Abstract
Rheumatoid arthritis (RA) is an autoimmune systemic disease characterized by long-standing inflammation and significant joint destruction. Despite significant research and success toward the treatment modalities, complete remission still remains a challenge. Even then a number of early and late extraarticular manifestations (EAMs) of the disease further complicate the disease progression. Various EAM encountered in RA can involve more than one organ or system in the body and their clinical features also show lot of variations, mostly involving cutaneous, cardiovascular, and pulmonary systems. The mortality associated with EAM may also be quite high in RA, sometimes more than the disease itself. These EAMs are difficult to diagnose and even more difficult to treat since no clear consensus exists among the rheumatologists as to their correct classification and also due to the fact that no clearcut guidelines are available for their treatment. With this background knowledge, the present review focuses on the various EAMs of RA, their classification, clinical features, and general management overview.
Keywords: Classification, extraarticular manifestation, EAM, remission, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is a common systemic inflammatory disease characterized by the presence of destructive polyarthritis with a predisposition for affecting the small joints of the hands and feet (though the disease process can virtually affect any synovial joint).[1] The prevalence of RA ranges from 0.5% to 2% among the general population, mostly affecting women who are two times more susceptible than men in the fourth to fifth decades of life.[2] There is neither exact definition of RA nor a pathognomonic test. The diagnosis of RA, therefore, rests on a composite of clinical and laboratory observations. RA is extremely heterogeneous with respect to severity and progression. Permanent remission can occur but is rare once there is a significant amount of joint damage. Although RA is predominantly an articular disease, it is important to remember that there are a number of “extraarticular manifestations” (EAMs) associated to the disease, for example, involvement of the eyes, lungs, skin, and nervous system. In fact, nearly 50% of patients with RA develop some kind of EAMs of RA.[3] Although RA itself results in significantly reduced survival among those affected, mortality rates are further increased in those having EAMs.[4] Proper diagnosis and appropriate management of EAM, therefore, play a crucial role in this scenario. This short review will give an overview of the various EAMs of RA and their appropriate management so as to minimize the added mortality due to the EAMs.
CLASSIFICATION OF THE EXTRAARTICULAR MANIFESTATIONS IN RHEUMATOID ARTHRITIS
EAM can affect various body organs; however, they can be quite distinct from the common ailments in that particular body organ or tissue. Although there is existence of the Malmö criteria [Table 1] that distinguishes the EAM into severe and not severe categories, however, there is a lot of debate and lack of clear consensus among rheumatologists as to the exact applicability of that criteria.[5] Although the common characteristic is that severe EAMs are usually associated with significantly more morbidity and mortality; however, that is not always true.[6]
Table 1.
Extraarticular manifestations in rheumatoid arthritis, according to Malmö criteria[5]
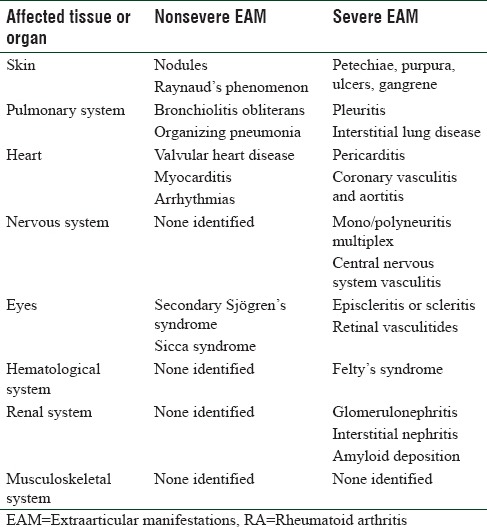
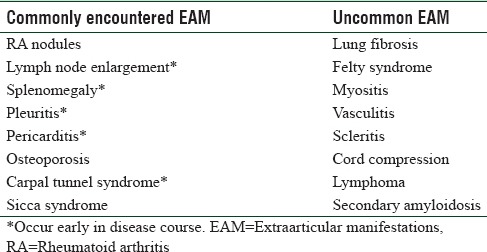
For the benefit of discussion and also to classify the EAMs in a simpler way, it might be a better option to classify them according to their prevalence in RA patients [Table 2].
