Abstract
HMGB1 (high mobility group box protein 1) is an architectural protein that facilitates formation of protein-DNA assemblies involved in transcription, recombination, DNA repair, and chromatin remodeling. Important to its function is the ability of HMGB1 to bend DNA non-sequence specifically. HMGB1 contains two HMG boxes that bind and bend DNA (the A box and the B box) and a C-terminal acidic tail. We investigated how these domains contribute to DNA bending by HMGB1 using single molecule FRET, which enabled us to resolve heterogeneous populations of bent and unbent DNA. We found that full length HMGB1 bent DNA more than the individual A and B boxes. Removing the C-terminal tail resulted in a protein that bent DNA to a greater extent than the full length protein. These data suggest that the A and B boxes simultaneously bind DNA in the absence of the C-terminal tail, but the tail modulates DNA binding and bending by one of the HMG boxes in the full length protein. Indeed, a construct composed of the B box and the C-terminal tail only bent DNA at higher protein concentrations. Moreover, in the context of the full length protein, mutating the A box such that it could not bend DNA resulted in a protein that bent DNA similarly to a single HMG box and only at higher protein concentrations. We propose a model in which the HMGB1 C-terminal tail serves as an intramolecular damper that modulates the interaction of the B box with DNA.
Keywords: FRET, single-molecule, TIRF microscopy, DNA bending, HMGB1
Introduction
In mammalian cells architectural proteins contribute to cellular regulation by mediating the proper assembly of nucleoprotein complexes on DNA. Among the known architectural proteins is the HMG (high mobility group) family, which contains HMG-box DNA binding domains that can bend DNA [1,2]. High mobility group box protein 1 (HMGB1) binds in the minor groove of DNA with little sequence specificity and induces the DNA to bend. HMGB1 plays important roles in several nuclear processes in mammalian cells, including transcription, recombination, DNA repair, and chromatin remodeling [1–4]. As part of these processes, HMGB1 facilitates the formation of nucleoprotein complexes via mechanisms that are not well understood, but likely involve its ability to induce conformational flexibility in DNA. For example, HMGB1 strongly enhances DNA binding by many transcription factors including p53, progesterone receptor, estrogen receptor, NF-κB/Rel, Oct, and HOX proteins [1–3]. Developing a fundamental understanding of how HMGB1 binds and bends DNA will provide insight into how architectural proteins regulate the formation and flexibility of nucleoprotein complexes that control a diversity of nuclear processes.
HMGB1 is composed of two highly conserved HMG box motifs, the A box and the B box, which are each composed of ~80 amino acids and can independently bind and bend DNA (Figure 1a) [1,5]. The HMG boxes are followed by an intrinsically disordered acidic C-terminal tail. The A and B boxes bind DNA in the minor groove and bend the DNA toward the major groove [6–8]. They are independent domains that do not interact with each other in the context of the full length protein [9,10]. The A and B boxes are similar in overall structure, consisting of three α-helices in an L shaped formation; however, their tertiary structures have slightly different shapes and orientations, and the residues that intercalate into DNA to facilitate structural distortion are different [3,5]. Specifically, the A box in human HMGB1 has a phenylalanine (Phe38) that intercalates into DNA, and the B box has 2 intercalating residues, a phenylalanine (Phe103) and an isoleucine (Ile122), suggesting that the two boxes employ different mechanisms of DNA bending [1,3]. The intercalating residues also allow high affinity binding between HMGB proteins and distorted DNA, for example cisplatinated DNA [11] or cruciforms [12].
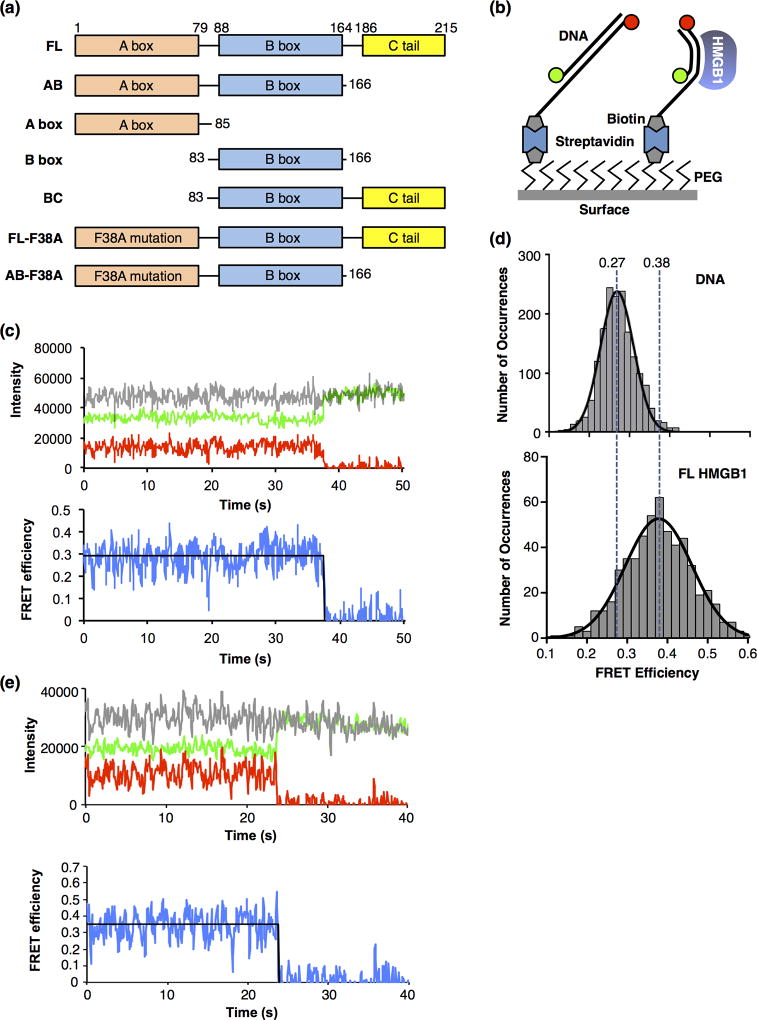
Figure 1.
Full length HMGB1 bends DNA molecules, as detected using smFRET. (a) Domain structures of full length human HMGB1 (FL) and the truncations and mutations used in this study. (b) Depiction of the surface used for the smFRET experiments. The glass coverslip was coated with polyethylene glycol (PEG) to prevent nonspecific interactions of the proteins and DNA with the surface. Some PEG molecules were functionalized with biotin, which allowed immobilization of streptavidin and biotinylated DNA labeled with donor and acceptor fluorophores (green and red circles). When HMGB1 bends the DNA (right side), the donor and acceptor fluorophores move closer together, resulting in a higher FRET efficiency than the unbent DNA (left side). (c) A representative time trace of the fluorescent signals from a single molecule of linear DNA. The upper plot shows emission from the donor fluorophore (green), acceptor fluorophore (red), and the sum of the donor and acceptor (grey) over time. The lower plot shows the FRET efficiency (blue) calculated from the donor and acceptor emissions, and the fit of the FRET efficiency (black line) over time. The acceptor dye photobleached at 38 s, resulting in a loss of FRET and an anticorrelated change in the donor fluorophore. For purposes of display, the signals were smoothed by averaging 3 adjacent time frames. (d) HMGB1 (FL) bends DNA to a single state. The upper plot shows 1701 FRET occurrences for DNA in the absence of protein. The data were fit to a Gaussian and the mean FRET efficiency is 0.27. The lower plot shows 553 FRET occurrences for DNA in the presence of 50 nM FL HMGB1. The data were fit to a Gaussian and the mean FRET efficiency is 0.38. The vertical dashed lines are centered over the mean FRET efficiencies and illustrate the HMGB1-induced increase in FRET due to DNA bending. (e) A representative time trace of the fluorescent signals from a single molecule of DNA bent by HMGB1. The upper plot shows emission from the donor fluorophore (green), acceptor fluorophore (red), and the sum of the donor and acceptor (grey) over time. The lower plot shows the FRET efficiency (blue) calculated from the donor and acceptor emissions, and the fit of the FRET efficiency (black line) over time. The acceptor dye photobleached at ~24 s, resulting in a loss of FRET and an anticorrelated change in the donor fluorophore. For purposes of display, the signals were smoothed by averaging 3 adjacent time frames.
