Abstract
We report the case of a 65-year-old woman who presented with a 1-month history of progressive paraparesia associated with a thoracic lesion with irregular ring-like gadolinium enhancement. Biopsy of the lesion confirmed the demyelinating origin and brain magnetic resonance imaging showed additional lesions demonstrative of dissemination in space. Immunomodulatory therapy with glatiramer acetate (GA) was started after having a second relapse 2 months later. Shortly after initiation, the patient developed acute hepatitis. Liver function tests returned to normal values 5 months after discontinuation and the patient was diagnosed with drug-induced liver injury (DILI) associated with GA. A literature review identified 11 previous cases of GA-related liver injury associated with two specific mechanisms: DILI (seven cases) and autoimmune hepatitis (four cases). Despite the fact that GA hepatic toxicity is uncommon and laboratory monitoring is not required during GA therapy, it should be considered at least in some special conditions such as comorbidities and previous history of DILI associated with other drugs.
Keywords: autoimmune hepatitis, drug-induced liver injury, glatiramer acetate, hepatitis, late onset, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system, which mainly affects young people. Glatiramer acetate (GA) is one of the most longstanding treatments, used for almost two decades as an immunomodulatory therapy for relapsing MS with an excellent safety profile.1–2 It is a mixture of synthetic polypeptides resembling the myelin basic protein and no laboratory monitoring is required during GA therapy.3 However, a few cases of hepatotoxicity have been described.2 We report a new case of hepatotoxicity in a patient with long-onset MS (LOMS) and review the proposed causal mechanisms described in the literature.
Case report
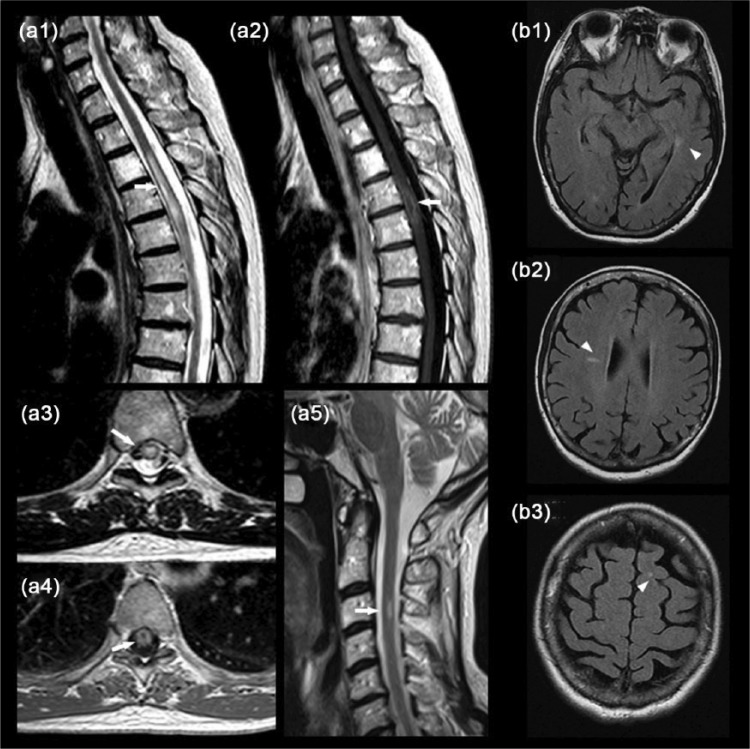
A 65-year-old woman with no relevant medical history presented with a 1-month history of progressive paraparesia and sensory disturbances followed by urinary urgency and the need to use a cane to walk. A spinal cord (SC) magnetic resonance imaging (MRI) scan showed T2 hyperintensity at the T4–T5 level with irregular ring-like gadolinium enhancement [Figure (a1–a4)]. The patient was referred to the Neurosurgery Department for biopsy suspecting an astrocytoma. The biopsy reported areas of demyelination with astrocytosis, and macrophage accumulation, suggestive of a demyelinating origin. A review of the SC MRI showed additional T2 hyperintensity at the C3 level [Figure (a5)] and brain MRI showed at least nine T2 hyperintense lesions without gadolinium enhancement in T1-weighted sequences fulfilling three Barkhof criteria4 [Figure (b1–b3)].
