Abstract
Background
The n-3 polyunsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) may prevent a range of chronic conditions through anti-inflammatory actions. However, since clinical trials using these fatty acids for primary prevention are yet unavailable their putative role in disease prevention rests, in part, on evidence of anti-inflammatory actions in healthy individuals.
Objective
To investigate in a double-blind, placebo-controlled clinical trial whether supplementation with a moderate dose of EPA+DHA reduces common biomarkers of chronic, systemic inflammation in healthy individuals.
Methods
A total of 261 healthy individuals aged 30–54 years who were free of inflammatory conditions and consumed ≤300 mg/day EPA+DHA were included in the study. Participants were randomly assigned to 18 weeks of either fish oil supplementation providing 1400 mg/day EPA+DHA or matching placebo. Outcome measures were serum levels of C-reactive protein (CRP) and interleukin (IL)-6. In a substudy, ex vivo cytokine production was measured. Missing data for CRP and IL-6 were estimated using regression imputation. Data analyses conformed to intention-to-treat principles.
Results
Participant blinding was verified. Is the meaning of this sentence still correct? EPA+DHA levels increased by 64% in the active treatment group, but serum levels of CRP and IL-6 were not affected by supplementation (P ≥ 0.20). Findings were consistent with and without imputed values and across subgroups. Similarly, EPA+DHA supplementation did not alter ex vivo production of four pro-inflammatory cytokines (P ≥ 0.20).
Conclusions
Supplementation with 1400 mg EPA+DHA did not reduce common markers of systemic inflammation in healthy adults. Whether this or a higher dose affects other measures of inflammation, oxidative stress, or immune function warrants examination.
Keywords: cytokines, docosahexaenoic acid, eicosapentaenoic acid, fish oils, inflammation
Introduction
The long-chain, n-3 polyunsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are available principally through eating fish or taking EPA- and DHA-containing supplements. Evidence from observational research and studies of disease pathophysiology suggest that greater daily intake of these nutrients may reduce the risk of chronic disorders, including cardiovascular disease, dementia, cancer, and arthritis [1–5], leading to speculation that greater EPA+DHA consumption can serve as a broadly effective disease prevention strategy. However, their true effects on major health outcomes remain unclear. Most clinical trials of raised EPA+DHA consumption examined the effects in patients with manifest disease. Further, in these trials, no reduction in major cardiovascular disease events was found [6], except perhaps as secondary prevention with doses of at least 1000 mg/day EPA+DHA [7], and cognitive decline was not reduced in elderly individuals with mild cognitive impairment, except possibly in APOE ε4 carriers [8, 9].
The role of these nutrients in true primary prevention requires support from research conducted in healthy, ‘pre-morbid’ adults (i.e. in the early phases of disease development). However, clinical trials of major health outcomes are impracticable due to the protracted time frame over which chronic disorders develop and become manifest. Nonetheless, raised intake of fish or EPA- and DHA-containing supplements should affect one or more of the key biological processes that predate and predict chronic disease. Evidence of such effects would support the biological plausibility of the effectiveness of long-chain, n-3 fatty acids in primary disease prevention.
Chronic inflammatory processes have a role in most degenerative diseases of aging, and it is believed that suboptimal lifestyles and environmental exposures commonly cause low-level chronic inflammation. In this regard, laboratory experiments have shown that EPA and DHA regulate multiple aspects of inflammation through generation of eicosanoids [10]. As recently discovered, these n-3 fatty acids also act as mediators of inflammation resolution and modulate transcription factors and gene expression [10–13]. In observational studies, low dietary consumption and low circulating levels of these n-3 fatty acids have been associated with elevated serum C-reactive protein (CRP) and interleukin (IL)-6 levels [14–17]. On the other hand, clinical trials of n-3 fatty acid supplementation in healthy adults have in most cases yielded null findings with regard to CRP levels and mixed findings for IL-6 [18–22]. However, trial data are limited by small sample sizes (typically fewer than 100 participants), and in most trials participants receive supplementation for 4–12 weeks whereas accretion of consumed n-3 fatty acids into tissue can take 4 months or more [23, 24]. Often, dietary sources of n-3 fatty acids in potential participants have not been considered in trials. In this regard, various organizations recommend that adults should ingest more than 250 or 500 mg/day EPA+DHA [25], but the mean intake among US adults is less than 100 mg/day [26].
Therefore, the aim of this study was to investigate in healthy, mid-life adults with relatively low dietary consumption of EPA+DHA (≤300 mg/day) whether supplementation for 18 weeks reduces two markers of chronic inflammation, serum CRP and IL-6. Given the role of the immune system in chronic inflammation, ex vivo pro-inflammatory cytokine production was also measured in fresh blood samples from a subset of trial participants.
Methods
Study design
This study was an exploratory single-site, randomized, double-blind, placebo-controlled trial to investigate the general health effects of supplemented dietary intake of long-chain, n-3 polyunsaturated fatty acids in healthy mid-life adults. The primary outcomes were: (i) preclinical markers of cardiovascular disease risk, including measures of chronic inflammation and immune function, and autonomic control of the heart (heart rate variability); and (ii) affect and related behaviors (mood, hostility, anger, impulsivity, and aggression). The secondary outcome measure was cognitive functioning, funded separately. The current report concerns only outcome measures of chronic inflammation and immune function; other outcomes will be reported separately. The trial is registered on ClinicalTrails.gov (RCT00663871), and the study protocol is available as an Online Supplement. The investigation was approved by the Investigational Review Board of the University of Pittsburgh, and was conducted between June 2008 and December 2011. All participants provided written informed consent and were paid for their participation.
