Abstract
Purpose of review
Ocular involvement in sarcoidosis is present in up to 80 percent of patients and is frequently manifested before diagnosis of the underlying systemic disease. Considering the therapeutic consequences, early diagnosis of the underlying disease is advantageous in patients presenting with ocular inflammation. There are several ocular findings suggestive of underlying sarcoidosis, such as granulomatous keratic precipitates, iris nodules, cells in the vitreous humor known as snowballs and snowbanks, and retinal periphlebitis. High suspicion is crucial for the diagnosis of sarcoidosis. This review on ocular sarcoidosis will mainly focus on new diagnostic and treatment modalities.
Recent findings
Recent studies found possible new diagnostic indicators for the diagnosis of ocular sarcoidosis which include not only serum profiles but also vitreous sample analysis. Ophthalmologic imaging techniques have improved to investigate the ocular structure in detail. Results from recent uveitis clinical trials have included sarcoidosis as an underlying cause and have reported positive results.
Summary
The diagnosis of ocular sarcoidosis can be challenging in some cases. High suspicion is important to diagnose ocular sarcoidosis with various laboratory and ophthalmic tools. There are many possible options for the treatment of ocular sarcoidosis including various biologic agents.
Keywords: ocular sarcoidosis, new diagnostic indicator, biologic agents
Introduction
Sarcoidosis can affect almost any portion of the eye or surrounding tissue. Examples include uveitis, scleritis, dry eye, optic neuritis, conjunctival granuloma, or exophthalmos. Uveitis can lead to visually significant complications such as cataract, glaucoma, retinal vasculitis, vitreous opacities and macular edema.[1]
Central nervous system involvement resulting from sarcoidosis can affect vision in a variety of ways including altering the function of cranial nerves.[2] In order to capture new developments in understanding sarcoidosis as it relates to eye disease, we searched the National Library of Medicine database using the key word, “ocular sarcoidosis”. We selected papers published since 2015 and ignored papers written in languages other than English or reports in the form of reviews. We also included publications on the differential diagnosis of uveitis or therapeutic advances if the report related to sarcoidosis. We report on 1) the frequency of sarcoidosis as a cause of uveitis based on surveys from a variety of countries; 2) the application of International Workshop of Uveitis criteria to diagnose uveitis; 3) novel observations on clinical features of uveitis or parameters that affect prognosis; 4) new insights into pathogenesis or biomarkers; 5) gene expression patterns associated with lacrimal or orbital involvement by sarcoidosis; and 6) results with biologic therapy to treat ocular sarcoidosis.
Dry eye is arguably the most common ocular manifestation of sarcoidosis. Dryness, however, usually does not result in severe visual loss. In contrast, uveitis is arguably the most common form of ocular involvement with the potential to affect visual function markedly. As most readers will be unfamiliar with clinical features of uveitis, we begin with a short overview of uveitis.
Overview of Uveitis
Uveitis is the term used to describe an inflammation of the uvea which includes iris, ciliary body and choroid. Uveitis is most commonly classified by anatomical location (anterior chamber, vitreous humor (intermediate uveitis), or choroid-retina (posterior uveitis)) of the observed inflammation using slit-lamp biomicroscopy and fundus examination. Sarcoidosis associated uveitis can present as anterior, intermediate, posterior or pan-uveitis. Frequent symptoms from uveitis include redness, eye pain, light sensitivity, vitreous floaters and blurry vision according to the extent, severity, location, and suddenness of the onset of inflammation.
The uveitis associated with sarcoid is generally bilateral and chronic. Anterior segment examination by slit-lamp biomicroscopy may show granulomatous keratic precipitates (inflammatory cellular deposit on the posterior surface of cornea), iris nodules, iris adhesion to cornea (anterior synechiae) or lens (posterior synechiae). Chronic uveitis can lead to cataract formation, secondary glaucoma and keratopathy. Fundus examination can reveal various degree of vitreous opacities, snow balls (aggregates of inflammatory cells in the vitreous humor posterior to the lens) and snow banks (accumulation of white exudates over the pars plana and ora serrata) in the vitreous cavity. These can be highly suggestive of granulomatous inflammation. Characteristic fundoscopic findings also include retinal periphlebitis and choroidal granuloma. Visual deterioration can be caused by media opacity (cataract and vitreous opacity), macular edema, and secondary glaucoma. The degree of anterior chamber inflammation and vitreous opacity can be assessed by standard guidelines and the treatment response can be judged also by the number of inflammatory cells in anterior chamber according to the standard guideline.[3,4]
Conjunctival involvement is common, but is frequently ignored since most conjunctival lesions are asymptomatic. These can present as nodules, cicatricial conjunctival scarring, and granuloma. Reportedly sarcoidosis-associated scleritis is uncommon but may present in older female patients. [5]
The lacrimal gland is a commonly affected orbital organ in sarcoidosis. Lacrimal gland inflammation which results in decreased aqueous tear production, can cause keratoconjunctivitis sicca (KCS). KCS in sarcoidosis patients is a different category from Sjogren’s syndrome by recent classification from the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR).[6]
Neurological involvement is observed in 5–26% of sarcoidosis cases[2]. Cranial neuropathy is the most frequent neurological manifestation; facial nerve and optic nerve are the most commonly affected.[2]
Various systemic disease can cause uveitis. The rate of sarcoidosis associated uveitis varies with ethnicities and countries. It can account for as much as 11.4% of patients in a referral clinical based on a survey of recent reports from around the world (Table 1). [7–15]
Table 1.
