Summary
Peripheral T-cell lymphomas (PTCL) have suboptimal outcomes using conventional CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. The anti-folate pralatrexate, the first drug approved for patients with relapsed/refractory PTCL, provided a rationale to incorporate it into the front-line setting. This phase 2 study evaluated a novel front-line combination whereby cyclophosphamide, etoposide, vincristine and prednisone (CEOP) alternated with pralatrexate (CEOP-P) in PTCL. Patients achieving a complete or partial remission (CR/PR) were eligible for consolidative stem cell transplantation (SCT) after 4 cycles. Thirty-three stage II–IV PTCL patients were treated: 21 PTCL-not otherwise specified (64%), 8 angioimmunoblastic T cell lymphoma (24%) and 4 anaplastic large cell lymphoma (12%). The majority (61%) had stage IV disease and 46% were International Prognostic Index high/intermediate or high risk. Grade 3–4 toxicities included anaemia (27%), thrombocytopenia (12%), febrile neutropenia (18%), mucositis (18%), sepsis (15%), increased creatinine (12%) and liver transaminases (12%). Seventeen patients (52%) achieved a CR. The 2-year progression-free survival and overall survial, were 39% (95% confidence interval 21–57) and 60% (95% confidence interval 39–76), respectively. Fifteen patients (45%) (12 CR) received SCT and all remained in CR at a median follow-up of 21.5 months. CEOP-P did not improve outcomes compared to historical data using CHOP. Defining optimal front line therapy in PTCL continues to be a challenge and an unmet need.
Keywords: T-cell lymphoma, therapy, clinical trials
Introduction
Peripheral Natural Killer (NK)/T cell lymphomas (PTCL) represent approximately 10% of all non-Hodgkin lymphomas (NHL) and, compared to B-cell NHL, are associated with a poorer prognosis (Savage 2005). In the World Health Organization (WHO) classification, mature T and NK neoplasms are subdivided into 21 histological sub-types (Swerdlow, et al 2008). The various sub-entities are molecularly and clinically heterogeneous and the three most common subtypes of nodal PTCL in the Western hemisphere include PTCL-not otherwise specified (NOS), anaplastic large cell lymphoma (ALCL) and angioimmunoblastic T cell lymphoma (AITL).
Currently, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) is considered a standard therapy for PTCL (Pinter-Brown, et al 2014). With the exception of anaplastic lymphoma kinase (ALK)-positive ALCL, most PTCL patients will either not achieve a complete remission (CR) or relapse after initial treatment with anthracycline-based regimens (Vose, et al 2008). In a meta-analysis of 31 studies of patients with PTCL treated with CHOP (n=2912), excluding ALCL cases, the estimated 5-year overall survival (OS) was only 37.3% [95% confidence interval (CI) 35.1–39.6] (Abouyabis, et al 2011). More intensive chemotherapy regimens have, at best, shown only modest improvement when compared to historical controls with CHOP and have not been definitively proven to be superior in randomized trials (Abouyabis, et al 2011, Simon, et al 2010).
The German High Grade Lymphoma Study Group analysed a subset of patients with PTCL treated on 7 different protocols in which etoposide was added to CHOP (CHOEP) administered every 14 days. The authors found that younger patients (< 60 years) with a normal lactic acid dehydrogenase who were treated with CHOEP had a significant improvement in event-free survival (EFS) compared to those treated with CHOP, although no difference in OS was observed. The greatest benefit was seen in the ALK-positive subset, with a trend towards improved EFS observed in the other nodal PTCLs (Schmitz, et al 2010).
Intensifying upfront therapy with high dose therapy and stem cell transplantation (HDT/SCT) has also been explored, suggesting some improvement in outcomes compared to historical results seen with CHOP. However, refractory disease to induction chemotherapy continues to be a challenge, limiting the proportion of patients able to undergo HDT/SCT (d’Amore, et al 2012, Reimer, et al 2009).
Pralatrexate, a novel anti-folate, was the first agent to receive US Food and Drug Administration (FDA) approval for the treatment of relapsed or refractory PTCL, with a 29% overall response rate (ORR) (O’Connor, et al 2011). In a multicentre phase 2 study of pralatrexate administered weekly for 6 weeks of a 7-week cycle, 63% of responders demonstrated reduction in disease burden by the end of cycle 1. The median duration of response and OS were 10.1 months (range, 1–673 days) and 14.5 months, respectively. Given the rarity and heterogeneity of PTCL, this was at the time the largest data set showing activity of a single agent in this disease.
With the goal to optimize the development of a new front line strategy, various approaches that individually had some success were combined. These included moving away from multi-drug resistance (MDR)-related anthracycline-based regimens, such as standard CHOP, and incorporating novel agents (pralatrexate) in up-front regimens. With these factors in mind, we tested a non-anthracycline containing regimen (cyclophosphamide, etoposide, vincristine and prednisone [CEOP]) alternating with pralatrexate (P). Consolidation with HDT/SCT for patients in remission as part of front line therapy for appropriate patients was at the discretion of the treating physician.
We hypothesized that this novel upfront regimen would result in a higher CR rate than historically observed from CHOP-like treatments and would thus allow more PTCL patients (if eligible) to receive HDT/SCT as consolidation.