Participants
The trial participants were drawn from the Adult Health and Behavior Project – Phase 2 (AHAB-II), a volunteer-based, cross-sectional study of psychosocial factors, behavioral and biological risk factors, and subclinical cardiovascular disease. The AHAB-II protocol included medical, demographic, and social histories, biomedical measures, psychosocial questionnaires, ambulatory monitoring of physical activity, mood and social interactions, and cognitive testing. AHAB-II participants were recruited through mass mailing of recruitment letters to individuals selected from voter registration and other public domain lists for the greater Pittsburgh metropolitan area.
To be eligible to participate in AHAB-II, individuals had to be between the ages of 30 and 54 years and work at least 25 hours per week outside of the home (because of a substudy focusing on occupational stress). Individuals were excluded if they: (i) had a history of cardiovascular disease, stage 2 hypertension (blood pressure ≥160/100 mmHg), schizophrenia or bipolar disorder, chronic hepatitis, renal failure, major neurological disorder, or chronic lung disease; (ii) reported drinking ≥35 units of alcohol per week; (iii) took fish oil supplements; (iv) were prescribed insulin or glucocorticoid, anti-arrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight-loss medications; (v) were pregnant; (vi) had less than an eighth grade reading level; or (vii) were shift workers.
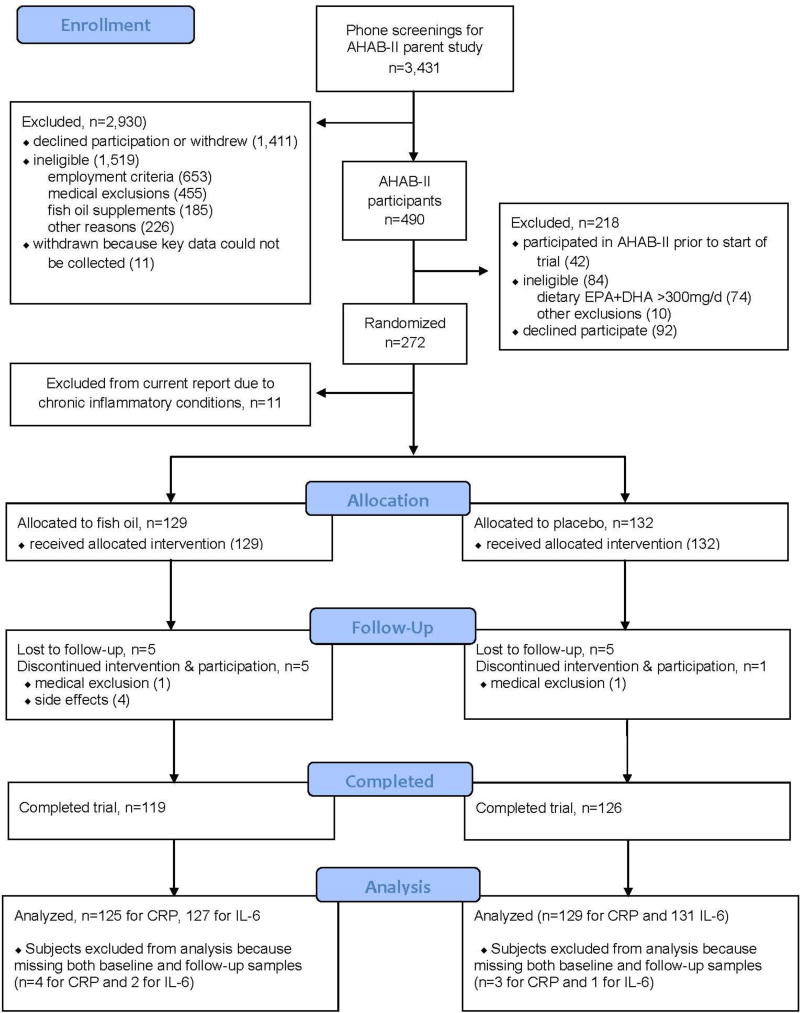
At total of 177,415 mailed recruitment letters for the AHAB-II parent study yielded 8957 study inquiries (response rate 5%). Of 3431 individuals who could be contacted for telephone screening, 490 completed the AHAB-II protocol and were considered for enrollment in the current trial if they met three additional eligibility criteria: (i) dietary EPA+DHA ≤300 mg/day, based upon the modified 2005 Block Food Frequency Questionnaire (NutritionQuest, Berkeley, CA, USA), (ii) lack of fish allergy, and (iii) fasting serum triglyceride concentration <400 mg/dL. A total of 272 individuals met these criteria, agreed to participate, and were randomly assigned to treatment or placebo. Eleven individuals were subsequently excluded from the present analyses because of an existing chronic inflammatory condition that would likely confound the interpretation of measures of inflammation and immune function (e.g. autoimmune disorder, asthma, or an allergic condition requiring ongoing therapy). Therefore, the sample for this report comprised 261 individuals (see Fig. 1).
Fig. 1.
CONSORT participant flow chart. CRP, C-reactive protein; IL-6, interleukin-6; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AHAB-II, Adult Health and Behavior Project – Phase 2.
Randomization, study intervention and follow-up
Subjects were randomly assigned at the time that trial eligibility was verified. Treatment assignments were computer-generated at randomization using minimization, an adaptive randomization approach which is securely implemented via the Internet using the clinical trial management system R-Track. Marginal treatment distribution is balanced through minimization within levels of stratification factors of race (white vs. non-white), age (<45 vs. ≥45 years), and sex. To assist with adherence, capsules were distributed in weekly blister packs, each labeled with the week number and a code for the treatment assignment. The assigned supplement was distributed by a blinded study nurse immediately after randomization. Through this standardized procedure, treatment allocation concealment was maintained. The duration of the treatment period was 18 weeks.