Structural and biochemical data support a model in which the C-terminal acidic tail is an important regulator of HMGB1 binding to DNA. NMR data show that the C-terminal tail makes extensive contacts with the DNA-binding surfaces of both the A box and the B box of HMGB1 [13,14], and the C-terminal tail can bind to the A and B boxes in trans [15,16]. Hence, the C-terminal tail has been suggested to limit or control the binding of HMGB1 to DNA. Indeed, structural models show that the A and B boxes sandwich the C-terminal tail such that their DNA binding domains are sheltered from other interactions, for example, with DNA [13,14]. This conformation, however, is thought to be dynamic in nature and therefore the DNA binding domains can be transiently exposed, which would enable them to make contacts with nucleic acid targets. Biochemical studies support this model and suggest that the intramolecular interactions between the C-terminal tail and the HMG box domains could fine-tune selectivity for either domain binding to other targets (i.e. DNA or other proteins) [16,17].
In addition to understanding how HMGB1 binds to DNA, biochemical and structural studies have investigated the mechanism by which HMGB1 bends DNA. Ligase-mediated circularization assays have revealed that HMGB1 can bend DNA molecules to enable their ends to be ligated [10,18–21]. In these assays, the single B box bends DNA more efficiently than the A box [18–21]. Dual-laser optical tweezer experiments showed that the linked A and B boxes of HMGB1 bend DNA to a somewhat lesser degree than a single HMG box [22], and similar results were obtained using atomic force microscopy [23]. Other studies have shown that deleting the acidic C-terminal tail from full length HMGB1 can enhance bending [18], while others reported that deleting the tail reduces bending [24]. Thus, the mechanism by which HMGB1 bends DNA, and the relative contributions of the A box, B box, and C-terminal tail to bending are not completely clear. A structure of full length HMGB1 bound to DNA has not been solved. An NMR structure of a DNA-bound chimeric protein, in which the A box of HMGB1 was replaced with the HMG box of SRY, revealed an overall bend angle of ~101° in the DNA, with the bend generated roughly equally from the SRY box and the B box of HMGB1 [25]. Other studies report moderately broad distributions of bend angles, which is inconsistent with either a static kink model for bending or a purely flexible hinge model [22,23]. Studies with nucleosome DNA have also provided insight into HMGB-induced DNA bending [26]. Binding of an HMGB protein at the edge of the DNA wrapped around a nucleosome could distort the DNA by imposing a bent region to prime remodeling or transcription factor binding.
To provide insight into the mechanism of HMGB1-induced DNA bending, we have utilized single-molecule FRET (smFRET). We found that full length human HMGB1 bends DNA more than the A box and B box individually. However, a truncated protein with the C-terminal tail deleted bends the DNA to a greater extent than the full length protein, suggesting that in the absence of the tail, both boxes bind and bend DNA together. When the C-terminal tail was linked to the B box alone, DNA bending was strongly reduced. The C-terminal tail also inhibited DNA bending in the context of a full length HMGB1 protein containing a point mutation in the A box that disrupted its ability to bend DNA. Together our data support a model in which HMGB1 can simultaneously use both the A and B boxes to bind and bend DNA in the absence of the C-terminal tail, however, in the full length protein the C-terminal tail acts as a damper to modulate the interaction of the B box with DNA.
Results
A single molecule system to monitor HMGB1-induced DNA bending
With the goal of understanding the relative contributions of the A box, the B box, and the C-terminal tail to DNA bending by HMGB1, we established an smFRET system to monitor protein-induced DNA bending. We designed a DNA construct with donor and acceptor fluorophores positioned such that when HMGB1 induced a bend, the distance between the donor and the acceptor fluorophores would decrease, and an increase in FRET efficiency would be observed (Figure 1b). The donor (Alexa 555) and acceptor (Alexa 647) dyes were attached to the 5’-ends of two oligos, that when annealed, formed an 18 bp dsDNA with a 19 nt single stranded 3' overhang containing a biotin on the end. This construct was attached to a glass surface through a biotin-streptavidin linkage. The sample chamber was placed on a microscope and imaged using total internal reflection fluorescence (TIRF) microscopy. In all experiments the donor fluorophore was excited with a 532 nm laser and the donor and acceptor emissions were monitored over time using two CCD cameras. Data were analyzed and apparent FRET efficiencies were calculated as previously described [27]. Figure 1c shows representative data obtained from a single molecule of DNA in the absence of protein. The upper plot shows a time trace of the donor emission in green, the acceptor emission in red, and the sum of the donor and acceptor emission in grey. At approximately 38 s, the acceptor dye photobleached, thus its emission went to zero and the donor emission exhibited an anticorrelated change, indicating that FRET had been occurring prior to photobleaching. The total fluorescence emission remained constant over time. The lower plot shows the apparent FRET efficiency calculated from the donor and acceptor emissions over time (blue line), and the FRET state that best fit the data prior to acceptor photobleaching (0.29, black line).
We obtained the average FRET efficiency for unbound DNA by determining the FRET states (i.e. occurrences) in a population of DNA molecules. The individual FRET states were binned to generate a histogram that was fit with a Gaussian, yielding a mean efficiency of 0.27 (Figure 1d, top plot). The mean FRET and confidence intervals of the Gaussian fit for these data and all subsequent data are provided in Table 1. To observe FRET changes due to bending by full length (FL) HMGB1, the protein was flowed into a chamber, the slide was imaged, and the FRET occurrences of individual molecules were calculated and histogrammed. In the presence of FL HMGB1, a single Gaussian was observed whose distribution was centered at a higher FRET efficiency (0.38) compared to free DNA (Figure 1d, lower plot). We conclude that HMGB1 bent the DNA, causing a decrease in the distance between the donor and acceptor dyes, resulting in an increase in FRET efficiency. We measured the affinity of HMGB1 binding the same 18 bp dsDNA sequence using electrophoretic mobility shift assays (EMSA), and found the KD to be 10 nM (Supplemental Figure). Therefore, at 50 nM HMGB1 used in the smFRET studies (Figure 1d, lower plot), a small fraction of the lower FRET occurrences likely arose from unbent (i.e. unbound) DNA, which was too small to resolve with a second Gaussian fit. It is formally possible that HMGB1 could bind DNA without bending, and smFRET will not detect this. Therefore, in describing our data we typically present the most conservative interpretation and describe results in terms of bending, not binding. When describing our models for HMGB1/DNA interactions we use binding and bending more interchangeably.
Table 1.