Figure 1.
Spinal cord magnetic resonance imaging (MRI) showed in sagittal sequences T2 hyperintensity at the T4–T5 level (a1, arrow) with irregular ring-like gadolinium enhancement (a2, arrow) and in axial sequences a bright spotty T2 lesion (a3, arrow) with a ring of enhancement (a4, arrow). Small T2 hyperintensity was observed at C3 (a5, arrow) with no gadolinium enhancement (not shown). (b) Brain MRI showed at least nine T2 hyperintensities in Fluid attenuated inversion recovery (FLAIR) sequence in specific locations: periventricular (b1, arrowhead), Dawson finger (b2, arrowhead) and yuxtacortical (b3, arrowhead).
Blood analysis including viral and bacterial serologies and autoimmunity (thyroid, anti-Ro, anti-La, complement, rheumatoid factor and angiotensin-converting enzyme) were normal or negative. Aquaporin-4 immunoglobulin G (IgG) and myelin oligodendrocyte glycoprotein IgG were analyzed by immunohistochemistry and in-house cell-based assay with live HEK293 transfected cells and were negative.5 Visual evoked potentials showed a prechiasmatic dysfunction in the patient’s left eye. The patient was diagnosed with a clinically isolated syndrome and almost completed recovered after treatment with oral dexamethasone 4 mg every 12 h for 5 days. Two months later, she had a second relapse of myelitis and treatment with intravenous methylprednisolone (1 g per day) was initiated. After the first dose, she developed a rash with pruritus suggestive of allergy to methylprednisolone and no further doses were administered. Blood analyses including liver tests were normal and treatment with GA (Copaxone, Teva Pharmaceutical Industries Ltd, Israel; 40 mg injected three times weekly) was started 1 month later. After three injections, she complained of petechial lesions on her ankles. Blood analyses showed a moderate hypertransaminasemia around 10 times the upper limit of normal [alanine transaminase (ALT) 481 units/liter (U/liter); normal values ⩽40 U/liter; aspartate transaminase (AST) 292 U/liter; normal ⩽40 U/liter] with normal parameters of alkaline phosphatase (AP) and γ-glutamyl transferase. Serum bilirubin was normal and there were no criteria of severe acute hepatitis, with normal coagulation tests at diagnosis. GA was stopped and she was referred to the Liver Unit for further evaluation. There was no evidence of metabolic or alcoholic hepatitis, and no abnormalities were observed in hematological panels. Ceruloplasmin, thyroid function and inflammatory markers, including C-reactive protein and γ globulins were normal. Serologic tests for hepatitis virus (A, B, C and D), Epstein–Barr virus and cytomegalovirus were also negative. She tested mildly positive for antinuclear antibody (ANA) with nuclear pattern and antismooth muscle antibody (SMA) (titer 1:40 for both), but no other immunological markers of autoimmune hepatitis (AIH), including antiliver/kidney antibody and antimitochondrial antibody, were found. Human leucocyte antigen (HLA) genotype was DRB1*07 and DRB1*14. Ultrasonography showed some simple hepatic cysts without signs of chronic liver disease or steatosis. Liver test abnormalities increased to maximum levels 1 month later [AST 448 U/liter, ALT 667 U/liter and AP 144 U/liter (normal value 46–116 U/liter)] and returned to normal values 5 months after the discontinuation of GA therapy. Due to the benign clinical course with recovery after GA withdrawal, liver biopsy was not performed. The patient was diagnosed with drug-induced liver injury (DILI), probably related to GA, according to the Adverse Drug Reaction Probability Scale6 and the Roussel Uclaf Causality Assessment Method.7 The patient provided written informed consent for publication of this case report.
Discussion
To compare our findings with those previously reported in the literature, we performed a comprehensive PubMed search using the terms ‘Glatiramer acetate’ and ‘hepatotoxicity’, ‘autoimmune hepatitis’ and ‘drug induced liver injury’ and identified 11 clinical cases published up to 15 February 2017. All published clinical cases were written in English and Spanish. The clinical information of these cases is summarized in Table 1.
