Abstract
Background
Brain-derived neurotrophic factor (BDNF) is produced by cleavage of proBDNF, and BDNF and proBDNF may play antagonistic roles in nervous system development, learning, memory and neuronal stress resistance. BDNF and proBDNF are present in blood, but the origin and relative contributions of soluble and extracellular vesicle (EV)-associated levels are unknown.
Methods
In this study we used validated immunoassays to measure proBDNF and BDNF levels in plasma, total plasma EVs and a subpopulation of EVs enriched for neuronal origin (expressing the neuronal marker L1CAM) in 150 Baltimore Longitudinal Study of Aging participants with and without decline in walking speed (reflecting aging-associated motor decline).
Results
Levels of BDNF and proBDNF were highest in L1CAM+ EVs. Participants with walking speed decline had higher levels of proBDNF in L1CAM+ EVs compared to non-decliners, but no differences in proBDNF levels in plasma and total EV.
Conclusions
Our findings suggest that levels of proBDNF and BDNF in circulating L1CAM+ EVs might be used as biomarkers for conditions involving altered BDNF signaling.
Keywords: BDNF, BLSA, aging, exosomes, extracellular vesicles, motor function, walking speed, proBDNF
Background
In the field of aging, one of the strongest phenotypic markers of health and function is walking speed. Walking speed has been shown to predict mortality, disability, cognitive decline, dementia and health care utilization (1-7). A meaningful decline in walking speed has been defined and shown to independently predict mortality (8, 9). Walking speed has also been shown to have shared underlying neurological processes with cognitive function, especially with executive function (10). Walking speed is thought to capture abnormalities in multiple organ systems (11, 12) and is sensitive to changes in brain structure and function (13-15). However, the specific mechanisms underlying neural contributions to walking speed are presently unknown.
Mature brain-derived neurotrophic factor (BDNF) is an extracellular signaling protein with neuroprotective and neuroregenerative functions that is well known for its prominent roles in nervous system development (16), learning and memory (17) and neuronal stress resistance (18). Additionally, BDNF increases with aerobic exercise and its increase has been implicated in neurocognitive and physical performance improvements that involve balance, coordination, flexibility and agility (19-22). Exercise interventions have also been associated with improvements in walking speed (23, 24). It is currently unknown whether changes in BDNF signaling are involved in walking speed decline.
BDNF is produced from a precursor protein (proBDNF) by proteolytic cleavage at amino acid 128 (25). BDNF and proBDNF often have opposing actions, with BDNF promoting synaptic long-term potentiation (LTP) and stress resistance, and proBDNF enhancing long-term depression (LTD) and endangering neurons (26-28). While BDNF and proBDNF can be distinguished by their molecular weights on immunoblots, the distinction between, and quantification of BDNF and proBDNF in higher throughput quantitative assays can be problematic because of potential cross-reactivity of BDNF antibodies with proBDNF.
There have been hundreds of studies in which levels of presumptive BDNF have been measured in plasma or serum samples from human subjects. Findings of these studies have often been inconsistent, in terms of differences in BDNF levels, in diseased versus control subjects, in response to environmental challenges such as exercise, or in observational studies versus clinical trials (29-34). However, very few studies have quantified levels of both BDNF and proBDNF in the same samples. Moreover, because proBDNF and BDNF are produced not only by neurons, but also by many other cell types including lymphocytes, megakaryocytes, and skeletal and vascular smooth muscle cells (35-38), the contribution of different cell types to levels of circulating BDNF and proBDNF are unknown.
Harvesting EVs enriched for neuronal origin from peripheral blood provides a novel platform for biomarker discovery and may allow us to follow biochemical and cellular changes occurring in neurons in a dynamic way. Our methodology for isolating EVs enriched for neuronal origin involves isolating total EVs from plasma or serum samples, followed by immunoprecipitation (IP) using antibodies against the neuronal cell adhesion molecule L1CAM to isolate a sub-population of L1CAM+ EVs (39). In a series of case-control studies, we showed that a priori selected proteins in plasma L1CAM+ EVs can be used as prognostic and diagnostic biomarkers for Alzheimer's disease (AD) (40-45). Neuronal origin EV-derived BDNF and proBDNF measures may better reflect neuronal neurotrophin production than circulating soluble levels and offer a tool for exploring neural mechanisms implicated in walking speed decline.
In this study we validated immunoassays that distinguish BDNF and proBDNF, and used the assays to quantify BDNF and proBDNF levels in plasma, total EVs and EVs of neuronal origin in samples from elderly subjects in the Baltimore longitudinal study of aing (BLSA) who either did or did not exhibit a decline in walking speed as they aged. We found that levels of BDNF and proBDNF are higher in EVs of neuronal origin compared to plasma and total EVs, and that proBDNF levels in EVs of neuronal origin are higher in subjects with walking speed decline compared to those without walking speed decline.
Design and Methods
Subjects and sample collection
We identified 150 participants from the BLSA, a longitudinal study of normative aging, age 65-97 years. All sample partiticipants were followed over time with repeated measures of walking speed. We selected 75 participants who had walking speed decline of at least 0.05 m/s/year and 75 who had stable walking speed. The follow-up period for this cohort ranged from 1 to 8 years, mean 1.93. The study protocol was approved by the NIH Institutional Review Board and all participants provided informed consent. Blood samples were collected from subjects after an overnight fast; plasma was separated by centrifugation, aliquoted and stored at -80°C. A total of 300 plasma samples (0.5 ml each) drawn from the 150 subjects at two time points were studied. In 116 samples from 58 subjects, we proceeded with EV isolation and L1CAM EV enrichment from the entire aliquot to generate enough material for future L1CAM+ EV-based studies. The remaining 184 samples from 92 subjects were used for BDNF and proBDNF assays in all types of samples (plasma, total EVs, L1CAM IP supernatant, and L1CAM+ EVs).