Patients and Methods
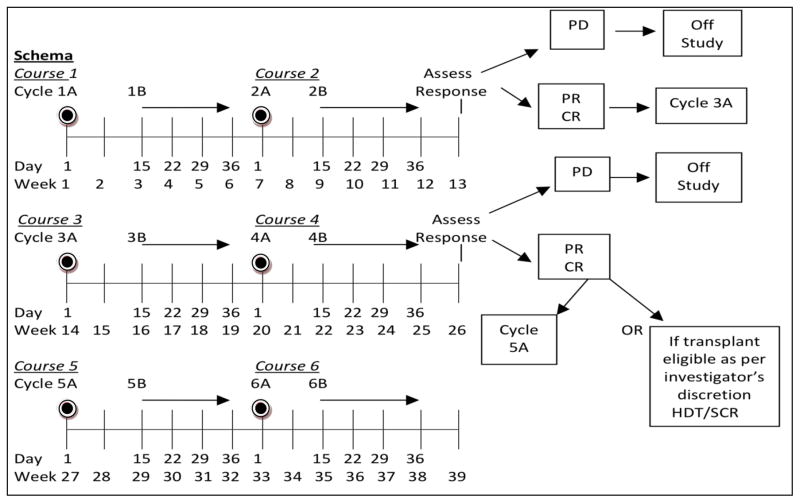
This open-label phase II study was conducted at academic sites participating in an informal working group, the “T Cell Consortium”, and approved by the institutional review board at each institution. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient. The University of Nebraska Medical Center provided data oversight. Patients ≥ 18 years with PTCL stages II–IV with no prior therapy, Karnofsky Performance Status > 70 and adequate end organ function were eligible. Eligible histologies included PTCL-NOS, AILT, ALCL (ALK positive patients were only allowed if the International Prognostic Index [IPI] was ≥3). Prior to each cycle the absolute neutrophil count was required to be > 1.0 × 109/l, and platelet count > 0.1 × 109/l. Detailed dose modification guidelines for hematological toxicities were built into the protocol (Supplemental Table 1). Each cycle consisted of CEOP (A) administered as: cyclophosphamide 750 mg/m2 IV day 1, etoposide 100 mg/m2 IV days 1–3 (or 100 mg/m2 IV day 1 and 200 mg/m2 PO days 2–3), vincristine 2 mg IV day 1 and prednisone 100 mg/day X 5 alternating with P (B) 30 mg/m2 IV days 15, 22 and 29. Growth factors were used to support both cycles of therapy (Figure 1). All patients received vitamin B12 (1 mg) intramuscular injection every 8–10 weeks and during B cycles oral folic acid (1.0–1.25 mg) daily. Patients with methylmalonic acid (MMA) levels ≥ 200 nmol/l or homocysteine (Hcy) ≥10 μmol/l at screening received supplementation > 10 days prior to the first pralatrexate dose (O’Connor, et al 2011).
Figure 1. CEOP-P Treatment Schema.
Cycle A: cyclophosphamide 750 mg/m2 day 1 IV; etoposide 100 mg/m2 days 1–3 IV (etoposide may be given PO on days 2 and 3 at double dose of 100 mg/m2 BID); vincristine 1.4 mg/m2 (capped at 2 mg) day 1 IV; prednisone 100 mg PO days 1–5; optional, per institutional standards, pegfilgrastim 6 mg day 4 of Week 1 of each course SQ.
Cycle B: pralatrexate 30 mg/m2 day 1 IV q week x 3; optional, per institutional standards, filgrastim (granulocyte colony-stimulating factor) 300 μg day 30 of each course SQ. Patients achieving stable disease after 4 courses (1,2,3,4) received 2 additional courses (5,6) and were then re-evaluated for response post-course 6.
PD, progressive disease; CR, complete response; PR, partial remission; HDT/SCR; High Dose Therapy/Stem Cell Rescue
Response assessment was performed by computerized tomography (CT) or positron emission tomography (PET)/CT based on the investigator’s preference after cycles 2, 4 and 6. Response was assessed by the treating physician according to the Cheson Revised response criteria (Cheson, et al 2007) or International Harmonization Project criteria (Cheson 2007), based on imaging modality used. Patients achieving a CR or partial remission (PR) were eligible for HDT/SCT after cycle 4B at physician discretion. Patients were followed until date of disease progression and /or death at 100 days and 2 years post consolidation of therapy.
Statistical Plan
The CR rate with CHOP has been variable and reported to be in the 30–73% range depending on the subtype of PTCL (Abouyabis, et al 2011, Reimer, et al 2009, Simon, et al 2010). The primary statistical aim of the present study was to improve the CR rate from 40% to 63% with CEOP-P and HDT/SCT. Secondary objectives included assessment of progression-free survival (PFS), OS and toxicity of the regimen. PFS was defined as time from the first therapy until relapse, progression, or death from any cause. OS was defined as time from the first chemotherapy administered on trial until death from any cause. A two-stage Simon design (alpha=0.10, 90% power) tested the null hypothesis that the CR rate would be greater than 40%. For the first stage of 20 evaluable patients, the trial would be terminated if 8 or fewer experienced a CR after course 2 of chemotherapy. For the second stage, a total of 34 patients were required with at least 17 patients achieving a CR at the end of therapy to consider the regimen useful.
All patients who received at least 2 complete courses of chemotherapy were evaluable for the response endpoint. Patients taken off study due to a global deterioration of health status without objective evidence of disease progression were counted as progressive disease (PD). Effort was made to document the objective progression even after discontinuation of treatment. Deaths were counted as treatment failure. CR rate was reported at the end of the CEOP-P (6 courses for patients not receiving transplant and 4–6 courses for patients receiving transplant). All eligible patients receiving at least one cycle of chemotherapy were evaluable for toxicity. All evaluable patients irrespective of the total number of cycles of therapy received were included in PFS and OS analyses.
Results
Thirty-four patients were enrolled and one withdrew consent before starting therapy, leaving 33 patients enrolled between July 2011 and January 2013. Characteristics are shown in Table I. The median age was 62 (range, 27–83) years. Twenty-one patients (64%) had PTCL, 8 (24%) AITL and 4 (12%) ALK-negative ALCL. The majority of patients (61%) had stage IV disease and 46% a high/intermediate or high risk IPI. The median number of chemotherapy cycles was 4 (range 1–6). Six patients received only 1 cycle due to either early PD (n=4) or adverse events (n=2). The number of patients receiving 4, 5 and 6 cycles was 9, 4 and 4, respectively.
Table I.
Patient Characteristics
| Variables | N (%) |
|---|---|
| N | 33 |
| Median age, years (range) | 62 (27 – 83) |
| Sex | |
| Female | 9 (27) |
| Male | 24 (73) |
| Karnofsky performance score | |
| 70 | 5 (15) |
| 80–100 | 28 (85) |
| Diagnosis | |
| PTCL-NOS | 21 (64) |
| AITL | 8 (24) |
| ALCL, T- and null cell types | 4 (12) |
| Ann Arbor Stage | |
| II | 4 (12) |
| III | 9 (27) |
| IV | 20 (61) |
| B symptoms | |
| No | 18 (55) |
| Yes | 15 (45) |
| IPI Score | |
| Low | 9 (27) |
| Low – intermediate | 9 (27) |
| High – intermediate | 9 (27) |
| High | 6 (19) |
| Lactate dehydrogenase | |
| Normal | 17 (52) |
| Elevated | 16 (48) |
| Extranodal involvement | |
| 0–1 | 24 (73) |
| 2 or more | 9 (27) |
| Median number of chemotherapy cycles (range) | 4 (1–6) |
| Median follow-up of survivors, months (range) | 20.4 (11.9 – 31.2) |
PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; IPI, International Prognostic Index.