Table 1.
Review of clinical characteristics of reported cases of GA associated liver injury.
| Case | Sex | Age (years) | Onset (days) | Symptoms | AST | ALT | AP | Bi | Ultrasound | Viral serologies (negative) |
Autoimmunity markers* | Biopsy | Recovery (days) | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neumann et al. (2007) | M | 71 | 60 | Jaundice, malaise | 178 | 317 | 138 | 7.6 | Normal | HV, HEV, EBV, CMV, VZV, HSV | ANA (+) 1:1280 | DILI | 30 | AIH |
| Von Kalckreuth et al. (2008) | F | 42 | 60 | 14.7 | ANA (+) SMA (+) |
Lymphocytic inflammation | IS | AIH | ||||||
| Deltrene et al. (2009) | F | 52 | 90 | No | 320 | 140 | Normal | Normal | Normal | HV, EBV, CMV | ANA (+) 1:320 SMA (+) 1:80 |
DILI | 90 | DILI |
| Subramaniam et al. (2012) | F | 31 | 37 | Jaundice, anorexia, lethargy | 276 | 1056 | 143 | 6.4 | Increased echogenicity | HV, EBV, CMV | SMA (+) 1:320 | DILI | 60 | DILI |
| Makhani et al. (2013) | F | 15 | 60 | Fatigue | 500 | 1150 | Normal | Fatty infiltration | HV, EBV | Negative (not AMA) |
DILI | 54 | DILI | |
| Onmez et al. (2013) | F | 36 | 90 | Fatigue, jaundice, nausea | 1834 | 1475 | 231 | 24.4 | Normal | HV, EBV, CMV | Negative | DILI | 36 | DILI |
| Sinagra et al. (2013) | F | 41 | 30 | 1612 | 4410 | 383 | 5 | HV (not A), EBV, CMV, HIV | ANA (+) 1:320 | Inflammation, eosinophilic infiltrate, fibrosis | 30 | AIH | ||
| Sinagra et al. (2013) | F | 29 | 30 | Jaundice, asthenia | 820 | 1260 | 342 | 4 | HV, EBV, CMV, HIV | ANA (+) 1:160 | Lymphocitic infiltrate, fibrosis | IS | AIH | |
| Antezana et al. (2014) | F | 28 | 180 | Jaundice | 905 | 1103 | 8 | HV | Negative | DILI | 30 | DILI | ||
| La Gioia et al. (2014) | F | 25 | 240 | Anorexia, constipation | 641 | 1433 | Normal | 1.5 | Reactive lymph nodes | HV, EBV, CMV | Negative | DILI | 56 | DILI |
| Fernández et al. (2015) | F | 42 | 180 | No | 383 | 602 | 2.2 | Normal | HV, HEV, EBV,CMV,HSV | ANA (+) 1:640 | DILI | 30 | DILI | |
| Almeida et al. (2016) | F | 65 | 7 | Petechiae | 448 | 667 | 151 | Normal | Simple cysts | HV, HDV, EBV, CMV | ANA (+) 1:40 SMA (+) 1:40 |
No | 147 | DILI |
AIH, autoimmune hepatitis; ALT, alanine aminotransferase (U/liter); AP, alkaline phosphatase (U/liter); AST, aspartate aminotransferase (U/liter); Bi, bilirubin (mg/dl); CMV, cytomegalovirus; DILI, drug-induced liver injury; EBV, Epstein–Barr virus; GA, glatiramer acetate; HDV, hepatitis D virus; HEV, hepatitis E virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HV, hepatotropic virus (includes A, B and C viruses; others are specified if tested); IS, immunosuppressive treatment; VZV, Varicella Zoster virus.
Includes antinuclear antibodies (ANAs), smooth muscle antibodies (SMAs), liver kidney microsome antibodies and antimitochondrial antibodies (AMAs), except for Makhani et al. (2013), who did not include AMAs. Von Kalckreuth et al. (2008) and Antezana et al. (2014) studies do not specify more antibodies than those written. In Deltrene et al. (2009) and Subramaniam et al. (2012) studies, antibodies turned negative during follow up.