Mean FRET efficiencies1
| Protein | Unbent mean FRET |
Bent mean FRET |
Unbent 95% CI |
Bent 95% CI |
|---|---|---|---|---|
| None | 0.27 | n/a | 0.266–0.271 | n/a |
| FL | – | 0.38 | – | 0.372–0.385 |
| A box | – | 0.32 | – | 0.317–0.327 |
| B box | – | 0.33 | – | 0.328–0.339 |
| B box (no His) | – | 0.34 | – | 0.334–0.351 |
| AB | – | 0.41 | – | 0.397–0.413 |
| A+B | – | 0.34 | – | 0.334–0.344 |
| AB immobilized | – | 0.43 | – | 0.428–0.439 |
| BC (low) | 0.27 | – | 0.272–0.278 | – |
| BC (high) | 0.28 | 0.33 | 0.277–0.284 | 0.316–0.346 |
| FL-F38A (low) | 0.27 | _ | 0.261–0.273 | _ |
| FL-F38A (high) | 0.26 | 0.34 | 0.241–0.281 | 0.337–0.351 |
| AB-F38A | – | 0.32 | – | 0.312–331 |
Abbreviations: n/a, not applicable; –, not detected; CI, confidence interval for the mean FRET; low and high refer to protein concentration.
Interestingly, we found that in the presence of HMGB1, the time traces for individual DNA molecules did not display dynamic switching between bent and unbent states over time. A representative time trace of a single HMGB1 bent molecule of DNA is shown in Figure 1e, which remained at a constant FRET state until ~24 s when the acceptor dye photobleached. This indicates that under the conditions and resolution (60 ms time frames) of our smFRET experiments, HMGB1 bends DNA stably.
The individual HMG boxes bend DNA to the same extent
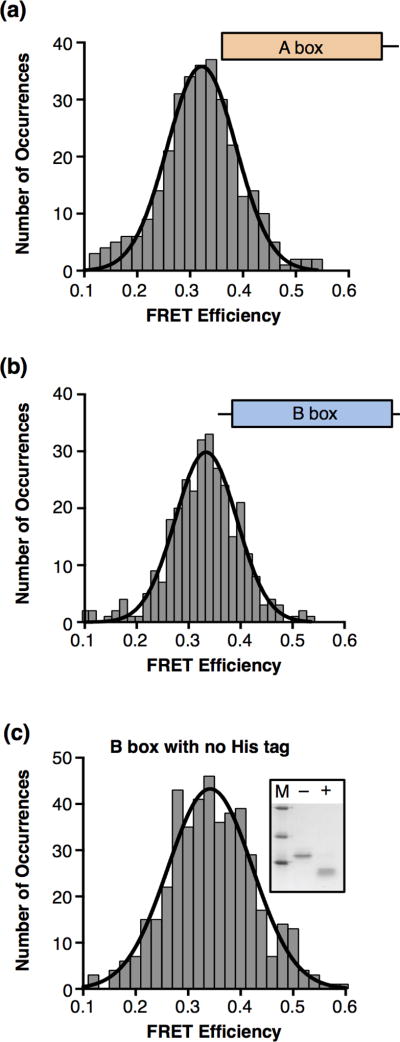
To determine how the individual A and B boxes bend DNA compared to the full length HMGB1 protein, we cloned, expressed, and purified the A and B box domains separately (see Figure 1a). We flowed the A box protein onto the surface-immobilized DNA, imaged single molecules of DNA and histogrammed the resulting FRET occurrences. The data fit to a single Gaussian with a mean FRET efficiency of 0.32 (Figure 2a). We also monitored DNA bending by the isolated B box, and observed a mean FRET efficiency of 0.33 (Figure 2b). These average FRET values for the A and B boxes individually are less than that obtained with full length HMGB1 (0.38), but similar to each other. We tested DNA binding with the isolated A box and B box proteins using EMSAs, but protein-DNA complexes were not cleanly resolvable in the gels, preventing us from estimating the affinity for DNA binding. Additional smFRET experiments showed that reducing the concentration of A box or B box protein from 100 nM to 50 nM did not change the mean FRET efficiency for bent DNA (data not shown), indicating that the DNA is predominately bound in Figures 2a and 2b.
Figure 2.
The HMGB1 A box and B box individually bend DNA to the same extent as the full length protein. (a) The HMGB1 A box bends DNA. Shown is a histogram of 307 occurrences obtained from single DNA molecules in the presence of 100 nM A box. The data were fit to a single Gaussian with a mean FRET efficiency of 0.32. (b) The HMGB1 B box bends DNA. The histogram shows 306 occurrences for DNA in the presence of 100 nM B box. The mean FRET efficiency obtained from the Gaussian fit is 0.33. (c) The B box with the His tag removed bends DNA to the same extent as the His-tagged B box protein. The inset gel shows the B box before (−) and after (+) incubation with enterokinase protease to cleave off the His tag; M designates markers of molecular weight 31, 21.5, and 14.4 kD. The histogram shows 440 occurrences for DNA in the presence of 100 nM cleaved B box. The mean FRET efficiency obtained from the Gaussian fit is 0.34.
Previous studies using ligation mediated circularization and supercoiling to monitor DNA bending by the B box have shown that the basic region between the B box and the C-terminal tail facilitates DNA bending by the B box [10,18,19]. This linker region was not present in our isolated B box construct. We asked whether the basic His tag residues on the N-terminus of the B box contributed to observing DNA bending in our experiments. The His tag was cleaved from the B box (see inset to Figure 2c), and the cleaved protein was tested in smFRET experiments. The mean bent state FRET efficiency (0.34) did not appreciably change upon removal of the His tag (Figure 2c). Together our data show that the individual boxes bend 18 bp of dsDNA to the same extent as one another and less than full length HMGB1. Lastly, like full length HMGB1, DNA bending by the A box or B box did not appear to be dynamic, since state changes between bent and unbent were seldom observed.
Deleting the HMGB1 C-terminal tail reveals a higher bent state
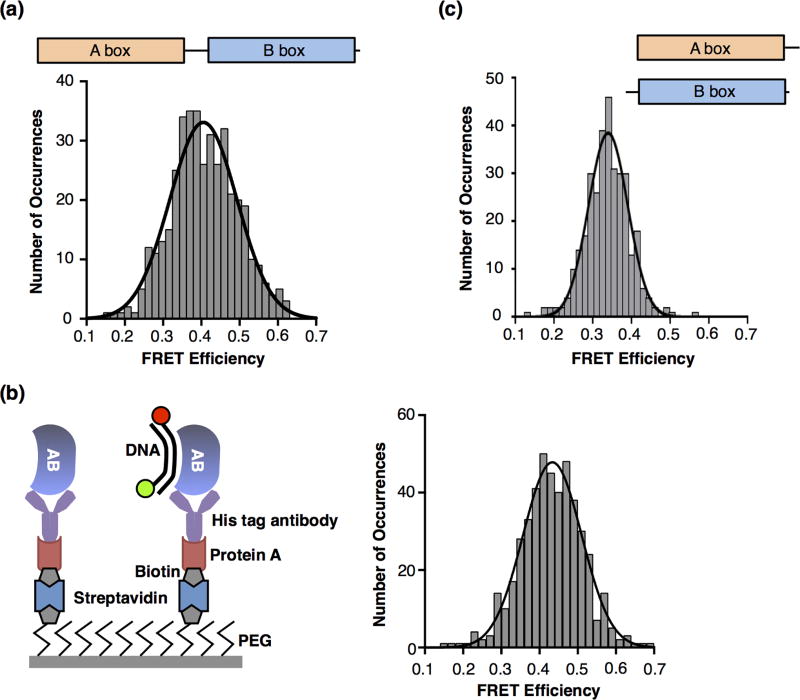
We next created a construct that lacked the acidic C-terminal tail, but contained the two HMG boxes (AB protein, Figure 1a) and asked how this protein bound and bent DNA. We measured a KD of 5 nM for the AB protein binding to DNA (Supplemental Figure), indicating that in the absence of the C-terminal tail the protein binds DNA with greater affinity. smFRET data obtained using a concentration of AB protein 4 times the KD revealed a relatively broad distribution of FRET occurrences; with lower FRET states likely arising from the small portion of unbent DNA that was not resolvable by a second Gaussian. The Gaussian fit of the data resulted in a mean FRET efficiency of 0.41. Therefore DNA molecules were bent to a greater extent by the AB protein compared to the full length protein, the A box, or the B box (see Table 1).