Table 2.
Classification of extraarticular manifestations in rheumatoid arthritis as per frequency
EAMs in RA remain a serious problem to the treating clinicians owing to their complex presentation and diagnostic difficulties, raising therefore, a need to treat them aggressively and also a need for intense and frequent monitoring of individual cases. EAM can present as a diagnostic dilemma as drug-induced toxicities and associated infections frequently mimic having similar presentations. EAMs occur in about 50% of RA patients, while the life expectancy is reduced by approximately 7 years in males and 3 years in females.[7] The incidence of EAM in RA varies widely among various studies mostly because of diverse nature of studies done. In some cases, there were lack of consensus about case definition as to the inclusion of all EAM or only severe ones, few inconsistencies are also due to variations between community-based and hospital-based studies as the hospital-based studies tend to include more severe and complex presentations and probably due to aggressive management.[8,9] EAM can occur at any time during the disease while few common EAM are even notorious for appearing early. Most cases of premature death in EAM have been associated with cardiovascular complications, lung diseases, and malignancy.[10] Unlike RA, there is no particular sex predilection in case of EAM, occurring equally in males and females and appearing at any age. Generally, those RA patients having high titers of rheumatoid factor,[11] antinuclear antibodies,[12] disease-associated human leukocyte histocompatibility antigen (HLA) genes (especially homozygous DRB1*04 subtype),[13] history of smoking,[14] etc., are most likely to have EAM which includes rheumatoid nodules, vasculitis, pulmonary, neurologic, cardiac, hematological, and cutaneous complications.[2,15] The presence of anti-CCP antibodies is also associated with a more progressive joint damage and severe EAM.[16] A genetic association that has been linked to EAM is homozygosity for HLA class II DRB1*04,[17] tyrosine-phosphatase gene PTPN22,[18] and epigenetic changes.[19] Although from various observational studies, it is suggested that RA is becoming a less severe disease now-a-days, probably because of the advent and early usage of newer and more efficacious disease-modifying antirheumatic drugs (DMARDs) or biologics,[20] unfortunately, the same cannot be said about the EAM which remains a serious challenge to the rheumatologists as well as to the patients.
ORGAN INVOLVEMENTS IN RHEUMATOID ARTHRITIS
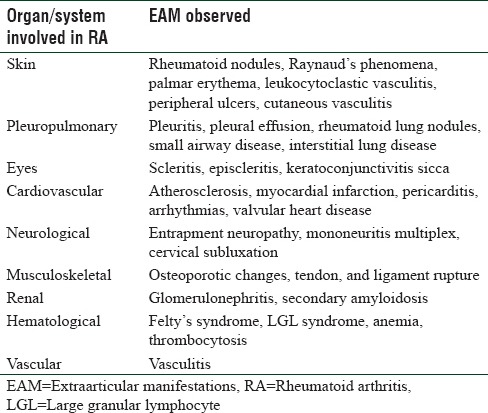
The various organ and systems involved in RA and the EAM seen thereof are listed in Table 3. However, details of such involvements including their pathophysiological features and prevalence are given below in a concise manner.
Table 3.
Organ/system specific extraarticular manifestations in rheumatoid arthritis
Cutaneous involvement
Rheumatoid nodules are the principal cutaneous manifestation occurring in up to 30% of RA patients with EAM. They are mainly found on the extensor surface of the forearm and also over pressure areas throughout the skin. Nodules, though not specific for RA, but are useful adjuncts in diagnosis and prognosis, which correlate with seropositivity, disease activity, and disease progression. It is mainly believed to occur due to small vessel vasculitis, other such manifestations being splinter hemorrhages, periungal infarcts, digital gangrene, and painful ulcers also,[21] presenting as Raynaud's phenomenon. Important point to note in this regard is that if Raynaud's phenomena appear long after the onset of RA, it generally leads to a more severe disease course.[22] Apart from those, palmar erythema is also fairly common in RA. Leukocytoclastic vasculitis also occurs and is seen as palpable purpura which mostly shows spontaneous improvement with RA treatment.