Motor Function
For measurement of walking speed, participants walked at their preferred speed on a 6 m walking course and were timed by a study staff member. The average of the two trials conducted was used for these analyses. Participants were defined as walking speed decliners if speed declined more than 0.05 m/s/year (8). Strength was assessed by 1) the average of three trials of maximum force grip strength by Jamar Hydraulic hand dynamometer and 2) the average of three trials of peak knee extension and flexion on a KinCom 125 AP dynamometer, each at angular velocities of 30°/s and 180°/s, as well as isometric knee strength at 120° and 140°. Physical activity was assessed by self-report of weekly activity yielding an estimate in total kilocalories per week (46).
Isolation of EVs
The following protocol was used for total EV isolation followed by L1CAM enrichment (numbers below refer to a starting volume of 0.5 ml and were adjusted linearly for decreased starting volumes) (39). Samples were defrosted and each sample received 0.2 ml of Thromboplastin-D (Thermo-Fisher Scientific Inc., Rockford, IL) followed by incubation for 30 minutes at room temperature (RT). After addition of 0.3 ml of Dulbecco's calcium and magnesium-free salt solution containing thrice the suggested concentrations of protease inhibitor cocktail (Complete Tablets Easy pack, Roche Applied Sciences, Inc., Indianapolis, IN) and phosphatase inhibitor cocktail (Pierce Halt, Thermo-Fisher Scientific, Inc., Rockford, IL), samples were mixed at RT and then centrifuged at 3000 × g for 20 minutes at 4°C. Supernatants were transferred to fresh tubes and gently mixed after addition of 252 μl of ExoQuick exosome solution (System Biosciences, Inc., Mountainview, CA). Suspensions with ExoQuick were incubated for 60 min at 4°C to precipitate total EVs and then centrifuged at 1,500 × g for 5 minutes at 4°C. Supernatants were discarded after centrifugation and the pellet containing EVs was re-suspended in 0.5 ml distilled water containing thrice the suggested concentrations of protease and phosphatase inhibitors. For L1CAM IP, suspensions were incubated for 1 hr at 4°C with 4 μg of mouse anti-human CD171 (L1CAM) biotinylated antibody (clone 5G3, eBioscience, San Diego, CA) in 50 μL of 3% BSA (1:3.33 dilution of Blocker BSA 10% solution in PBS [Thermo Scientific, Inc.]) per tube with mixing, followed by addition of 18.75 μl of streptavidin-agarose Ultralink resin (Thermo Scientific, Inc.) in 21.25 μL of 3% BSA and incubation for 30 min at 4°C with continuous mixing. After centrifugation at 200 × g for 5 min at 4°C and removal of the supernatant, each pellet was re-suspended in 200 μL of 0.1 M glycine-HCl by mixing for 10 sec and centrifuged at 4,500 × g for 10 min at 4°C. 10 μL of the supernatant was aliquoted for EV counts using a nanoparticle tracking analysis instrument (NS500). To the remainder of the sample, which was used to measure BDNF and proBDNF levels, was added 15 μL of the supernatant. To lyse EVs, each tube received 300 μL of M-PER mammalian protein extraction reagent (Thermo Scientific, Inc.), containing thrice the suggested concentrations of protease and phosphatase inhibitors. These suspensions were stored at -80°C until used for assays.
Enzyme-linked immunosorbent assays (ELISAs)
Mature BDNF (BDNF Emax® ImmunoAssay System, Promega, cat. #G7611) and proBDNF (Pro BDNF [Human, Mouse, Rat] ELISA Kit, Aviscera Bioscience, cat#s SK00752-08 and SK00752-09) ELISAs were performed on aliquots of plasma, and total EVs or L1CAM+ EVs isolated from the plasma, using the protocols of the assay manufacturer's. Assays procedures were performed according to protocols outlined by the manufacturers. Plasma and supernatant samples were run on SK00752-08 plates, and the total EV and L1CAM+ EV samples were run on SK00752-09 plates.
For each type of sample, one third of the total sample was run in duplicate (plasma, total EVs, L1CAM IP supernatant, and L1CAM+ EVs). Members of the research team who performed EV isolation and assays (CS, EE, DK) remained blinded to decliner/non-decliner status until all measurements were completed and data were handed off to unblinded collaborators (NC, TQ, SD) for statistical analysis. Each plate included both samples (time points) from a given subject. We chose to optimize comparability within and between participants for each type of sample rather than comparability between types of sample within participants. Therefore, we opted to run separate plates for plasma, total EVs, L1CAM IP supernatant, and L1CAM+ EVs fitting as many participants as possible in each plate rather than fit all types of samples from a given participant in the same plate. To correct for between-plates variability, plasma samples from three control subjects (not part of the BLSA cohort), two with high BDNF levels and one with a high proBDNF level, were included as external standard in all ELISAs. Raw signal values were normalized to the average signal value of these three control subjects. To further examine whether between plate variability affects the findings, plate number was used in logistic regression as a covariate (adjustment did not affect the magnitude of the associations and is included as supplemental analysis).
Anthropometric and body composition measures
Body mass index (BMI) (kg/m2) was obtained from physical examination. Lean mass and fat mass were assessed via dual-energy X-ray absorptiometry (DXA) whole body scans from the Prodigy Scanner using Encore Software. In addition to absolute values of lean mass and fat mass, we assessed appendicular lean mass (ALM), the sum of lean mass from the arms and legs, ALM relative to height (kg/m2), percent lean mass, and percent fat mass.
Comorbidity Index
A summary measure was created to account for the presence of 14 comorbidities: hypertension, diabetes, coronary artery disease, congestive heart failure, stroke, chronic obstructive pulmonary disease, cancer, Parkinson's disease, arthritis, anemia, chronic kidney disease, peripheral arterial disease, cognitive impairment, and depression (47). Anemia was defined as having a hemoglobin less than 12g/dl in women and 13g/dl in men (47). Cognitive impairment was defined as a Mini Mental State Examination score less than 24 (48). A Center for Epidemiologic Depression Scale score greater than or equal to 16 defined depression (49). All other conditions for the comorbidity index were determined from self-report from the BLSA medical questionnaire.