Participants assigned to the fish oil group received a daily dose of two 1000 mg fish oil capsules, providing a total of 1000 mg EPA and 400 mg DHA; those assigned to the placebo group received a daily dose of two identical-appearing 1000 mg soybean oil capsules. The placebo capsules contained 1% fish oil, and both supplements contained mint flavor to help maintain participant blinding. All supplements also contained 10 IU vitamin E. Pre-trial chemical analysis established that the capsules were devoid of vitamin D. Study staff assessed side effects and adherence by contacting participants by telephone during Weeks 2 and 12, and at a brief clinic visit during Week 7 when additional supplement was distributed. If capsule counts revealed more than one dose missed every 2 weeks (<93%), participants were guided through problem-solving and habit-forming approaches to help improve adherence. If adherence was 80% or lower, staff additionally conducted a follow-up interview by telephone 2 weeks later. During the final 2 weeks of the supplement period participants attended appointments for end-of-study procedures and measures.
Study measures
At baseline and at the end of the 4-month intervention period, self-reported diet and side effect information were obtained, and a fasting morning venous blood sample was collected. All staff involved in collection of study measures remained blind to group assignment. Participants were instructed not to use tobacco products, drink alcohol, or engage in formal exercise in the 2 h preceding blood collection.
Because acute illness and immunization can affect peripheral markers of immune function and inflammation, blood samples were not collected if the participant reported having had an infection or received antibiotics, antiviral medication or vaccination in the past 2 weeks, or symptoms of acute infection in the preceding 24 h. Where feasible, clinic visits were rescheduled for at least 2 weeks after such an event.
Diet was assessed at baseline and post-supplementation using the Block Food Frequency Questionnaire, modified by the addition of nine items concerning fish and enriched egg consumption to increase the accuracy of omega-3 fatty acid intake. Participants completed this online survey with instruction from and supervision by research staff trained in the use of Block Dietary Data Systems. The reference period was the previous 4 months.
To determine the fatty acid composition of red blood cells (RBCs), hemoglobin-free RBC ghost membranes were prepared as previously described [27] and stored at −70°C until required for further analysis. Lipids were extracted from RBC ghost membranes [28] and fatty acid methyl esters were prepared using methanolic KOH reagent [29]. Diheptadecanoyl lecithin (Matreya, Inc. Pleasant Gap, Pennsylvania, USA) was used as an internal standard. Quantitative determination of fatty acid distribution was performed by capillary gas chromatography with flame ionization detector using a Hewlett-Packard capillary gas chromatograph (Model 5890 Series II, Palo Alto, California, USA) equipped with a hydrogen flame ionization detector. The method used for the analysis has been previously described [30]. The results of RBC fatty acids are expressed in nmol/mL packed RBCs. The intra- and inter-assay coefficients of variation (CV) were 1.98% and 3.88%, respectively, for fatty acids at a mean concentration of >300 nmol/mL and 3.57% and 8.62%, respectively, at <150 nmol/mL.
Serum high-sensitivity CRP was measured at the Laboratory of Clinical Biochemistry Research at the University of Vermont with a BNII nephelometer (Dade Behring, Deerfield, Illinois, ISA) utilizing a particle-enhanced immunonepholometric assay. The assay range is 0.175–1100 mg/L. Intra-assay CVs ranged from 2.3% to 4.4% and inter-assay CVs from 2.1% to 5.7%. Values >10 pg/mL were considered to reflect a substantial but undetected inflammatory process, and were treated as missing data. Serum IL-6 concentration was determined at the Behavioral Immunology Laboratory at the University of Pittsburgh using a high-sensitivity, quantitative sandwich enzyme immunoassay kit, according to the manufacturer’s instructions (R & D Systems, Minneapolis, MN, USA). The assay standard range is from 0.156 to 10 pg/mL. Intra- and inter-assay CVs were <10%.
Ex vivo stimulated production of IL-6, IL-1β, IL-8, and tumor necrosis factor-alpha (TNF-α) was determined using citrate-treated whole blood. Within 60 min of collection, lipopolysaccharide (LPS; serotype 026:B6; Sigma, Sigma-Aldrich Corp. St Louis, Missouri, USA) was added to whole blood, to achieve a concentration of 2.5 µg/mL, without antibiotics in polypropylene tubes under sterile conditions. Control samples, containing whole blood without LPS, were prepared in parallel to measure spontaneous levels of cytokine production. The samples were incubated at 37°C for 24 h. Following incubation, the tubes were centrifuged at 1000 g for 10 min and supernatants were frozen at −80°C until the completion of the study. Samples were assayed using a multiplex analysis system as previously described [31]. Briefly, multiplex bead kits, based on the principle of solid phase sandwich immunoassays, were employed and samples were read within 24 h using a Bio-Plex reader (Luminex 100; Bio-rad Corporation, Hercules, CA, USA). Stimulated levels of cytokines were determined using the Bio-Plex Manager Software, interpolating from the standard curve (Logistic-5PL curve fit). Inter- and intra-assay CVs were less than 10%.
Adverse events were measured with an eight-item non-serious side effect questionnaire with severity ratings (none, mild, moderate, severe) completed by participants at baseline, at Weeks 2, 7, and 12, and at the end of the supplementation period.
Trial monitoring and oversight
Trial data safety and monitoring oversight was provided by an external reviewer, François Lespérance, MD (Chief of Psychiatry, Centre Hospitalier de l’Université de Montréal), working in conjunction with the trial statistician Susan Sereika, Ph.D. On an annual basis, Dr Lespérance reviewed information on data security and participant confidentiality, recruitment, non-completion, protocol adherence, and adverse events. No interim analyses of study outcomes were conducted.
Statistical analysis
This study had a target sample size of 250 subjects (125 per treatment group) in order to achieve a power of at least 0.80 to detect differences in mean changes in CRP and IL-6 levels from baseline to post-supplementation in terms of the standardized mean difference (d) as small as d = 0.433 between treatment groups. Sample size determination assumed a test-wise significance level of 0.01 when conducting two-sided hypothesis testing using linear contrasts within a repeated measures framework.