The Frequency of Sarcoidosis as an underlying cause for Uveitis in International Clinics
| Location | Size of series | conclusions |
|---|---|---|
| Germany[7] | 474 | 11.4 %, sarcoidosis was most commonly associated systemic disease |
| Saudi Arabia[8] | 888 | 0.5 % of uveitis had sarcoidosis as underlying disease |
| Taiwan[9] | 405 | Sarcoidosis account for 2.7 % of uveitis |
| Korea [10] | 602 | 2.7 % |
| Japan [11] | 695 | 8.1 %, sarcoidosis was most common systemic cause |
| India [12] | 980 | 2 % |
| New Zealand[13] | 1148 | 5% of patients had sarcoidosis and panuveitis (17.5%) was most common type of uveitis. |
| Turkey [14] | 4863 | 2.8 % of uveitis caused by sarcoidosis with female predominance (M:F=1:4) |
| Australia[15] | 1165 | Sarcoidosis was associated in 5.8% of patients younger than 60 year-old and 8.9 % of patients older than 60 year-old |
Two peaks of incidence are reported: the first in those aged 20–30 years, and the second in those aged 50–60 years.[16] Females (76%) are more likely to develop ocular involvement compared to males (24%) in a study with biopsy-proven systemic sarcoidosis.[1]
Sarcoidosis is relatively rare in children but may affect childhood and cause uveitis in young age also.[17] However, most of patients previously diagnosed as early onset sarcoidosis are now considered as having Blau syndrome with de novo mutation. [18]
Sarcoidosis can be included on the differential diagnosis of almost any presentation of uveitis. Diagnostic criteria for sarcoidosis associated uveitis have been proposed at first International Workshop on Ocular Sarcoidosis (IWOS) in 2009 (Table 2). [19] In order to make a diagnosis of ocular sarcoidosis, three conditions must be met. First, the patient must have clinical findings consistent with sarcoidosis by IWOS (International Workshop on Ocular Sarcoidosis) criteria.[19] Second, evidence of noncaseating granulomas must be demonstrated histologically on a tissue biopsy. Third, other causes of granulomatous inflammation must be excluded such as tuberculosis or syphilis. Many patients with clinical features of ocular sarcoidosis are unable to undergo biopsy or do not have an appropriate site for biopsy. In these cases, a diagnosis of probable or presumed ocular sarcoidosis is made based on clinical features, laboratory findings and chest imaging results according to the IWOS criteria.[19] The IWOS criteria have been recently validated in several countries and shown high reliability for the clinical diagnosis of ocular sarcoidosis. [20,21] However, the criteria have some limitations. First, abnormal liver enzyme tests are not helpful to detect ocular sarcoidosis. Second, a negative tuberculin skin test was not supported in an international validation study. On the other hand, positive TB tests are very useful to differentiate TB from sarcoidosis in TB endemic countries. Therefore, TB tests should be modified appropriately. Third, categories in the classification of ocular sarcoidosis such as probable ocular sarcoid and possible ocular sarcoid do not help to detect ocular sarcoidosis patients. For these reasons, current IWOS criteria will be modified at 6th international workshop on ocular sarcoidosis on April, 2017.
Table 2.
Diagnostic criteria for ocular sarcoidosis (from IWOS[19]).
| Intraocular signs suggestive for the diagnosis of ocular sarcoidosis |
|---|
| 1. Mutton-fat keratic precipitates (large or small) and or iris nodules (Koeppe/Busacca) |
| 2. Trabecular meshwork nodules and/or tent-shaped peripheral anterior synechia |
| 3. Snowballs/strings of pearls’ vitreous opacities |
| 4. Multiple chorioretinal peripheral lesions (active and/or atrophic) |
| 5. Nodular and/or segmental periphlebitis (± candle-wax drippings) and/or retinal macro-aneurysm in an inflamed eye |
| 6. Optic-disk nodule(s)/granuloma(s) and/or a solitary choroidal nodule |
| 7. Bilateral inflammation (evident on clinical examination or on investigational imaging) |
| Investigational tests supportive of ocular sarcoidosis |
|---|
| 1. Negative tuberculin test in a BCG-vaccinated patient or in a patient who has a previous positive tuberculin skin test |
| 2. Elevated serum angiotensin converting-enzyme levels and/or elevated serum lysozyme |
| 3. Positive chest X-ray (bilateral hilar lymphadenopathy<comma> BHL) |
| 4. Abnormal liver–enzyme tests (any two of alkaline phosphatase<comma> aspartate transaminase<comma> alanine transaminase) |
| 5. Abnormal chest CT scan in patients with a negative chest X-ray |
| Diagnostic criteria of ocular sarcoidosis |
|---|
Diagnostic criteria of ocular sarcoidosis were worked out in four levels of certainty:
|
Ophthalmologic tools for diagnostic imaging include fundus photo camera, fluorescein angiography, fundus autofluorescence (FAF), and optical coherence tomography (OCT). These technologies are evolving rapidly and changing how patients with uveitis are evaluated.