Statistical analyses
T-tests and chi-square were used to assess differences between decliners and non-decliners for covariates including age, anthropometric and body composition measures, comorbidities, physical function, and muscle strength. Covariates that were statistically significant, or biologically plausible, were included in the regression analyses. Logistic regression was used separately for each time point to predict odds of being a decliner, with and without adjustment for age, sex, percent fat mass, baseline walking speed, plate number, and comorbidity index. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
BDNF and proBDNF ELISAs have little or no cross-reactivity
We used recombinant BDNF (7.8-4000 pg/ml) and proBDNF (7.8-4000 pg/ml) protein to generate the standard curves for the ELISA assays of proBDNF (Figure 1A) and BDNF (Figure 1B) and assess potential cross-reactivity. Adding standard concentrations of recombinant BDNF to the proBDNF ELISA generated a linear curve with a significantly lower slope compared to the one achieved by recombinant proBDNF; the measured concentrations of BDNF were approximately 20-fold lower than the known concentrations added across all seven standards and in three separate proBDNF ELISA plates (Figure 1A). Conversely, recombinant proBDNF was detected with the BDNF ELISA only in concentrations higher than 5 ng/ml (Figure 1B) and even then the measured concentrations in all repeats were about 25 times lower than the concentrations actually added to the assay. In order to further examine if proBDNF interferes with BDNF detection and vise-versa, BDNF and proBDNF were combined on each plate to ensure that the mixture of the two does not modify the expected signal (for example, on the proBDNF plate, BDNF was added to the proBDNF internal standard). Adding increasing amounts of BDNF, up to 500 pg, did not interfere with the measurements of either low or high concentrations of proBDNF (Figure 1C) (each concentration level serves as a technical repeat and the whole experiment was repeated on three separated ELISA plates). Similarly, adding up to 100 ng of proBDNF had no effect on BDNF measurements (Figure 1D). Moreover, reproducibility was shown in technical duplicates throughout the study (data not shown). We conclude that although there is some signal cross-reactivity, it is negligible.
Figure 1.
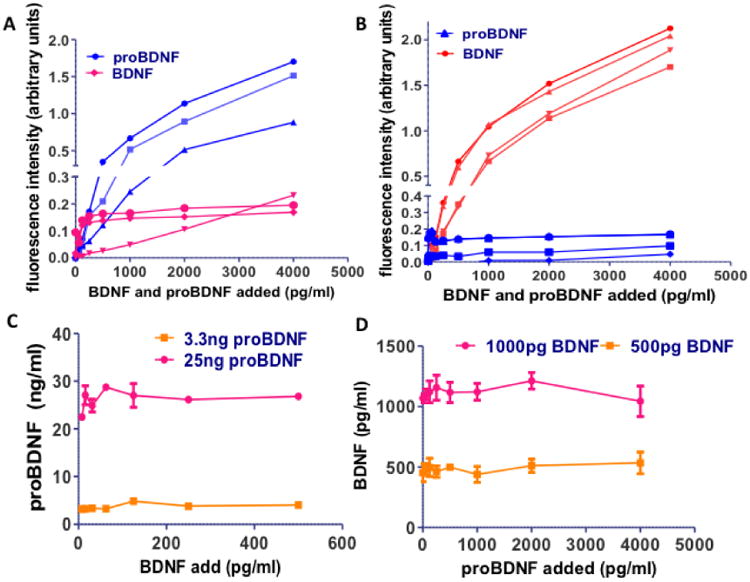
Recombinant BDNF was added in concentrations of 62-4000 pg/dl to proBDNF ELISA plates (A) and recombinant proBDNF was added in concentrations of 62 – 4000 pg to BDNF ELISA plates (B). The signal produced was compared to the signal produced by the same concentration of recombinant proBDNF (A) or recombinant BDNF (B). Each curve represents a separate experiment on a separate ELISA plate, while the samples were run in duplicates. C. On a proBDNF ELISA plate, increasing concentrations of recombinant BDNF were added to 3.3 or 25 ng of recombinant proBDNF and the measured concentration of proBDNF was calculated based on the standard curve produced by pure recombinant proBDNF. The fact that the line remains almost horizontal at the excpected concentration shows the reproducibility of the ELISA and its validity when measuring mixtures of BDNF and proBDNF. D. On a BDNF ELISA plate, increasing concentrations of recombinant proBDNF were added to 500 or 1000pg of recombinant BDNF and the measured concentration of BDNF was calculated based on the standard curve produced by pure recombinant BDNF. The fact that the line remains almost horizontal at the excpected concentration shows the reproducibility of the ELISA and its validity when measuring mixtures of BDNF and proBDNF.
BDNF and proBDNF levels are highest in L1CAM+ EVs
To determine the range of BDNF and proBDNF values in plasma, total EVs and L1CAM+ EVs, a preliminary cohort of 20 subjects was analyzed (Figure 2). BDNF levels were significantly different in the three fluid types (F = 6.868, p = 0.002). Specifically, BDNF levels in L1CAM+ EVs were significantly higher compared to levels in total EVs (p = 0.016) and plasma (p = 0.001) and were comparable between total EVs and plasma (p = 0.254) (Figure 2A). ProBDNF levels were also significantly different in the three fluid types (F = 4.410, p = 0.017). Specifically, proBDNF levels in L1CAM+ EVs were significantly higher compared to levels in total EVs (p = 0.026) and plasma (p = 0.007) and were comparable between total EVs and plasma (p = 0.628) (Figure 2B).
Figure 2.