Baseline characteristics were compared between treatment groups using chi-squared and Fisher’s exact tests for categorical variables and independent samples t-tests for continuous data. Adherence to treatment was quantified as the proportion of capsules ingested based on pill counts, and the treatment groups were compared using an independent samples t-test. As the severity of side effects was rated on an ordinal scale, treatment groups were compared using the Wilcoxon rank-sum test with exact estimation. Participant blinding was expressed as the proportion of subjects who reported their blinded treatment assignment correctly, and the two groups were compared using a chi-squared test.
Baseline CRP and IL-6 values were missing for 13 participants (lost samples, n = 2; acute infections or inflammatory processes, n = 11), and post-supplementation values were missing for 32 participants (failed phlebotomy, n = 2; acute infections or other inflammatory processes at the final study visit, n = 30). Additionally, six CRP values at baseline and six at follow-up were >10 ng/mL, indicating undiagnosed inflammatory processes; these results were discarded. Finally, the CV was >20% for one IL-6 value at follow-up, and was discarded. CRP and IL-6 values were log10-transformed to provide a better approximation of a normal distribution. Linear regression was employed to adjust IL-6 values for plate-to-plate variation based upon pooled serum control samples run on each plate.
Outcome testing used all available data and, for CRP and IL-6, was also performed using imputed missing data. In addition, missing data for CRP and IL-6 were estimated and replaced using regression imputation (i.e. a method of single imputation). The regression imputation models were conducted separately by treatment condition. Missing values for baseline CRP were imputed by the predicted values yielded from a fitted regression model with baseline CRP as the dependent variable and age, sex, body mass index (BMI), smoking status, and follow-up CRP value as predictor variables. Similarly, the missing values for follow-up CRP were imputed based on a treatment group-specific regression model using age, sex, BMI, smoking status, and baseline CRP as predictor variables. The same procedures were used for imputing missing values of baseline and follow-up IL-6. Seven and three participants, respectively, were missing both baseline and follow-up CRP and IL-6 values. As shown in the CONSORT flow chart (Fig. 1), these missing data could not be imputed. Mahalanobis distance was used to identify multivariate outliers stratified by treatment group. Potential influential cases for the variables of interest were identified from studentized residual versus centered leverage plots. Test of Box–Cox transformation was performed to assess all possible pairs of linearity tests. White’s test was used to assess homoscedasticity (i.e. constant error variance).
Outcome analyses were performed according to intention-to-treat principles, and were conducted both with and without imputed missing values. Repeated measures analyses of variance were used to test for change in immune parameters. Treatment group (fish oil vs. placebo) was used as a random effects, between-subjects factor and time (baseline vs. post-supplementation) was used as a fixed, within-subjects factor, with the interaction term of treatment group and time. Possible moderation of treatment effect by sex, baseline inflammation (CRP <2.0 vs. 2.0 ng/dL and median split of baseline serum IL-6), background n-3 fatty acid exposure (baseline RBC EPA+DHA) and BMI were each tested as a three-way interaction between time, treatment group, and the suspected moderating variable. Model assessment included residual analysis and the determination of possible influential observations. The level of significance was set at 0.01 for two-sided hypothesis testing. All statistical analyses were performed using SPSS 21 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics are presented in Table 1. Comparison of the 11 excluded subjects and the remaining 261 participants revealed no differences in baseline characteristics (Supplemental Table 1). Randomization resulted in treatment groups that were comparable in terms of baseline characteristics, with the exception of lower CRP levels in those assigned to placebo compared to the fish oil group (median 0.65 vs. 1.03 ng/mL; P = 0.008). Nonetheless, most participants in both treatment groups had a normal CRP concentration of <2.0 ng/mL (80% vs. 73%, P = 0.24). The average duration of supplementation was 129 days (18.4 weeks).
Table 1.
Baseline characteristics of study participants in the placebo and active treatment groups
| Placebo (n = 132) | Fish oil (n = 129) | P-value | |
|---|---|---|---|
| Race (% white) | 84.1 | 80.6 | 0.38 |
| Sex (% female) | 57 | 58 | 0.90 |
| Age (years) | 42.6 ± 7.0 | 43.2 ± 7.6 | 0.55 |
| BMI (kg/m2) | 26.8 ± 4.6 | 27.5 ± 6.0 | 0.31 |
| Current smoking (%) | 13.6 | 17.1 | 0.49 |
| Dietary EPA+DHA (mg/day) | 109 ± 72 | 104 ± 69 | 0.51 |
| Red blood cell EPA+DHA (nmol/mL) | 2.91 ± 1.01 | 3.02 ± 1.05 | 0.43 |
| CRP (mg/L)a | 0.65 (1.38) | 1.03 (1.74) | 0.008 |
| IL-6 (pg/mL)a | 0.99 (0.79) | 1.15 (1.11) | 0.84 |
Due to distribution skew, the median and interquartile range are presented; data were log10 transformed for analysis.
Data are presented as mean ± SD/SEM, unless stated otherwise.
BMI, body mass index; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; CRP, C-reactive protein; IL-6, interleukin-6.
Complete capsule count data were available for 93% of participants, in whom the minimum adherence was 70% and the median adherence as 98.5%. The two supplementation groups had similar levels of adherence (P = 0.19). ‘Per protocol’ analyses were not performed because adherence was high. Mean EPA+DHA concentration in RBCs among fish oil-treated individuals increased by 64% to 4.95 ± 1.59 nmol/mL (P < 0.001), whereas EPA+DHA in the placebo group remained unchanged at 2.85 nmol/mL (P = 0.89).