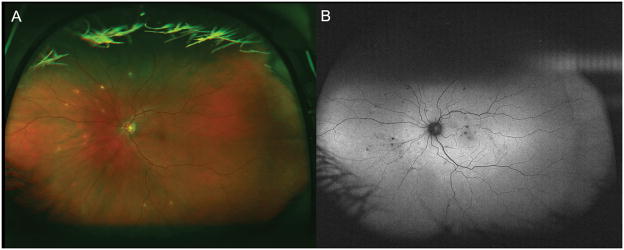
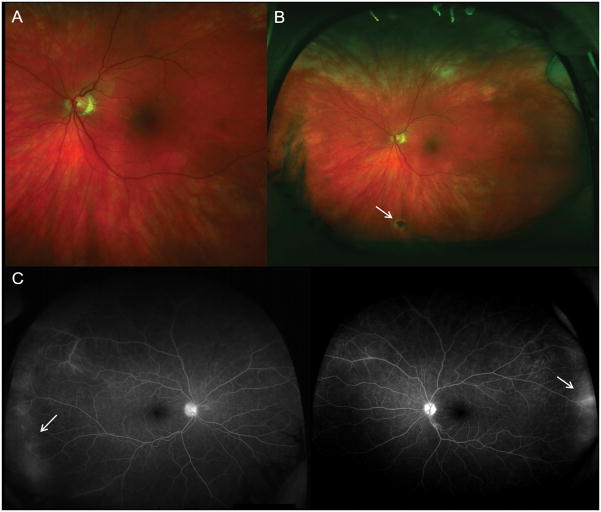
Fundus photography for the uveitis patient with vitreous opacities may be helpful for the documentation and objective future comparisons. Ultra-wide field retinal imaging has gained popularity and can provide a wider view of approximately 200°, which covers 82% of the retina in a single image, while conventional fundus photography provides a 30° field of view. (Figure 1-A). [22] This allows for documentation and comparison of peripheral retinal findings. The area of apparent fluorescein leakage due to retinal vasculitis can be also greater and more diagnostic with wide field images than in conventional 9-field montage images. (Figure 2) [23] Fundus autofluorescence (FAF) has proven to be a valuable tool for the detection of subclinical retinal pigment epithelium changes in chorioretinal pathologies which are not seen in fundoscopic examination and usual color fundus photography. FAF may become an essential tool to detect subtle inflammatory change and monitor treatment response in uveitis patient with chorioretinal lesions (Figure 3).[24]
Figure 1. wide field color fundus photo (A) and wide field fundus autofluorescence (B).
Color fundus photo(A) shows multiple yellowish spots (sarcoid spots) whereas autofluorescence (B) shows more mall black (hypofluorescence) spots compared to color fundus photo. This indicates there are more retinal pigmentary change due to outer retinal and choroidal inflammation as seen in color fundus photo and fundus examination.
Figure 2. wide field fundus photo and fluorescein angiography.
Figure A indicates the area covering conventional photography. Wide field photo can reveal far peripheral retinal lesion (B, white arrow) and vascular Leakage (C, white arrows) and enables the diagnosis of peripheral chorioretinal lesions and vasculitis. It also determines the extent of affected area.
Figure 3. Wide field fundus autofluorescence of left eye.
This patient denied any deterioration of left eye vision, but there was increased hyperfluorescence intensity (B) temporal and inferotemporal to the macular area (white arrows) compared to previous visit(A). This finding can reflect active subclinical inflammation
Severity of macular edema correlates with severity of ocular inflammation and loss of visual acuity. [25] Macular edema can be assessed and monitored with optical coherence tomography (OCT) (Figure 4). The integrity of the photoreceptor inner segment and outer segment junction and external limiting membrane on optical coherence tomography (OCT) may predict functional outcome (visual acuity, central distortions and scotomas) following treatment of the macular edema.[26] OCT images can detect microstructural changes of retina and choroid in a noninvasive manner. This enables detection of subclinical disease activity and monitoring of patients with uveitic macular edema.
Figure 4. Optical coherence tomography (OCT) with cystoid macular edema.
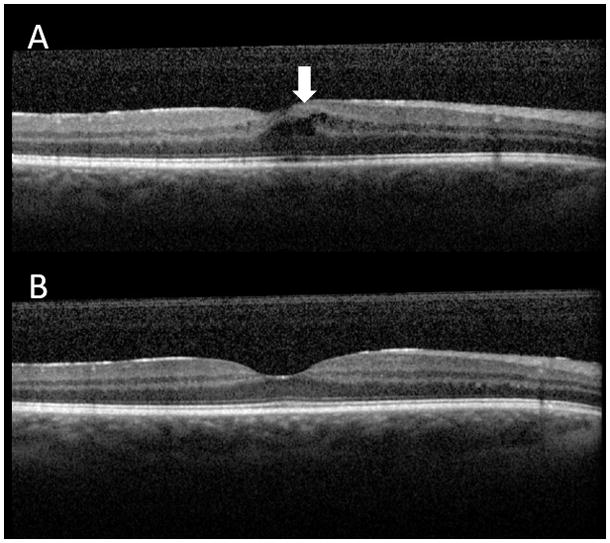
A. The area indicated by white arrow shows several black cystic spaces (cystoid macular edema). Intraretinal fluid accumulation due to inflammation result in retinal thickening and visual deterioration.
B. The cystoid macular edema resolved after the treatment. OCT shows normal concave health macular contour. OCT is useful imaging tool for detection of macular edema and assessing treatment response.