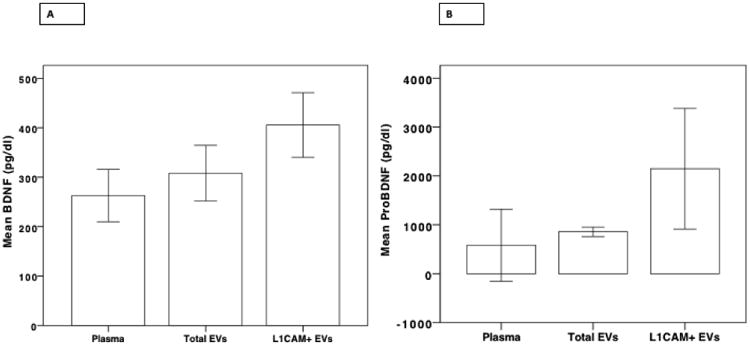
A. BDNF concentrations in L1CAM+ EVs, plasma, and total EVs, in a preliminary cohort of 20 participants showing higher levels of BDNF within L1CAM+ EVs. B. ProBDNF concentrations in L1CAM+ EVs, plasma, and total EVs, in a preliminary cohort of 20 participants showing higher levels of BDNF within L1CAM+ EVs.
proBDNF levels in L1CAM+ EVs differ between decliners and non-decliners
Participant characteristics are described in Table 1. Decliners were more likely to be male, with greater height, higher lean mass, lower fat mass, and faster baseline walking speed. There were no other significant differences between decliners and non-decliners.
Table 1.
Basic characteristics of the cohort at time 1.
| Non-Decliners | Decliners | p-value | |||
|---|---|---|---|---|---|
| n | Mean(SD) | n | Mean(SD) | ||
| Interval | 75 | 2.05 (0.98) | 75 | 1.80 (0.97) | 0.12 |
| Age, yrs | 75 | 75.45 (8.21) | 75 | 77.64 (8.66) | 0.11 |
| Female Sex, % | 75 | 46.67 | 75 | 17.33 | <0.01 |
| Height, m | 75 | 1.68 (0.10) | 75 | 1.72 (0.10) | <0.01 |
| BMI, kg/m2 | 75 | 27.78 (4.93) | 75 | 26.87 (4.28) | 0.23 |
| Gait Speed, m/s | 75 | 0.99 (0.23) | 75 | 1.15 (0.29) | <0.01 |
| Comorbidity Index (0-14) | 75 | 2.36 (1.16) | 75 | 2.07 (1.46) | 0.18 |
| Physical Activity, total kk/wk | 67 | 88.30 (67.48) | 71 | 85.96 (51.39) | 0.82 |
| ALM, kg | 65 | 20.33 (5.04) | 70 | 22.32 (4.84) | 0.02 |
| ALM/ht2, kg/m2 | 64 | 7.09 (1.21) | 70 | 7.41 (1.14) | 0.11 |
| Percent Lean Mass | 64 | 60.33 (7.99) | 70 | 64.51 (6.96) | <0.01 |
| Fat Mass, kg | 64 | 29.45 (11.48) | 70 | 25.90 (9.24) | 0.05 |
| Percent Fat Mass | 65 | 36.09 (8.29) | 70 | 31.69 | <0.01 |
| Grip Strength, kg | 71 | 29.93 (11.33) | 70 | 32.40 (10.47) | 0.18 |
| Knee Extension 30 d/s, Nm | 46 | 115.20 (48.03) | 49 | 117.4 (35.64) | 0.80 |
| Knee Extension 180 d/s, Nm | 45 | 75.53 (33.25) | 49 | 77.57 (24.50) | 0.74 |
| Knee Flexion 30 d/s, Nm | 46 | 55.44 (22.57) | 49 | 57.80 (20.11) | 0.59 |
| Knee Flexion 180 d/s, Nm | 45 | 60.87 (24.99) | 48 | 63.71 (20.98) | 0.55 |
| Isometric 120 d, Nm | 44 | 129.00 (49.75) | 48 | 127.90 (35.75) | 0.90 |
| Isometric 140 d, Nm | 44 | 90.98 (37.67) | 48 | 92.53 (26.01) | 0.82 |
Participant characteristics at time 2 are detailed in Table 2. All of the differences observed at time 1 were present at time 2. Additionally, at time 2 decliners had fewer comorbidities. There were no other significant differences between decliners and non-decliners.
Table 2.
Basic characteristics of the cohort at time 2.
| Non-Decliners | Decliners | p-value | |||
|---|---|---|---|---|---|
| n | Mean(SD) | n | Mean(SD) | ||
| Interval | 75 | 2.05 (0.98) | 75 | 1.80 (0.97) | 0.12 |
| Age, yrs | 75 | 77.51 (7.80) | 75 | 79.44 (8.40) | 0.15 |
| Female Sex, % | 75 | 46.67 | 75 | 17.33 | <0.01 |
| Height, m | 75 | 1.67(0.10) | 75 | 1.72 (0.09) | <0.01 |
| BMI, kg/m2 | 75 | 28.28 (5.04) | 75 | 27.12 (4.82) | 0.15 |
| Baseline Gait Speed, m/s | 75 | 0.99 (0.23) | 75 | 1.15 (0.29) | <0.01 |
| Time Two Gait Speed, m/s | 75 | 1.00 (0.22) | 75 | 0.97 (0.28) | 0.34 |
| Comorbidity Index (0-14) | 75 | 2.67 (1.24) | 75 | 2.19 (1.41) | 0.03 |
| Physical Activity, total kk/wk | 65 | 80.26 (56.54) | 64 | 86.11 (77.41) | 0.63 |
| ALM, kg | 62 | 19.97 (4.66) | 63 | 22.19 (4.66) | 0.01 |
| ALM/ht2, kg/m2 | 62 | 7.06 (1.13) | 63 | 7.43 (1.14) | 0.07 |
| Percent Lean Mass | 62 | 59.99 (8.43) | 63 | 64.90 (7.33) | <0.01 |
| Fat Mass, kg | 62 | 29.44 (11.77) | 63 | 25.05 (9.09) | 0.02 |
| Percent Fat Mass | 62 | 36.46 (8.73) | 63 | 31.29 (7.63) | <0.01 |
| Grip Strength, kg | 70 | 28.66 (11.08) | 72 | 30.78 (10.24) | 0.24 |
| Knee Extension 30 d/s, Nm | 41 | 115.8 (43.53) | 32 | 117.8 (41.72) | 0.84 |
| Knee Extension 180 d/s, Nm | 41 | 75.06 (27.80) | 32 | 73.72 (25.32) | 0.83 |
| Knee Flexion 30 d/s, Nm | 41 | 61.23 (24.52) | 32 | 61.93 (19.33) | 0.90 |
| Knee Flexion 180 d/s, Nm | 41 | 61.59 (23.24) | 32 | 65.01 (20.10) | 0.51 |
| Isometric 120 d, Nm | 40 | 134.8 (46.91) | 32 | 125.1 (48.43) | 0.39 |
| Isometric 140 d, Nm | 40 | 98.02 (37.69) | 32 | 95.67 (35.67) | 0.79 |
Within subject differences in measures between time 2 and time 1 show that non-decliners at time 2 had a decline in proBDNF from L1CAM+ EVs, were older, shorter, had a higher BMI, more comorbidities, and less perecent lean mass (Supplemental Table 1). Decliners at time 2 were also older and shorter, and additional had a slower gait speed, less ALM, less grip strength, less knee extension strength at 180°/s, and less isometric strength at 120°. The only measures that displayed significant different change between decliners and non-decliners was gait speed.
