Abstract
Hyperacusis is a loudness hypersensitivity disorder in which moderate-intensity sounds are perceived as extremely loud, aversive and/or painful. To assess the aversive nature of sounds, we developed an Active Sound Avoidance Paradigm (ASAP) in which rats altered their place preference in a Light/Dark shuttle box in response to sound. When no sound (NS) was present, rats spent more than 95% of the time in the Dark Box versus the transparent Light Box. However, when a 60 or 90 dB SPL noise (2–20 kHz, 2–8 kHz, or 16–20 kHz bandwidth) was presented in the Dark Box, the rats’ preference for the Dark Box significantly decreased. Percent time in the dark decreased as sound intensity in the Dark Box increased from 60 dB to 90 dB SPL. Interestingly, the magnitude of the decrease was not a monotonic function of intensity for the 16–20 kHz noise and not related to the bandwidth of the 2–20 kHz and 2–8 kHz noise bands, suggesting that sound avoidance is not solely dependent on loudness but the aversive quality of the noise as well. Afterwards, we exposed the rats for 28 days to a 16–20 kHz noise at 102 dB SPL; this exposure produced a 30–40 dB permanent threshold shift at 16 and 32 kHz. Following the noise exposure, the rats were then retested on the ASAP paradigm. High-frequency hearing loss did not alter Dark Box preference in the no-sound condition. However, when the 2–20 kHz or 2–8 kHz noise was presented at 60 or 90 dB SPL, the rats avoided the Dark Box significantly more than they did before the exposure, indicating these two noise bands with energy below the region of hearing loss were perceived as more aversive. In contrast, when the 16–20 kHz noise was presented at 60 or 90 dB SPL, the rats remained in the Dark Box presumably because the high-frequency hearing loss made 16–20 kHz noise less audible and less aversive. These results indicate that when rats develop a high-frequency hearing loss, they become less tolerant of low frequency noise, i.e., high intensity sounds are perceived as more aversive and should be avoided.
Keywords: Hyperacusis, Avoidance, Noise-Induced Hearing Loss, Light-Dark Box
1. Introduction
Hyperacusis, or loudness intolerance, is an auditory hypersensitivity disorder in which an individual perceives moderate-intensity, everyday sounds as abnormally or uncomfortably loud (Blaesing et al., 2012). Approximately 6–9% of adults have loudness intolerance problems, but the percentage is likely higher because many are unaware of their condition. Hyperacusis is often associated with hearing loss and tinnitus. Approximately 40% of tinnitus patients have hyperacusis whereas up to 86% of hyperacusis patients also have tinnitus (Baguley, 2003). This comorbidity suggests that the neural substrate that gives rise to tinnitus and hyperacusis has some common underlying mechanisms.
Hyperacusis, like many other neurological disorders, has considerable phenotypic diversity (Pienkowski et al., 2014; Tyler et al., 2014). The simplest form is pure loudness hyperacusis, a condition in which moderate-intensity sounds are perceived as too loud. A second more complex form, referred to as avoidance hyperacusis, is a condition in which sounds are not only too loud, but also extremely annoying, dangerous or fear-evoking. Those with avoidance hyperacusis attempt to escape from moderately intense sounds by wearing earplugs or avoiding noisy social situations (Ashkenazi et al., 2010; Blaesing et al., 2012). Finally, some hyperacusis patients not only experience moderate-intensity sounds as too loud, but also painful, a condition referred to as pain hyperacusis (Tyler et al., 2014). For these patients, sound-evoked otalgia evokes painful sensations within and/or around the ear that can begin almost immediately or develop slowly over a few hours (McFerran et al., 2007; Van Campen et al., 1999). Thus, in many cases, it is possible that the affective qualities of sound are just as critical as its acoustic properties for inducing hyperacusis.
While some progress has been made toward identifying the neural mechanisms involved in hyperacusis (Chen et al., 2016; Chen et al., 2015; Diehl et al., 2015), advances have been hampered by the paucity of animal behavioral models that can both identify individual animals experiencing hyperacusis and determine the nature of the disturbance (i.e. if sounds are perceived as too loud, aversive, or painful). Enhanced acoustic startle reflex (ASR) amplitudes have often been used to infer the presence of hyperacusis in animals that have been exposed to noise or ototoxic drugs (Hickox et al., 2014; Salloum et al., 2014; Sun et al., 2009; Turner et al., 2008). Partial support for this metric comes from human studies in which the amplitude of the acoustic startle reflex was inversely correlated with loudness discomfort level (LDL). However, the acoustic startle amplitude was not correlated with another metric of hyperacusis, namely scores on a sound level tolerance questionnaire. These results suggest that LDL and ASR may only reflect loudness, but not other aspects of hyperacusis such as annoyance, fear or avoidance of loud sounds (Knudson et al., 2016).
Reaction time-intensity (RT-I) functions have also been used to assess normal and abnormal loudness growth. Reaction time (RT) decreases with intensity and in humans RT-I measures are closely correlated with numerical estimates of loudness growth and equal loudness contours (Marshall et al., 1980; Pfingst et al., 1975; Stebbins, 1966). In some animal models of noise- or drug-induced hearing loss, RTs initially decrease rapidly with increasing intensity, but the function decelerates eventually catching up to normal RTs observed at high intensities. Thus, RT-I curves recapitulate the basic features of loudness recruitment. Hyperacusis-like RT-I functions have been observed in canaries with a genetic high-frequency hearing loss; RTs in this model were faster than normal-hearing controls at moderate to high intensities (Lauer et al., 2007). Hyperacusis-like RT-I functions have also been observed in rats treated with high-dose salicylate known to cause 20–30 dB hearing loss, tinnitus and neural hyperactivity in the central auditory pathway (Chen et al., 2014a; Chen et al., 2015; Radziwon et al., 2017). However, because collecting RT-I measures typically requires operant training using positive reinforcement, it is difficult to assess if animals with hyperacusis-like RT-I curves also find moderate/high intensity sounds unpleasant or aversive. Because rats and canaries with hyperacusis did not avoid but continued to respond at high stimulus intensities, it is reasonable to assume that RT-I functions are best suited for assessing pure loudness hyperacusis, rather than avoidance hyperacusis.