We asked whether the higher FRET state would be observed when AB protein was immobilized on the surface as opposed to immobilizing the DNA (see schematic in Figure 3b). Immobilizing the protein and flowing in limiting amounts of DNA ensured a 1:1 stoichiometry and that the DNA observed was all bound to HMGB1. Moreover, we used an antibody against the His tag to immobilize the AB protein thereby also minimizing the possibility that the basic His tag contributed to observing the higher bent FRET state. As shown in the histogram in Figure 3b, immobilized AB protein bent DNA to a mean FRET state of 0.43, slightly higher than that observed with immobilized DNA. A separate unbent population of DNA was not resolvable from the bent state in the histogram, suggesting that most of the DNA molecules that bound AB were bent, and few, if any, molecules of DNA were bound but unbent. This accounts for the higher mean FRET state compared to AB protein binding immobilized DNA, and also supports the idea that binding typically results in bending. Lastly, we asked whether the higher FRET state obtained with the AB protein was dependent on the covalent linkage between the A and the B boxes. A box protein and B box protein were mixed and then flowed over the surface-immobilized fluorescent DNA. The mean FRET state observed was 0.34 (Figure 3c), which is similar to that observed with either box alone. Therefore, the A box and the B box need to be covalently connected to bend DNA to the higher FRET state that was observed with the AB protein.
Figure 3.
The AB construct, which lacks the C-terminal tail, bends DNA to a higher FRET state, suggesting that both the A and the B boxes together bind and bend the DNA. (a) AB protein bends DNA to a higher FRET. 403 occurrences for DNA in the presence of 20 nM AB protein were histogrammed and fit to a Gaussian with mean FRET efficiency of 0.41. (b) AB protein immobilized on the slide surface bent DNA to a high FRET state. The schematic on the left depicts the surface used to immobilize the AB protein and observe bound and bent DNA via smFRET. The histogram on the right shows 466 occurrences after flowing in 0.5 nM DNA. The data were fit to a single Gaussian with a mean FRET efficiency of 0.43. (c) Observing the higher FRET state requires that the A box and B box be covalently linked. The histogram shows 333 occurrences after 75 nM A box plus 50 nM B box were flowed into the chamber. The data were fit to a single Gaussian with a mean FRET efficiency of 0.34.
Together the data in Figure 3 support a model in which both of the HMG boxes in the AB protein can bind and bend the DNA simultaneously, resulting in a higher mean FRET state for bent DNA and a higher affinity for binding DNA compared to the full length protein. This suggests that in the full length protein the C-terminal tail dampens DNA binding and bending by one or both of the HMG boxes. This is consistent with previous data showing that the C-terminal tail can interact with both HMG boxes [13–16].
The C-terminal tail of HMGB1 dampens DNA binding by the B box
To test the model that the C-terminal tail dampens DNA binding and bending in HMGB1, we first asked whether the C-terminal tail affects DNA bending by the B box, which is proximal to the C-terminal tail. We generated a construct that contains the B box connected to the C-terminal tail (BC), and the purified BC protein was flowed into the imaging chamber at a concentration at which DNA bending is observed with the isolated B box. As shown in Figure 4a, the mean FRET efficiency was 0.27, which is indistinguishable from the value observed for DNA in the absence of protein. The B box alone bent DNA to an average FRET state of 0.33 (Figure 2b), hence, the simplest interpretation of the data in Figure 4a is that the C-terminal tail impedes DNA binding by the B box.
Figure 4.
The C-terminal tail reduces the apparent affinity with which the B box binds and bends DNA. (a) The BC protein does not bend DNA when used at the same concentration at which the B box alone bent DNA. BC protein (the B box plus the C-terminal tail) was flowed into the chamber at 50 nM; 292 occurrences were histogrammed. The Gaussian fit revealed a mean FRET efficiency of 0.27, consistent with unbent DNA. (b) A population of bent DNA is observed occurs at a higher concentration of the BC protein. In the presence of 200 nM BC protein, 190 occurrences were histogrammed. The histogram revealed two FRET populations that were fit with two Gaussians to yield mean FRET efficiencies of 0.28 and 0.33.
Structural models for HMGB1 indicate that intramolecular interactions that occur between the C-terminal tail and the HMG boxes are dynamic [13,14], suggesting that the presence of the C-terminal tail would reduce affinity of the B box for DNA, as opposed to completely blocking the interaction. To address this we asked whether increasing the concentration of the BC protein would result in detectable DNA bending. We introduced a higher concentration of BC into the flow cell and monitored the FRET states of the DNA. The data revealed two distinct FRET populations with mean efficiencies of 0.28 and 0.33 (Figure 4b). The lower FRET state is consistent with unbent DNA, whereas the higher FRET state is the same as that observed for DNA bent by the B box alone. Unlike previous histograms obtained in the presence of protein, here the unbent DNA population was clearly resolvable from the bent population due to the greater fraction of unbound DNA. Based on these data we conclude that the C-terminal tail of HMGB1 decreases the affinity of the B box for DNA. The BC/DNA complex was not resolvable in EMSA, so we were unable to measure a KD; however, our smFRET histograms and the EMSA data for full length HMGB1 and AB protein are consistent with DNA bending closely reflecting DNA binding. Interestingly, we did not observe single molecules of DNA switching between bent and unbent states. This suggests that although the C-terminal tail might dynamically interact with the B box in the BC construct, once the DNA is bent, it remains in that state.