Primary outcomes
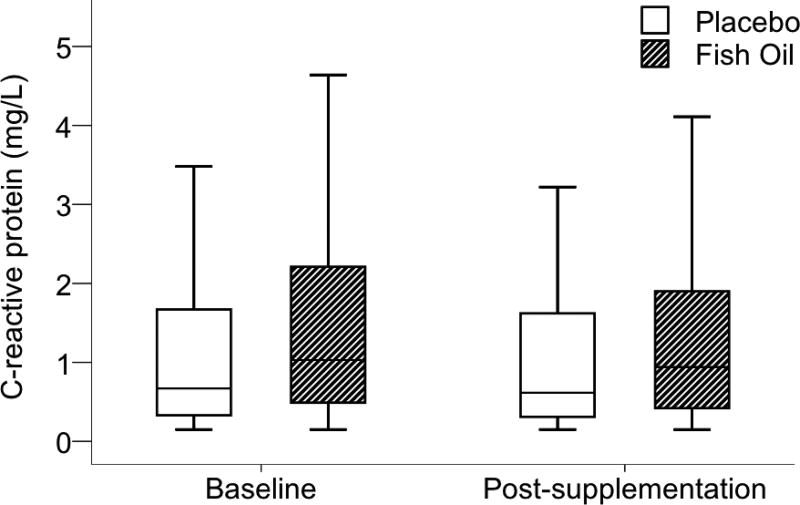
Relative to placebo, fish oil did not affect serum CRP concentration. This result was evident in analyses with [Mdiff 0.05 mg/L, 95% confidence interval (CI) −0.24, 0.19; P = 0.21] and without imputed missing data (Mdiff 0.04 mg/L, 95% CI −0.21, 0.29; P = 0.37). Fig. 2 shows baseline and post-supplementation CRP values. We found no evidence of heterogeneity of the effect of fish oil on CRP according to sex, BMI, baseline RBC EPA+DHA or baseline CRP (<2 vs. ≥2 ng/dL) (three-way interaction P values > 0.10). Finally, when divided by tertiles of post-supplementation RBC EPA+DHA, individuals attaining higher tissue levels of n-3 fatty acids did not exhibit lower CRP (one-way ANOVA, P > 0.10).
Fig. 2.
Serum C-reactive protein concentration at baseline and post-supplementation. No effect of fish oil was found (Mdiff 0.183 mg/L, 95% confidence interval −0.098, 0.464; P = 0.21). Data are presented as box and whisker plots in which the horizontal bar represents the median, the box demarcates the 25th and 75th percentiles, and the whiskers indicate ± 1.5× the interquartile range.
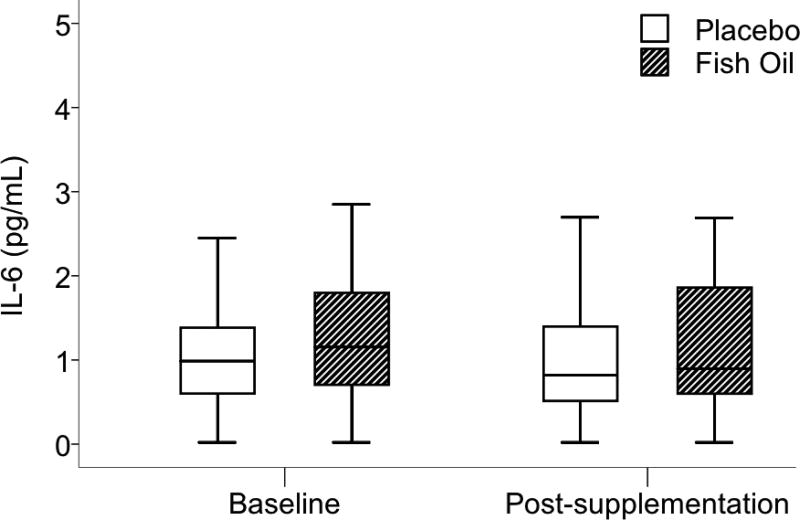
Fish oil supplementation had no effect on serum IL-6 concentrations. This result was evident in analyses with (Mdiff −0.04 pg/mL, 95% CI −0.27, 0.19; P = 0.59) and without imputed missing values (Mdiff 0.01 mg/L, 95% CI −0.06, 0.04; P = 0.93). Fig. 3 shows baseline and post-supplementation IL-6 values. As with CRP, we found no evidence of heterogeneity of the effect of fish oil on IL-6 levels according to sex, BMI, baseline IL-6, or post-supplementation EPA+DHA in RBCs (interaction P values > 0.10). There was a non-statistically significant trend toward heterogeneity of the effect as a function of baseline RBC EPA+DHA (three-way interaction P = 0.089). When further examined in subgroup analyses, however, IL-6 concentration was not affected by supplementation in the low or high EPA+DHA groups.
Fig. 3.
Serum interleukin-6 (IL-6) at baseline and post-supplementation. No effect of fish oil was found (Mdiff −0.037 pg/mL, 95% confidence interval −0.268, 0.194; P = 0.59). Data are presented as box and whisker plots in which the horizontal bar represents the median, the box demarcates the 25th and 75th percentiles, and the whiskers indicate ± 1.5× the interquartile range.
Cytokine production substudy
As a substudy, we also measured ex vivo stimulated production of four cytokines: IL-6, IL-1β, IL-8, and TNF-α. Collection of these samples began after the trial was partially completed, and in several instances samples were not tested due to acute inflammatory conditions, phlebotomy failure, or lack of technician availability to set-up fresh blood incubation. As a result, 116 participants took part in this substudy. Among these 116 individuals, there was a greater proportion of current smokers than among the remaining 145 participants (P < 0.05); otherwise, the two groups were comparable in terms of demographic characteristics and general health indicators (see Supplemental Table 2). A number of assay results were out of range or were considered unreliable based on a CV of >20% between duplicate samples. As a consequence, the number of subjects with results available for analysis at baseline and post-supplementation varied from 74 to 107 across the four cytokines. Fish oil supplementation did not affect ex vivo stimulated production of IL-6 (Mdiff −12.2 ng/mL, 95% CI −89.8, 65.4; P = 0.43), IL-1β (Mdiff 13.3 pg/mL, 95% CI −70.9, 97.4; P = 0.99), IL-8 (Mdiff −1.65 ug/mL, 95% CI −12.16, 8.85; P = 0.20), or TNF-α (Mdiff 313 pg/mL, 95% CI −1673, 2298; P = 0.81).
