Abstract
Objective:
To analyze available evidence on the safety of different biological response modifiers which are used for a treatment of rheumatoid arthritis (RA).
Materials and Methods:
We searched systematically for randomized controlled clinical trials on treatment of RA with different biological response modifiers, followed by a systematic review with meta-analysis. Trials were searched from MEDLINE and Cochrane Library databases. The following safety parameters reported in the selected trials were analyzed: number of patients suffering any adverse event (AE), withdrawal due to AEs, serious AE (SAEs), infections, serious infections, infusion reactions, injection site reactions, malignancies, and overall mortality. Undesired effects were estimated using combined relative risks (RR) and number needed to harm (NNH). Heterogeneity was evaluated by Cochrane's Q and I2 statistics.
Results:
According to inclusion criteria, a total of 43 trials (20,504 patients) were included in this study. A total number of AEs were found more with abatacept (RR: 1.05, NNH: 21.93). Withdrawal due to AEs was found with all biologicals, highest with anakinra (RR: 3.48, NNH: 15.70). Patients receiving newer tumor necrosis factor-alpha inhibitors, golimumab, were more likely to develop SAEs (RR: 2.44, NNH: 12.72) and infection (RR: 1.25, NNH: 10.09), and in certolizumab, serious infections (RR: 2.95, NNH: 37.31) were found more. Infusion reaction develops more with rituximab (RR: 1.52, NNH: 8.47). Etanercept showed the highest risk to develop infusion site reaction (RR: 5.33, NNH: 4.65). Biologicals showed no difference to their control counterparts in malignancy and mortality risk.
Conclusion:
This meta-analysis helps to clarify some frequently encountered and unanswered safety questions of different biological response modifiers, a new class of drugs, in the clinical care of RA patients.
Keywords: Adverse event, biological response modifiers, meta-analysis, number needed to harm, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease, in which there is joint inflammation, synovial proliferation, and destruction of articular cartilage.[1] Uncontrolled active RA causes disability, decreases the quality of life, and increases comorbidity. These, in turn, result in loss of work, high medical and social costs, and substantial morbidity and mortality.[2]
There is no curative treatment of RA. To date, the goal of treatment in RA is to reduce joint inflammation and pain, maximize joint function, and prevent joint destruction and deformity. The treatment of RA optimally involves a combination of nonpharmacological intervention (patient education, rest and exercise, and joint protection), a pharmacological intervention (such as medications nonsteroidal anti-inflammatory drugs [NSAIDs], disease-modifying antirheumatic drugs [DMARDs], tumor necrosis factor [TNF]-alpha inhibitors, immunosuppressant, and steroids) and occasionally surgery. There are two classes of medications which are used in treatment of RA: Fast acting (first-line drugs) such as NSAIDs and corticosteroids are used to reduce pain, inflammation, and swelling. Slow acting (second line drugs) such as methotrexate (MTX) and DMARDs are used to promote disease remission and prevent progressive joint destruction.[3]
Now, newer second-line drug biological response modifiers (biologics) are available. Biologics are genetically engineered antibodies derived from human genes. They are designed to inhibit specific components of the immune system that play pivotal roles in fueling inflammation, which is a central feature of RA.[4] In comparison with traditional DMARDs, the biological response modifiers have a much more rapid onset of action and can have powerful effects on stopping the progressive joint damage. Their method of action is also more directed, defined, and targeted.[3]
Although there is an increasing appreciation of evidence-based medicine, the data sources for this are still in their infancy. Guidelines and algorithms have been developed to help determine the appropriate choices of treatment, but they are not applicable to every patient. Moreover, new information from clinical trials is being published at too fast a rate for textbooks to remain current. The challenge is to translate the clinical research data into a format suitable for use by busy clinicians in practice. One key item of information needed for an informed decision is an easily understood estimate of the magnitude of benefit (risk of adverse effects) that can be used by doctors and other caregivers.[5]
Biological response modifiers' use is limited by their cost and toxicity due to interfering with the immune system responses. Data from clinical trials and postmarketing surveillance studies have raised a wide range of safety signals including a risk of infections, malignancy, demyelinating disorders, congestive heart failure, gastrointestinal perforations, hepatic impairment, dyslipidemia, autoimmune syndromes, and infusion reactions.[6] However, many questions about the safety of this new class of drugs still remain unanswered. To date, there is no published article on direct head-to-head comparative studies of different biologics. An alternative approach to answering the safety-related questions is to perform a systematic review with meta-analysis of relevant research.[7]
In this study, we have conducted a systematic review of various clinical trials of biologics in RA followed by a meta-analysis of the safety of it at their different doses.