The A box predominantly binds and bends DNA in context of full length HMGB1
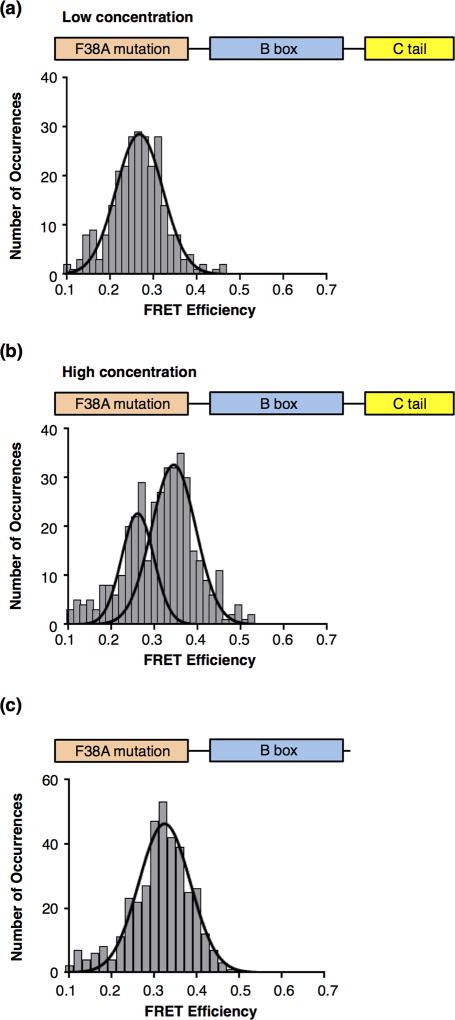
One prediction from the model that the C-terminal tail dampens DNA binding by the B box is that in the context of full length HMGB1, the A box contributes most to DNA binding and bending. To test this we made a point mutation in the A box that would disrupt its ability to bend DNA [11,28]. The phenylalanine residue that intercalates into the DNA to mediate bending (F38) was mutated to an alanine (F38A) in the context of the full length protein. We anticipated the mutant protein (FL-F38A) would behave similarly to the BC protein. Accordingly, FL-F38A was used in smFRET experiments at low and high concentrations (Figures 5a and 5b, respectively). At a low concentration of protein we observed a single population of DNA molecules with a FRET state of 0.27, which corresponds to unbound/unbent DNA (Figure 5a). Therefore, the F38A mutation in the A box eliminated DNA bending by the full length protein, presumably because the C-terminal tail was engaged in intramolecular interactions with the B box. At a high concentration of FL-F38A, two FRET states were observed: a lower state at 0.26 and a higher state at 0.34 (Figure 5b). The average FRET value of the lower state reflects the fraction of unbound DNA, while the higher state is consistent with DNA bent by a single box; therefore, FL-F38A behaves similarly to the BC protein. Given these data, it follows that removing the C-terminal tail in the context of the F38A mutation should allow DNA binding by the B box at a low concentration of the protein. Hence we generated a construct in which the Cterminal tail was removed from the mutant protein (i.e. AB-F38A); measurements of DNA binding affinity for both the F38A mutant proteins are in the Supplemental Figure. Indeed, AB-F38A protein bent the population of DNA molecules with a mean FRET state of 0.32, consistent with DNA being bent by the single B box (Figure 5c). Moreover, these data support our earlier conclusion that the high FRET state of 0.41–0.43 observed with the wild-type AB protein (see Figure 3) arose from both HMG boxes simultaneously binding and bending DNA. Together the data in Figure 5 support the model that in the context of full length HMGB1, the A box binds and bends the DNA while the C-terminal tail makes intramolecular interactions with the B box that impede its ability to bind and bend DNA.
Figure 5.
The C-terminal tail impedes DNA bending by the B box in the context of full length HMGB1 containing a mutant A box. (a) Removing the ability of the A box to bend DNA in the context of the full length protein resulted in no DNA bending at low protein concentrations. The plot shows 269 occurrences for DNA in the presence of 32 nM FL-F38A protein. The data were fit to a Gaussian with a mean FRET efficiency of 0.27. (b) At a higher concentration of FLF38A, a population of bent DNA is observed. 402 occurrences observed in the presence of 5.5 µM FL-F38A were histogrammed to reveal two populations with mean FRET efficiencies of 0.26 and 0.34, corresponding to unbent and bent DNA, respectively. (c) Removing the C-terminal tail from the protein with the mutant A box enabled DNA binding and bending at low concentrations of protein. AB-F38A (56 nM) was flowed into the chamber, 369 occurrences were histogrammed. The data revealed a single FRET state with a mean efficiency of 0.32, indicative of bent DNA.
Discussion
Here we used smFRET to investigate how HMGB1 bends DNA. This technique allowed us to resolve heterogeneous populations of unbent DNA and bent DNA. We found that the individual A and B boxes bent DNA less than full length HMGB1. With the AB protein, which lacked the C-terminal tail, we observed the DNA was bent to a greater extent than full length protein. This suggests that both HMG boxes can bind and bend DNA simultaneously in the absence of the C-terminal region. We investigated the role of the C-terminal tail in the context of the isolated B box (BC protein versus B box) and in the context of full length protein containing a mutant A box (FL-F38A protein). In both cases the C-terminal tail reduced interaction of the B box with DNA, requiring higher concentrations of protein in order to observe bending. We propose a model that in the context of the full length protein, the A box predominantly binds and bends DNA, whereas intramolecular interactions with the acidic C-terminal tail inhibit the B box from binding and bending DNA. In the absence of a C-terminal tail, both boxes can together bind and bend DNA, resulting in a greater level of bending.
Our smFRET data show that individually the A box and B box of HMGB1 bend DNA to the same extent (Figure 2). This is perhaps not surprising given their similarities in structure [3,5], yet previous data have shown that the B box bends DNA to a greater extent than the A box [18,19,21]. DNA bending by the B box can be enhanced by (and in some cases require) the short, basic linker region between the B box and the C-terminal tail [10,18,19]; this linker region was not present in our isolated B box construct. It is possible that addition of the basic linker region to the B box and to the AB protein would reveal higher smFRET states. Our observation that the B box, in the absence of the linker residues, bends DNA to the same extent as the A box could be attributable to the use of short DNAs. Most other studies of HMGB1-induced DNA bending used longer DNAs, and observed more heterogeneity in the extent to which HMG boxes bend DNA [22,23]. In systems using longer DNAs, multiple HMG proteins can bind and bend a single DNA; our data are most consistent with 1:1 stoichiometry on a short piece of DNA (Figure 3b). Perhaps as the protein:DNA stoichiometry increases, more heterogeneity in bending becomes apparent. A recent crystal structure depicts two independent (i.e. not covalently linked) A boxes simultaneously bound to DNA, each inserting a phenylalanine between the same CG base pair, thereby causing a dramatic kink in the DNA [29]. This structure suggests a model for how DNA bending might occur when multiple HMGB1 proteins bind.
In our smFRET system, HMG box bending of DNA was stable (i.e. switching between a bent FRET state and an unbent FRET state on a single molecule of DNA over time was seldom observed). Some studies have shown that DNA binding by HMGB1 is dynamic in vitro [30,31] and also in cells [32], while other studies measured slower rates of dissociation [33]. A recent single molecule kinetic study using optical tweezers proposed that microscopic and macroscopic dissociation constants control interactions between HMGB proteins and DNA [34]. The microscopic dissociation events are rapid and involve breaking short range protein-DNA contacts, while the macroscopic events can be measured as slow depending on the experimental conditions, and involve complete dissociation and escape of the protein into solution. It is possible that observing unbending via a decrease in smFRET requires complete dissociation of HMGB1 and is slow under our assay conditions. smFRET studies similar to ours monitored DNA bending dynamics by yeast Nhp6A, a single HMGB box protein [35]. Using physiological buffer conditions, they observed single molecules of DNA switching between bent and unbent states, with the lifetime of a bent state lasting a few seconds. It is possible that human HMGB1 might exhibit dynamic bending and unbending at physiological salt concentrations.