DILI, defined as an elevation in the serum concentration of ALT, conjugated bilirubin or AP exceeding two times the upper normal limit, is one of the most frequent reasons for drug withdrawal from the market.8 Liver injury has been more frequently described in women and the elderly due to changes in drug metabolism, hepatic detoxification and clearance mechanisms, blood flow or hepatic structure.9 However, data about safety in elderly populations regarding all drugs approved for MS are scarce. Despite the fact that GA pivotal trials included patients younger than 60 years old,10 no evidence of hematological, hepatic or renal dysfunction were observed after 15 years of follow up.1,3 In fact, no laboratory monitoring is required during GA therapy.
According to Antezana and colleagues,11 the United States Food and Drug Administration Adverse Event Reporting System database contained 95 reports of liver damage possibly related to GA by 2013, and GA was the only drug administered in 51 of these cases. The literature review of the 11 reported cases (Table 1) identifies two main etiologies for GA-associated liver injury, according to clinical course, biopsy findings and autoimmunity markers: seven patients (64%) developed a DILI and four (36%) developed an AIH. The mean age (standard deviation) of the reported patients was 37.5 (15) years and only one of them was older than 65 years. Thus, other factors beyond age may be relevant in developing this complication.
In GA-induced DILI, the time of presentation ranged from 1 to 8 months. In four cases,7,11–13 specific autoimmune markers were negative and liver histology showed hepatocellular necrosis compatible with toxic hepatitis. Although GA is not metabolized in the liver,11 mitochondrial damage has been suggested as the mechanism responsible for the toxic injury.12–13 The other three patients14–16 had a transient elevation of autoantibodies (ANAs, SMAs or both), suggesting the contribution of an immune-mediated unknown mechanism. All patients with GA-induced DILI recovered completely in 1–5 months after drug withdrawal (Table 1).
In AIH related to GA, the time interval from drug exposure to clinical symptoms ranged from 30 to 60 days,17–19 and significant titers of autoantibodies (especially ANAs and SMAs) were detected at baseline and follow up. Liver biopsy showed a specific pattern of inflammatory infiltrates, including mononuclear cells, eosinophils and lymphocytes. Two patients recovered spontaneously, but the other two required long-term immunosuppressive therapy.18–19 The exact mechanism of GA-induced AIH is unknown. The hypothesis is that GA may induce T-helper type 2 cells, leading to the release of cytokines, interleukin (IL)-4, IL-6 and IL-10, and autoantibody production in predisposed patients.14–15,17 In fact, AIH seems to be 10 times more frequent in patients with MS than in the general population.20 Recurrences of new episodes of DILI are more likely in those patients who harbor high titers of specific autoantibodies (>1:80).21 Our patient was diagnosed with DILI due to GA for several reasons. Liver test abnormalities appeared after GA initiation and improved after its discontinuation. Moreover, normal IgG levels, and the positivity of ANAs and SMAs at low titers argued against the diagnosis of AIH.22 Nevertheless, since a liver biopsy was not performed, we could not exclude completely the possibility of an AIH, since liver histology was not assessed and the diagnostic scores of AIH could not be calculated. It is worth mentioning, however, that our patient previously developed an allergic reaction to methylprednisolone. Allergic reactions to systemically administered corticosteroids are infrequent23 and in our experience with more than 800 patients with MS followed in our MS unit, only two other patients have been diagnosed with this complication in the last 20 years. Whether the single methylprednisolone dose contributed through a synergic effect or it merely reflects a particular predisposition for the patient to develop drug toxicity is a matter of discussion. In fact, case reports of DILI and AIH have been described in patients treated with repetitive pulses of methylprednisolone.24 Despite the lack of HLA-DRB1*07 association with GA-induced liver injury, it has been associated with other forms of liver injury such as type 2 AIH and other drug-induced toxicities.25–26
Finally, the current case highlights that the diagnosis and therapy approach in subjects with late onset MS (>50 years)27–28 is challenging because the differential diagnosis is wider and they are not usually included in randomized clinical trials.10
To conclude, liver injury associated with GA therapy is an uncommon side effect, with no such cases reported in clinical trials. This clinical case and the literature review highlight that GA-associated hepatotoxicity can exist and be serious. Therefore, we recommend liver test monitoring for at least the first 6 months,29 especially in older patients, those with previous chronic liver disease, and patients with specific antibodies or a previous history of DILI associated with other drugs.19
Acknowledgments
The authors wish to thank the patient whose case is reported. Javier Almeida and Nuria Solà-Valls contributed equally.