MATERIALS AND METHODS
Study selection criteria
We carried out a search of all randomized controlled clinical trials of biological response modifiers for treating patients with RA. Clinical trials were excluded if they either used administration routes other than recommended or included no treatment group with recommended doses. Only information published in the trial reports was assessed.
Safety parameters
The following safety parameters reported in the selected trials were analyzed: number of patients suffering any adverse event (AE), withdrawal due to AEs, serious AE (SAEs), infections, serious infections, infusion reactions, infusion-site reactions, malignancies, and overall mortality.
Search strategy
Trials were searched in scientific journals. Information from the MEDLINE and Cochrane Library databases was checked using a high-sensitivity strategy. The descriptors used were RA, biological response modifiers, randomized controlled trial, and meta-analysis. The computerized search was completed with a manual search of reference lists from the articles retrieved and from rheumatological journal articles published.
Data extraction
Trials with information only in the abstract format were excluded. Data were extracted using key items for each trial: study design, patients' characteristics (sex, age, and duration of disease evolution), patient inclusion criteria, drugs and doses used, treatment duration, and safety parameters.
Statistical analysis
We have used the relative risk (RR) with 95% confidence intervals (95% CI) to estimate the risk of AEs. RR is the ratio of the probability of an event occurring in an exposed group to the probability of the event occurring in a comparison, nonexposed group. RR of 1 means there is no difference in risk between exposed and nonexposed groups. RR of <1 means the event is less likely to occur in the exposed group than in the nonexposed group. RR of >1 means the event is more likely to occur in the exposed group than in the nonexposed group.
We estimated the number needed to harm (NNH) defined as the number of patients receiving active treatment that would harm one patient compared to controls. We used the specific statistical software MedCalc Trial Version 17.2 (MedCalc, Ostend, Belgium) which is available online from http://www.medcalc.org for analysis and presentation of main results.
To determine statistical significance, we have considered P < 0.05 as statistically significant.
RESULTS
A total of 43 publications which met the selection criteria were included in the meta-analysis. We analyzed the entire set of 20,504 patients recruited for the 43 trials selected: Five using adalimumab (2585 patients), four using certolizumab (2062 patients), four using etanercept (1823 patients), three using abatacept (1148 patients), seven using golimumab (2998 patients), two using anakinra (661 patients), seven using infliximab (3448 patients), three using rituximab (1062 patients), and eight using tocilizumab (4717 patients). An overview of the AEs reported in selected trials is displayed in Table 1.
Table 1.