A recent human study found a strong correlation between self-reported avoidance of loud sounds and distress (Blaesing et al., 2012). Based on these human data, we decided to investigate potentially more complex aspects of hyperacusis in which animals avoid intense sounds because of the annoyance or distress it evokes. To accomplish this, we developed an Active Sound Avoidance Paradigm (ASAP) that takes advantage of a rat’s innate aversion to bright, open-spaces and preference to stay in a dark, enclosed box (Bourin et al., 2003; Crawley, 1981). Sound avoidance was quantified by measuring the shift in the rat’s preference from the Dark Box to the Light Box as sound level in the Dark Box increased. We then induced a high-frequency hearing loss in the same rats and found that they avoided sounds at a significantly lower intensity than before the noise exposure.
2. Materials and methods
2.1 Subjects
Six male Sprague-Dawley (Charles River Lab, Inc.) rats (~4 months old) were used in the study. The rats were housed in the Laboratory Animal Facility at the University at Buffalo and given free access to food and water. The colony was maintained at 22 °C with a 12 h light/dark cycle. All procedures in this project were approved by the Institutional Animal Care and Use Committee (HER05080Y) at the University at Buffalo and carried out in accordance with NIH guidelines.
2.2 Noise exposure
Details of the noise exposure can be found in our previous study (Chen et al., 2014b). Briefly, the six rats were housed individually in their home cages in the Lab Animal Facility and exposed to a 102 dB SPL (+/− 2.5 dB), 16–20 kHz noise 24 h per day for 4 weeks. The noise exposure was delivered from a speaker mounted above the center of each cage. Sound levels were measured at several locations within each cage at the height of the animals head using a sound level meter (Larson Davis System 824 sound level meter, Larson Davis, half-inch free-field microphone model 2540). The sound level in the 1/3 octave band centered at 16 kHz was 102 dB SPL (+/− 2.5 dB).
2.3 ABR
Our auditory brainstem response (ABR) procedures are described in detail in our earlier publications (Chen et al., 2014b; Chen et al., 2010; Kane et al., 2012). Rats were anesthetized with a ketamine (50 mg/kg)/xylazine (6 mg/kg) cocktail (i.p.) and placed on a regulated heating pad (FHC, model 40-90-2) set to maintain the core body temperature at 37 °C. Subdural electrodes were placed at the vertex (non-inverting), ipsilateral mastoid (inverting) and hind limb (ground). Stimuli were delivered to the ear ipsilateral to the mastoid electrode through the sound delivery tube on the IHS loudspeaker. ABRs were collected using a computerized stimulus presentation and data acquisition system from Intelligent Hearing Systems (IHS, Miami Florida). ABRs were filtered, amplified and digitized (1024 presentations, 40 kHz sampling rate, 30–3000 Hz, 100X) in response to tone bursts presented at 4, 12, 16 and 32 kHz (1 ms rise/fall, cosine gated, 5 ms duration, 21/s). Stimulus presentations began at a high sound level to obtain a clear and consistent ABR waveform and then the intensity was lowered in 10 dB steps until the response completely disappeared. ABR threshold was defined as the lowest intensity at which an ABR waveform could be visually identified.
2.4 ASAP Equipment
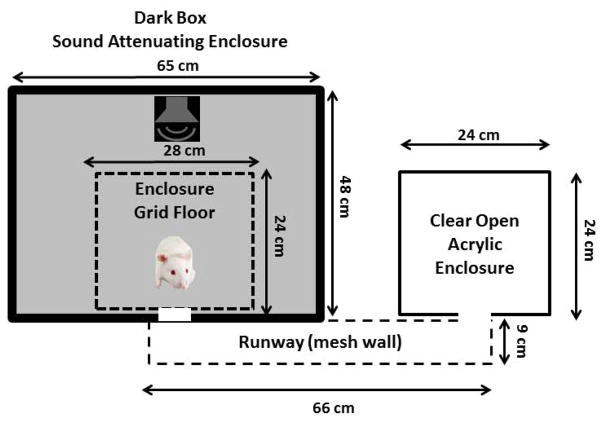
The ASAP paradigm is based on a rodent’s innate aversion to brightly illuminated open areas and preference for a dark enclosed space (Bourin et al., 2003). The ASAP apparatus is schematized in Figure 1A with the nominal dimensions of the three main parts. (1) The dark enclosure (Dark Box) consisted of a sound attenuating box (MDF board, 2.54 cm thick) lined with sound absorbing foam panels mounted on the interior walls (Sonex Acoustics, thickness 5 cm). An enclosure with a grid floor (Med Associates, ENV-007) was located near one wall of the Dark Box; rats were confined to the grid floor enclosure to maintain consistent sound levels during testing. A loudspeaker (Fostex FT 28D) was mounted on the roof of the grid floor enclosure. The entry/exit port (square opening, 6.4 cm) in the wall of the grid floor enclosure was connected to an entry/exit port of similar size on the wall of the outer Dark Box. (2) The entry/exit port led to a runway with a plastic floor and wire mesh walls; the runway ran alongside the wall of the Dark Box. (3) An entry/exit port of the clear, open acrylic enclosure (Light Box) was connected to the opposite end of the runway. Two bright lights located above the apparatus illuminated the runway and transparent Light Box (300–350 lux).