Toxicity
Toxicities during CEOP-P were moderate. The most frequent grade 3–4 toxicities seen in ≥ 10% of patients and attributed to therapy included; anaemia (27%), thrombocytopenia (12%), febrile neutropenia (18%), mucositis (18%), sepsis (15%), elevated creatinine (12%) and liver transaminases (12%). These were largely reversible with supportive care and treatment delay. Two patients discontinued treatment due to adverse events.
Response
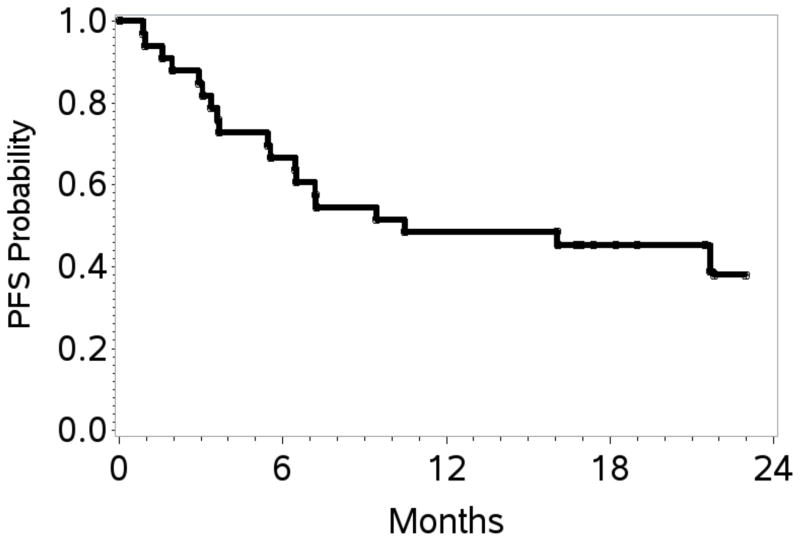
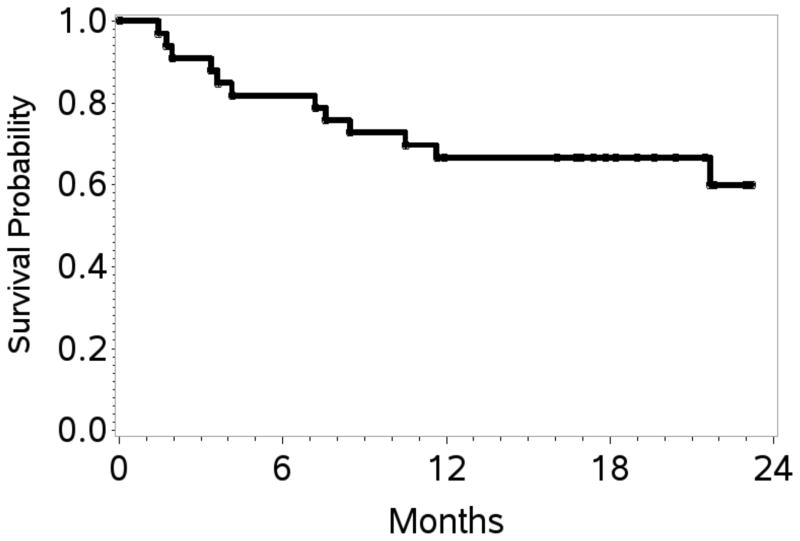
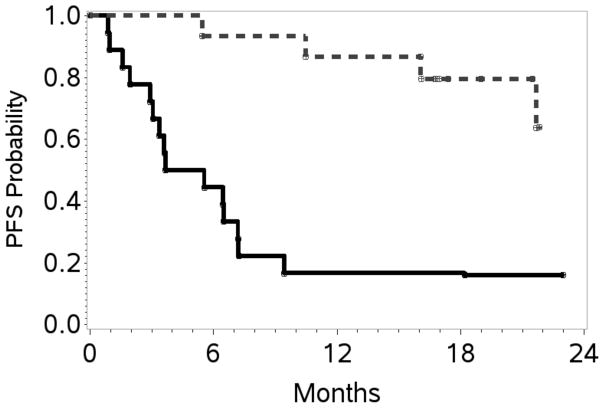
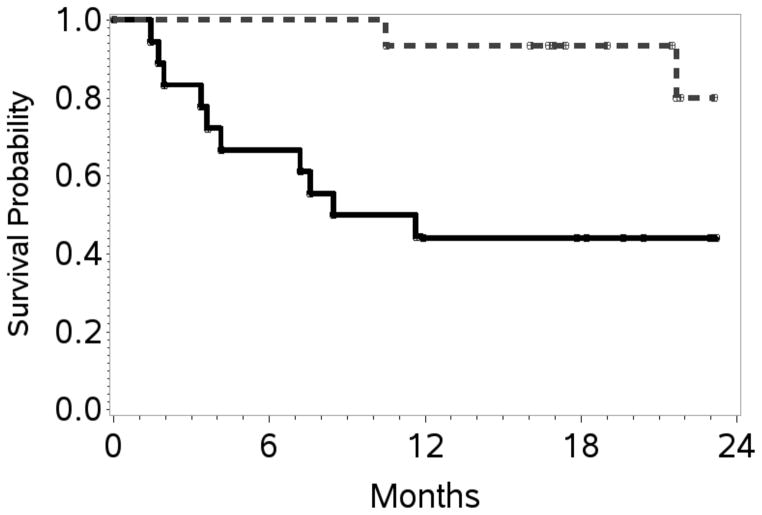
At the end of stage 1, 10 of 20 patients (50%) achieved a CR; therefore accrual proceeded, per protocol design, to stage 2. At the end of study, the overall response rate (ORR) was 70% with 17 patients (52%) achieving a CR. At a overall median follow up of 20 months, the estimated 1- and 2-year PFS / OS rates were 48% (95% CI 31–64) / 39% (95% CI 21–57), and 67% (95% CI 48–80) / 60% (95% CI 39–76), respectively (Table II; Figures 2A, 2B). Table III shows response rates by histological subtypes, IPI and for patients treated with versus without HDT/SCT. The ORR/CR for PTCL-NOS, AITL and ALCL were 76%/48%, 38%/25% and 100%/75% respectively. Fifteen patients (12 CR, 2 PR, 1 stable disease) received consolidation with HDT/SCT and have sustained complete remissions post-transplantation. With a median follow-up of 21.5 months, the estimated 2-year OS and PFS was 80% (95% CI 37–95) and 64% (95% CI 25–86), respectively. The PFS and OS were significantly better in these patients compared to those who did not receive HDT/SCT. The latter group had an estimated 2-year PFS of 17% (95% CI 4–36) and an OS of 44% (95% CI 22–65) (Figure 3A, 3B). Characteristics of patients treated versus those not treated with HDT/SCT are shown in Table IV. Patients who proceeded to SCT were younger (58 versus 64 years) but other characteristics did not differ. On exploratory bivariate analyses, age <60 years, absence of B symptoms, low IPI score (0,1), achieving a CR and receiving a HDT/SCT were the strongest predictors associated with better PFS (Table V). For OS, lack of B symptoms, low IPI score, achieving a CR and receiving a SCT were significant. In a comparison of patients in a CR with (n=12) or with out HDT/SCT (n=5), both PFS and OS were similar (p=0.26).