Sarcoidosis can affect ocular and periocular structures with variable rate depending on countries and ethnicities. One report from Serbia with 88 biopsy-proven sarcoidosis showed 32 patients (36.4% of all) manifested ocular sarcoidosis.[1] Posterior uveitis was the most common (15.9%), followed by neuro-ophthalmologic manifestations (9.1%), conjunctival lesions (7.9%), panuveitis (5.7%), eyelid skin lesions (2.3%), orbital inflammation (2.3%), anterior uveitis (2.3%), and intermediate uveitis (1.1%). Complications included cataract (20.4%), glaucoma (5.7%), cystoid macular edema (3.4%), epiretinal membrane formation (4.5%); macular atrophy (2.3%), and choroidal neovascularization (1.1%). Binocular visual impairment was present in one patient (1.1%), due to complications of posterior uveitis (macular scars). In constrast, another study from Serbia with 55 ocular sarcoidosis patients reported that the most frequent manifestation was anterior uveitis (64.6%). Macular edema and periphlebitis associated with periarteritis were frequently observed.[25] One retrospective study with 83 cases of biopsy-proven sarcoidosis uveitis from France showed that 52 out of 83 patients initially presented with isolated sarcoid uveitis, whereas 31 out of 83 patients had original systemic sarcoidosis in addition to ocular involvement. [27] This study showed a favorable visual outcome for sarcoidosis uveitis. 60.2% of all patients had complete visual recovery and 89.2% retrieved best-corrected visual acuity (BCVA) >20/50 in both eyes. A unilateral loss of BCVA of worse than 20/200 was documented in only two patients. There were no patients with bilateral severe visual impairment or blindness. Factors related with a poor visual prognosis (BCVA ≤20/50) were chronic macular edema and persistent ocular inflammation. Sarcoidosis patients with ocular involvement needed systemic therapy more frequently compared to isolated ocular sarcoidosis. Interestingly, visual outcome was better in systemic sarcoidosis with ocular involvement patients. This result might be because more systemic treatment was undertaken in systemic sarcoidosis.
There were some recent prognostic features regarding ocular sarcoidosis. To date, there were few dedicated investigations of risk factors for ocular sarcoidosis. Recently cigarette smoking was considered as a risk factor for development of ocular sarcoidosis in a retrospective study with 109 patients. [28▪ ].
One retrospective study of 3364 veteran patients with sarcoidosis reported that ocular inflammation had significantly lower 1-year all-cause mortality than those without ocular inflammation.[29▪]
Diagnosis of ocular sarcoidosis
Sarcoidosis can be included on the differential diagnosis of almost any presentation of uveitis. A chest X-ray is the best and easiest screening tool for the diagnosis of sarcoidosis, because most of patients with sarcoidosis have pulmonary involvement. One of the main diseases in the differential diagnosis is tuberculosis which also causes pulmonary changes. Babu et al. showed hilar lymphadenopathy and fissural nodules were significantly more common in ocular sarcoidosis patients compared to ocular tuberculosis on high resolution chest computerized tomography. [30]
Recently published articles have shown that there were newly investigated laboratory findings helping diagnose uveitis associated with sarcoidosis.[31] Gundlach et al showed that an elevated level of soluble interleukin 2 receptor (sIL2R) suggests sarcoidosis with uveitis more convincingly than ACE, making sIL2R a more effective marker for sarcoidosis than ACE or chest x-ray in uveitis patients.[32▪] Jones et al. suggested that significant lymphopenia (<1.0×10(9)/L) is an independent predictor of sarcoidosis in new patients presenting with sarcoidosis associated uveitis in a large (n=129) retrospective study.[33▪]
Newly developed techniques have been investigated to show specific findings for uveitis. These findings might prove helpful for differential diagnosis for a certain type of uveitis in the future. Enhanced depth imaging OCT is suitable to visualize choroidal granulomas and to describe their characteristics.[34] OCT angiography is the most advanced technology which allows visualization of microvascular changes in superficial and deep layer in retina. Visual prognosis and disease course can be assessed and monitored by quantifying retinal capillary changes.[35] Rose-Nussbaumer et al. noninvasively evaluated the characteristic findings of inflammatory cells in the anterior chamber using optical coherence tomography Inflammatory cells of sarcoidosis uveitis showed a predominantly mononuclear pattern. This technique may become a useful adjunct to guide the diagnosis and treatment. [36]
In limited cases, vitrectomy can be done and vitreous samples can be obtained for diagnostic analysis. Masaru et al. showed that high-mobility group box-1 (HMGB1), which is secreted by activated leukocytes and acts as a primary inflammatory cytokine was detected in the vitreous of 23 of 24 patients (95.8%) with ocular sarcoidosis and the level of HMGB was high as 52.5 ng/ml in ocular sarcoidosis compared to proliferative diabetic retinopathy patients (85.2%, 9.84 ng/ml) and epiretinal membrane patients (66.7%, 6.99ng/ml). The authors showed it is associated with vitreous levels of Th1- and regulatory T-related cytokines, but not with inflammatory or Th17-related cytokines. [37▪] These investigations suggest that cytopathological examination of vitreous samples may contribute to the diagnosis of intraocular sarcoidosis. Interestingly, Goto H. et al suggested Propionibacterium acne as a possible pathogen of granuloma in ocular sarcoidosis patients by showing this organism in a removed epiretinal membrane. [38▪]
Siasos et al. reported that ocular involvement in sarcoidosis patients was associated with impaired endothelial function (estimated the flow mediated dilation in the brachial artery) and increased arterial stiffness (measured carotid-femoral pulse wave velocity). These results strengthened the vascular theory which considers uveitis a consequence of vascular dysfunction in sarcoidosis patients and revealed a possible clinical importance of the use of endothelial function tests. [39] Ocular inflammation (chorioretinal lesions) can be a marker of microcirculatory damage in sarcoidosis patients and choroidal involvement is associated with an increased risk of cardiac disease.