Figure 1.
Figure 1a. Overhead view of ASAP apparatus with nominal dimensions: (1) Dark Box, sound attenuating enclosure (height: 55 cm) equipped with loudspeaker located above the grid floor enclosure (height: 30 cm), (2) runway with acrylic floor and wire mesh walls (height: 10 cm) and (3) clear open acrylic enclosure (height: 30 cm). Runway connects Dark Box and bright Light Box.
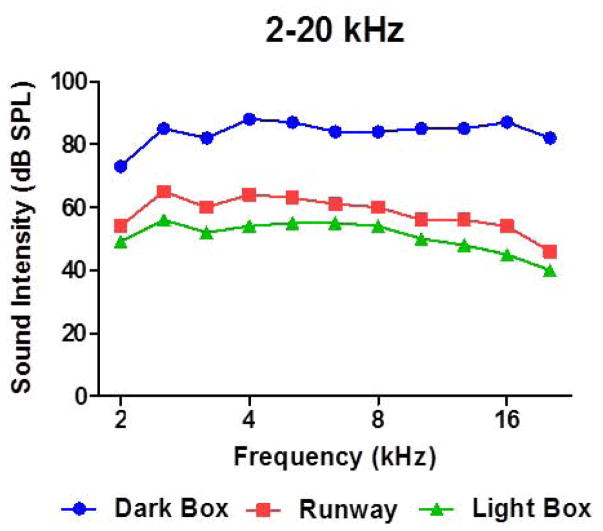
Figure 1b. Differences in the sound intensity between the Dark Box (blue), runway (red) and Light Box (green) for 2–20 kHz noise at high intensity (90 dB).
The loudspeaker in the Dark Box was connected to a Crown amplifier (XLS202). The output of the sound card in a personal computer was fed to the input of the amplifier. The intensity and bandwidth of the three bands of noise (2–8 kHz; 2–20 kHz and 16–20 kHz) used in the study were digitally synthesized with Adobe Audition software. AnyMaze software was used to control the presentation order and duration of the sounds presented through the speaker in the Dark Box. Sound levels were measured at the height of the rat’s head at several locations in Light Box, runway and Dark Box using a sound level meter (Larson Davis, model 824) and half-inch condenser microphone (Larson Davis, model 2540). Sounds were attenuated by 30–40 dB in the Light Box with the greatest attenuation at higher frequencies (Figure 1B). Attenuation in the runaway was slightly less but still considerable at 25–35 dB (Figure 1B).
2.5 ASAP Procedures
Prior to ASAP testing, the rats were acclimated to handling by experimenters and the test environment. Following acclimation to handling and the testing room, animals were habituated to the testing chambers (without bright lights on) over three days with three consecutive 10 minute trials/day. Next, baseline light-dark preference was measured over an additional three days with three consecutive 10 minute sessions/day. All ASAP testing was performed at a similar time each day (9am–12pm) by the same experimenters, all of whom had extensively handled and habituated the rats.
Following the acclimation period, rats were tested with ASAP on three consecutive days using one of the three noise bands on each day. Noise band order was randomized between subjects. Each daily session consisted of three consecutive 10-minute trials. On each trial, one of three possible acoustic conditions occurred: (1) No sound (NS), (2) 60 dB SPL or (3) 90 dB SPL noise. Trial condition was randomized between subjects and across sessions. The apparatus was cleaned between sessions. The location of the rat was monitored by a camera located above the apparatus. The camera output was displayed on a computer monitor located in an adjacent room where the experimenter recorded the time spent in the Dark Box on each trial using a stopwatch. Because the runway and Light Box had similar luminance and attenuation levels, time spent in the Dark Box was our primary metric for light/dark preference. A complete set of ASAP measurements was typically collected in 10–14 days. Time spent in the Dark Box is expressed as a percentage of each 10-minute trial.
2.6 Experimental Design
In Phase 1 of the study, a complete set of baseline ASAP measurements was obtained to determine the rat’s avoidance behavior to the three types of noise (2–20 kHz, 2–8 kHz, 16–20 kHz) presented at 60 and 90 dB SPL. Afterwards, ABR thresholds were measured at 4, 12, 16 and 32 kHz to establish the rat’s hearing sensitivity prior to noise exposure. In Phase II of the study, the rats were exposed to 16–20 kHz narrowband noise presented 24 h/d for 4 weeks at 102 dB SPL. The rats were allowed to recover from the noise exposure for 4–5 weeks and then the ASAP was repeated to determine if the high frequency hearing loss had altered their sound avoidance behavior. Afterwards, ABR measurements were repeated in order to determine the amount of hearing loss at the four test frequencies.