We performed logistic regression analyses to assess the association between BDNF and proBDNF measures and walking speed decline. The significant differences in lean mass measures and height between decliners and non-decliners were fully attenuated upon sex adjustment; therefore, these covariates were not included in further regression analyses. Percent fat mass showed a greater difference between decliners and non-decliners than total fat mass, therefore it was included as a covariate in the final regression analyses. We included comorbidity index even though it did not differ significantly between decliners and nondecliners because comorbidity has a strong influence on multiple aspects of aging and health.
To evaluate potential correlations among key factors, we additionally examined the associations between the multiple strength measures and BDNF and proBDNF levels, adjusting for age and height. At time 1, isokinetic peak torque during knee flexion at 30°/s was significantly associated with BDNF in L1CAM+ EVs and isometric peak torque at 140° was significantly associated with the ratio of BDNF/proBDNF from total EVs (both p-values < 0.05). At time 2, more strength measures were associated with neurotrophin levels: isokinetic peak torque during knee extension at 180°/s with the BDNF/proBDNF ratio from plasma; grip strength with BDNF from total EVs; and isokinetic peak torque during knee extension at 30°/s, knee extension at 180°/s, knee flexion at 30°/s, and isometric peak torque at 140°, all with the BDNF/proBDNF ratio from total EVs (all p-values <0.05). All of the strength measures were significantly correlated with walking speed at times 1 and 2 (p-values <0.01), except for isokinetic peak torque during knee flexion at 180°/s, which supports walking speed as a marker for motor function.
Full regression models were adjusted for age, sex, plate number, percent fat mass, baseline walking speed, and the comorbidity index. Results with and without adjustment for covariates did not affect the magnitude of the associations between BDNF and proBDNF measures and walking speed decline.
In the logistical regression analyses, only proBDNF levels from the L1CAM+EVs are associated with an increased risk of being a walking speed decliner at both time points (Table 3). Mean differences in BDNF measures, adjusted for age and sex, are shown in Supplemental Table 2 by decliner status and time. In L1CAM+ EVs, BDNF levels (Figure 3A) did not differ between decliners and nondecliners or between visits but proBDNF levels (Figure 3B) were different between decliners and nondecliners at both time points but not between visits. The BDNF/proBDNF ratio is also not different between decliners and nondecliners or between visits. In plasma samples, the level of BDNF (Supplemental Figure 1A, S1A), proBDNF (Figure S1B), and the BDNF/proBDNF ratio (Figure S1C) did not differ between decliners and non-decliners or between visits. In total EVs, the level of BDNF (Figure S2A), proBDNF (Figure S2B), and the BDNF/proBDNF ratio (Figure S2C) did not differ between decliners and non-decliners or between visits.
Table 3.
A. Adjusted odds ratio (OR) for time 1, where only proBDNF L1CAM+ EVs predict decliners. Models adjusted for age, sex, plate number, percent fat mass, baseline gait speed, and comorbidity index. B. Adjusted OR for time 2, where only proBDNF L1CAM+ EVs predict decliners. Models adjusted for age, sex, plate number, percent fat mass, baseline gait speed, and comorbidity index.
| A. | |||
|---|---|---|---|
| Time One | Measurement | OR | 95% CI |
| Plasma | BDNF | 0.86 | (0.58, 1.26) |
| proBDNF | 1.14 | (0.91, 1.44) | |
| BDNF:proBDNF | 0.96 | (0.87, 1.06) | |
| Total Evs | BDNF | 0.08 | (<0.001, >999.99) |
| proBDNF | 3.01 | (0.31, 29.75) | |
| BDNF:proBDNF | 0.86 | (0.19, 3.83) | |
| L1CAM + EVs | BDNF | 0.07 | (<0.01, 1.88) |
| proBDNF | 2.12 | (1.09, 4.15) | |
| BDNF:proBDNF | 0.45 | (0.15, 1.36) | |
| B. | |||
| Time Two | Measurement | OR | 95% CI |
| Plasma | BDNF | 0.91 | (0.56, 1.46) |
| proBDNF | 1.13 | (0.93, 1.36) | |
| BDNF:proBDNF | 1 | (0.96, 1.05) | |
| Total Evs | BDNF | 1.82 | (<0.001, >999.99) |
| proBDNF | 13.5 | (0.32, 567.73) | |
| BDNF:proBDNF | 0.53 | (0.10, 2.81) | |
| L1CAM + EVs | BDNF | 0.84 | (0.10, 6.97) |
| proBDNF | 2.6 | (1.07, 6.29) | |
| BDNF:proBDNF | 0.74 | (0.26, 2.13) | |
Figure 3.