Table II.
Primary and Secondary Outcomes
| Outcomes | N (%) |
|---|---|
| Best Response | |
| CR | 17 (52) |
| PR | 6 (18) |
| PD | 8 (24) |
| SD | 2 (6) |
| Proceeded to HDT/SCT | |
| No | 18 (55) |
| Yes | 15 (45) |
| Probability | |
| Progression-free survival | |
| 100 days | 82 (95% CI, 64–91) |
| 6 months | 67 (95% CI, 48–80) |
| 1 year | 48 (95% CI, 31–64) |
| 2 years | 39 (95% CI, 21–57) |
| Overall survival | |
| 100 days | 91 (95% CI, 74–97) |
| 6 months | 82 (95% CI, 64–91) |
| 1 year | 67 (95& CI, 48–80) |
| 2 years | 60 (95% CI, 39–76) |
CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; HDT/SCT, high dose therapy/stem cell transplantation.
Figure 2. Progression-free survival and overall survival.
A. Kaplan–Meier curves for estimated 1- and 2-year progression-free survival: 48% (95% confidence interval [CI] 31–64) and 39% (95% CI 21–57) respectively.
B. Kaplan–Meier curves for estimated 1- and 2-year overall survival: 67% (95% CI 48–80) and 60% (95% CI 39–76) respectively.
Table III.
Overall complete remission rates according to risk factors
| Variables | CR | CR + PR (ORR) |
|---|---|---|
| Diagnosis | N (%) | N (%) |
| PTCL-NOS | 12/21 (48) | 16/21 (76) |
| AITL, lymphoma | 2/8 (25) | 3/8 (38) |
| ALCL, T- and null cell types | 3/4 (75) | 4/4 (100) |
| IPI Score | ||
| Low | 8/9 (89) | 9/9 (100) |
| Low – intermediate | 3/9 (33) | 5/9 (56) |
| High – intermediate | 4/9 (44) | 5/9 (56) |
| High | 2/6 (33) | 4/6 (67) |
| Auto - Transplant | ||
| No | 5/18 (28) | 9/18 (50) |
| Yes | 12/15 (80) | 14/15 (93) |
CR, complete remission; PR, partial remission; ORR, overall response rate; PTCL-NOS, Peripheral T-cell lymphoma, not otherwise specified; AITL, Angioimmuno-blastic T-cell lymphoma; ALCL, Anaplastic large cell lymphoma; IPI, International Prognostic Index
Figure 3. Progression-free survival and overall survival in patients who received HDT/SCT compared to those who did not.
A. Kaplan–Meier curves at 24 months for patients treated with high dose therapy and stem cell transplantation (HDT/SCT) (n=15) and without HDT/SCT (n=18): progression-free survival with HDT/SCT: 63% (95% confidence interval [CI] 25–86) and without HDT/SCT: 17% (95% CI 4–36) log rank p-value = 0.0002.
B. Kaplan–Meier curves at 24 months for patients treated with high dose therapy and stem cell transplantation (HDT/SCT) (n=15) and without HDT/SCT (n=18): Overall survival with HDT/SCT: 80% (95% CI 37–95) and without HDT/SCT: 44% (95% CI 22–65) log rank p-value = 0.007.
Table IV.
Comparison of patients who did receive HDT/SCT to those who did not
| Variable | No HDT/SCT N=18 N (%) |
Received HDT/SCT N=15 N (%) |
p-value |
|---|---|---|---|
| Median age, years (range) | 68 (34–83) | 59 (27–69) | 0.03 |
| Age at diagnosis | 0.06 | ||
| ≤60 years | 5 (28) | 9 (60) | |
| >60 years | 13 (72) | 6 (40) | |
| Sex | 0.48 | ||
| Female | 4 (22) | 5 (33) | |
| Male | 14 (78) | 10 (67) | |
| Karnofsky performance score | 0.79 | ||
| 70 | 3 (17) | 2 (13) | |
| 80–100 | 15 (83) | 13 (87) | |
| Diagnosis | 0.64 | ||
| PTCL-NOS | 10 (56) | 11 (73) | |
| AITL, | 5 (28) | 3 (20) | |
| ALCL, T- and null cell types | 3 (17) | 1 (7) | |
| Ann Arbor Stage | 0.84 | ||
| II | 2 (11) | 2 (13) | |
| III – IV | 16 (89) | 13 (87) | |
| B symptoms | 0.57 | ||
| No | 9 (50) | 9 (60) | |
| Yes | 9 (50) | 6 (40) | |
| IPI Score | 0.65 | ||
| Low | 4 (22) | 5 (33) | |
| Low – intermediate | 4 (22) | 5 (33) | |
| High – intermediate | 6 (33) | 3 (20) | |
| High | 4 (22) | 2 (13) | |
| Lactate dehydrogenase | 0.85 | ||
| Normal | 9 (50) | 8 (53) | |
| Elevated | 9 (50) | 7 (47) | |
| Extranodal involvement | 0.39 | ||
| 0–1 | 12 (67) | 12 (80) | |
| 2 or more | 6 (33) | 3 (20) | |
| Median number of chemotherapy cycles (range) | 2 (1–6) | 4 (1–6) | 0.03 |
HDT/SCT, high dose therapy and stem cell transplantation, PTCL, peripheral T-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; IPI, International Prognostic Index.