3. Results
3.1 Dark Box Preference
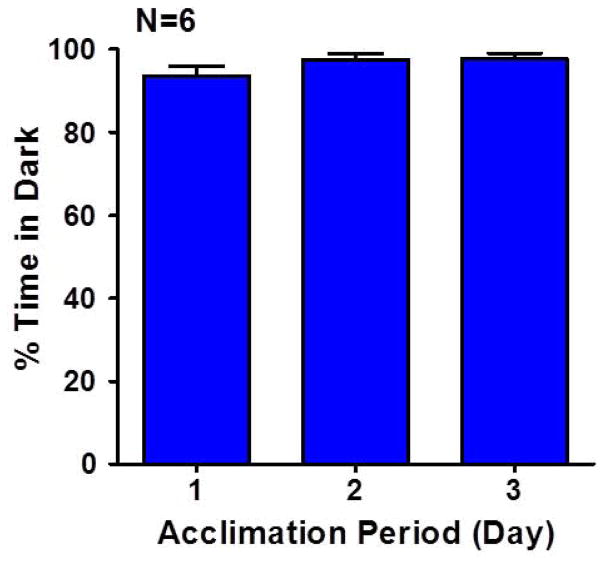
To determine if rats had a preference for the Dark Box in our ASAP apparatus, the animals were tested on three 10-minute trials per day for three days during the acclimation period. Figure 2 shows the mean (n=6 rats, +/− SEM) percent time spent in the Dark Box on days 1–3. The rats showed a strong preference for the Dark Box on all three days consistent with previous reports (Bourin et al., 2003; Crawley, 1981). On average, the rats spent between 93% and 98% of the time in the Dark box on days 1–3.
Figure 2.
Mean (N=6, + SEM) percent time spent in Dark Box during the three day acclimation period.
3.2 ASAP
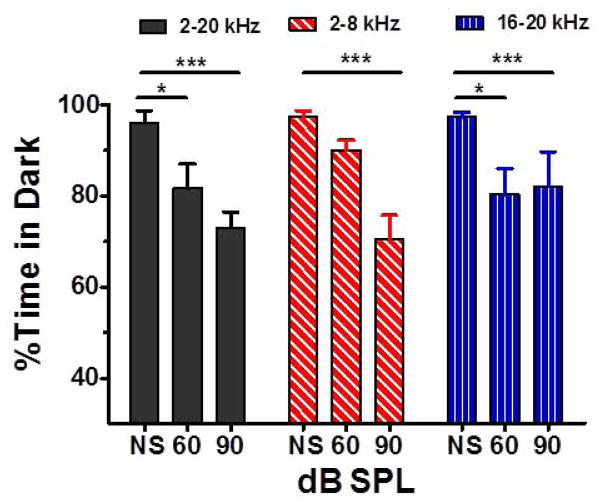
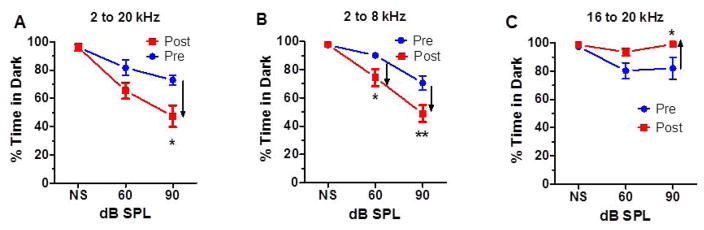
To determine if moderate or high intensity noises would cause the rats to avoid the Dark Box, we compared the percent time spent in the Dark Box with no sound (NS) to the percent time spent in the Dark Box when one of the three noise bands (2–20 kHz, 2–8 kHz or 16–20 kHz) was presented at 60 or 90 dB SPL in the Dark Box (Figure 3). For the 2–20 kHz condition, the mean percent time in the dark decreased from 96% with no sound to 82% when the sound intensity was 60 dB SPL and to 73% when the intensity was 90 dB SPL. There was a significant effect of sound stimulation (One-way repeated measure ANOVA, F(2, 5)= 20.09, p=0.0003). The 60 dB and 90 dB conditions were significantly different from the no sound condition (Tukey post-hoc, p<0.05; Cohen’s d = 1.22 for 60 dB and 2.79 for 90 dB). For the 2–8 kHz condition, the mean percent time in the dark decreased from 97% with NS to 90% when the intensity was 60 dB SPL and to 71% when the intensity was 90 dB SPL. There was a significant effect of intensity (One-way repeated measure ANOVA, F(2, 5)=33.99, p<0.0001). The 90 dB condition was significantly different from the 60 dB and the NS condition (p<0.05; Cohen’s d = 1.56 for 90 dB compared to 60 dB and 2.15 for 90 dB compared to NS). For the 16–20 kHz condition, the mean percent time in the dark decreased from 97% with NS to 80% when the intensity was 60 dB SPL and to 82% when the intensity was 90 dB SPL. There was a significant effect of sound stimulation (One-way repeated measure ANOVA, F(2, 5)=4.257, p=0.46,); however, none of the between group comparisons were significant (p>0.05). Overall, there was clear trend for the rats to avoid the Dark Box when noise was present. Moreover, time spent in the Dark Box decreased as intensity increased, except for the 16–20 kHz noise band.
Figure 3.
Mean (n=6 rats) percent (+SEM) time spent in Dark Box as a function of intensity (NS: no sound, 60 or 90 dB SPL) for 2–20 kHz noise, 2–8 kHz noise and 16–20 kHz noise. Horizontal bars show conditions that were significantly different (**** p<0.001; ** p<0.01, * p<0.05) from 0 dB (no stimulus presented).
3.3 Noise-Induced Hearing Loss
After the baseline ASAP tests were completed, ABR thresholds were measured in all six rats (Figure 4, Baseline from all six rats). Afterwards, the six rats were exposed for 4 weeks to a 102 dB SPL, 16–20 kHz noise. Approximately 4 weeks after the noise exposure ended, their ABR thresholds were re-measured (note: Post-exposure ABR data from two rats were lost due to technical problems, therefore pre- and post-exposure thresholds reflect the data from only the four rats tested both times). As shown in Figure 4, post-exposure thresholds were unaffected at 4 kHz, but were elevated approximately 10 dB at 12 kHz, 40 dB at 16 kHz and 30 dB at 32 kHz. Thus, hearing thresholds were elevated significantly at the high frequencies.