Sarcoidosis can affect various components of the orbit including the lacrimal gland or adipose tissue. Using formalin fixed biopsies provided by an international consortium, we have characterized the pattern of gene expression in this tissue. Our observations show that distinct diagnoses such as sarcoidosis, thyroid associated eye disease, or granulomatosis with polyangiitis generally have unique and recognizable patterns of gene expression.[40▪] Furthermore, mRNA transcripts which are either up or down regulated in lacrimal gland or orbital adipose tissue are often similarly altered in whole blood.[41] This suggests the possibility that the measurement of gene expression in peripheral blood could ultimately become an accurate diagnostic modality for sarcoidosis or for identifying the cause of orbital inflammatory disease. Pathway analsysis suggests that transcripts which are upregulated in blood, orbital fat and lacrimal gland from patients with sarcoidosis are frequently regulated by the transcription factors, interferon response factors 1 and 2 and nuclear factor kappa B [42▪].
Management of Ocular Sarcoidosis
The primary purposes for the management of ocular sarcoidosis are to restore vision and to prevent complications from inflammation related ocular structural changes.
Corticosteroids
The first-line treatment for ocular sarcoidosis is corticosteroids. Anterior uveitis is treated with topical corticosteroids with/without cycloplegics to prevent synechiae. 0.05% difluprednate is relatively new and more potent topical medication. Its potency is at least double prednisolone acetate 1% (the most popular topical corticosteroid used for uveitis therapy in the US) for the treatment of anterior uveitis.[43] Regional corticosteroid injections and implants can be considered in posterior uveitis or cases of poor responders to topical steroids. Those include subconjunctival, periocular or subtenon injection, and intravitreal injections. A biodegradable intraocular implant containing dexamethasone given through the pars plana with a 22-gauge applicator has been approved for the treatment of uveitis involving the posterior segment of the eye. A single dexamethasone implant was effective in improving vision and macular edema in the majority of the patients with noninfectious causes of uveitis. However, recurrence of macular edema was observed in approximately 65% of the patients at 6 months.[44] A sustained-release fluocinolone acetonide implant has been approved in the US for chronic noninfectious posterior uveitis since 2005. All patients who receive this implant eventually require cataract surgery and one-third require filtering surgery to control glaucoma not controlled by topical medications.[45] The Multicenter Uveitis Steroid Treatment (MUST) trial has recently compared the effectiveness of systemic corticosteroids in combination with immunosuppression versus the fluocinolone acetonide implant for non-infectious uveitis. This study suggests that implant therapy is valuable for patients with uveitis who do not require systemic treatment for non-ocular indications.[46]
Systemic immunosuppressive agents
These agents are indicated in corticosteroid-dependent or –intolerant cases as a steroid sparing purpose. Commonly used antimetabolite agents for long-term control of systemic and ocular inflammation include methotrexate, mycophenolate mofetil, or azathioprine. Among T cell inhibitors, cyclosporine and tacrolimus, have been shown efficacy in decreasing inflammation in uveitis. However, voclosporin failed to meet its primary endpoint in the majority of clinical trials.[47] Subconjunctival/intravitreal sirolimus has demonstrated dose dependent benefit (the highest dose was not as effective as a lower dose used in the trial),[48] but it is not an FDA approved option at the time of this writing.
Biologic agents
Patients who are refractory to steroid-sparing agents often benefit from biologics. Interferons showed possible beneficial for the treatment of macular edema associated with uveitis.[47] Interferons, however, can also cause systemic granulomatous disease. Biologic agents studied for the treatment of uveitis related to sarcoidosis are Tumor Necrosis Factor (TNF)-α inhibitors, including infliximab, adalimumab, etanercept, golimumab and Certolizumab. Among those, etanercept showed no significant difference between etanercept and placebo groups.[47]. Among TNF-α inhibitors, Adalimumab, is the only systemic non-corticosteroid agent which has been approved by the US Food and Drug Administration (FDA) for the treatment of non-infectious uveitis. Two successful trials with adalimumab for non-infectious, intermediate, posterior or panuveitis uveitis have been reported. Jaffe et al. reported the result of a multinational phase 3 trial (VISUAL I) involved adults who had active non-infectious intermediate uveitis, posterior uveitis, or pan-uveitis despite having received prednisone treatment for 2 or more weeks. [49▪ ▪] This study included 217 patients, 16 patients were associated with sarcoidosis as underlying cause. In this trial, adalimumab was found to be associated with a lower risk of uveitic flare or visual impairment and it demonstrated a steroid sparing effect. Nguyen et al. published another multicenter, double-masked, randomized, placebo-controlled phase 3 trial (VISUAL II) with adalimumab. [50▪ ▪] This study included 229 patients (aged ≥ 18 years) with inactive, non-infectious intermediate, posterior, or pan- uveitis controlled by 10–35 mg/day of prednisone. Patients were randomly assigned with 1:1 ratio to placebo or adalimumab. From week 2, all patients underwent a mandatory prednisone taper to 0 mg by week 19. Thirty two patients had sarcoidosis associated uveitis. This study showed that adalimumab significantly lowered the risk of uveitic flare or loss of visual acuity on corticosteroid withdrawal with inactive uveitis patients. Adalimumab also showed the efficacy for pediatric patients with anterior uveitis.[51] Leyre et al. showed the effectiveness of adalimumab in refractory sarcoidosis associated uveitis with 17 patients. [52] Overall, adalimumab can be a next step treatment for refractory uveitis cases on anti-metabolites or T-cell inhibitors. And this dose not mean for only sarcoidosis associated uveitis. Due to limited long term safety and efficacy data, biologic therapy is reserved as secondary or tertiary treatment of uveitis associated with sarcoidosis.