Table V.
Probability of 2 year PFS and OS according to risk factors
| Variable | PFS (95% CI) | p-value | OS (95% CI) | p-value |
|---|---|---|---|---|
| Age at diagnosis | 0.007 | 0.14 | ||
| ≤60 years | 71 (41–88) | 78 (47–92) | ||
| >60 years | 17 (4–40) | 48 (22–70) | ||
| Sex | 0.53 | 0.33 | ||
| Female | 56 (20–80) | 78 (36–94) | ||
| Male | 33 (14–54) | 53 (29–73) | ||
| Karnofsky performance score | 0.10 | 0.10 | ||
| 70 | 0 | 40 (5–75) | ||
| 80–100 | 43 (22–62) | 63 (39–80) | ||
| Diagnosis | 0.31 | 0.62 | ||
| PTCL-NOS (n=21) | 39 (14–64) | 59 (29–80) | ||
| AITL (n=8) | 25 (4–56) | 50 (15–77) | ||
| ALCL, T- and null cell types (n=4) | 50 (6–84) | 75 (13–96) | ||
| Ann Arbor Stage | 0.16 | 0.26 | ||
| II | 75 (13–96) | 100 | ||
| III | 44 (7–78) | 52 (8–84) | ||
| IV | 29 (11–50) | 55 (31–73) | ||
| B symptoms | 0.16 | 0.04 | ||
| No | 44 (18–68) | 71 (37–89) | ||
| Yes | 32 (11–56) | 47 (21–69) | ||
| IPI Score | 0.01 | 0.007 | ||
| Low (n=9) | 88 (43–98) | 100 | ||
| Low – intermediate (n=9) | 44 (14–72) | 78 (36–94) | ||
| High – intermediate (n=9) | 11 (1–39) | 22 (3–51) | ||
| High (n=9) | 0 | 50 (11–80) | ||
| Lactate dehydrogenase | 0.11 | 0.03 | ||
| Normal | 59 (32–78) | 82 (55–94) | ||
| Elevated | 15 (1–44) | 33 (8–63) | ||
| Extranodal involvement | 0.05 | 0.21 | ||
| 0–1 | 50 (26–70) | 66 (38–83) | ||
| 2 or more | 0 | 44 (13–72) | ||
| Best Response | <0.0001 | 0.01 | ||
| CR | 70 (36–89) | 70 (36–89) | ||
| PR | 17 (1–52) | 83 (27–97) | ||
| PD | 0 | 25 (4–56) | ||
| SD | 0 | 50 (1–91) | ||
| Autologous Transplant | 0.0002 | 0.007 | ||
| No (n=18) | 17 (4–36) | 44 (22–65) | ||
| Yes (n=15) | 66 (26–88) | 80 (37–95) |
PFS, progression free survival; OS, overall survival; 95% CI, 95% confidence interval; PTCL, Peripheral T-cell lymphoma; AITL, Angioimmunoblastic T-cell lymphoma; ALCL, Anaplastic large cell lymphoma; IPI, International Prognostic Index; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
Overall there were 12 deaths, due to disease progression (n=6), sepsis (n=3), congestive heart failure (n=1), renal failure (n=1) and subdural haematoma (n=1).
Discussion
In the absence of randomized clinical trials, CHOP or CHOP-like chemotherapy is considered a standard therapy for PTCLs but typically has disappointing outcomes (Savage, et al 2004, Vose, et al 2008). The advantage of a CHOP “like” regimen is that it is widely used in the community setting where most patients are treated. Data from the Vancouver Cancer Agency suggested that similar outcomes were obtained when etoposide was substituted for doxorubicn (adriamycin) in DLBCL patients who were unable to receive anthracyclines due to a variety of reasons (Moccia, et al 2009). In order to develop a non-anthracycline platform, we substituted etoposide for doxorubicn in part A of the regimen. Etoposide has commonly been used in other regimens for PTCL, such as cisplatinum, etoposide, gemcitabine and solumedrol (PEGS), CHOEP and steroids, methotrexate, Ifosfamide, lasparaginase and etoposide (SMILE) with activity in haemophagocytic syndromes, which is often seen in patients with aggressive PTCL (Mahadevan, et al 2013, Pfreundschuh, et al 2008, Yamaguchi, et al 2011). Furthermore, the addition of etoposide to CHOP improves EFS in younger PTCL patients, as discussed above. Thus, the CEOP backbone was considered both rational and promising. When the study design was conceived, pralatrexate, a novel anti-folate, was the only FDA-approved drug for relapsed and refractory PTCL. The overall response rate per International Workshop Criteria (IWC) by independent central review was 29% (n=32) across a variety of PTCL subtypes (O’Connor, et al 2011). We hypothesized that adding pralatrexate, as a non-cross resistant agent, to a predictable backbone in the front line setting might be beneficial. Our regimen sequenced pralatrexate with CEOP to avoid overlapping toxicity. Unlike other front line studies in PTCL in the US which have often taken > 3–5 years to complete, our T Cell Consortium study accrued rapidly in 1.5 years, suggesting that novel strategies for this rare disease can be tested in a reasonable time frame with committed investigators. The frequency of neutropenia and thrombocytopenia was not significantly increased compared to historical data with CHOEP (Schmitz, et al 2010). While our interim analysis showed that CEOP-P met the pre-defined stage 1 response criteria with a CR rate of 52% compared with 31% reported in prospective studies with CHOP (Reimer, et al 2009), the 2-year PFS and OS of 39% and 60%, respectively, do not appear to be a significant improvement over historic outcomes reported with CHOP-like regimens. It is plausible that, in our study, pralatrexate alone between the CEOP doses may actually have decreased the intensity of treatment and hence the overall efficacy.