Number of patients who presented adverse effects in trial with different biologics
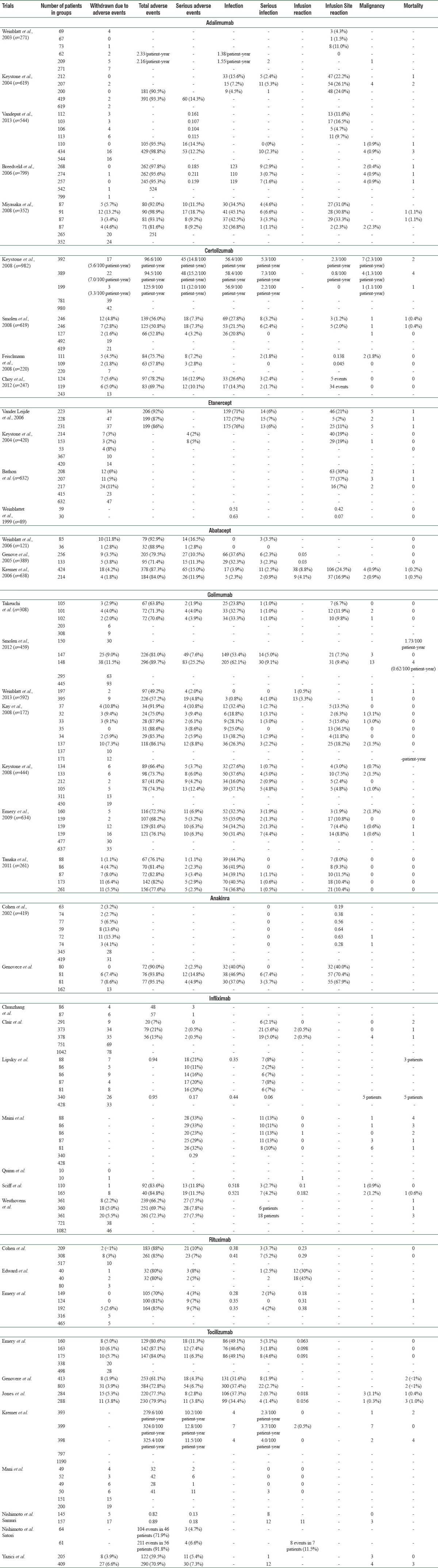
Information on the incidence of SAEs, serious infections, malignancies, and mortality is provided, specifying whether patients were in the experimental or control arms. Other important safety information (number of total AEs, total number of infection, infusion reaction, and infusion site reactions) was provided much less consistently.
Safety analysis
Individual event-wise safety analysis (RR, NNH, and heterogeneity) of different biologicals as shown in Table 2, which was interpreted as following:
Table 2.
Overall and individual drug wise adverse events relative risk, number needed to harm and heterogeneity results
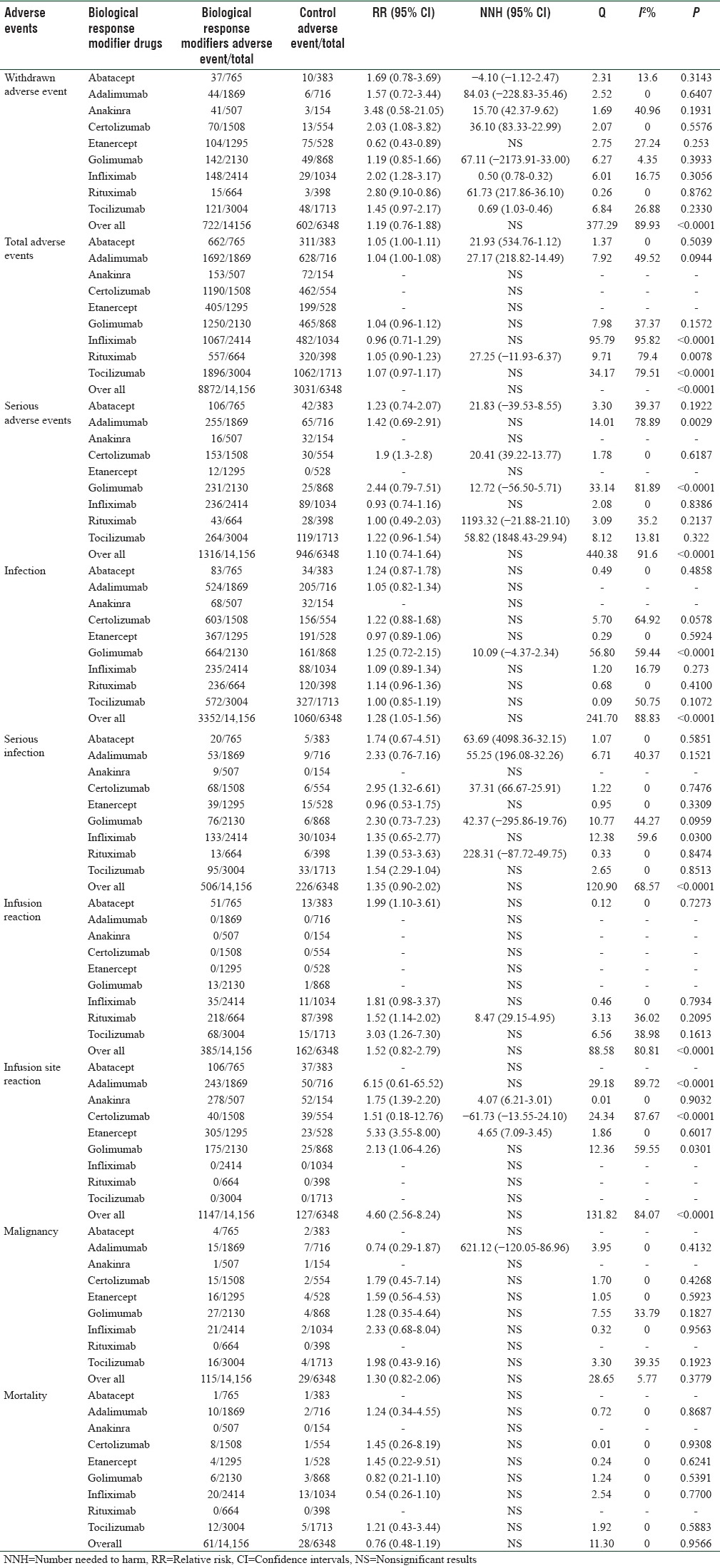
Withdrawal due to adverse events
The number of withdrawals due to AEs according to treatment arm was reported in all trials. We found no significant overall difference between the experimental and control groups, with a pooled RR (95% CI) of 1.19 (0.76–1.88). There was statistically significant heterogeneity among the drugs (Q = 377.29; P ≤ 0.0001, I2: 89.93) but not within the groups given each specific drug.
Result differed depending on the specific biologic response modifier given: patients in anakinra arms were more likely to withdraw from AEs than their control counterparts (RR [95% CI]: 3.48 [0.58–21.05]). Patients in the abatacept arms were less likely to withdraw from AEs than their control counterparts (Negative NNH), but the opposite was the case for all others except the etanercept, all those comparisons reaching statistical significance. No statistically significant difference found in a patient taking etanercept as compared to their control counterparts.
Total adverse events
Overall, there was no statistically significant difference found between the experimental and control group. There was statistically significant heterogeneity among the drugs.
Profile of individual drug showed risk to develop total AE were almost similar in abatacept, adalimumab, and rituximab treatment arms compared to their control. No significant differences were found in other drugs. Infliximab, rituximab, and tocilizumab showed within the group statistically significant heterogeneity.
Serious adverse events
We found that there was no significant overall difference between experimental and control groups in SAEs, with a pooled RR (95% CI) of 1.10 (0.74–1.64).
In terms of individual drug-wise SAEs, patients in adalimumab, anakinra, etanercept, and infliximab arms showed no statistically significant differences compared to their control parts. Patients in golimumab arms showed the highest risk to develop SAEs. There was statistically significant heterogeneity among the drugs (Q = 440.38; P ≤ 0.0001, I2: 91.6) but not within the groups given each specific drug except adalimumab and golimumab.
Infection
Overall, there was no significant difference between experimental and control groups in infection, with a pooled RR (95% CI) of 1.28 (1.05–1.56). There was statistically significant heterogeneity among the drugs (Q = 241.70; P ≤ 0.0001, I2: 88.83).