Surgery
Surgical interventions may be needed in cases resistant to medical treatment to remove media opacity such as vitreous opacity and cataract. Surgery can exacerbate ocular inflammation, microincision vitrectomy surgery (MIVS) using wide-viewing system is minimal invasive and showed favorable outcome for complicated ocular sarcoidosis cases without aggravation of ocular inflammation by surgical stress. [53]
Conclusion
The diagnosis of ocular sarcoidosis is important for not only the ophthalmologist but also the rheumatologist and pulmonologist. Traditional laboratory, radiologic and pathologic findings are crucial and should be evaluated initially. There are no standardized therapeutic guidelines for ocular sarcoidosis. Traditionally used systemic immunosuppressive agents should be considered as primary steroid sparing agents. In refractory cases, adalimumab is now a therapeutic option.
Key points.
Sarcoidosis should be included on the differential diagnosis of almost any presentation of uveitis. Ocular and ocular adnexa inflammation can be the first or only clinically important manifestation for sarcoidosis.
Suggestive ocular findings include mutton-fat keratic precipitates, iris nodules, snow balls in vitreous cavity, multiple chorioretinal peripheral lesions, retinal periphlebitis and bilateral involvement. Various ophthalmic imaging tools should be considered as ancillary diagnostic modalities.
Recently published findings which include elevated serum levels of CXCL9, CXCL10 and soluble interleukin 2 receptor (sIL2R) might suggest sarcoidosis with uveitis more accurately than ACE.
Detection of high-mobility group box-1 (HMGB1) in vitreous sample may be diagnostic.
Adalimumab is a therapeutic option for refractory ocular sarcoidosis (intermediate, posterior and panuveitis).
Acknowledgments
Financial support and sponsorship
This research was supported by the William and Mary Bauman Foundation, the Stan and Madelle Rosenfeld Family Trust, Research to Prevent Blindness (New York), and National Institutes of Health RO-1 Grant, EY 020249.
Footnotes
Conflicts of interest
None.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Radosavljevic A, Jaksic V, Pezo L, et al. Clinical features of ocular sarcoidosis in patients with biopsy-proven pulmonary sarcoidosis in serbia. Ocul Immunol Inflamm. 2016:1–5. doi: 10.3109/09273948.2016.1167224. [DOI] [PubMed]
- 2.Ibitoye RT, Wilkins A, Scolding NJ. Neurosarcoidosis: a clinical approach to diagnosis and management. J Neurol. 2016 doi: 10.1007/s00415-016-8336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis JL, Madow B, Cornett J, et al. Scale for photographic grading of vitreous haze in uveitis. Am J Ophthalmol. 2010;150:637–641. e631. doi: 10.1016/j.ajo.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu K, Kini R, Mehta R. Scleral nodule and bilateral disc edema as a presenting manifestation of systemic sarcoidosis. Ocul Immunol Inflamm. 2010;18:158–161. doi: 10.3109/09273941003753416. [DOI] [PubMed] [Google Scholar]
- 6.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9–16. doi: 10.1136/annrheumdis-2016-210571. [DOI] [PubMed] [Google Scholar]
- 7.Grajewski RS, Caramoy A, Frank KF, et al. Spectrum of uveitis in a German tertiary center: Review of 474 consecutive patients. Ocul Immunol Inflamm. 2015:1–7. doi: 10.3109/09273948.2014.1002567. [DOI] [PubMed] [Google Scholar]
- 8.Al Dhibi HA, Al Shamsi HN, Al-Mahmood AM, et al. Patterns of uveitis in a tertiary care referral institute in Saudi Arabia. Ocul Immunol Inflamm. 2016:1–8. doi: 10.3109/09273948.2015.1133836. [DOI] [PubMed]
- 9.Chen SC, Chuang CT, Chu MY, et al. Patterns and etiologies of uveitis at a tertiary referral center in Taiwan. Ocul Immunol Inflamm. 2016:1–8. doi: 10.1080/09273948.2016.1189577. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Kim DY, Woo SJ, et al. Clinical patterns of uveitis in tertiary ophthalmology centers in Seoul, South Korea. Ocul Immunol Inflamm. 2016:1–7. doi: 10.1080/09273948.2016.1206203. [DOI] [PubMed]
- 11.Nakahara H, Kaburaki T, Tanaka R, et al. Frequency of Uveitis in the Central Tokyo Area (2010–2012) Ocul Immunol Inflamm. 2016:1–7. doi: 10.3109/09273948.2015.1133840. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh P, Gogia V, Shah B, et al. Patterns of uveitis at the Apex Institute for Eye Care in India: Results from a prospectively enrolled patient data base (2011–2013) Int Ophthalmol. 2016;36:365–372. doi: 10.1007/s10792-015-0128-9. [DOI] [PubMed] [Google Scholar]
- 13.Wong A, McKelvie J, Slight C, et al. Land of the long white cloud: The spectrum of uveitis at a tertiary referral center in New Zealand. Ocul Immunol Inflamm. 2016:1–7. doi: 10.1080/09273948.2016.1203957. [DOI] [PubMed]