Lymph node enlargement
lymph nodes are often enlarged in RA but are rarely palpable. Epitrochlear lymph nodes are commonly enlarged in those who have significant arthritis involving joints of hands. In a few cases, RA may present with widespread nodes mimicking Hodgkin's disease.
Pleuropulmonary involvement
Pleuritis is a common EAM in RA, often in milder form but difficult to identify clinically, affecting almost 5%–10% of RA patients.[23] A diverse variety of clinical pictures may represent the pulmonary system involvement in RA that encompass pleural effusion, interstitial lung disease (ILD), rheumatoid pulmonary nodules, small airway disease, etc.[24] Exudative pericarditis and ILD very often are found along with RA. But like other pulmonary and cardiac manifestations, they are more common in the older patients. Clinically significant ILD is prevalent in about 7.7%–12% of RA cases.[25,23] The primary patterns of ILD in RA patients happen to be usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP) that are observed both histologically as well as radiologically.[26] With regard to the prognostic point of view, the presence of UIP in RA has been associated with a poor prognosis than NSIP, more RA disease severity, and a reduced survival.[27] The management options in most cases of rheumatoid ILD consist of biological DMARDs with a careful monitoring with high-resolution computerized tomography (HRCT) scans. A combined approach of a rheumatologist, pulmonogist, and radiologist ensures a better and effective care to such cases.[28,29,30] Pleural effusion if occur is mostly bilateral. Rheumatoid pulmonary nodules are another important finding which are asymptomatic and found almost exclusively in seropositive RA patients. Radiographically, they are coin-shaped lesions that can be difficult to differentiate from malignancy. To confirm the diagnosis, therefore, in patients in whom malignancy is clinically suspected, further imaging or tissue biopsy may be warranted. Diffuse interstitial fibrosis and fibrosing alveolitis are rarely associated with RA. In few extremely rare cases, methotrexate use is also associated with the development of pulmonary fibrosis. HRCT is by far the best approach for early diagnosis of pulmonary EAM in RA.[31] The mainstay of treatment in pulmonary EAM is based on systemic steroids and immunosuppressive therapy in the form of mycophenolate or cyclophosphamide and azathioprine although in very severe presentations or in case of an unresponsive disease, rituximab therapy is also common.
Cardiovascular system
There is increasing evidence that patients with RA suffer from the increased cardiovascular disease independent of the traditional risk factors. The more prevalent manifestations include atherosclerosis, myocardial infarction, pericarditis, arrhythmias, and valvular heart disease.[32] The most common EAM in cardiovascular system is pericarditis, usually associated with seropositive RA patients.[33] In fact, symptomatic pericarditis can even be the first sign of RA.[34] Most of the cardiac manifestations are silent and rarely need treatment. In symptomatic pericardial disease, steroids or nonsteroidal anti-inflammatory drugs (NSAIDs) may bring about rapid improvement.[35] Inflammation is thought to play a key role in the development of atherosclerosis and the systemic inflammatory response in RA may explain the link. An earlier work by the same authors has established possible beneficial role of adjunct statins in the management of RA; statins by their pleotropic actions on a variety of inflammatory and immune mediators may be particularly helpful not only in the RA disease amelioration but also reducing the cardiologic and vascular EAM in RA.[36] Epidemiological studies reveal that cardiovascular mortality is increased in patients with early and established disease, and is surprisingly, worse in women, a group traditionally put at lower risk of having cardiovascular mortality.[37] Another important manifestation that is clinically less evident but frequently associated with RA is congestive heart failure.[33] Formation of rheumatoid nodules in mitral or aortic valve can sometimes lead to valvular heart disease.