Except for golimumab, all other drugs showed no significant difference between experimental and control groups in infection and did not show statistically significant heterogeneity.
Serious infection
There was no significant overall difference between experimental and control groups in serious infection, with a pooled RR (95% CI) of 1.35 (0.90–2.02). There was statistically significant heterogeneity among the drugs (Q = 120.9; P ≤ 0.0001, I2: 68.57).
Except anakinra, adalimumab, infliximab, and tocilizumab, all other experimental drugs showed a significant difference to their control counterparts. The risk to develop serious infection was found in a certolizumab group (RR [95% CI]: 2.95 [1.32–6.61] and NNH [95% CI]: 37.31 [66.67–25.91]). Except for infliximab, no other drugs showed statistically significant heterogeneity.
Infusion reaction
There was no significant overall difference between experimental and control groups in infusion reaction, with a pooled RR (95% CI) of 1.52 (0.82–2.79). There was statistically significant heterogeneity among the drugs (Q = 88.58; P ≤ 0.0001, I2: 80.81) but not within the groups given each specific drug.
Except rituximab, no other drug showed a significant difference to their control counterparts. Risk to develop infusion reaction was found highest in tocilizumab (RR [95% CI]: 3.03 [1.26–7.30]), but it showed no significant difference to their control counterparts.
Infusion site reaction
There was no significant overall difference between the experimental and control groups in the development of infusion site reaction with a pooled RR (95% CI) of 4.60 (2.56–8.24). There was statistically significant heterogeneity among the drugs (Q = 131.82; P ≤ 0.0001, I2: 84.07).
Adalimumab showed the highest risk to develop infusion site reaction (RR [95% CI]: 6.15 [0.61–65.52]), but it showed no significant difference to their control. Anakinra, certolizumab, and etanercept showed a significant difference to their control. Among them, etanercept showed highest risk to develop infusion site reaction (RR [95% CI]: 5.33 [3.55–8.00]). Except adalimumab and certolizumab, no group showed statistically significant heterogeneity.
Malignancy
There was no significant overall difference between the experimental and control groups in the development of malignancy with a pooled RR of 1.30 (0.82–2.06). There was no statistically significant heterogeneity among the drugs (Q = 28.65; P ≤ 0.0001, I2: 5.77) and not within the groups given each specific drug.
Risk to malignancy was found highest in infliximab group, but it showed no significant difference to their control group. Except adalimumab, all other drugs showed no significant difference to their control.
Mortality
There was no significant difference found between the experimental and control groups in terms of mortality during the treatment with overall and individual drugs. There was no statistically significant heterogeneity among the drugs (Q = 11.30; P = 0.9566, I2: 0) and not within the groups given each specific drug.
DISCUSSION
Guidelines for the treatment of RA recommend early aggressive therapy with one or more DMARDs to prevent joint destruction, disability, and loss of work capacity. Conventional DMARDs are usually considered the standard of care for most patients. The emergence of biologic agents has provided effective therapeutic options for patients with inadequate response to conventional DMARDs. Despite the efficacy of biologic agents, their immunomodulatory properties have raised many safety concerns; prompting careful evaluation in clinical trials and intensive postmarketing surveillance.[8] This meta-analysis will discuss the safety of currently available biologic agents in patients with RA.