- 14.Yalcindag FN, Ozdal PC, Ozyazgan Y, et al. Demographic and clinical characteristics of uveitis in Turkey: The first national registry report. Ocul Immunol Inflamm. 2016:1–10. doi: 10.1080/09273948.2016.1196714. [DOI] [PubMed] [Google Scholar]
- 15.Zagora SL, Symes R, Yeung A, et al. Etiology and clinical features of ocular inflammatory diseases in a tertiary referral centre in Sydney, Australia. Ocul Immunol Inflamm. 2016:1–8. doi: 10.1080/09273948.2016.1247871. [DOI] [PubMed] [Google Scholar]
- 16.Rothova A, Alberts C, Glasius E, et al. Risk factors for ocular sarcoidosis. Doc Ophthalmol. 1989;72:287–296. doi: 10.1007/BF00153496. [DOI] [PubMed] [Google Scholar]
- 17.Engelhard SB, Bajwa A, Reddy AK. Causes of uveitis in children without juvenile idiopathic arthritis. Clin Ophthalmol. 2015;9:1121–1128. doi: 10.2147/OPTH.S83950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose CD, Wouters CH, Meiorin S, et al. Pediatric granulomatous arthritis: an international registry. Arthritis Rheum. 2006;54:3337–3344. doi: 10.1002/art.22122. [DOI] [PubMed] [Google Scholar]
- 19.Herbort CP, Rao NA, Mochizuki M, et al. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17:160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 20.Meneses CF, Egues CA, Uriarte M, et al. Diagnostic categorization according to the First International Workshop on Ocular Sarcoidosis (FIWOS) criteria in a series of 11 patients. Reumatol Clin. 2017;13:25–29. doi: 10.1016/j.reuma.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal R, Gonzalez-Lopez JJ, Meier F, et al. Ocular and systemic features of sarcoidosis and correlation with the International Workshop for Ocular Sarcoidosis diagnostic criteria. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:237–245. [PubMed] [Google Scholar]
- 22.Dickson D, Agarwal A, Sadiq MA, et al. Assessment of vitreous haze using ultra-wide field retinal imaging. J Ophthalmic Inflamm Infect. 2016;6:35. doi: 10.1186/s12348-016-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson BP, Nigam D, Miller D, et al. Comparison of wide-field fluorescein angiography and 9-field montage angiography in uveitis. Am J Ophthalmol. 2014;157:673–677. doi: 10.1016/j.ajo.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reznicek L, Seidensticker F, Stumpf C, et al. Systematic analysis of wide-field fundus autofluorescence (FAF) imaging in posterior uveitis. Curr Eye Res. 2014;39:164–171. doi: 10.3109/02713683.2013.834938. [DOI] [PubMed] [Google Scholar]
- 25.Paovic J, Paovic P, Sredovic V, et al. Clinical manifestations, complications and treatment of ocular sarcoidosis: correlation between visual efficiency and macular edema as seen on optical coherence tomography. Semin Ophthalmol. 2016:1–8. doi: 10.1080/08820538.2016.1206576. [DOI] [PubMed] [Google Scholar]
- 26.Pakzad-Vaezi K, Or C, Yeh S, et al. Optical coherence tomography in the diagnosis and management of uveitis. Can J Ophthalmol. 2014;49:18–29. doi: 10.1016/j.jcjo.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Rochepeau C, Jamilloux Y, Kerever S, et al. Long-term visual and systemic prognoses of 83 cases of biopsy-proven sarcoid uveitis. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2016-309767. [DOI] [PubMed] [Google Scholar]
- 28▪.Janot AC, Huscher D, Walker M, et al. Cigarette smoking and male sex are independent and age concomitant risk factors for the development of ocular sarcoidosis in a New Orleans sarcoidosis population. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:138–143. First study about the relationship between ocular sarcoidosis and smoking. [PMC free article] [PubMed] [Google Scholar]
- 29▪.Birnbaum AD, French DD, Mirsaeidi M, et al. Sarcoidosis in the national veteran population: association of ocular inflammation and mortality. Ophthalmology. 2015;122:934–938. doi: 10.1016/j.ophtha.2015.01.003. First study which adress the association with ocular inflammation and mortality in sarcoidosis patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babu K, Shukla SB, Philips M. High resolution chest computerized tomography in the diagnosis of ocular sarcoidosis in a high TB endemic population. Ocul Immunol Inflamm. 2016:1–6. doi: 10.3109/09273948.2015.1115082. [DOI] [PubMed] [Google Scholar]
- 31.Sahin O, Ziaei A, Karaismailoglu E, et al. The serum angiotensin converting enzyme and lysozyme levels in patients with ocular involvement of autoimmune and infectious diseases. BMC Ophthalmol. 2016;16:19. doi: 10.1186/s12886-016-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Gundlach E, Hoffmann MM, Prasse A, et al. Interleukin-2 receptor and angiotensin-converting enzyme as markers for ocular sarcoidosis. PLoS One. 2016;11:e0147258. doi: 10.1371/journal.pone.0147258. Recent study for new biologic marker for ocular sarcoidosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪.Jones NP, Tsierkezou L, Patton N. Lymphopenia as a predictor of sarcoidosis in patients with uveitis. Br J Ophthalmol. 2016;100:1393–1396. doi: 10.1136/bjophthalmol-2015-307455. Recent study for a new hematologic finding as a predictor for sarcoidosis. [DOI] [PubMed] [Google Scholar]