Our data indicate that in the absence of the C-terminal tail, both the A box and the B box can together bind and bend DNA. When the C-terminal tail was removed the AB protein bent the DNA to a higher FRET state compared to the full length protein. The higher FRET state was not observed with either HMG box alone or with the two HMG boxes added together, suggesting that when covalently connected the A and B boxes can both simultaneously bind and bend a single DNA molecule. Moreover, when the intercalating residue in the A box was mutated in the context of the AB construct, a lower bent FRET state was observed (Figure 5c), further supporting the model that both HMG boxes bind DNA together in the wild-type AB construct. Previous studies have also suggested that both boxes can bend DNA together in the absence of the C-terminal tail. In circularization assays the AB protein bent DNA more effectively than either of the single HMG box domains [18,19], and the full length protein at low concentrations [18]. In contrast to our findings, previous ensemble FRET experiments found that the AB protein bent DNA to a lesser extent than full length HMGB1 [24]. It is possible, however, that the DNA in these experiments was not fully occupied by the AB protein; therefore the measured FRET would be the average of the FRET from unbound DNA and bent DNA in the population, resulting in an apparent lower level of bending for the population.
Our data support a model in which intramolecular interactions between the C-terminal tail and the B box partially interfere with DNA binding and bending, resulting in the A box making the primary contributions to DNA binding and bending in the context of the full length protein. Moreover, once the A box binds and bends DNA, the local DNA concentration is high enough that it can partially invade the intramolecular BC interaction, thereby allowing the B box to bend the DNA part of the time. This model arises from synthesizing several observations. First, to observe DNA bending, the BC protein had to be added at a much higher concentration than the B box alone, but the bent FRET state was similar for the two proteins (Figure 4). Second, the FL-F38A protein acted similarly to the BC protein, only bending DNA at a higher concentration, but the bent FRET state was similar to that of the B box alone (Figures 5a and 5b). Third, we found that the AB-F38A protein, which lacked the C-terminal tail, bound DNA at low concentrations and bent DNA similarly to the B box alone. These observations imply that in the absence of a functional A box, the C-terminal tail impeded DNA binding and bending by the B box. Fourth, the mean FRET of the bent state observed with FL-F38A mutation was lower than that obtained with the wild-type full length protein. This suggests that in the wild-type protein, the A box predominantly binds and bends DNA, and the B box can make some contributions to DNA bending, although these are dampened by the C-terminal tail. Previous studies also support a model in which the C-terminal tail can regulate DNA binding and bending by the HMG boxes. Structural, biochemical, and thermodynamic experiments indicate that the C-terminal tail can interact with both the A and B boxes [9,13–16,36]. A structural model has been proposed in which the A and B boxes sandwich the C-terminal tail, thereby restricting the boxes from making other interactions [13]. Our data adds to these models, suggesting that the C-terminal tail primarily acts as a damper for the B box in the context of the full length protein.
Factors that can bind the C-terminal tail have the potential to impact the mechanism by which HMGB1 binds and bends DNA. For example, studies have shown that the C-terminal tail can bind to linker histone H1 [37]. If the tail binds to histone H1, then HMGB1 could bind the surrounding nucleosomal DNA using both the A box and the B box, resulting in a larger bend. Moreover, the C-terminal tail/histone H1 interaction could facilitate displacement of the histone by HMGB1, thereby aiding in nucleosome remodeling [37]. Others have shown that the C-terminal tail can interact with the tail of histone H3, which stimulates transcription in a cellular reporter assay and allows the binding of HMGB1 to chromatin [36,38]. Coupling of biochemical, structural, and functional assays will provide new insight into how the mechanism of HMGB1 bending controls its ability to act as an architectural protein and mediate important nuclear events such as transcription, recombination, DNA repair, and chromatin remodeling.
Materials and methods
Oligonucleotides
smFRET experiments were performed using a double-stranded biotinylated DNA construct containing a donor (Alexa555) and acceptor (Alexa647) fluorophore pair. The sequences (5’ to 3’) of the two oligos (Integrated DNA Technologies) were Alexa555-CAGGCTATAAAAGGGACG and Alexa647-CGTCCCTTTTATAGCCTGATACAAGCAGCAGTCACCA-biotin, where the underlined region of the Alexa647-labeled oligo annealed to the Alexa555-labeled oligo to yield 18 bp dsDNA. The oligos were annealed by heating to 95°C for 3 minutes, incubating the DNA at 65°C for 45 minutes, and then cooling to 4°C at a rate of 0.1°C/sec.
Protein expression and purification
The sequence encoding human full length HMGB1 (1–215) was cloned using first strand cDNA generated from reverse transcription of RNA isolated from Jurkat cells. The HMGB1 cDNA was cloned into a pET19b vector after mutating the native Nde1 site in the HMGB1 nucleotide sequence. The following HMGB1 truncations or mutations were made using PCR from the plasmid containing full length HMGB1: A box (1–85), B box (83–166), AB (1–166), BC (83–215), FL-F38A (1–215), and AB-F38A (1–166). In each construct, the natural cysteine codons were changed to serine codons and a single cysteine codon was added to enable future protein-labeling experiments. DNA bending between the wild-type and mutant full length HMGB1 was indistinguishable. The proteins contained the following amino acid changes: FL HMGB1 (C23S, C45S, C106S, E204C), A box (C23S, C45S, S35C), B box (C106S, A137C), AB (C23S, C45S, C106S, S35C), and BC (C106S), FL-F38A (C23S, F38A, C45S, C106S, E204C), AB (C23S, F38A, C45S, C106S, S35C).
The HMGB1 expression plasmids were transformed into E. coli BL-21 DE3 pLysS cells and grown overnight at 37°C on LB plates containing 100 µg/mL ampicillin and 50 µg/ mL chloramphenicol. For each construct, one colony was picked and grown in a 5 mL culture containing LB, 2 g/L D-glucose, ampicillin, and chloramphenicol at the concentrations above with overnight shaking at 200 rpm at 37°C. 2 mL of the 5 mL starter culture was used to inoculate 400–500 mL of LB containing glucose, ampicillin (100 µg/mL), and chloramphenicol (50 µg/ mL) and cells were grown to OD600 nm 0.4–0.5. Protein expression was then induced with 0.5 mM IPTG for 2 hours, and cells were pelleted by centrifugation at 5000 rpm for 15 min.