Ocular manifestations
The most common ocular manifestation in RA is the presence of sicca syndrome or keratoconjunctivitis sicca, affecting at least 10% of patients, frequently associated with dry mouth, salivary gland swelling, and secondary Sjögren's syndrome.[10] Treatment of which is often symptomatic with artificial tear and/or saliva supplements based on evaluation. Rheumatoid vasculitis also gives rise to a severe form of painful scleritis, leading to scleromalacia. Prompt treatment with high-dose steroids can salvage vision in such cases. On the other hand, episcleritis, which is an inflammation in the superficial layer to sclera, is benign and usually resolves spontaneously. Recent studies reveal reduction in ocular manifestations with the use of rituximab and other biologic agents, but clinical trial data are not yet available.[38]
Neurological involvement
The most common neurological involvement in RA is entrapment neuropathy developing secondary to synovitis. Median nerve compression can occur early in the disease. In fact in every patient with carpal tunnel syndrome,[39] the diagnosis of RA should be kept in mind. Other nerves getting entrapped in RA are rare and include posterior tibial nerve at tarsal tunnel and ulnar nerve at elbow. Mononeuritis multiplex, a peripheral but often bilateral neuropathy, can also present acutely in RA.[40] This is a manifestation of systemic rheumatoid vasculitis that warrants urgent evaluation and intervention. Clinically, this presents with wrist drop and foot drop. A sudden onset of peripheral sensorimotor neuropathy can signal the presence of aggressive rheumatoid vasculitis and thereby poorer prognosis. Treatment includes high doses of intravenous (IV) steroids with IV cyclophosphamide. Cervical subluxation at the atlantoaxial level[41] can be present in about one-third of the long-standing RA patients but is usually asymptomatic. Another rare but serious EAM is cervical myelopathy due to cervical instability which can be fatal. Symptoms include paresthesia, weakness, paralysis, sensory loss, incontinence, and syncope.
Osteoporosis and fractures
Various cytokines which are generated as a result of inflammatory process in RA, encourage bone resorption by inducing osteoclast action, and lead to osteoporotic changes.[42] However, other additional risk factors such as physical inactivity, difficulty in walking with deformed lower limb joints with increased incidence of falls, Vitamin D deficiency, and preexisting osteoporosis contribute to spontaneous fractures. Use of systemic as well as intraarticular corticosteroids further increases fracture risk due to osteoporosis.
Tendons and ligaments
Spontaneous rupture of tendons and ligaments, most commonly at the wrist, hand, and rotator cuff are occasionally encountered in RA. More often, tenosynovitis and weakening of ligaments lead to joint instability and subluxation.
Renal involvement
Renal involvement is relatively rare in RA. This EAM might be a consequence of antirheumatic drugs itself, especially of NSAIDs and DMARDs.[43] Apart from that, long duration of disease and poor response to therapy are important risk factors for this EAM. Mesangial glomerulonephritis is the hallmark of renal involvement in RA in about 60% of cases.[44] In patients in whom renal involvement is present, secondary amyloidosis appears to be the most common feature. However, intensive antirheumatic therapy now gives a more favorable outcome, rituximab being a good choice for this condition.
Felty's syndrome
This is the most common type of hematological EAM in RA that may be present at the time of diagnosis or can occur during treatment. There is association of splenomegaly and neutropenia in typically those patients who have rather destructive RA.[45] Systemic disease, hepatomegaly, and lymphadenopathy are also common. Most of the patients having this EAM generally have rheumatoid factor positivity.[46] In uncomplicated cases, treatment is mainly conservative with methotrexate, splenectomy remaining a controversial option and often offer only transient benefit in these patients.[47]
Large granular lymphocyte syndrome
Although similar in presentation with Felty's syndrome, it is differentiated by the presence of large granular lymphocytes and a clonal lymphocytosis.[48,49] The arthritis that accompanies LGL syndrome is typically less destructive than that seen with Felty's although this is not always the case.