We have compared the following nine biologics with their placebo. TNF inhibitors (adalimumab, certolizumab, etanercept, golimumab, and infliximab), interleukin-1 receptor antagonist (anakinra), interleukin-6 receptor antagonist (tocilizumab), selective co-stimulation modulator of T-cells (abatacept), and anti-B-cell (rituximab) therapies.[9] We focused solely on published results from well-designed randomized controlled trials; our review shows that patients receiving biological response modifiers are more prone to experience AEs. Although some of the relative safety estimates are statistically significant, their magnitude is rather small and their clinical relevance should also be addressed. All comparisons performed in this analysis were conducted by comparing the event rate in the medication of interest group with that of the placebo group in the selected studies. Negative values for NNH imply that the treatment of interest is more likely to result in harm than benefit. For example, a negative NNH indicates that a patient assigned to placebo has a lower risk for the AE than a patient assigned to the medication of interest. Infinity values for number needed to treat (NNT which is the number of patients needed to treat to prevent one additional bad outcome such as death and stroke) or NNH indicate that an infinite number of patients would be required to show any benefit or harm. If the 95% CI for NNT or NNH includes infinity, the finding is considered statistically insignificant.[10]
In regards to withdrawal due to AEs found in all trials, which was similar to meta-analysis done by Alonso-Ruiz et al. on TNF-alpha drugs in RA (13 trials: 7087 patients).[7] Patients in anakinra arms were more likely and in etanercept arm were less likely (NNH: no significant) to withdraw from AEs than their control counterparts. Similar results were found in meta-analysis done by Singh et al. which showed that higher rate of withdrawals because of AEs with infliximab, anakinra, and adalimumab compared with placebo or etanercept.[11] A negative NNH in abatacept showed that withdrawal was more in placebo arms than intervention arm.
In this study, a total number of AEs showed that there was no statistically significant difference between experimental and control group. Abatacept was associated with a statistically significantly higher number of AEs compared with placebo. Apart from abatacept, adalimumab and rituximab also showed a significant difference compared to their control parts. This finding was different from a study done by Codreanu and Damjanov which showed that biologics were associated with more AEs than placebo and infliximab was associated with a statistically significantly higher number of AEs (odds ratio [OR] = 1.55; 95% CI: 1.01–2.35) compared with placebo.[12] However, the number of AEs for the other eight biologics was not statistically different from those observed in the placebo groups.
SAEs result of this study showed similarity with a study done by Alonso-Ruiz et al. that there was no significant overall difference between experimental and control groups.[7] Furthermore, both studies showed overall similarity in RR and statistically significant heterogeneity. Risk to develop SAE was highest in newer TNF alpha inhibitors golimumab (RR [95% CI]: 2.44 [0.79–7.51]) followed by certolizumab (RR [95% CI]: 1.9 [1.3–2.8]). A study done by Codreanu and Damjanov showed that the number of SAEs observed during treatment with any of the nine biologics was not significantly different than the number of SAEs observed during treatment with placebo. Pair-wise comparisons between the biologics showed that certolizumab pegol was associated with a statistically significant increase in the number of SAEs compared with adalimumab (OR = 1.63; 95% CI: 1.01–2.62).[12]
Biological therapies targeting key components of the immune system allows efficient suppression of the pathologic inflammation cascade that gives rise to RA symptoms and subsequent joint destruction. As flip side of the coin, treatment with biologicals leaves the patient more susceptible to infection by inducing a certain extent of immunosuppression. Infectious complications of biological therapy include bacterial infections such as tuberculosis, Streptococcus pneumoniae and Listeria monocytogenes and potential reactivation of viral infections such as hepatitis B or C, herpes, and varicella zoster.[13] Serious infections were defined as infections associated with death, hospitalization, or the use of intravenous antibiotics.