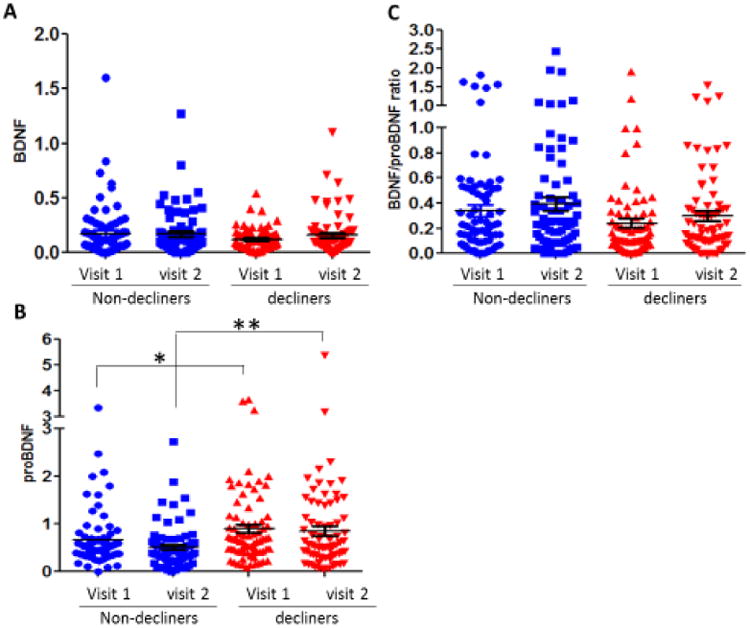
A. BDNF readings from L1CAM+ EVs are not significantly different between decliners and non-decliners or visits. B. proBDNF readings from L1CAM+ EVs are significantly different between decliners and non-decliners at both time points. C. The ratio of BDNF to proBDNF in L1CAM+ EVs is not significantly different between decliners and non-decliners or time points.
Discussion
This study has three main findings: 1) There were low levels of cross-reactivity between BDNF and proBDNF using two widely used commercially available assays; 2) BDNF and proBDNF levels were higher in L1CAM+ EVs than in plasma or total plasma EVs; and 3) walking speed decliners had higher levels of proBDNF in L1CAM+ EVs compared to nondecliners, even after adjustment for multiple factors affecting walking speed.
Research on EVs in recent years has documented an impressive variety of cargo molecules (collated in the combined ExoCarta/EVpedia dataset (50, 51)), which can potentially be used as disease biomarkers for various conditions and disease states (52, 53). Our team has pioneered a new approach in biomarker discovery for AD and other neurodegenerative diseases by measuring candidate biomarkers in plasma L1CAM+ EVs, including pathogenic molecules (42), markers of metabolic and lysosomal dysfunction (40, 41), but also intracellular signaling molecules that promote cellular resistance to various stressors (39, 45). The concentration of some of these molecules (e.g tau) can be many-fold higher in L1CAM+ EVs compared to plasma (39). Therefore, our findings of higher BDNF and proBDNF in L1CAM+ EVs compared to plasma and total plasma EVs are not surprising. Importantly, biomarker levels in this enriched EV sub-population may more closely reflect brain changes compared to plasma, especially when the biomarker of interest is produced by the brain as well as by peripheral cells, as is the case for BDNF. The latter findings suggest that neuronal alterations contribute to walking speed decline during aging, and further suggest a potential value for analyses based on plasma EV subpopulations in elucidating underlying cellular and molecular mechanisms in their cells of origin. Our finding that proBDNF levels are elevated in plasma EVs of neuronal cell origin suggests a role for perturbed BDNF signaling in age-related decline in walking speed. More broadly, our findings suggest that molecular interrogation of L1CAM+ EVs may serve as a platform for investigating a wide range of aging related processes and diseases. Further developments in isolation of cell-origin specific EVs is warranted to increase our ability to study diseases and age related alterations in humans in a minimally invasive manner.
BDNF plays important roles in learning and memory, and motor functions. Experiments in both rodents and humans have found that exercise increases BDNF levels, and can reverse the effects of a high-fat diet through BDNF signaling (54-58). Associations between motor function and BDNF were reported in a previous study (59). In addition, serum levels of BDNF are correlated with poor motor function in Parkinson's disease patients (60). Moreover, mice with BDNF haploinsufficiency exhibit a decline of motor performance and in synaptic markers in their motor cortex, but no differences in learning and memory or hippocampal synaptic markers (61). Clinical studies of exercise using plasma or serum BDNF measures have reported contrasting findings in that clinical trials suggest that BDNF increases with exercise while observational studies have found an inverse relationship (34). Our analysis of plasma and plasma-derived EVs revealed no associations of plasma BDNF or proBDNF levels on motor decline, whereas proBDNF in L1CAM+ EVs uniquely discriminated between decliners and nondecliners. Given that L1CAM+ EVs more closely reflect brain processes, the association of elevated L1CAM+ EV proBDNF levels with motor decline suggests that a neural contributor may be a prominent factor in walking speed decline.
BDNF is cleaved from proBDNF by different proteases and in response to stimulation (62, 63). Though proBDNF has been shown to have opposite effects of BDNF in learning and memory, and despite the plethora of information tying BDNF to motor function, many articles investigating motor function and BDNF ignore proBDNF. However those that include plasma measurements of proBDNF have found that the serum levels are negatively correlated with motor function (for instance, in recovery after stroke (64). There has been evidence of BDNF being produced in smooth and skeletal muscle (65, 66), but no studies have specifically examined the effects of proBDNF on muscle. However, several studies discuss the role of proBDNF in cell death and atrophy of motoneurons (63, 67). Here we found that higher proBDNF only in L1CAM+ EVs is associated with walking speed decline, suggesting that proBDNF plays a role in motor function and aging-associated motor decline. Decliners may either be producing an excess of proBDNF, have reduced cleavage of proBDNF, or an increase in release into the circulation (packed predominantly into L1CAM+ EVs). However, the fact that we see only minimal reduction in BDNF levels between groups suggests that an alteration in cleavage is not the main culprit.