Figure 4.
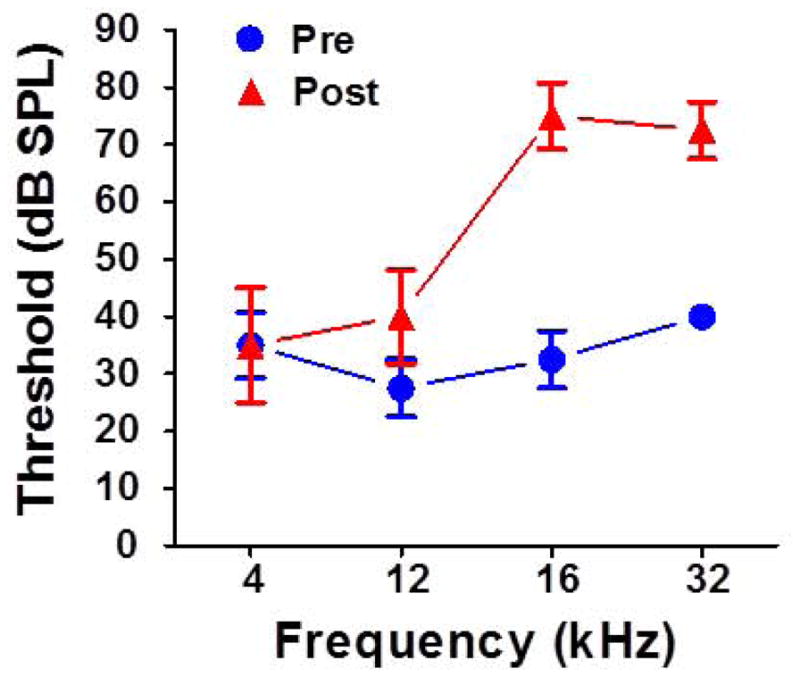
Mean (+/− SEM) pre and post-exposure ABR thresholds (+/− SEM) only for four rats tested before and after the exposure. (note: ABR data from the other two rats were lost due technical problems post-exposure).
3.4 Avoidance Hyperacusis
To determine if the high-frequency hearing loss in the 16–32 kHz range would alter sound avoidance behavior, we repeated the ASAP test approximately 1 month after the end of the 28 day noise-exposure. The high-frequency hearing loss did not alter the rat’s preference for the Dark Box during the NS conditions (Figure 5A–C); the rats continued to spend more than 95% time in the Dark Box. For the 2–20 kHz noise (Figure 5A), percent time in the Dark Box progressively decreased as intensity increased from NS to 90 dB SPL, but the decrease was much larger post-exposure than pre-exposure. At 90 dB SPL, preference decreased from approximately 75% pre-exposure to roughly 50% post-exposure. There was a statistically significant (two-way repeated measures ANOVA) effect of noise treatment (F(1,10)=9.213, p<0.05), stimulus level (F(2, 10)=34.14; p< 0.0001) and treatment x level interaction (F(2,10)=4.32, p<0.05). At 90 dB SPL, percent time in the Dark Box was significantly less post-exposure than pre-exposure (p<0.05, Cohen’s d = 1.90). These results suggest that the rats found the 2–20 kHz noise more aversive after developing a high frequency hearing loss and therefore diminished their natural tendency to stay in the Dark Box.
Figure 5.
Mean (n=6 rats) percent (+/− SEM) time spent in Dark Box as a function of intensity (NS: no sound, 60 or 90 dB SPL) measured pre- and post-noise exposure. Data shown for: (A) 2–20 kHz noise, (B) 2–8 kHz noise and (C) 16–20 kHz noise (** p<0.01, * p<0.05).
For the 2–8 kHz noise (Figure 5B), percent time in the Dark Box progressively decreased as intensity increased from NS to 90 dB SPL, but the decrease was much larger post-exposure than pre-exposure. At 90 dB SPL, percent time in the dark decreased from roughly 70% to approximately 50%. There was a statistically significant (two-way repeated measures ANOVA) effect of noise treatment (F (1,10)=7.705, 1, 10 DF, p<0.05), stimulus level (F(2,10)=63.52; p< 0.0001) and treatment x level interaction (F(2,10)=5.687, p<0.05). At 60 and 90 dB SPL, percent time in the Dark Box was significantly less post-exposure than pre-exposure (p<0.05; Cohen’s d = 1.58 for 60 dB SPL & 1.61 for 90 dB SPL). After the rats developed a high-frequency hearing loss in the 16–32 kHz range, they spent significantly less time in the Dark Box when the 2–8 kHz noise was presented presumably because it was perceived as more aversive.
The noise exposure created a hearing loss of approximately 40 dB at 16 kHz. Therefore, the 16–20 kHz noise stimulus was far less audible post-exposure than pre-exposure resulting in significant changes in the post-exposure ASAP function. Before the noise exposure, the percent time in the Dark Box decreased approximately 20% from NS to the 90 dB condition (Figure 5C). After the noise exposure, percent time in the dark showed little decline from NS to the 60 and 90 dB conditions, presumably because of the substantial high-frequency hearing loss. At 90 dB SPL, percent time in the dark increased from approximately 80% to 98%; the direction of the effect was the opposite that seen for the 2–20 kHz and 2–8 kHz noise bands. There was a statistically significant (two-way repeated measures ANOVA) effect of noise treatment (F (1,10)=6.072, p<0.05) and stimulus level (F (2,10)=5.725; p< 0.05). At 90 dB SPL, percent time in the Dark Box was significantly greater post-exposure than pre-exposure (p<0.05; Cohen’s d = 1.72). After the rats developed a high-frequency hearing loss in the 16–32 kHz range, they spent more time in the Dark Box when the 16–20 kHz noise was presented post-exposure, presumably because the stimulus was far less audible due to the high-frequency hearing loss.