Participant blinding
At the final trial visit, participants were asked to indicate whether they suspected that they were receiving fish oil or placebo, or whether they did not know which they were receiving. Overall, 33% in the fish oil group and 28% in the placebo group guessed correctly (P = 0.58).
Side effects
Compared to those receiving placebo, participants assigned to fish oil reported significantly greater severity ratings of ‘fishy belch or aftertaste‘ at both the Week 2 and final study visits and ’loose stool, bloating, or gas pains‘ only at the final study visit (P values < 0.05). By contrast, higher ratings of the severity of ‘minty belch or aftertaste’ were reported by the placebo group at both the Week 2 and final study visits (P < 0.05). No statistically significant differences were found between the groups for the remaining non-serious side effects (rash/reaction or easy bruising, change in taste of food, heartburn/stomach ache, back pain, flu-like symptoms, or infection). No serious adverse events were reported.
Discussion
The long-chain, n-3 polyunsaturated fatty acids are believed to have wide-ranging health benefits, and millions of individuals worldwide use fish oil supplements in the absence any official dietary reference intake [25]. Inhibition of inflammatory processes is generally cited as the primary mechanism of action. To examine the role of EPA and DHA in primary disease prevention, we enrolled healthy adults in the current randomized, placebo-controlled clinical trial to test the effects of 1400 mg/day on two markers of chronic inflammation, serum CRP and IL-6. We reasoned that even in healthy adults suboptimal lifestyles and environmental exposures result in prevalent, low-level chronic inflammation. No effect of supplementation on chronic inflammation markers was detected overall, or in analyses in which subjects were stratified by either key characteristics or RBC EPA+DHA concentration. Additionally, in a substudy to examine leukocyte function in whole blood we detected no effects on ex vivo production of inflammatory cytokines. Of note, the effects of supplementation were investigated in community dwelling volunteers in whom mean EPA+DHA intake was about 100 mg/day, which is near the US adult average yet well below the minimum consumption of 250–500 mg/day recommended by various organizations [25].
EPA and DHA are incorporated into the phospholipids of cell membranes throughout the human body and serve a range of roles. As membrane constituents, EPA and DHA modify lipid raft formation, which in turn modulates intracellular signaling in T cells [32]. The n-6 polyunsaturated fatty acid arachidonic acid (AA) is also found in membrane phospholipids. AA, EPA, and DHA are released from membranes into the cytosol by phospholipase A2 and serve as the obligate precursors of eicosanoids (prostaglandins, leukotrienes, and thromboxanes), which are widely studied mediators and regulators of inflammation. AA gives rise to pro-inflammatory eicosanoids whereas those derived from EPA and DHA tend to be less potent or even anti-inflammatory. In addition, EPA and DHA are the key substrates for specialized pro-resolving mediators (e.g. resolvins and protectins) [11] and a subset of potently anti-inflammatory endocannabinoids [33]. These n-3 fatty acids further inhibit inflammation through transcription factors, including restraining nuclear factor kappa B and activating peroxisome proliferator activated receptor-γ [10].
Several previous clinical trials have examined CRP levels following fish oil supplementation in healthy, non-elderly adults (mean age <65 years) [19, 20, 34–39]. Each study included between eight and 50 subjects per group and most involved supplementation for 8–12 weeks. All but one found no reduction in serum CRP level [34]. Our larger and longer trial further supports the conclusion that moderate-dose EPA+DHA supplementation does not affect serum CRP concentration in healthy adults.
Serum IL-6 was reduced by high-dose fish oil supplementation in a trial of healthy but overweight adults who had low fish intake at baseline [22] and in a small trial in women [34]. We did not find evidence for such an effect in the current trial overall, nor any evidence of effect modification by sex or BMI. Similarly, no decline in IL-6 was reported in other trials of healthy adults [20, 40, 41], nor in a study of overweight adults treated with high-dose supplementation for 6 months [36]. The present findings are also consistent with the majority of published research.
In addition to measurement of circulating inflammatory markers, which originate from adipocytes and other tissues, blood leukocyte function is also of interest in studies of systemic inflammation. Early, small uncontrolled studies using freshly collected whole blood samples demonstrated blunted ex vivo stimulated cytokine production following fish oil supplementation [42, 43]. Similar findings have been reported from some but not all randomized placebo-controlled investigations including 20–34 participants per treatment condition [21, 39]. In the current, somewhat larger, substudy of ex vivo production of four inflammatory cytokines by leukocytes, there was no effect of supplementation. However, our dose of 1400 mg/day EPA+DHA was smaller than the daily dose of 2.5–5.0 g used in those trials with positive results [21, 42, 43].
Changes in other circulating markers of inflammation, such as TNF-α and adhesion molecules, have not been detected in several studies in healthy adults [20, 36, 39, 41, 44]. However, mixed findings were reported from some of the larger such trials and those in patient populations [22, 37, 45, 46]. Limited evidence suggests that raising EPA+DHA intake in humans can lower natural killer cell activity [47], affect inflammatory gene expression [12, 13, 46], lower oxidative stress, and slow telomere shortening [48]. Finally, in a substudy of the current trial we found evidence that the anti-inflammatory actions of EPA+DHA supplementation may involve the endogenous generation of electrophilic n-3 fatty acid ketone derivatives [49].