Infection and serious infection results of this study showed that there was no significant overall difference between the experimental and control groups. Regarding infection, golimumab only showed the highest risk, significant difference to their control, and statistically significant heterogeneity. Risk to develop serious infection was found more in patient taking certolizumab and golimumab: newer TNF-alpha inhibitors. A recent study using data from the North American CORRONA registry indicates that MTX and TNF inhibitor therapy and the combination of both are all associated with a comparable increase in the incidence of overall infections as well as opportunistic infections.[13] In a study done by Codreanu and Damjanov, certolizumab pegol demonstrated a statistically significant increase in serious infections compared with placebo (OR = 4.75; 95% CI: 1.52–18.45).[12] However, data on the infectious complication risk with the newer TNF inhibitors such as golimumab and certolizumab are still limited.[13] A study done by Keyser showed that overall increase in infection particularly serious infection risk under rituximab, abatacept, tocilizumab, and anakinra are somewhat lower or seems to be associated more with concomitant other biologic therapies.[13] Clinicians considering starting biological therapy for an RA patient should be aware that biological therapy further increases the already moderately increased infection risk of the RA patient. Precautions needed before the start of biological therapy include checking and updating the patient's vaccination status and screening for latent tuberculosis.[13]
Infusion reaction result of this study showed that the risk was high (RR [95% CI]: 3.03 [1.26–7.30]) but no significant difference to their control in tocilizumab. Only rituximab showed statistically significant higher risk compared to their control. Rituximab is an effective and relatively safe option to be considered in patients who are refractory or intolerant to the anti-TNF biologics. Infusion reactions appear to be a disadvantage of the drug when compared to other available biological agents, but their incidence is reduced with glucocorticoids premedication and in subsequent infusions. The immunogenicity of rituximab does not seem to correlate with efficacy or the incidence of infusion reactions. Pooling of data from randomized controlled trials of rituximab in RA revealed that first infusion reactions occurred in approximately 25% of patients. Most reactions were mild to moderate in severity, with the most common symptoms being headache, skin itchiness, throat irritation, flushing, rash, hypertension, and pyrexia. The rates of infusion reaction in the second, third, fourth, and fifth course of rituximab were 13%, 9%, 9%, and 3%, respectively.[14]
Regarding infusion site reaction, though adalimumab showed the highest risk (RR [95% CI]: 6.15 [0.61–65.52]), it is not statistically significant when compared to control. Hence etanercept which is statistically significant when compared to control has the highest risk to develop infusion site reaction (RR [95% CI]: 5.33 [3.55–8.00]). Statistically significant heterogeneity found only in adalimumab and certolizumab. A meta-analysis done by Alonso-Ruiz et al. showed adalimumab had a risk to develop infusion site reaction which was 1.7 (1.0–3.0) and showed statistically significant heterogeneity.[7] Highest risk to develop infusion site reactions was found with etanercept 5.1 (2.9–8.8) which was similar to our finding.[7]
Regarding malignancy, no significant overall difference between the experimental and control groups in the development of malignancy, with a pooled RR (95% CI) of 1.30 (0.82–2.06). There was no statistically significant heterogeneity among the drugs (Q = 28.65; P ≤ 0.0001, I2: 5.77) and not within the groups given each specific drug. A meta-analysis done by Alonso-Ruiz et al. showed similar result of no significant overall difference between experimental and control group, with a pooled RR (95% CI) of 1.5 (0.8–3.0) and no statistically significant heterogeneity.[7] Risk to malignancy was found highest in infliximab group (RR [95% CI]: 2.33 [0.68–8.04]), but it showed no significant difference to their control group similar to meta-analysis done by Alonso-Ruiz et al.[7] In this study, only adalimumab showed a significant difference to their control counterparts, this finding is contrary to Alonso-Ruiz et al.[7] Study done by Codreanu and Damjanov showed treatment with TNF inhibitors may increase the risk of skin cancer in patients with RA which was revealed from long-term safety data were obtained from the registries established in Europe, the USA, and Asia.[12]
RA is associated with reduced life expectancy. Whether the development of RA initiates this process of premature aging or is part of it is not clear. The excess mortality is apparent within the first few years of disease and increases with RA disease duration. Most of the excess deaths are attributable to infection, cardiovascular disease (in particular coronary heart disease), and respiratory disease.[15] Mortality results also showed similarity with meta-analysis of Alonso-Ruiz et al. that there was no significant difference found between the experimental and control groups in terms of mortality during the treatment with overall and individual drug.[7] There was no statistically significant heterogeneity among the drugs a nd not within the groups given each specific drug. A study done by Nakajima et al. showed that mortality in RA patients exposed to biologics did not exceed that in patients not exposed to biologics, but death from pulmonary manifestations was proportionally increased in RA patients exposed to biologics.[16]
This overview has some limitations. The studies included were randomized, controlled trials with strict inclusion and exclusion criteria, which may not represent the patient population in a clinical setting. The included reviews consist of randomized controlled trials that differed in patient population characteristics such as the duration of RA disease, prior failed therapy, concomitant MTX use, and trial duration. Furthermore, delayed and rare adverse effects would not be detected by these controlled trials. Long-term monitoring of patients and postmarketing surveillance may reveal a different picture, and pharmacists and other health-care professionals involved in the treatment of RA should remain aware and educated in this area.