In this study we found a novel association between proBDNF and aging-related walking speed decline using L1CAM+ EVs as a diagnostic platform. This supports the broader hypothesis that increased proBDNF levels are associated with motor impairment including with aging-related motor impairment. Though previous studies have found associations between motor function and BDNF plasma levels, we did not find any such association, raising doubts about any specific implication of plasma BDNF levels in aging-related motor impairment.
While our study was designed to assess potential causality (operating within the timeframe between the two time points) by including “before and after” measures of gait speed and neurotrophins, we were unable to achieve this goal. While we anticipated that associations between the neurotrophins and walking speed would only develop at time 2, we observed an association between LICAM+ EVs proBDNF at both time 1 and time 2. Our walking speed decliner group had a slower baseline gait speed, and even though we accounted for this in our analyses, the associations between L1CAM+ EVs and walking speed at time 1 persisted. Therefore, the neurotrophin changes may have preceded our time 1 observations and the direction of effect cannot be determined.
Many factors affect change in walking speed. While changes in muscle strength are a potential contributing factor, and soluble plasma levels of BDNF are known to be associated with muscle (68, 69), our study found no differences in strength measures between decliners and non-decliners, and found no associations between strength and proBDNF from L1CAM+ EVs. The association we found between between neuronal origin EV-derived proBDNF, but not circulating soluble proBDNF, and decline in walking speed provides supporting evidence for a contribution from the nervous system to decline in walking speed.
Conclusion
With the increase in lifespan, healthspan becomes of paramount importance, especially the preservation of cognitive and motor function. Multiple lines of evidence suggest that cognitive and motor functions are inter-related and BDNF signaling is perhaps the most prominent mechanism evoked to support this connection. Our findings highlight the complexity of measuring candidate biomarkers in biological fluids, as they may be variably present in different fractions in mature and precursor forms. Moreover, clinical studies where levels of a single molecule are used to characterize a complex and likely multifactorial phenomenon (typical for all aging-associated changes) may oversimplify this inherent complexity. More research should be done to advance EV research, and, in particular, studies of cell type-specific subsets of EVs that can further improve knowledge of complex phenomena of aging.
Supplementary Material
Highlights.
There was little or no cross-reactivity between assays for BDNF and proBDNF
BDNF levels in extracellular vesicles, particularly L1CAM+ EVs (enriched for neuronal origin) were higher than in plasma
Levels of proBDNF in L1CAM+ EV were higher in older adults with walking speed decline compared to those without walking speed decline
Acknowledgments
This work was supported entirely by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Artaud F, Singh-Manoux A, Dugravot A, Tzourio C, Elbaz A. Decline in Fast Gait Speed as a Predictor of Disability in Older Adults. Journal of the American Geriatrics Society. 2015;63:1129–1136. doi: 10.1111/jgs.13442. [DOI] [PubMed] [Google Scholar]
- 2.Best JR, Liu-Ambrose T, Boudreau RM, Ayonayon HN, Satterfield S, Simonsick EM, et al. An Evaluation of the Longitudinal, Bidirectional Associations Between Gait Speed and Cognition in Older Women and Men. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71:1616–1623. doi: 10.1093/gerona/glw066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumurgier J, Artaud F, Touraine C, Rouaud O, Tavernier B, Dufouil C, et al. Gait Speed and Decline in Gait Speed as Predictors of Incident Dementia. The journals of gerontology Series A, Biological sciences and medical sciences. 2016 doi: 10.1093/gerona/glw110. [DOI] [PubMed] [Google Scholar]
- 4.Perera S, Patel KV, Rosano C, Rubin SM, Satterfield S, Harris T, et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71:63–71. doi: 10.1093/gerona/glv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. Jama. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. Journal of the American Geriatrics Society. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 7.White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the Health, Aging and Body Composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:456–464. doi: 10.1093/gerona/gls197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) The journal of nutrition, health & aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 10.Tian Q, An Y, Resnick SM, Studenski S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: Results from the Baltimore Longitudinal Study of Aging. Age and ageing. 2016 doi: 10.1093/ageing/afw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. Journal of the American Geriatrics Society. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-Related Change in Mobility: Perspectives From Life Course Epidemiology and Geroscience. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71:1184–1194. doi: 10.1093/gerona/glw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosso AL, Studenski SA, Longstreth WT, Jr, Brach JS, Boudreau RM, Rosano C. Contributors to Poor Mobility in Older Adults: Integrating White Matter Hyperintensities and Conditions Affecting Other Systems. The journals of gerontology Series A, Biological sciences and medical sciences. 2016 doi: 10.1093/gerona/glw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, et al. Brain structural change and gait decline: a longitudinal population-based study. Journal of the American Geriatrics Society. 2013;61:1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- 15.Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR. Neurological Gait Abnormalities Moderate the Functional Brain Signature of the Posture First Hypothesis. Brain topography. 2016;29:334–343. doi: 10.1007/s10548-015-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray PS, Holmes PV. An overview of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus. International journal of peptides. 2011;2011:654085. doi: 10.1155/2011/654085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, et al. BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends in endocrinology and metabolism: TEM. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. Journal of psychiatric research. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 21.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]
- 22.Vaughan S, Wallis M, Polit D, Steele M, Shum D, Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age and ageing. 2014;43:623–629. doi: 10.1093/ageing/afu010. [DOI] [PubMed] [Google Scholar]