Several features of this study should be noted. First, randomization resulted in a somewhat higher average baseline CRP level in the active group compared to placebo-treated control subjects, but this was taken into account in data analysis and is unlikely to have biased towards the null hypothesis. Secondly, in order to address primary prevention of disorders involving chronic systemic inflammation, we specifically enrolled healthy individuals and attempted to minimize the extraneous influence of acute and chronic inflammatory states. Consequently, over 75% of participants had a baseline CRP level below 2 ng/mL, and detecting treatment effects may be difficult in such individuals. However, in subgroup analyses we found no change in participants with CRP ≥2 ng/mL or IL-6 above the median. Finally, while this study addressed primary prevention in healthy unselected groups, investigations of the effects of n-3 fatty acids on CRP and IL-6 in high-risk populations and secondary prevention have also produced largely null findings [50–52]. For example, supplementation reduced major cardiovascular disease events and mortality in a large heart failure trial [53] yet did not alter CRP concentration [54].
In summary, in this randomized, placebo-controlled clinical trial we found no effect of 1400 mg/day EPA+DHA supplementation on serum levels of CRP and IL-6 in healthy adults. This finding corroborates previous reports. On the other hand, a range of biological actions of the n-3 fatty acids within inflammatory cascades is well documented, and other measures of immune function, regulators of inflammation, and biomediators of oxidative stress may reveal actions not detected by serum CRP and IL-6 levels. Furthermore, daily doses of ≥2000 mg/day, in contrast to lower amounts, may have measureable physiological effects. If this is the case, however, the requirement of such a high dose would diminish the feasibility of widespread primary prevention through raised consumption of fish or n-3 polyunsaturated fatty acid supplements.
Supplementary Material
Acknowledgments
Funding sources
The study was funded by US Public Health Service Awards P01 HL40962 and R21 HL081282. The US Public Health Service had no role in the study design or implementation, data collection, statistical analysis, or interpretation, or manuscript composition.
We acknowledge the efforts of Dr François Lespérance, MD, who served as the trial data safety monitor. The University of Pittsburgh Cancer Institute Luminex facility, which is supported in part by award P30CA047904, was used for this project.
Footnotes
Trial registration: ClinicalTrails.gov, number RCT00663871.
Author contributions
Drs Muldoon, Marsland, Sereika, and Manuck designed the study (project conception, development of overall research plan, and study oversight). Dr Muldoon and Ms Kuan performed the statistical analyses, with assistance from Drs Laderian and Sereika. Drs Muldoon and Laderian wrote the paper. Drs Muldoon, Marsland, and Manuck share primary responsibility for the final content.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Dangour AD, Andreeva VA, Sydenham E, Uauy R. Omega 3 fatty acids and cognitive health in older people. Br J Nutr. 2012;107(Suppl 2):S152–8. doi: 10.1017/S0007114512001547. [DOI] [PubMed] [Google Scholar]
- 2.Solfrizzi V, Frisardi V, Capurso C, D'Introno A, Colacicco AM, Vendemiale G, et al. Dietary fatty acids in dementia and predementia syndromes: epidemiological evidence and possible underlying mechanisms. Ageing Res Rev. 2010;9(2):184–99. doi: 10.1016/j.arr.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 4.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364(25):2439–50. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 5.Lopez LB, Kritz-Silverstein D, Barrett Connor E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: the Rancho Bernardo study. J Nutr Health Aging. 2011;15(1):25–31. doi: 10.1007/s12603-011-0009-5. [DOI] [PubMed] [Google Scholar]
- 6.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308(10):1024–33. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 7.Casula M, Soranna D, Catapano AL, Corrao G. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, placebo controlled trials [corrected] Atheroscler Suppl. 2013;14(2):243–51. doi: 10.1016/S1567-5688(13)70005-9. [DOI] [PubMed] [Google Scholar]
- 8.Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2010;91(6):1725–32. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 9.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–8. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 10.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75(3):645–62. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maskrey BH, Megson IL, Rossi AG, Whitfield PD. Emerging importance of omega-3 fatty acids in the innate immune response: molecular mechanisms and lipidomic strategies for their analysis. Mol Nutr Food Res. 2013;57(8):1390–400. doi: 10.1002/mnfr.201200723. [DOI] [PubMed] [Google Scholar]
- 12.Vedin I, Cederholm T, Freund-Levi Y, Basun H, Garlind A, Irving GF, et al. Effects of DHA-rich n-3 fatty acid supplementation on gene expression in blood mononuclear leukocytes: the OmegAD study. PLoS One. 2012;7(4):e35425. doi: 10.1371/journal.pone.0035425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver KL, Ivester P, Seeds M, Case LD, Arm JP, Chilton FH. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J Biol Chem. 2009;284(23):15400–7. doi: 10.1074/jbc.M109.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205(2):538–43. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He K, Liu K, Daviglus ML, Jenny NS, Mayer-Davis E, Jiang R, et al. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;103(9):1238–43. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith KM, Barraj LM, Kantor M, Sahyoun NR. Relationship between fish intake, n-3 fatty acids, mercury and risk markers of CHD (National Health and Nutrition Examination Survey 1999–2002) Public Health Nutr. 2009;12(8):1261–9. doi: 10.1017/S1368980008003844. [DOI] [PubMed] [Google Scholar]
- 17.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46(1):120–4. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Myhrstad MC, Retterstol K, Telle-Hansen VH, Ottestad I, Halvorsen B, Holven KB, et al. Effect of marine n-3 fatty acids on circulating inflammatory markers in healthy subjects and subjects with cardiovascular risk factors. Inflamm Res. 2011;60(4):309–19. doi: 10.1007/s00011-010-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93(2):243–52. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Root M, Collier SR, Zwetsloot KA, West KL, McGinn MC. A randomized trial of fish oil omega-3 fatty acids on arterial health, inflammation, and metabolic syndrome in a young healthy population. Nutr J. 2013;12(1):40. doi: 10.1186/1475-2891-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain, Behavior, and Immunity. 2011;25:1725–34. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain, Behavior, and Immunity. 2012;26:988–95. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38(10):2012–22. [PubMed] [Google Scholar]
- 24.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467S–76S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 25.Flock MR, Harris WS, Kris-Etherton PM. Long-chain omega-3 fatty acids: time to establish a dietary reference intake. Nutr Rev. 2013;71(10):692–707. doi: 10.1111/nure.12071. [DOI] [PubMed] [Google Scholar]
- 26.NHANES. 2011 Pages http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/1112/Table_1_NIN_GEN_11.pdf on July 13 2015.