Drug toxicities
Although not a true EAM of RA, drug toxicities of medications used in RA need special consideration. Various adverse drug reactions are also common as the drugs have to be used continuously and for prolonged period. Clinical and laboratory monitoring for the side effects of DMARDs,[50,51] and also for NSAIDs are extremely important. Complete blood counts and liver and renal function tests are mandatory as per the guidelines, initially every month for the first 3 months and every 3 monthly thereafter.[52]
CONCLUSION
In spite of the common notion that severe EAM in RA may be on the declining trend because of the current biologic therapy, there is a significant lack of published reference and guidelines in its support. Major therapeutic guidelines are mostly based on nonrandomized trials as the patients with EAM were mostly excluded from controlled clinical trials where newer biologics were compared head to head against conventional DMARDs. Even there is no clear consensus till now as to a universal classification of EAM in RA. Keeping in mind, the diverse EAMs that are encountered in RA, a clear consensus regarding its definition, classification, and management should be the need of the hour.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O'Fallon WM, et al. Survival in rheumatoid arthritis: A population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–8. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 3.Turesson C, O'Fallon WM, Crowson CS, Matteson EL. Occurrence of extra-articular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–7. [PubMed] [Google Scholar]
- 4.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: Incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turesson C, Jacobsson L, Bergström U. Extra-articular rheumatoid arthritis: Prevalence and mortality. Rheumatology (Oxford) 1999;38:668–74. doi: 10.1093/rheumatology/38.7.668. [DOI] [PubMed] [Google Scholar]
- 6.Turesson C, McClelland RL, Christianson TJ, Matteson EL. Multiple extra-articular manifestations are associated with poor survival in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1533–4. doi: 10.1136/ard.2006.052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimmino MA, Salvarani C, Macchioni P, Montecucco C, Fossaluzza V, Mascia MT, et al. Extra-articular manifestations in 587 Italian patients with rheumatoid arthritis. Rheumatol Int. 2000;19:213–7. doi: 10.1007/pl00006853. [DOI] [PubMed] [Google Scholar]
- 8.Turesson C, McClelland RL, Christianson TJ, Matteson EL. No decrease over time in the incidence of vasculitis or other extraarticular manifestations in rheumatoid arthritis: Results from a community-based study. Arthritis Rheum. 2004;50:3729–31. doi: 10.1002/art.20590. [DOI] [PubMed] [Google Scholar]
- 9.Bartels C, Bell C, Rosenthal A, Shinki K, Bridges A. Decline in rheumatoid vasculitis prevalence among US veterans: A retrospective cross-sectional study. Arthritis Rheum. 2009;60:2553–7. doi: 10.1002/art.24775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronstein BN. Interleukin-6 – A key mediator of systemic and local symptoms in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(Suppl 1):S11–5. [PubMed] [Google Scholar]
- 11.Voskuyl AE, Zwinderman AH, Westedt ML, Vandenbroucke JP, Breedveld FC, Hazes JM. Factors associated with the development of vasculitis in rheumatoid arthritis: Results of a case-control study. Ann Rheum Dis. 1996;55:190–2. doi: 10.1136/ard.55.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turesson C, Jacobsson L, Bergström U, Truedsson L, Sturfelt G. Predictors of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2000;29:358–64. doi: 10.1080/030097400447552. [DOI] [PubMed] [Google Scholar]