4. Discussion
4.1 ASAP Paradigm
During the NS condition, rats displayed a strong place preference for the Dark Box consistent with prior reports (Figure 2) (Miller et al., 2011). Introducing moderate to high intensity noise into the Dark Box caused the rats to avoid the noise by moving to the Light Box, an effect seen for all three noise bands (Figure 3). The strongest, intensity-dependent shift from the Dark Box to the Light Box occurred with the 2–20 kHz and 2–8 kHz noise bands. A much smaller decline occurred with the 16–20 kHz noise, where the magnitude of the shift was the similar for 60 and 90 dB SPL. One interpretation of these sound-evoked avoidance behaviors is that the rats found the noise aversive and the avoidance typically increased with increasing stimulus intensity. In addition, the magnitude of the place-preference shift also varied with the frequency and/or bandwidth of the noise. During baseline testing, larger place-preference shifts occurred with the 2–20 kHz and 2–8 kHz noise bands versus the 16–20 kHz noise band. The fact that avoidance behavior was greater for larger bandwidth noise is consistent with the known effects of bandwidth on perceived loudness (Scharf, 1970). However, although the 2–20 kHz noise is more than an octave wider than the 2–8 kHz noise band, the results of the two noise bands were nearly identical. If ASAP was purely dependent on loudness, greater avoidance of the Dark Box would be expected for the 2–20 kHz signal than the 2–8 kHz signal because wide band signals are perceived as louder than narrow band signals of equal intensity (Scharf, 1970). The lack of intensity-dependent changes in the 16–20 kHz noise band data provides additional clues on the nature of the task. If ASAP was solely a loudness-dependent task, percent time in the Dark Box should have decreased substantially between 60 and 90 dB SPL as it does for reaction time measures of loudness (Chen et al., 2015; Radziwon et al., 2017). Since avoidance behavior cannot be explained exclusively by the psychoacoustic perception of sound intensity and bandwidth, this suggests that ASAP may be more indicative of the aversive nature of the noise rather than its loudness per se.
Our results suggest that ASAP may be a useful tool for assessing sound avoidance behavior in the rat. One positive aspect of the method is that it takes advantage of a rodent’s innate behavior and therefore does not require time-consuming operant training. A similar place preference task was recently developed to measure avoidance behavior in response to noxious sound stimuli (100–120 dB) (Flores et al., 2015). We have extended these findings to more moderate sound intensities (60–90 dB) that are loud but not acutely harmful or damaging. This has important clinical implications, as decreased tolerance to everyday, moderate-intensity sounds is the defining characteristic of hyperacusis. Moreover, because there is an innate difference in place preference in our paradigm (light vs dark), the changes in observed behavior are likely a robust indicator of the aversive qualities of sound, as animals must actively eschew their natural preference for a dark enclosed space by moving to an innately aversive bright open space. We therefore think ASAP is ideally suited for examining sound tolerance disturbances, which are associated with a wide-range of neurological disorders. However, further work is needed to determine if ASAP can be used to assess sound avoidance in the expanding array of normal and genetically modified mice as well as other strains of rats. Some examples where ASAP might provide important insights include animal models of anxiety, autism and Williams syndrome (Gomes et al., 2008; Juris et al., 2013; Klein et al., 1990). In addition to intensity and noise bandwidth, further work is needed to identify the key acoustic parameters that drive sound avoidance behavior. Such information could be used to identify brain regions that underlie avoidance behavior to acoustically complex sounds with innate affective features (Davis, 2006; Sander et al., 2001; Seifritz et al., 2003) or neutral signals that acquire affective content as a result of conditioning (Davis, 1986).
4.2 Noise-Induced Hearing Loss and Avoidance Hyperacusis
The 16–20 kHz noise exposure induced a 30–40 dB high-frequency (16–32 kHz) hearing loss (Figure 4); however, the hearing loss did not alter the preference for the Dark Box during the NS condition. These results suggest that Dark Box place preference remains stable over many months and is not affected by high-frequency hearing loss, consistent with previous results (Yang et al., 2011). In contrast, the noise-exposure caused a significant, frequency-dependent change in sound avoidance behavior on the ASAP task. When the 2–20 kHz and 2–8 kHz noise bands were presented at 60 or 90 dB SPL, significantly less time was spent in the Dark Box post-exposure than pre-exposure. One interpretation of these results is that the rats perceived these noise bands as more aversive, annoying or fear-evoking due to the noise-induced hearing loss and/or the result of being exposed for 4 weeks to a very intense noise. Clinically, many hyperacusis patients with high frequency hearing loss have an aversion to noisy environments such as restaurants. One explanation for the aversion to noisy environments comes from learning that it is extremely difficult to communicate in these situations (Zhou et al., 2012). Accordingly, moderate to high intensity noise takes on aversive or annoying connotations because it impedes social interactions. For an animal, moderate to high intensity noise could mask relevant acoustic cues that warn of an approaching predator, social communications or pup calls to its mother (Brouette-Lahlou et al., 1992; Brudzynski, 2009; Tennessen et al., 2016). Because high intensity noise increases corticosterone/cortisol stress hormones (Alario et al., 1987; De Boer et al., 1989; Hasson et al., 2013), sound-induced avoidance behavior may be linked to the degree of stress evoked by our 60 and 90 dB noise stimuli. Long-term noise exposures alter the adrenal cortex and corticosterone plasma levels (Soldani et al., 1999). This raises the possibility that the post-exposure shifts in place-preference (Figure 5A–B) may be related to noise-induced changes in the hypothalamic-pituitary-adrenal axis and alterations in the amount of corticosterone released during ASAP sound stimulation. This hypothesis is consistent with previous results showing that salicylate-induced hyperacusis is associated with an increase in plasma corticosterone levels (Chen et al., submitted 7.10. 2016). While it is possible that an increased stress response may also alter light sensitivity (Digre et al., 2012), the fact that animals were driven from the dark side (for 2–8 or 2–20 kHz noise bands) more robustly following hearing loss suggests even if they did find the light more aversive, the changes to sound perception were much more severe. Future studies can vary both sound and light intensity to determine the interaction between these different sensory modalities.