- 27.Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963;100:119–30. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 28.Rose HG, Oklander M. Improved Procedure for the Extraction of Lipids from Human Erythrocytes. J Lipid Res. 1965;6:428–31. [PubMed] [Google Scholar]
- 29.Ichihara K, Shibahara A, Yamamoto K, Nakayama T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids. 1996;31(5):535–9. doi: 10.1007/BF02522648. [DOI] [PubMed] [Google Scholar]
- 30.Yao JK, van Kammen DP, Welker JA. Red blood cell membrane dynamics in schizophrenia. II. Fatty acid composition. Schizophr Res. 1994;13(3):217–26. doi: 10.1016/0920-9964(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 31.Marsland AL, Sathanoori R, Muldoon MF, Manuck SB. Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain Behav Immun. 2007;21(2):218–28. doi: 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Stulnig TM, Zeyda M. Immunomodulation by polyunsaturated fatty acids: impact on T-cell signaling. Lipids. 2004;39(12):1171–5. doi: 10.1007/s11745-004-1344-x. [DOI] [PubMed] [Google Scholar]
- 33.Balvers MG, Verhoeckx KC, Plastina P, Wortelboer HM, Meijerink J, Witkamp RF. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochim Biophys Acta. 2010;1801(10):1107–14. doi: 10.1016/j.bbalip.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Ciubotaru I, Lee YS, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr.Biochem. 2003;14(9):513–21. doi: 10.1016/s0955-2863(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 35.Geelen A, Brouwer IA, Schouten EG, Kluft C, Katan MB, Zock PL. Intake of n-3 fatty acids from fish does not lower serum concentrations of C-reactive protein in healthy subjects. Eur J Clin Nutr. 2004;58(10):1440–2. doi: 10.1038/sj.ejcn.1601986. [DOI] [PubMed] [Google Scholar]
- 36.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, et al. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30(10):1535–44. doi: 10.1038/sj.ijo.0803309. [DOI] [PubMed] [Google Scholar]
- 37.Krysiak R, Gdula-Dymek A, Okopien B. The effect of bezafibrate and omega-3 fatty acids on lymphocyte cytokine release and systemic inflammation in patients with isolated hypertriglyceridemia. Eur J Clin Pharmacol. 2011;67(11):1109–17. doi: 10.1007/s00228-011-1063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. British Journal of Nutrition. 2003;89(4):517–22. doi: 10.1079/BJN2002815. [DOI] [PubMed] [Google Scholar]
- 39.Vega-Lopez S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with omega3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53(2):236–40. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen MS, Gammelmark A, Madsen T, Obel T, Aardestrup I, Schmidt EB. The effect of low-dose marine n-3 fatty acids on the biosynthesis of pro-inflammatory 5-lipoxygenase pathway metabolites in overweight subjects: a randomized controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2012;87(1):43–8. doi: 10.1016/j.plefa.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Pot GK, Brouwer IA, Enneman A, Rijkers GT, Kampman E, Geelen A. No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: a randomized controlled trial in healthy, middle-aged individuals. Eur J Clin Nutr. 2009;63(11):1353–9. doi: 10.1038/ejcn.2009.63. [DOI] [PubMed] [Google Scholar]
- 42.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. New England Journal of Medicine. 1989;320(5):265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 43.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63(1):116–22. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 44.Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. 2011;141(12):2166–71. doi: 10.3945/jn.111.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulo MC, Andrade AM, Andrade ML, Morais MG, Kiely M, Parra D, et al. Influence of n-3 polyunsaturated fatty acids on soluble cellular adhesion molecules as biomarkers of cardiovascular risk in young healthy subjects. Nutr Metab Cardiovasc Dis. 2008;18(10):664–70. doi: 10.1016/j.numecd.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Kolahi S, Ghorbanihaghjo A, Alizadeh S, Rashtchizadeh N, Argani H, Khabazzi AR, et al. Fish oil supplementation decreases serum soluble receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio in female patients with rheumatoid arthritis. Clin Biochem. 2010;43(6):576–80. doi: 10.1016/j.clinbiochem.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am J Clin Nutr. 2001;73(3):539–48. doi: 10.1093/ajcn/73.3.539. [DOI] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, et al. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav Immun. 2013;28:16–24. doi: 10.1016/j.bbi.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cipollina C, Salvatore SR, Muldoon MF, Freeman BA, Schopfer FJ. Generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives. PLoS One. 2014;9(4):e94836. doi: 10.1371/journal.pone.0094836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Troseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism. 2009;58(11):1543–9. doi: 10.1016/j.metabol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 51.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35(7):772–81. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 52.Grundt H, Nilsen DW, Mansoor MA, Hetland O, Nordoy A. Reduction in homocysteine by n-3 polyunsaturated fatty acids after 1 year in a randomised double-blind study following an acute myocardial infarction: no effect on endothelial adhesion properties. Pathophysiol Haemost Thromb. 2003;33(2):88–95. doi: 10.1159/000073852. [DOI] [PubMed] [Google Scholar]
- 53.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–30. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 54.Masson S, Marchioli R, Mozaffarian D, Bernasconi R, Milani V, Dragani L, et al. Plasma n-3 polyunsaturated fatty acids in chronic heart failure in the GISSI-Heart Failure Trial: relation with fish intake, circulating biomarkers, and mortality. Am Heart J. 2013;165(2):208–15. e4. doi: 10.1016/j.ahj.2012.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.