- 13.Weyand CM, Xie C, Goronzy JJ. Homozygosity for the HLA-DRB1 allele selects for extraarticular manifestations in rheumatoid arthritis. J Clin Invest. 1992;89:2033–9. doi: 10.1172/JCI115814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Struthers GR, Scott DL, Delamere JP, Sheppeard H, Kitt M. Smoking and rheumatoid vasculitis. Rheumatol Int. 1981;1:145–6. doi: 10.1007/BF00541260. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC, Johnston SS, John AK. The incidence and prevalence of extra-articular and systemic manifestations in a cohort of newly-diagnosed patients with rheumatoid arthritis between 1999 and 2006. Curr Med Res Opin. 2008;24:469–80. doi: 10.1185/030079908x261177. [DOI] [PubMed] [Google Scholar]
- 16.Turesson C, Jacobsson L, Sturfelt G, Matteson E, Mathsson L, Ronnelid J. Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;66:59–64. doi: 10.1136/ard.2006.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojas-Villarraga A, Diaz FJ, Calvo-Páramo E, Salazar JC, Iglesias-Gamarra A, Mantilla RD, et al. Familial disease, the HLA-DRB1 shared epitope and anti-CCP antibodies influence time at appearance of substantial joint damage in rheumatoid arthritis. J Autoimmun. 2009;32:64–9. doi: 10.1016/j.jaut.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Tobón GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J Autoimmun. 2010;35:10–4. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Brooks WH, Le DC, Pers JO, Youinou P, Renaudineau Y. Epigenetics and autoimmunity. J Autoimmun. 2010;34:J207–19. doi: 10.1016/j.jaut.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Bergström U, Book C, Lindroth Y, Marsal L, Saxne T, Jacobsson L, et al. Lower disease activity and disability in Swedish patients with rheumatoid arthritis in 1995 compared with 1978. Scand J Rheumatol. 1999;28:160–5. doi: 10.1080/03009749950154239. [DOI] [PubMed] [Google Scholar]
- 21.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular manifestations in rheumatoid arthritis. Maedica (Buchar) 2010;5:286–91. [PMC free article] [PubMed] [Google Scholar]
- 22.Saraux A, Allain J, Guedes C, Baron D, Youinou P, Le Goff P. Raynaud's phenomenon in rheumatoid arthritis. Br J Rheumatol. 1996;35:752–4. doi: 10.1093/rheumatology/35.8.752. [DOI] [PubMed] [Google Scholar]
- 23.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2010;62:1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prete M, Racanelli V, Digiglio L, Vacca A, Dammacco F, Perosa F, et al. Extra-articular manifestations of rheumatoid arthritis: An update. Autoimmun Rev. 2011;11:123–31. doi: 10.1016/j.autrev.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183:372–8. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HK, Kim DS, Yoo B, Seo JB, Rho JY, Colby TV, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127:2019–27. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 27.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics – A large multicentre UK study. Rheumatology (Oxford) 2014;53:1676–82. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 28.Mori S. Management of rheumatoid arthritis patients with interstitial lung disease: Safety of biological antirheumatic drugs and assessment of pulmonary fibrosis. Clin Med Insights Circ Respir Pulm Med. 2015;9:41–9. doi: 10.4137/CCRPM.S23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814–24. doi: 10.1378/chest.12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lauretis A, Veeraraghavan S, Renzoni E. Review series: Aspects of interstitial lung disease: Connective tissue disease-associated interstitial lung disease: How does it differ from IPF? How should the clinical approach differ? Chron Respir Dis. 2011;8:53–82. doi: 10.1177/1479972310393758. [DOI] [PubMed] [Google Scholar]
- 31.Lioté H. Pulmonary manifestation of rheumatoid arthritis. Rev Mal Respir. 2008;25:973–88. doi: 10.1016/s0761-8425(08)74414-0. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 33.Ortega-Hernandez OD, Pineda-Tamayo R, Pardo AL, Rojas-Villarraga A, Anaya JM. Cardiovascular disease is associated with extra-articular manifestations in patients with rheumatoid arthritis. Clin Rheumatol. 2009;28:767–75. doi: 10.1007/s10067-009-1145-8. [DOI] [PubMed] [Google Scholar]