5. Conclusions
There is a growing realization that hyperacusis is more complicated than just loudness intolerance. The perceived loudness of a sound can be accompanied by other conditions such as pain, avoidance, annoyance and negative emotional responses (e.g., misophonia) (Pienkowski et al., 2014; Tyler et al., 2014). We found that sound avoidance measures varied with sound intensity, suggesting that loudness is indeed a major determinant for this behavior. However, avoidance behavior did not vary with noise bandwidth or intensity in a manner consistent with the known psychoacoustic properties of loudness. Thus, we conclude that, while the task is related to loudness, ASAP measures are strongly affected by the aversive qualities of a given sound. Sound avoidance behavior could be related to annoyance or fear evoked by sound stimulation. It is unclear if these sound avoidance behaviors are innate, as in a baby’s scream (Arnal et al., 2015), learned, as in the case of auditory fear conditioning (Tang et al., 2001), or some combination of the two. By varying the acoustic properties of the stimuli, the genetic background of the animals, and prior sound exposure history of the subjects used in ASAP testing, it may be possible disentangle the relative contribution of these factors to sound intolerance.
Acknowledgments
Supported in part by grants from NIH (R01DC014452 & R01DC014693) and HHF (72364).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alario P, Gamallo A, Villanua MA, Trancho G. Chronic noise stress and dexamethasone administration on blood pressure elevation in the rat. J Steroid Biochem. 1987;28:433–6. doi: 10.1016/0022-4731(87)91062-4. [DOI] [PubMed] [Google Scholar]
- Arnal LH, Flinker A, Kleinschmidt A, Giraud AL, Poeppel D. Human screams occupy a privileged niche in the communication soundscape. Curr Biol. 2015;25:2051–6. doi: 10.1016/j.cub.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Yang I, Mushtaq A, Oshinsky ML. Is phonophobia associated with cutaneous allodynia in migraine? Journal of neurology, neurosurgery, and psychiatry. 2010;81:1256–60. doi: 10.1136/jnnp.2009.198481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM. Hyperacusis. Journal of the Royal Society of Medicine. 2003;96:582–5. doi: 10.1258/jrsm.96.12.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesing L, Kroener-Herwig B. Self-reported and behavioral sound avoidance in tinnitus and hyperacusis subjects, and association with anxiety ratings. International journal of audiology. 2012;51:611–7. doi: 10.3109/14992027.2012.664290. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. European journal of pharmacology. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Brouette-Lahlou I, Vernet-Maury E, Vigouroux M. Role of pups’ ultrasonic calls in a particular maternal behavior in Wistar rat: pups’ anogenital licking. Behav Brain Res. 1992;50:147–54. doi: 10.1016/s0166-4328(05)80296-7. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches. ILAR J. 2009;50:43–50. doi: 10.1093/ilar.50.1.43. [DOI] [PubMed] [Google Scholar]
- Chen GD, Sheppard A, Salvi R. Noise trauma induced plastic changes in brain regions outside the classical auditory pathway. Neuroscience. 2016;315:228–45. doi: 10.1016/j.neuroscience.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Radziwon KE, Kashanian N, Manohar S, Salvi R. Salicylate-induced auditory perceptual disorders and plastic changes in nonclassical auditory centers in rats. Neural plasticity. 2014a;2014:658741. doi: 10.1155/2014/658741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Decker B, Krishnan Muthaiah VP, Sheppard A, Salvi R. Prolonged noise exposure-induced auditory threshold shifts in rats. Hear Res. 2014b;317:1–8. doi: 10.1016/j.heares.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265:63–9. doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Auerbach BD, Manohar S, Radziwon K, Salvi R. Tinnitus and Hyperacusis: Contributions of Paraflocculus, Reticular Formation and Stress. Hear Res. 2016 Jul 10; doi: 10.1016/j.heares.2017.03.005. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife. 2015;4:e06576. doi: 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav. 1981;15:695–9. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci. 1986;100:814–24. doi: 10.1037//0735-7044.100.6.814. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–56. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Van der Gugten J, Slangen JL. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiol Behav. 1989;45:789–95. doi: 10.1016/0031-9384(89)90296-5. [DOI] [PubMed] [Google Scholar]
- Diehl PU, Schaette R. Abnormal Auditory Gain in Hyperacusis: Investigation with a Computational Model. Front Neurol. 2015;6:157. doi: 10.3389/fneur.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digre KB, Brennan KC. Shedding light on photophobia. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2012;32:68–81. doi: 10.1097/WNO.0b013e3182474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores EN, Duggan A, Madathany T, Hogan AK, Marquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, Garcia-Anoveros J. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol. 2015;25:606–12. doi: 10.1016/j.cub.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E, Pedroso FS, Wagner MB. Auditory hypersensitivity in the autistic spectrum disorder. Pro Fono. 2008;20:279–84. doi: 10.1590/s0104-56872008000400013. [DOI] [PubMed] [Google Scholar]