The cell pellets were resuspended in 10 mL buffer A (20 mM Tris (pH 7.9), 5 mM 2-mercaptoethanol, 500 mM NaCl, 10% (w/v) glycerol, 1X EDTA-free protease inhibitors (Roche), 0.2 mM PMSF, and 10 mM imidazole) and sonicated (5 times with 30 s on and 30 s off). Samples were centrifuged at 15,000 rpm for 15 min at 4°C. The supernatant was added to 1.2 mL of HisPur Ni-NTA (Thermofisher) that was pre-equilibrated in buffer A. The sample was nutated at 4°C for 1 h. The resin was placed in a column and allowed to settle before washing thoroughly with buffer A, followed by buffer A with 50 mM imidazole. Protein was eluted from the column with buffer A containing 250 mM imidazole. The eluate was dialyzed in buffer B (20 mM HEPES (pH 7.9), 50 mM KCl, 1 mM EDTA, 1 mM DTT, 10% glycerol, 0.2 mM PMSF) at 4°C. The full length protein was then placed over a Uno Q column that was equilibrated in buffer B and the flow through was collected. All proteins except BC were further purified over a dsDNA cellulose column as follows. The dsDNA resin (Sigma) was pre-equilibrated in buffer C (20 mM HEPES (pH 7.9), 50 mM KCl, 10% glycerol, 5 mM MgCl2, 1 mM DTT) using ~0.5 mL swelled resin per protein sample. Protein was added to the resin and nutated at 4°C for 1 hr. The dsDNA cellulose was then placed into a column, allowed to settle, and washed thoroughly with buffer C. Protein was eluted in buffer C with 500 mM KCl. Eluates were dialyzed in buffer C at 4°C. All proteins were flash frozen in liquid nitrogen in small aliquots and stored at −80°C. Protein concentrations were determined from Coomassie stained SDS-PAGE gels in which several different volumes of an HMGB1 protein were run alongside a standard curve with known amounts of BSA. The band intensities were quantitated in ImageJ, and the equation for the line that fit the BSA data was used to calculate the concentration of the HMGB1 construct. The His tag was cleaved off the B box (Figure 2c) using an enterokinase cut site. B box protein (15 µg) was incubated in 20 mM Tris (pH 7.9), 50 mM NaCl, 2 mM CaCl2, and 2 ng enterokinase (New England Biolabs) for 4 hrs at 4°C. The enterokinase was inactivated using trypsin inhibitor beads (Sigma) equilibrated in the same buffer as the protease digestion. The proteins were run on a 15% SDS-PAGE gel to observe digestion.
Preparation of surfaces and smFRET reaction conditions
Glass slides and coverslips (VWR) were prepared as described previously [27]. Briefly, slides and coverslips were cleaned using sequential sonications in a 1% alconox, ethanol, and 1 M KOH. Slides were silanated in methanol containing 2% aminosilane [N-(2-aminoethyl) 3-aminopropyltrimethoxysilane (UCT)], followed by rinsing and baking at 110°C. A solution of 0.38% (w/v) biotin-PEG-SC (MW 5000, Laysan Bio, Inc.) and 20% (w/v) mPEG-SVA (MW 5000, Laysan Bio, Inc.) in 0.1 M sodium bicarbonate was allowed to react with the silanated glass. To assemble reaction chambers, coverslips were attached to slides with double-sided tape.
Streptavidin (0.2 mg/mL resuspended in 10 mM Tris (pH 7.9), 10% glycerol, 50 mM KCl, and 5 mM MgCl2) was flowed into the sample chamber in buffer D (10% (v/v) glycerol, 25 mM Tris (pH 7.9), 50 mM KCl, 1 mM DTT, 0.05 mg/mL BSA, 5 mM MgCl2, 0.1% NP-40) and incubated for 5 min. Unbound streptavidin was flushed from the chamber with excess buffer D. For DNA immobilization, 200–400 pM double stranded DNA (prepared as described above) was flowed into the chamber in buffer D and incubated for 10 min, followed by washing with excess buffer D. After immobilized DNA surfaces were assembled, imaging solution was flowed into the chamber with or without protein (concentrations indicated in the Figure legends), and fluorescence measurements were taken as described below. Imaging solution consisted of buffer D containing 2 mM Trolox, 1 mg/ml glucose oxidase, 11 µg/mL catalase, and 0.8% (w/v) D-glucose. Trolox was prepared by dissolving 6 mM Trolox into 30% (v/v) glycerol, 150 mM Tris (pH 7.9), 150 mM KCl, and 15 mM MgCl2 with overnight nutation in the dark, then 3 mM DTT, 0.15 mg/mL BSA, and 0.3% NP-40 were added (final concentrations).
For protein immobilization (Figure 3c), 10 nM biotinylated Protein A (Millipore) was flowed into the chamber in buffer D, incubated for 10 min, and unbound Protein A was removed by washing. The following were incubated in buffer D for 10 min: His-tag antibody (Santa Cruz, sc-803) at 5 µg/mL, 185 nM AB protein, 0.5 nM double stranded 18 bp DNA with Alexa555 and Alexa 647 on each 5' end, and 2 mM Trolox. After incubation, 1 mg/ml glucose oxidase, 11 µg/mL catalase, and 0.8% (w/v) D-glucose were added and the solution was flowed on the slide for imaging.
smFRET data collection and analysis
FRET data were collected on an objective-based total internal reflection fluorescence microscope (Nikon TE-2000U) equipped with a 1.49 NA immersion objective, a 532 nm continuous wave laser, a Cascade II CCD camera for collecting data in the donor channel, and an Evolve CCD camera for collecting data in the acceptor channel. Donor and acceptor emission data were collected with NIS Elements software (Molecular Devices) using a 60 ms exposure time. Data were analyzed as described previously [27]. Briefly, in-house software was used to pair donor and acceptor spot pairs from individual molecules. Data were corrected for acceptor emission resulting from bleed through of the donor dye into the acceptor channel, and the ratio of the donor and acceptor detection efficiencies (γ). Apparent FRET efficiencies over time were calculated from the corrected donor and acceptor emissions using the equation E = Acorr/(Acorr+Dcorr). In-house software was used to determine the FRET states in time traces from individual molecules. FRET states for a given experimental condition were histogrammed using a bin of 0.015 or 0.02. The data were fit with Gaussians to extract mean FRET efficiencies and their 95% confidence intervals.
Supplementary Material
Acknowledgments
This work was supported by grant MCB-1244518 from the National Science Foundation. A.H. was partially supported by training grant T32 GM-065103 from the National Institutes of Health.
