011. doi:10.1016/j.aspen.2010.11.011.
- 14.Giday M, Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J Ethnopharmacol. 2010. doi:10.1016/j.jep.2010.07.046. [DOI] [PubMed]
- 15.Kalayou S, Haileselassie M, Gebre-egziabher G, Tiku’e T, Sahle S, Taddele H, et al. In-vitro antimicrobial activity screening of some ethno veterinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray region. Ethiopia Asian Pac J Trop Biomed. 2012. doi:10.1016/S2221-1691(12)60088-4. [DOI] [PMC free article] [PubMed]
- 16.Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA. Screening of crude extracts of six medicinal plants used in south-west Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med. 2005. doi:10.1186/1472-6882-5-6. [DOI] [PMC free article] [PubMed]
- 17.Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in-vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010. doi:10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed]
- 18.Finney D. Probit Analysis. 3rd Ed. New York: Cambridge University Press; 1971.
- 19.Weber C. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Cincinnati: Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency; 1991.
- 20.Otieno G, Waititu GA, Salifu D. Generalized estimating equations for repeated measures logistic regression in mosquito dose-response. OJS. 2013;3:293–298. doi: 10.4236/ojs.2013.35034. [DOI] [Google Scholar]
- 21.Hammami H, Mezghani-Jarraya R, Damak M, Ayadi A. Molluscicidal activity of various solvent extracts from Solanum nigrum Var. Villosum L. aerial parts against Galba truncatula. Parasite. 2011;18(1):63–70. doi: 10.1051/parasite/2011181063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira AR, McCue C, Gerwick WH. Cyanolide a, a glycosidic macrolide with potent molluscicidal activity from the Papua New Guinea cyanobacterium Lyngbya bouillonii. J Nat Prod. 2010. doi:10.1021/np900812873(2):217. [DOI] [PMC free article] [PubMed]
- 23.Rawani A, Ghosh A, Chandra G. Laboratory evaluation of molluscicidal & mosquito larvicidal activities of leaves of Solanum nigrum L. Indian J Med Res. 2014;140(2):285–295. [PMC free article] [PubMed] [Google Scholar]
- 24.Kartik R, Rao CV, Trivedi SV, Pushpangadan P, Reddy GD. Amelioration effects against N-nitrosodiethylamine and CCl 4-induced hepatocarcinogenesis in Swiss albino rats by whole plant extract of Achyranthes aspera. Indian J Pharmacol. 2010. doi:10.4103/0253-7613.71921. [DOI] [PMC free article] [PubMed]
- 25.Barua CC, Talukdar A, Begum SA, Borah P, Lahkar M. Anxiolytic activity of methanol leaf extract of Achyranthes aspera Linn in mice using experimental models of anxiety. Indian J Pharmacol. 2012. doi:10.4103/0253-7613.91869. [DOI] [PMC free article] [PubMed]
- 26.Bhosale UA, Yegnanarayan R, Pophale P, Somani R. Effect of aqueous extracts of Achyranthes Aspera Linn. On experimental animal model for inflammation. An sci life. 2012. doi:10.4103/0257-7941.107362. [DOI] [PMC free article] [PubMed]
- 27.Molla E, Giday M, Erko B. Laboratory assessment of the molluscicidal and cercariacidal activities of Balanites aegyptiaca. Asian Pac J Trop Biomed. 2013. doi:10.1016/S2221-1691(13)60132-X. [DOI] [PMC free article] [PubMed]
- 28.Utzinger J, Tanner M. Microhabitat preferences of Biomphalaria pfeifferi and Lymnaea natalensis in a natural and a manmade habitat in southeastern Tanzania. Mem Inst Oswaldo Cruz Rio Jan. 2000;95:287–294. doi: 10.1590/S0074-02762000000300002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