- 23.Hortobagyi T, Lesinski M, Gabler M, VanSwearingen JM, Malatesta D, Granacher U. Effects of Three Types of Exercise Interventions on Healthy Old Adults' Gait Speed: A Systematic Review and Meta-Analysis. Sports medicine (Auckland, NZ) 2015;45:1627–1643. doi: 10.1007/s40279-015-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregory MA, Boa Sorte Silva NC, Gill DP, McGowan CL, Liu-Ambrose T, Shoemaker JK, et al. Combined Dual-Task Gait Training and Aerobic Exercise to Improve Cognition, Mobility, and Vascular Health in Community-Dwelling Older Adults at Risk for Future Cognitive Decline1. Journal of Alzheimer's disease : JAD. 2017 doi: 10.3233/JAD-161240. [DOI] [PubMed] [Google Scholar]
- 25.Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, et al. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Molecular brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science (New York, NY) 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 27.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature neuroscience. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 29.Teixeira AL, Barbosa IG, Diniz BS, Kummer A. Circulating levels of brain-derived neurotrophic factor: correlation with mood, cognition and motor function. Biomarkers in medicine. 2010;4:871–887. doi: 10.2217/bmm.10.111. [DOI] [PubMed] [Google Scholar]
- 30.Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, et al. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PloS one. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes BS, Molendijk ML, Kohler CA, Soares JC, Leite CM, Machado-Vieira R, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC medicine. 2015;13:289. doi: 10.1186/s12916-015-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Frontiers in human neuroscience. 2014;8:985. doi: 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore Longitudinal Study of Aging. PloS one. 2010;5:e10099. doi: 10.1371/journal.pone.0010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scandinavian journal of medicine & science in sports. 2014;24:1–10. doi: 10.1111/sms.12069. [DOI] [PubMed] [Google Scholar]
- 35.Chacon-Fernandez P, Sauberli K, Colzani M, Moreau T, Ghevaert C, Barde YA. Brain-derived Neurotrophic Factor in Megakaryocytes. 2016;291:9872–9881. doi: 10.1074/jbc.M116.720029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. The American journal of pathology. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- 37.Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H. Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. Journal of neuroscience research. 1995;42:21–33. doi: 10.1002/jnr.490420104. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura S, Ochi H, Isobe N, Matsushita T, Motomura K, Matsuoka T, et al. Altered production of brain-derived neurotrophic factor by peripheral blood immune cells in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2010;16:1178–1188. doi: 10.1177/1352458510375706. [DOI] [PubMed] [Google Scholar]
- 39.Mustapic M, Eitan E, Werner JK, Jr, Berkowitz ST, Lazaropoulos MP, Tran J, et al. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front Neurosci. 2017;11:278. doi: 10.3389/fnins.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11:600–607.e601. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016 doi: 10.1096/fj.201600756R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer's disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016 doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Low neural exosomal levels of cellular survival factors in Alzheimer's disease. Annals of clinical and translational neurology. 2015;2:769–773. doi: 10.1002/acn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. Journal of the American Geriatrics Society. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 47.Fabbri E, An Y, Zoli M, Tanaka T, Simonsick EM, Kitner-Triolo MH, et al. Association Between Accelerated Multimorbidity and Age-Related Cognitive Decline in Older Baltimore Longitudinal Study of Aging Participants without Dementia. Journal of the American Geriatrics Society. 2016;64:965–972. doi: 10.1111/jgs.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 49.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 50.Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amiral J, Seghatchian J. Measurement of extracellular vesicles as biomarkers of consequences or cause complications of pathological states, and prognosis of both evolution and therapeutic safety/efficacy. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2016;55:23–34. doi: 10.1016/j.transci.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Park YH, Shin HW, Jung AR, Kwon OS, Choi YJ, Park J, et al. Prostate-specific extracellular vesicles as a novel biomarker in human prostate cancer. Scientific reports. 2016;6:30386. doi: 10.1038/srep30386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of neurochemistry. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 57.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 58.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiology of learning and memory. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cellular and molecular neurobiology. 2010;30:493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson's disease. Journal of neurology. 2010;257:540–545. doi: 10.1007/s00415-009-5357-2. [DOI] [PubMed] [Google Scholar]
- 61.Carreton O, Giralt A, Torres-Peraza JF, Brito V, Lucas JJ, Gines S, et al. Age-dependent decline of motor neocortex but not hippocampal performance in heterozygous BDNF mice correlates with a decrease of cortical PSD-95 but an increase of hippocampal TrkB levels. Experimental neurology. 2012;237:335–345. doi: 10.1016/j.expneurol.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 62.Cao W, Duan J, Wang X, Zhong X, Hu Z, Huang F, et al. Early enriched environment induces an increased conversion of proBDNF to BDNF in the adult rat's hippocampus. Behavioural brain research. 2014;265:76–83. doi: 10.1016/j.bbr.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Je HS, Yang F, Ji Y, Nagappan G, Hempstead BL, Lu B. Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15924–15929. doi: 10.1073/pnas.1207767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niimi M, Hashimoto K, Kakuda W, Miyano S, Momosaki R, Ishima T, et al. Role of Brain-Derived Neurotrophic Factor in Beneficial Effects of Repetitive Transcranial Magnetic Stimulation for Upper Limb Hemiparesis after Stroke. PloS one. 2016;11:e0152241. doi: 10.1371/journal.pone.0152241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, et al. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia Implications for paracrine and target-derived Neurotrophic functions. The American journal of pathology. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 67.Taylor AR, Gifondorwa DJ, Robinson MB, Strupe JL, Prevette D, Johnson JE, et al. Motoneuron programmed cell death in response to proBDNF. Developmental neurobiology. 2012;72:699–712. doi: 10.1002/dneu.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C. Autocrine and immune cell-derived BDNF in human skeletal muscle: implications for myogenesis and tissue regeneration. The Journal of pathology. 2013;231:190–198. doi: 10.1002/path.4228. [DOI] [PubMed] [Google Scholar]
- 69.Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5739–5749. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