- 34.Invernizzi A, Mapelli C, Viola F, et al. Choroidal granulomas visualized by enhanced depth imaging optical coherence tomography. Retina. 2015;35:525–531. doi: 10.1097/IAE.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 35.Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;171:101–112. doi: 10.1016/j.ajo.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose-Nussbaumer J, Li Y, Lin P, et al. Aqueous cell differentiation in anterior uveitis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56:1430–1436. doi: 10.1167/iovs.14-15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Takeuchi M, Taguchi M, Sato T, et al. Association of high-mobility group box-1 with Th cell- related cytokines in the vitreous of ocular sarcoidosis patients. Invest Ophthalmol Vis Sci. 2017;58:528–537. doi: 10.1167/iovs.16-20324. Recent study for the diagnosis of ocular sarcoidosis with vitreous analysis. [DOI] [PubMed] [Google Scholar]
- 38▪.Goto H, Usui Y, Umazume A, et al. Propionibacterium acnes as a possible pathogen of granuloma in patients with ocular sarcoidosis. Br J Ophthalmol. 2017 doi: 10.1136/bjophthalmol-2016-309248. Recent study showing microrganism may be pathogen of granolma in ocular sarcoidosis. [DOI] [PubMed]
- 39.Siasos G, Paraskevopoulos T, Gialafos E, et al. Vascular function and ocular involvement in sarcoidosis. Microvasc Res. 2015;100:54–58. doi: 10.1016/j.mvr.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 40▪.Rosenbaum JT, Choi D, Wilson DJ, et al. Orbital pseudotumor can be a localized form of granulomatosis with polyangiitis as revealed by gene expression profiling. Exp Mol Pathol. 2015;99:271–278. doi: 10.1016/j.yexmp.2015.07.002. Genetic analysis for convincing orbital pseudotumor associated sarcoidosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbaum JT, Sibley CH, Choi D, et al. Molecular diagnosis: Implications for ophthalmology. Prog Retin Eye Res. 2016;50:25–33. doi: 10.1016/j.preteyeres.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪.Rosenbaum JT, Choi D, Wilson DJ, et al. Parallel gene expression changes in sarcoidosis involving the lacrimal gland, orbital tissue, or blood. JAMA Ophthalmol. 2015;133:770–777. doi: 10.1001/jamaophthalmol.2015.0726. Genetic study showing different expression of genes in orbital sarcoidosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheppard JD, Toyos MM, Kempen JH, et al. Difluprednate 0. 05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study. Invest Ophthalmol Vis Sci. 2014;55:2993–3002. doi: 10.1167/iovs.13-12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khurana RN, Porco TC. Efficacy and safety of dexamethasone intravitreal implant for persistent uveitic cystoid macular edema. Retina. 2015;35:1640–1646. doi: 10.1097/IAE.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 45.Jaffe GJ, Martin D, Callanan D, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Multicenter Uveitis Steroid Treatment Trial Research G. Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JS, Knickelbein JE, Nussenblatt RB, et al. Clinical trials in noninfectious uveitis. Int Ophthalmol Clin. 2015;55:79–110. doi: 10.1097/IIO.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen QD, Merrill PT, Clark WL, et al. Intravitreal sirolimus for noninfectious uveitis: A phase III sirolimus Study Assessing Double-masKed Uveitis TReAtment (SAKURA) Ophthalmology. 2016;123:2413–2423. doi: 10.1016/j.ophtha.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 49▪▪.Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–943. doi: 10.1056/NEJMoa1509852. Large clinical trial for the treatment of noninfectious uveitis with adalimumab. [DOI] [PubMed] [Google Scholar]
- 50▪▪.Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–1192. doi: 10.1016/S0140-6736(16)31339-3. Recent clinical trial for the treatment of noninfectious uveitis with adalimumab. [DOI] [PubMed] [Google Scholar]
- 51.Castiblanco C, Meese H, Foster CS. Treatment of pediatric uveitis with adalimumab: the MERSI experience. J AAPOS. 2016;20:145–147. doi: 10.1016/j.jaapos.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Riancho-Zarrabeitia L, Calvo-Rio V, Blanco R, et al. Anti-TNF-alpha therapy in refractory uveitis associated with sarcoidosis: Multicenter study of 17 patients. Semin Arthritis Rheum. 2015;45:361–368. doi: 10.1016/j.semarthrit.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Takayama K, Tanaka A, Shibata M, et al. Evaluation of microincision vitrectomy surgery using wide-viewing system for complications with ocular sarcoidosis. Medicine (Baltimore) 2015;94:e559. doi: 10.1097/MD.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]