- Hasson D, Theorell T, Bergquist J, Canlon B. Acute stress induces hyperacusis in women with high levels of emotional exhaustion. PLoS One. 2013;8:e52945. doi: 10.1371/journal.pone.0052945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? Journal of neurophysiology. 2014;111:552–64. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juris L, Andersson G, Larsen HC, Ekselius L. Psychiatric comorbidity and personality traits in patients with hyperacusis. International journal of audiology. 2013;52:230–5. doi: 10.3109/14992027.2012.743043. [DOI] [PubMed] [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear Res. 2012;283:80–8. doi: 10.1016/j.heares.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AJ, Armstrong BL, Greer MK, Brown FR., 3rd Hyperacusis and otitis media in individuals with Williams syndrome. The Journal of speech and hearing disorders. 1990;55:339–44. doi: 10.1044/jshd.5502.339. [DOI] [PubMed] [Google Scholar]
- Knudson IM, Melcher JR. Elevated Acoustic Startle Responses in Humans: Relationship to Reduced Loudness Discomfort Level, but not Self-Report of Hyperacusis. J Assoc Res Otolaryngol. 2016;17:223–35. doi: 10.1007/s10162-016-0555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Dooling RJ. Evidence of hyperacusis in canaries with permanent hereditary high-frequency hearing loss. Seminars in Hearing. 2007;28:319–326. [Google Scholar]
- Marshall L, Brandt JF. The relationship between loudness and reaction time in normal hearing listeners. Acta Otolaryngol. 1980;90:244–9. doi: 10.3109/00016488009131721. [DOI] [PubMed] [Google Scholar]
- McFerran DJ, Baguley DM. Acoustic shock. The Journal of laryngology and otology. 2007;121:301–5. doi: 10.1017/S0022215107006111. [DOI] [PubMed] [Google Scholar]
- Miller SM, Piasecki CC, Lonstein JS. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol Biochem Behav. 2011;100:130–7. doi: 10.1016/j.pbb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Hienze R, Kimm J, Miller J. Reaction-time procedure for measuring hearing. I. Suprathreshold functions. The Journal of the Acoustical Society of America. 1975;57:421–430. doi: 10.1121/1.380465. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Tyler RS, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Coelho CB, Andersson G, Keiner AJ, Cacace AT, Martin N, Moore BC. A review of hyperacusis and future directions: part II. Measurement, mechanisms, and treatment. Am J Audiol. 2014;23:420–36. doi: 10.1044/2014_AJA-13-0037. [DOI] [PubMed] [Google Scholar]
- Radziwon K, Holfoth D, Lindner J, Kaier-Green Z, Bowler R, Urban M, Salvi R. Salicylate-induced hyperacusis in rats: Dose- and frequency-dependent effects. Hear Res. 2017;350:133–138. doi: 10.1016/j.heares.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum RH, Yurosko C, Santiago L, Sandridge SA, Kaltenbach JA. Induction of enhanced acoustic startle response by noise exposure: dependence on exposure conditions and testing parameters and possible relevance to hyperacusis. PLoS One. 2014;9:e111747. doi: 10.1371/journal.pone.0111747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res. 2001;12:181–98. doi: 10.1016/s0926-6410(01)00045-3. [DOI] [PubMed] [Google Scholar]
- Scharf B. Critical Bands. In: Tobias J, editor. Foundations of Modern Auditory I. Academic Press; New York: 1970. pp. 157–202. [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–75. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Soldani P, Gesi M, Lenzi P, Natale G, Fornai F, Pellegrini A, Ricciardi MP, Paparelli A. Long-term exposure to noise modifies rat adrenal cortex ultrastructure and corticosterone plasma levels. Journal of submicroscopic cytology and pathology. 1999;31:441–8. [PubMed] [Google Scholar]
- Stebbins WC. Auditory reaction time and the derivation of equal loudness contours for the monkey. J Exp Anal Behav. 1966;9:135–42. doi: 10.1901/jeab.1966.9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–34. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Wotjak CT, Wagner S, Williams G, Schachner M, Dityatev A. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain research. 2001;919:232–41. doi: 10.1016/s0006-8993(01)03020-7. [DOI] [PubMed] [Google Scholar]
- Tennessen JB, Parks SE, Langkilde TL. Anthropogenic Noise and Physiological Stress in Wildlife. Adv Exp Med Biol. 2016;875:1145–8. doi: 10.1007/978-1-4939-2981-8_142. [DOI] [PubMed] [Google Scholar]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–92. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Dauman N, Andersson G, Keiner AJ, Cacace AT, Martin N, Moore BC. A review of hyperacusis and future directions: part I. Definitions and manifestations. Am J Audiol. 2014;23:402–19. doi: 10.1044/2014_AJA-14-0010. [DOI] [PubMed] [Google Scholar]
- Van Campen LE, Dennis JM, Hanlin RC, King SB, Velderman AM. One-year audiologic monitoring of individuals exposed to the 1995 Oklahoma City bombing. Journal of the American Academy of Audiology. 1999;10:231–47. [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14974–9. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XM, Merzenich MM. Environmental noise exposure degrades normal listening processes. Nat Commun. 2012:3. doi: 10.1038/ncomms1849. [DOI] [PubMed] [Google Scholar]