Footnotes
Funding: This study is supported in part by a grant from Instituto de Salud Carlos III, Spain (FI16/00251).
Conflict of interest statement: Javier Almeida declares that there is no conflict of interest. Nuria Solà-Valls has received compensation for consulting services and speaker honoraria rom Sanofi-Aventis and Bayer-Schering. Elisa Pose declares that there is no conflict of interest. Yolanda Blanco has received compensation for consulting services and speaker honoraria from Sanofi-Aventis, Teva Pharmaceutical Industries Ltd and Novartis. María Sepúlveda has received compensation for consulting services and speaker honoraria from Sanofi-Aventis and Novartis. Sara Llufriu has received compensation for consulting services and speaker honoraria from Sanofi-Aventis, Novartis, Teva Pharmaceutical Industries Ltd and Biogen-Idec. Pere Ginés declares that there is no conflict of interest. Albert Saiz has received compensation for consulting services and speaker honoraria from Bayer-Schering, Merck-Serono, Biogen-Idec, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd and Novartis.
Contributor Information
Javier Almeida, Neurology Department, Cruces University Hospital, Barakaldo, Bizkaia, Spain and Neuroimmunology Program, Service of Neurology, Hospital Clinic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Universitat de Barcelona, Barcelona, Spain.
Nuria Solà-Valls, Service of Neurology, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain.
Elisa Pose, Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS), Barcelona, Spain.
Yolanda Blanco, Neuroimmunology Program, Service of Neurology, Hospital Clinic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Universitat de Barcelona, Barcelona, Spain.
María Sepúlveda, Neuroimmunology Program, Service of Neurology, Hospital Clinic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Universitat de Barcelona, Barcelona, Spain.
Sara Llufriu, Neuroimmunology Program, Service of Neurology, Hospital Clinic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Universitat de Barcelona, Barcelona, Spain.
Pere Gines, Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS), Barcelona, Spain.
Albert Saiz, Neuroimmunology Program, Service of Neurology, Hospital Clinic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Universitat de Barcelona, Barcelona, Spain.
References
- 1. Ziemssen T, Ashtamker N, Rubinchick S, et al. Long-term safety and tolerability of glatiramer acetate 20 mg/ml in the treatment of relapsing forms of multiple sclerosis. Expert Opin Drug Saf 2017; 16: 247–255. [DOI] [PubMed] [Google Scholar]
- 2. Comi G, Amato MP, Bertolotto A, et al. The heritage of glatiramer acetate and its use in multiple sclerosis. Mult Scler Demyelinating Disord 2016; 1: 6. [Google Scholar]
- 3. Scott LJ. Glatiramer acetate: a review of its use in patients with relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. CNS Drugs 2013; 27: 971–988. [DOI] [PubMed] [Google Scholar]
- 4. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sepúlveda M, Armangué T, Sola-Valls N, et al. Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm 2016; 14; 3: e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 7. Onmez A, Eminler AT, Ergenç H, et al. Drug-induced liver injury by glatiramer acetate used for treatment of multiple sclerosis: a case report. J Investig Med High Impact Case Rep 2013; 14: 2324709613517493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol 1990; 11, 272–276. [DOI] [PubMed] [Google Scholar]
- 9. Stine JG, Sateesh P, Lewis JH. Drug-induced liver injury in the elderly. Curr Gastroenterol Rep 2013; 15: 299. [DOI] [PubMed] [Google Scholar]