Abbreviations
- AB
protein containing the HMGB1 A box and B box domains
- AB-F38A
AB protein with a point mutation changing Phe38 to Ala
- BC
protein containing the HMGB1 B box domain and C-terminal tail
- CCD
charge-coupled device
- FL
full length HMGB1
- FL-F38A
full length HMGB1 with a point mutation changing Phe38 to Ala
- FRET
fluorescence resonance energy transfer
- HMGB1
high mobility group box protein 1
- smFRET
single molecule FRET
- TIRF
total internal reflection fluorescence
References
- 1.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim. Biophys. Acta. 2010;1799:101–113. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Agresti A, Bianchi ME. HMGB proteins and gene expression. Curr. Opin. Genet. Dev. 2003;13:170–178. doi: 10.1016/s0959-437x(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JO, Travers AA. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem. Sci. 2001;26:167–174. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- 4.Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol. Carcinog. 2009;48:571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malarkey CS, Churchill ME. The high mobility group box: the ultimate utility player of a cell. Trends Biochem. Sci. 2012;37:553–562. doi: 10.1016/j.tibs.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardman CH, Broadhurst RW, Raine AR, Grasser KD, Thomas JO, Laue E. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry. 1995;34:16596–16607. doi: 10.1021/bi00051a007. [DOI] [PubMed] [Google Scholar]
- 7.Weir HM, Kraulis PJ, Hill CS, Raine AR, Laue ED, Thomas JO. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993;12:1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Read CM, Cary PD, Crane-Robinson C, Driscoll PC, Norman DG. Solution structure of a DNA-binding domain from HMG1. Nucl. Acids Res. 1993;21:3427–3436. doi: 10.1093/nar/21.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramstein J, Locker D, Bianchi ME, Leng M. Domain-domain interactions in high mobility group 1 protein (HMG1) Eur. J. Biochem. 1999;260:692–700. doi: 10.1046/j.1432-1327.1999.00185.x. [DOI] [PubMed] [Google Scholar]
- 10.Grasser KD, Teo SH, Lee KB, Broadhurst RW, Rees C, Hardman CH, Thomas JO. DNA-binding properties of the tandem HMG boxes of high-mobility-group protein 1 (HMG1) Eur. J. Biochem. 1998;253:787–795. doi: 10.1046/j.1432-1327.1998.2530787.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohndorf UM, Rould MA, He Q, Pabo CO, Lippard SJ. Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature. 1999;399:708–712. doi: 10.1038/21460. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989;243:1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- 13.Stott K, Watson M, Howe FS, Grossmann JG, Thomas JO. Tail-mediated collapse of HMGB1 is dynamic and occurs via differential binding of the acidic tail to the A and B domains. J. Mol. Biol. 2010;403:706–722. doi: 10.1016/j.jmb.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Watson M, Stott K, Thomas JO. Mapping intramolecular interactions between domains in HMGB1 using a tail-truncation approach. J. Mol. Biol. 2007;374:1286–1297. doi: 10.1016/j.jmb.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 15.Knapp S, Muller S, Digilio G, Bonaldi T, Bianchi ME, Musco G. The long acidic tail of high mobility group box 1 (HMGB1) protein forms an extended and flexible structure that interacts with specific residues within and between the HMG boxes. Biochemistry. 2004;43:11992–11997. doi: 10.1021/bi049364k. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Zeng M, Wang W, Tang J. The HMGB1 acidic tail regulates HMGB1 DNA binding specificity by a unique mechanism. Biochem. Biophys. Res. Commun. 2007;360:14–19. doi: 10.1016/j.bbrc.2007.05.130. [DOI] [PubMed] [Google Scholar]
- 17.Lee KB, Thomas JO. The effect of the acidic tail on the DNA-binding properties of the HMG1,2 class of proteins: insights from tail switching and tail removal. J. Mol. Biol. 2000;304:135–149. doi: 10.1006/jmbi.2000.4206. [DOI] [PubMed] [Google Scholar]
- 18.Stros M. DNA bending by the chromosomal protein HMG1 and its high mobility group box domains. Effect of flanking sequences. J. Biol. Chem. 1998;273:10355–10361. [PubMed] [Google Scholar]
- 19.Teo SH, Grasser KD, Thomas JO. Differences in the DNA-binding properties of the HMG-box domains of HMG1 and the sex-determining factor SRY. Eur. J. Biochem. 1995;230:943–950. doi: 10.1111/j.1432-1033.1995.tb20640.x. [DOI] [PubMed] [Google Scholar]
- 20.Pil PM, Chow CS, Lippard SJ. High-mobility-group 1 protein mediates DNA bending as determined by ring closures. Proc. Natl. Acad. Sci. U S A. 1993;90:9465–9469. doi: 10.1073/pnas.90.20.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paull TT, Haykinson MJ, Johnson RC. The nonspecific DNA-binding and -bending proteins HMG1 and HMG2 promote the assembly of complex nucleoprotein structures. Genes Dev. 1993;7:1521–1534. doi: 10.1101/gad.7.8.1521. [DOI] [PubMed] [Google Scholar]
- 22.McCauley MJ, Zimmerman J, Maher LJ, Williams MC. HMGB binding to DNA: single and double box motifs. J. Mol. Biol. 2007;374:993–1004. doi: 10.1016/j.jmb.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, McCauley MJ, Maher LJ, Williams MC, Israeloff NE. Mechanism of DNA flexibility enhancement by HMGB proteins. Nucl. Acids Res. 2009;37:1107–1114. doi: 10.1093/nar/gkn1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belgrano FS, de Abreu da Silva IC, Bastos de Oliveira FM, Fantappie MR, Mohana-Borges R. Role of the acidic tail of high mobility group protein B1 (HMGB1) in protein stability and DNA bending. PLoS ONE. 2013;8:e79572. doi: 10.1371/journal.pone.0079572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stott K, Tang GS, Lee KB, Thomas JO. Structure of a complex of tandem HMG boxes and DNA. J. Mol. Biol. 2006;360:90–104. doi: 10.1016/j.jmb.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 26.Travers AA. Priming the nucleosome: a role for HMGB proteins. EMBO Rep. 2003;4:131–136. doi: 10.1038/sj.embor.embor741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair RH, Goodrich JA, Kugel JF. Single-molecule fluorescence resonance energy transfer shows uniformity in TATA binding protein-induced DNA bending and heterogeneity in bending kinetics. Biochemistry. 2012;51:7444–7455. doi: 10.1021/bi300491j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stros M, Polanská E, Struncová S, Pospísilová S. HMGB1 and HMGB2 proteins upregulate cellular expression of human topoisomerase IIalpha. Nucl. Acids Res. 2009;37:2070–2086. doi: 10.1093/nar/gkp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sánchez-Giraldo R, Acosta-Reyes FJ, Malarkey CS, Saperas N, Churchill ME, Campos JL. Two high-mobility group box domains act together to underwind and kink DNA. Acta Crystallogr D Biol Crystallogr. 2015;71:1423–1432. doi: 10.1107/S1399004715007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman J, Maher LJ. Transient HMGB protein interactions with B-DNA duplexes and complexes. Biochem. Biophys. Res. Commun. 2008;371:79–84. doi: 10.1016/j.bbrc.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czapla L, Peters JP, Rueter EM, Olson WK, Maher LJ. Understanding apparent DNA flexibility enhancement by HU and HMGB architectural proteins. J. Mol. Biol. 2011;409:278–289. doi: 10.1016/j.jmb.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agresti A, Scaffidi P, Riva A, Caiolfa VR, Bianchi ME. GR and HMGB1 interact only within chromatin and influence each other’s residence time. Mol. Cell. 2005;18:109–121. doi: 10.1016/j.molcel.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Skoko D, Wong B, Johnson RC, Marko JF. Micromechanical analysis of the binding of DNA-bending proteins HMGB1, NHP6A, and HU reveals their ability to form highly stable DNA-protein complexes. Biochemistry. 2004;43:13867–13874. doi: 10.1021/bi048428o. [DOI] [PubMed] [Google Scholar]
- 34.McCauley MJ, Rueter EM, Rouzina I, Maher LJ, Williams MC. Single-molecule kinetics reveal microscopic mechanism by which High-Mobility Group B proteins alter DNA flexibility. Nucl. Acids Res. 2013;41:167–181. doi: 10.1093/nar/gks1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coats JE, Lin Y, Rueter E, Maher LJ, Rasnik I. Single-molecule FRET analysis of DNA binding and bending by yeast HMGB protein Nhp6A. Nucl. Acids Res. 2013;41:1372–1381. doi: 10.1093/nar/gks1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawase T, Sato K, Ueda T, Yoshida M. Distinct domains in HMGB1 are involved in specific intramolecular and nucleosomal interactions. Biochemistry. 2008;47:13991–13996. doi: 10.1021/bi8013449. [DOI] [PubMed] [Google Scholar]
- 37.Cato L, Stott K, Watson M, Thomas JO. The interaction of HMGB1 and linker histones occurs through their acidic and basic tails. J. Mol. Biol. 2008;384:1262–1272. doi: 10.1016/j.jmb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Ueda T, Chou H, Kawase T, Shirakawa H, Yoshida M. Acidic C-tail of HMGB1 is required for its target binding to nucleosome linker DNA and transcription stimulation. Biochemistry. 2004;43:9901–9908. doi: 10.1021/bi035975l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.