Intensifying upfront therapy with HDT/SCT may improve the generally poor outcomes seen with standard CHOP induction chemotherapy; however, the major limitation is that significant subsets of patients never manifest sufficient chemosensitivity in order to undergo consolidative HDT/SCT. Recent prospective trials assessing the role of consolidative HDT/SCT in patients achieving a CR/PR, report that only 66–72% of enrolled patients actually receive the planned HDT/SCT (d’Amore, et al 2012, Reimer, et al 2009). Despite these limitations, cumulatively these prospective studies suggest a moderately better PFS and OS than population-based series with CHOP (Ellin, et al 2014). In our study, patients who received HDT/SCT had improved outcomes when compared to patients who did not, which reflects the poor prognosis of patients who are chemo-refractory and do not receive HDT/SCT. Younger patients and those with a low IPI did particularly well. Interestingly, within the caveats of small sample size, no statistically significant difference in PFS and OS was noted in patients who achieved a CR and proceeded to HDT/SCT versus those with a CR and no HDT/SCT. This is similar to results from a retrospective review in which the most dominant prognostic factor was response to initial therapy (CR versus other), with no OS difference based on choice of upfront regimen or SCT in first remission (Abramson, et al 2014). Unfortunately, all transplant studies have similar limitations due to selection biases with a tendency to include mainly younger patients with chemosensitive disease and exclude frail patients who are unable to tolerate HDT (Pedersen, et al 2014). Therefore, the question still remains whether or not the HDT/SCT as consolidation after primary therapy improves outcome. Randomized studies comparing chemotherapy to chemotherapy with HDT/SCT are unfortunately lacking.
Many studies have investigated combining novel treatment regimens with CHOP as the backbone chemotherapy in PTCL. Thus far, none have demonstrated a significant improvement in outcomes when compared to CHOP alone. As serum concentration of VEGF has been shown to be an independent predictor of poor outcome in patients with NHL (Salven, et al 1998), the Eastern Cooperative Oncology Group (ECOG) 2404 trial evaluated the combination of an antiangiogenic agent bevacizumab (Avastin) and CHOP (ACHOP) followed by maintenance bevacizumab (Salven, et al 1998). Despite a high CR rate, the 1-year PFS was only 44% at a median follow-up of 3 years and the combination was quite toxic, with grade 3 congestive heart failure reported in 18% of patients (Advani, et al 2012, Ganjoo, et al 2014). Combinations of bortezomib/CHOP, alemtuzamab/CHOP or CHOEP and denileukin difitox/CHOP have also been evaluated and results do not report durable responses (Binder, et al 2013, Enblad, et al 2004, Foss, et al 2013, Gallamini, et al 2007, Kim, et al 2012).
The Southwestern Oncology Group (SWOG) 0350 trial evaluated PEGS, a novel non-CHOP regimen, based on the premise that the poor efficacy of CHOP therapy may be due to T cells expressing high levels of p-glycoprotein, resulting in MDR (Mahadevan, et al 2013). Although the heterogeneous patient population, which included relapsed disease, confounded the intended interpretation, the 2-year PFS of 12% with an ORR of 31% to frontline treatment was disappointing. A UK group is currently evaluating another gemcitabine-based regimen in combination with cisplatin (GEM-P) versus CHOP in a randomized phase 2 study (NCT01719835).
Since our study inception, several other novel agents have been approved for relapsed PTCL (Dupuis, et al 2014, Lee, et al 2015, Pro, et al 2012). The encouraging single agent activity of brentuximab vedotin in relapsed and refractory ALCL (Pro, et al 2012), as well as in other PTCLs (Horwitz, et al 2014), has led to its evaluation in combination with CHOP (Fanale, et al 2014). The latter study has shown promising phase 1 results and a phase 3 study comparing brentuximab vedotin with modified CHOP (without vincristine) versus CHOP (ECHELON-2) is ongoing in patients with CD30+ PTCL (NCT 01777152). Romidepsin and Belinostat are histone deacetylase inhibitors, approved for relapsed PTCL with activity across multiple subtypes (Coiffier, et al 2012, Lee, et al 2015). Romidepsin has been evaluated in combination with CHOP in the front line setting with an ORR/CR of 68% and 51%, respectively. With a median follow-up of 17.5 months, the estimated PFS is 57% at 18 months (Dupuis, et al 2014). This combination is also being tested in a randomized phase 3 trial (NCT01796002).
Recent studies have identified molecular subsets with improved prognostication among PTCL-NOS, ALK-positive and ALK-negative lymphomas (Iqbal, et al 2010, Parrilla Castellar, et al 2014, Piccaluga, et al 2013). Additional mutations (i.e. TET2 and RHOA) have been identified in AITL. These advances provide a rationale for the development of novel pathway targeted regimens that specifically target distinct subsets of PTCL (Cairns, et al 2012, Sakata-Yanagimoto, et al 2014).
In conclusion, the sequential addition of pralatrexate to a CEOP backbone did not demonstrate sufficient activity to warrant further exploration. It is unclear whether a different schedule that would not de-intensify chemotherapy may be superior. The overall management of front-line PTCL remains challenging, and currently there is no “home run” in any front line therapeutic approach. Clearly, investigating additional novel approaches is critical and defining the optimal front line therapy in PTCL continues to be a challenge and an unmet need.
Supplementary Material
Supplemental Table 1: Dose modifications for hematological toxicities
Acknowledgments
This study was supported by Allos Therapeutics/Spectrum Pharmaceuticals Inc.
Grant Number:P30 CA008748.
Footnotes
Author contributions
Ranjana Advani designed the research study, enrolled patients, analysed data, and wrote the manuscript. Stephen Ansell, Mary Jo Lechowicz, Anne Beaven, Ken Carson, Andrew Evens and Julie Vose designed the research study, enrolled patients and critically revised manuscript. Fausto Loberiza designed the research study, analysed data and critically revised the manuscript. Francine Foss, Steven Horwitz, Barbara Pro, Lauren Pinter Brown, Sonali Smith, Andrei Shustov and Kerry Savage designed the research study and critically revised manuscript. All authors approved the final version of the paper for publication.