- 34.Voskuyl AE. The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology (Oxford) 2006;45(Suppl 4):iv4–7. doi: 10.1093/rheumatology/kel313. [DOI] [PubMed] [Google Scholar]
- 35.Kitas G, Banks MJ, Bacon PA. Cardiac involvement in rheumatoid disease. Clin Med (Lond) 2001;1:18–21. doi: 10.7861/clinmedicine.1-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S, Mohanty M, Padhan P. Outcome of rheumatoid arthritis following adjunct statin therapy. Indian J Pharmacol. 2015;47:605–9. doi: 10.4103/0253-7613.169585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turesson C, Jarenros A, Jacobsson L. Increased incidence of cardiovascular disease in patients with rheumatoid arthritis: Results from a community based study. Ann Rheum Dis. 2004;63:952–5. doi: 10.1136/ard.2003.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iaccheri B, Androudi S, Bocci EB, Gerli R, Cagini C, Fiore T, et al. Rituximab treatment for persistent scleritis associated with rheumatoid arthritis. Ocul Immunol Inflamm. 2010;18:223–5. doi: 10.3109/09273941003739928. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal V, Singh R, Wiclaf F, Chauhan S, Tahlan A, Ahuja CK, et al. A clinical, electrophysiological, and pathological study of neuropathy in rheumatoid arthritis. Clin Rheumatol. 2008;27:841–4. doi: 10.1007/s10067-007-0804-x. [DOI] [PubMed] [Google Scholar]
- 40.Muramatsu K, Tanaka H, Taguchi T. Peripheral neuropathies of the forearm and hand in rheumatoid arthritis: Diagnosis and options for treatment. Rheumatol Int. 2008;28:951–7. doi: 10.1007/s00296-008-0630-8. [DOI] [PubMed] [Google Scholar]
- 41.Ito H, Neo M, Sakamoto T, Fujibayashi S, Yoshitomi H, Nakamura T, et al. Subaxial subluxation after atlantoaxial transarticular screw fixation in rheumatoid patients. Eur Spine J. 2009;18:869–76. doi: 10.1007/s00586-009-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. Mechanisms of disease: The link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol. 2005;1:47–54. doi: 10.1038/ncprheum0036. [DOI] [PubMed] [Google Scholar]
- 43.Mielants H, Van den Bosch F. Extra-articular manifestations. Clin Exp Rheumatol. 2009;27:S56–61. [PubMed] [Google Scholar]
- 44.Korpela M, Mustonen J, Teppo AM, Helin H, Pasternack A. Mesangial glomerulonephritis as an extra-articular manifestation of rheumatoid arthritis. Br J Rheumatol. 1997;36:1189–95. doi: 10.1093/rheumatology/36.11.1189. [DOI] [PubMed] [Google Scholar]
- 45.Balint GP, Balint PV. Felty's syndrome. Best Pract Res Clin Rheumatol. 2004;18:631–45. doi: 10.1016/j.berh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Turesson C. Rheumatoid vasculitis: An update. Curr Opin Rheumatol. 2015;27:63–70. doi: 10.1097/BOR.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 47.Sordet C, Gottenberg JE, Hellmich B, Kieffer P, Mariette X, Sibilia J, et al. Lack of efficacy of rituximab in Felty's syndrome. Ann Rheum Dis. 2005;64:332–3. doi: 10.1136/ard.2004.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 49.Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand J Rheumatol. 2002;31:251–9. doi: 10.1080/030097402760375124. [DOI] [PubMed] [Google Scholar]
- 50.McCain ME, Quinet RJ, Davis WE. Etanercept and infliximab associated with cutaneous vasculitis. Rheumatology (Oxford) 2002;41:116–7. doi: 10.1093/rheumatology/41.1.116. [DOI] [PubMed] [Google Scholar]
- 51.Kekow J, Welte T, Kellner U, Pap T. Development of rheumatoid nodules during anti-tumor necrosis factor alpha therapy with etanercept. Arthritis Rheum. 2002;46:843–4. doi: 10.1002/art.10096. [DOI] [PubMed] [Google Scholar]
- 52.Chakravarty K, McDonald H, Pullar T, Taggart A, Chalmers R, Oliver S, et al. BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British association of dermatologists. Rheumatology (Oxford) 2008;47:924–5. doi: 10.1093/rheumatology/kel216a. [DOI] [PubMed] [Google Scholar]