- 10. Bornstein MB, Miller A, Slagle S, et al. A placebo-controlled, double-blind, randomized, two-center, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology 1991; 41: 533–539. [DOI] [PubMed] [Google Scholar]
- 11. Antezana A, Herbert J, Park J, et al. Glatiramer acetate-induced acute hepatotoxicity in an adolescent with MS. Neurology 2014; 82: 1846–1847. [DOI] [PubMed] [Google Scholar]
- 12. Makhani N, Ngan BY, Kamath BM, et al. Glatiramer acetate-induced acute hepatotoxicity in an adolescent with MS. Neurology 2013; 81: 850–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. La Gioia S, Bacis G, Sonzogni A, et al. Glatiramer acetate-induced hepatitis in a young female patient with multiple sclerosis. Mult Scler Relat Disord 2014; 3: 732–734. [DOI] [PubMed] [Google Scholar]
- 14. Deltenre P, Peny MO, Dufour A, et al. Acute hepatitis induced by glatiramer acetate. BMJ Case Rep 2009; bcr09.20080913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Subramaniam K, Pavli P, Llewellyn H, et al. Glatiramer acetate induced hepatotoxicity. Curr Drug Saf 2012; 7: 186–188. [DOI] [PubMed] [Google Scholar]
- 16. Fernández N, Joao Matias D, Pisabarros Blanco C, et al. Hepatitis asociada a acetato de glatirámero. Gastroenterol Hepatol 2015; 38: 280–286. [DOI] [PubMed] [Google Scholar]
- 17. Neumann H, Csepregi A, Sailer M, et al. Glatiramer acetate induced exacerbation of autoimmune hepatitis in a patient with multiple sclerosis. J Neurol 2007; 254: 816–817. [DOI] [PubMed] [Google Scholar]
- 18. Von Kalckreuth V, Lohse AW, Schramm C. Unmasking autoimmune hepatitis under inmunomodulatory treatment of multiple sclerosis – not only Beta interferon. Am J Gastroenterol 2008; 103: 2147–2148. [DOI] [PubMed] [Google Scholar]
- 19. Sinagra E, Raimondo D, Cottone S, et al. Does glatiramer acetate provoke hepatitis in multiple sclerosis? Mult Scler Relat Disord 2014; 3: 266–268. [DOI] [PubMed] [Google Scholar]
- 20. De Seze J, Delalande S, Michelin E, et al. Autoimmune hepatitis and multiple sclerosis: a coincidental association? Mult Scler 2005; 11: 691–693. [DOI] [PubMed] [Google Scholar]
- 21. Castiella A, Zapata E, Lucena MI, et al. Drug-induced autoimmune liver disease: a diagnostic dilemma of an increasingly reported disease. World J Hepatol 2014; 6: 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liberal R, Grant CR, Longhi MS, et al. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev 2014; 13: 435–440. [DOI] [PubMed] [Google Scholar]
- 23. Aranda A, Mayorga C, Ariza A, et al. IgE-mediated hypersensitivity reactions to methylprednisolone. Allergy 2010; 65: 1376–1380. [DOI] [PubMed] [Google Scholar]
- 24. Uppal R, Lau D, Challies T, et al. Steroids may not be immune to causing hepatotoxicity. World J Med 2012; 7: 301–306. [Google Scholar]
- 25. Muratori P, Lalanne C, Fabbri A, et al. Type 1 and type 2 autoimmune hepatitis in adults share the same clinical phenotype. Aliment Pharmacol Ther 2015; 41: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 26. Schaid DJ, Spraggs CF, McDonnell SK, et al. Prospective validation of HLA-DRB1*07:01 allele carriage as a predictive risk factor for lapatinib-induced liver injury. J Clin Oncol 2014; 32: 2296–2303. [DOI] [PubMed] [Google Scholar]
- 27. Kis B, Rumberg B, Berlit P. Clinical characteristics of patients with late-onset multiple sclerosis. J Neurol 2008; 255: 697–702. [DOI] [PubMed] [Google Scholar]
- 28. De Seze J, Delalande S, Michelin E, et al. Brain MRI in late-onset multiple sclerosis. Eur J Neurol 2005; 12: 241–244. [DOI] [PubMed] [Google Scholar]
- 29. Andrade RJ, Lucena MI, Fernández MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005; 129: 512–521. [DOI] [PubMed] [Google Scholar]