Conflicts of interest
RHA has reported research funding from Seattle Genetics and Allos Therapeutics. MJL has reported research funding from Allos Therapeutics, Celgene, Seattle Genetics and Millennium; consultancy for Millennium, Allos Therapeutics and Seattle Genetics. AB has reported GlaxoSmithKline (GSK) stock and family member is an employee of GSK; research funding from Spectrum Pharmaceuticals; speakers board for Celgene and Seattle Genetics. KC has reported research funding and honoraria from Spectrum Pharmaceuticals. FF has reported research funding from Merck, Spectrum Pharmaceuticals, Celgene and Seattle Genetics; membership on Board of Directors or advisory committees for Eisai and Millennium; honoraria received from Celgene and Millennium. SH has reported research funding from Celgene, Spectrum Pharamaceuticals, Genzyme, Seattle Genetics, Janssen and Millennium; Consultancy for Spectrum Pharamaceuticals, Celgene, Seattle Genetics, Kyowa Hakko Kirn Pharma, Infinity Pharmaceuticals and Millennium. BP has reported honoraria from Spectrum Pharmaceuticals. LPB has reported consultancy/honoraria from Spectrum Pharmaceuticals. SMS has reported consultancy for Allos Therapeutics, Celgene, Seattle Genetics, Onyx, Genentech, Micromet and Gilead; speakers bureau for Janssen. AS has reported research funding from Celgene; consultancy honoraria from Celgene and Spectrum Pharamaceuticals. KJS has reported research funding from Spectrum Pharmaceuticals. JV has reported research funding from Spectrum Pharmaceuticals. All remaining authors have declared no conflicts of interest.
Additional supporting information may be found in the online version of this article
References
- Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A Systematic Review and Meta-Analysis of Front-line Anthracycline-Based Chemotherapy Regimens for Peripheral T-Cell Lymphoma. ISRN Hematol. 2011;2011:623924. doi: 10.5402/2011/623924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson JS, Feldman T, Kroll-Desrosiers AR, Muffly LS, Winer E, Flowers CR, Lansigan F, Nabhan C, Nastoupil LJ, Nath R, Goy A, Castillo JJ, Jagadeesh D, Woda B, Rosen ST, Smith SM, Evens AM. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol. 2014;25:2211–2217. doi: 10.1093/annonc/mdu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani RH, Hong F, Horning SJ, Kahl BS, Manola J, Swinnen LJ, Habermann TM, Ganjoo K. Cardiac toxicity associated with bevacizumab (Avastin) in combination with CHOP chemotherapy for peripheral T cell lymphoma in ECOG 2404 trial. Leuk Lymphoma. 2012;53:718–720. doi: 10.3109/10428194.2011.623256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C, Ziepert M, Pfreundschuh M, Duhrsen U, Eimermacher H, Aldaoud A, Rosenwald A, Loeffler M, Schmitz N, Truemper L German High Grade Non-Hodgkin’s Lymphoma Study Group. CHO(E)P-14 followed by alemtuzumab consolidation in untreated peripheral T cell lymphomas: final analysis of a prospective phase II trial. Ann Hematol. 2013;92:1521–1528. doi: 10.1007/s00277-013-1880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais JP, Parrens M, Martin A, Xerri L, Brousset P, Chan LC, Chan WC, Gaulard P, Mak TW. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119:1901–1903. doi: 10.1182/blood-2011-11-391748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21:841–854. doi: 10.1016/j.hoc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Borchmann P, Morschhauser F, Wilhelm M, Pinter-Brown L, Padmanabhan S, Shustov A, Nichols J, Carroll S, Balser J, Balser B, Horwitz S. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, Holte H, Osterborg A, Merup M, Brown P, Kuittinen O, Erlanson M, Ostenstad B, Fagerli UM, Gadeberg OV, Sundstrom C, Delabie J, Ralfkiaer E, Vornanen M, Toldbod HE. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Morschhauser F, Ghesquieres H, Tilly H, Casasnovas O, Amorim S, Ribrag V, Coiffier B. Final Analysis of the RO-CHOP Phase Ib/II Study: Romidepsin in Association with CHOP in Patients with Peripheral T-Cell Lymphoma (PTCL) Blood (ASH Annual Meeting Abstracts) 2014;124:504. [Google Scholar]
- Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124:1570–1577. doi: 10.1182/blood-2014-04-573089. [DOI] [PubMed] [Google Scholar]
- Enblad G, Hagberg H, Erlanson M, Lundin J, MacDonald AP, Repp R, Schetelig J, Seipelt G, Osterborg A. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- Fanale MA, Horwitz SM, Forero-Torres A, Bartlett NL, Advani RH, Pro B, Chen RW, Davies A, Illidge T, Huebner D, Kennedy DA, Shustov AR. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32:3137–3143. doi: 10.1200/JCO.2013.54.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss FM, Sjak-Shie N, Goy A, Jacobsen E, Advani R, Smith MR, Komrokji R, Pendergrass K, Bolejack V. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leuk Lymphoma. 2013;54:1373–1379. doi: 10.3109/10428194.2012.742521. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Zaja F, Patti C, Billio A, Specchia MR, Tucci A, Levis A, Manna A, Secondo V, Rigacci L, Pinto A, Iannitto E, Zoli V, Torchio P, Pileri S, Tarella C. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–2323. doi: 10.1182/blood-2007-02-074641. [DOI] [PubMed] [Google Scholar]
- Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ, Habermann TM, Kahl BS, Advani RH. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404) Leuk Lymphoma. 2014;55:768–772. doi: 10.3109/10428194.2013.816700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O’Connor OA, Siddiqi T, Kennedy DA, Oki Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095–3100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Weisenburger DD, Greiner TC, Vose JM, McKeithan T, Kucuk C, Geng H, Deffenbacher K, Smith L, Dybkaer K, Nakamura S, Seto M, Delabie J, Berger F, Loong F, Au WY, Ko YH, Sng I, Armitage JO, Chan WC International Peripheral TCLP. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115:1026–1036. doi: 10.1182/blood-2009-06-227579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Yoon DH, Kang HJ, Kim JS, Park SK, Kim HJ, Lee J, Ryoo BY, Ko YH, Huh J, Yang WI, Kim HK, Min SK, Lee SS, Do IG, Suh C, Kim WS Consortium for Improving Survival of Lymphoma i. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: a multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48:3223–3231. doi: 10.1016/j.ejca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Lee HZ, Kwitkowski VE, Del Valle PL, Ricci MS, Saber H, Habtemariam BA, Bullock J, Bloomquist E, Li Shen Y, Chen XH, Brown J, Mehrotra N, Dorff S, Charlab R, Kane RC, Kaminskas E, Justice R, Farrell AT, Pazdur R. FDA Approval: Belinostat for the Treatment of Patients with Relapsed or Refractory Peripheral T-cell Lymphoma. Clin Cancer Res. 2015;21:2666–2670. doi: 10.1158/1078-0432.CCR-14-3119. [DOI] [PubMed] [Google Scholar]
- Mahadevan D, Unger JM, Spier CM, Persky DO, Young F, LeBlanc M, Fisher RI, Miller TP. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2013;119:371–379. doi: 10.1002/cncr.27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia AA, Schaff K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Gascoyne RD, Connors JM, Sehn LH. R-CHOP with Etoposide Substituted for Doxorubicin (R-CEOP): Excellent Outcome in Diffuse Large B Cell Lymphoma for Patients with a Contraindication to Anthracyclines. Blood (ASH Annual Meeting Abstracts) 2009;114:408. [Google Scholar]
- O’Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, Lechowicz MJ, Savage KJ, Shustov AR, Gisselbrecht C, Jacobsen E, Zinzani PL, Furman R, Goy A, Haioun C, Crump M, Zain JM, Hsi E, Boyd A, Horwitz S. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla Castellar ER, Jaffe ES, Said JW, Swerdlow SH, Ketterling RP, Knudson RA, Sidhu JS, Hsi ED, Karikehalli S, Jiang L, Vasmatzis G, Gibson SE, Ondrejka S, Nicolae A, Grogg KL, Allmer C, Ristow KM, Wilson WH, Macon WR, Law ME, Cerhan JR, Habermann TM, Ansell SM, Dogan A, Maurer MJ, Feldman AL. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124:1473–1480. doi: 10.1182/blood-2014-04-571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MB, Hamilton-Dutoit SJ, Bendix K, Moller MB, Norgaard P, Johansen P, Ralfkiaer E, Brown PD, Hansen PB, Jensen BA, Madsen J, Schollkopf C, d’Amore F. Evaluation of clinical trial eligibility and prognostic indices in a population-based cohort of systemic peripheral T-cell lymphomas from the Danish Lymphoma Registry. Hematol Oncol. 2014 doi: 10.1002/hon.2153. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Zwick C, Zeynalova S, Duhrsen U, Pfluger KH, Vrieling T, Mesters R, Mergenthaler HG, Einsele H, Bentz M, Lengfelder E, Trumper L, Rube C, Schmitz N, Loeffler M German High-Grade Non-Hodgkin’s Lymphoma Study G. Dose-escalated CHOEP for the treatment of young patients with aggressive non-Hodgkin’s lymphoma: II. Results of the randomized high-CHOEP trial of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Ann Oncol. 2008;19:545–552. doi: 10.1093/annonc/mdm514. [DOI] [PubMed] [Google Scholar]
- Piccaluga PP, Fuligni F, De Leo A, Bertuzzi C, Rossi M, Bacci F, Sabattini E, Agostinelli C, Gazzola A, Laginestra MA, Mannu C, Sapienza MR, Hartmann S, Hansmann ML, Piva R, Iqbal J, Chan JC, Weisenburger D, Vose JM, Bellei M, Federico M, Inghirami G, Zinzani PL, Pileri SA. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31:3019–3025. doi: 10.1200/JCO.2012.42.5611. [DOI] [PubMed] [Google Scholar]
- Pinter-Brown L, Foss FM, Carson KR, Horwitz SM, Rosen ST, Pro B, Federico M, Gisselbrecht C, Hsi ED, Shustov AR, Advani R, Feldman T, Lechowicz MJ, Smith S, Tulpule A, Greer JP, Kahl BS, Leach J, Morganstein N, Casulo C, Park SI. Patient Characteristics and Initial Treatment Patterns in the United States for the Most Common Subtypes of Peripheral T-Cell Lymphoma (PTCL) Blood. 2014;124:4434–4434. [Google Scholar]
- Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Yang Y, Sievers EL, Kennedy DA, Shustov A. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, Engert A, Einsele H, Muller-Hermelink HK, Wilhelm M. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, Muto H, Tsuyama N, Sato-Otsubo A, Okuno Y, Sakata S, Kamada Y, Nakamoto-Matsubara R, Tran NB, Izutsu K, Sato Y, Ohta Y, Furuta J, Shimizu S, Komeno T, Sato Y, Ito T, Noguchi M, Noguchi E, Sanada M, Chiba K, Tanaka H, Suzukawa K, Nanmoku T, Hasegawa Y, Nureki O, Miyano S, Nakamura N, Takeuchi K, Ogawa S, Chiba S. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:171–175. doi: 10.1038/ng.2872. [DOI] [PubMed] [Google Scholar]
- Salven P, Ruotsalainen T, Mattson K, Joensuu H. High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int J Cancer. 1998;79:144–146. doi: 10.1002/(sici)1097-0215(19980417)79:2<144::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Savage KJ. Aggressive peripheral T-cell lymphomas (specified and unspecified types) Hematology Am Soc Hematol Educ Program. 2005:267–277. doi: 10.1182/asheducation-2005.1.267. [DOI] [PubMed] [Google Scholar]
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- Simon A, Peoch M, Casassus P, Deconinck E, Colombat P, Desablens B, Tournilhac O, Eghbali H, Foussard C, Jaubert J, Vilque JP, Rossi JF, Lucas V, Delwail V, Thyss A, Maloisel F, Milpied N, le Gouill S, Lamy T, Gressin R. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151:159–166. doi: 10.1111/j.1365-2141.2010.08329.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, Stein H, Thiele J, Vardiman JWE. WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on CAncer (IARC); Lyon, France: 2008. [Google Scholar]
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, Suzumiya J, Kodama T, Kimura H, Hyo R, Nakamura S, Oshimi K, Suzuki R. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Dose modifications for hematological toxicities