CONCLUSION
We have concluded that a total number of AEs was found more with abatacept followed by adalimumab and rituximab. Withdrawal due to AE found more with anakinra. The risk to develop SAEs, infection and serious infection, was more with newer TNF-alpha inhibitors: golimumab and certolizumab. Infusion reaction develops more with rituximab. Etanercept showed the highest risk to develop infusion site reaction. Biological response modifiers showed no difference to their control counterparts in malignancy and mortality risk. Our meta-analysis helps to clarify some frequently encountered safety questions in the clinical care of RA patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tripathi KD. Essentials of Medical Pharmacology. 7th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2013. p. 210. [Google Scholar]
- 2.Scott DL. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther. 2012;91:30–43. doi: 10.1038/clpt.2011.278. [DOI] [PubMed] [Google Scholar]
- 3.Shiel WC. Rheumatoid Arthritis (RA) [Cited on 2016 May 31]. Available from: http://www.medicinenet.com/rheumatoid_arthritis/page7.htm .
- 4.Biologics for Rheumatoid Arthritis Treatment. [Cited on 2016 Jun 01]. Available from: http://www.webmd.com/rheumatoid.arthritis/guide/biologics .
- 5.Osiri M, Suarez-Almazor ME, Wells GA, Robinson V, Tugwell P. Number needed to treat (NNT): Implication in rheumatology clinical practice. Ann Rheum Dis. 2003;62:316–21. doi: 10.1136/ard.62.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danila MI, Curtis JR. CME Activity: Biologics and Newer Therapies for Rheumatoid Arthritis: A Primer for Primary Care Physicians. 2014. Jan 27, [Last accessed 25 May 2016]. Available from: http://www.opencme.org/course/biologic-andnewer-therapies-rheumatoid-arthritis-primer-primary-care-physicians .
- 7.Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calbozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis. BMC Muskuloskelet Disord. 2008;9 doi: 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubbert-Roth A. Assessing the safety of biologic agents in patients with rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl 5):V38–47. doi: 10.1093/rheumatology/kes114. [DOI] [PubMed] [Google Scholar]
- 9.Tvete IF, Natvig B, Gåsemyr J, Meland N, Røine M, Klemp M, et al. Comparing effects of biologic agents in treating patients with rheumatoid arthritis: A multiple treatment comparison regression analysis. PLoS One. 2015;10:e0137258. doi: 10.1371/journal.pone.0137258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopal S, Berwaerts J, Nuamah I, Akhras K, Coppola D, Daly E, et al. Number needed to treat and number needed to harm with paliperidone palmitate relative to long-acting haloperidol, bromperidol, and fluphenazine decanoate for treatment of patients with schizophrenia. Neuropsychiatr Dis Treat. 2011;7:93–101. doi: 10.2147/NDT.S17177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: A cochrane overview. CMAJ. 2009;181:787–96. doi: 10.1503/cmaj.091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Codreanu C, Damjanov N. Safety of biologics in rheumatoid arthritis: Data from randomized controlled trials and registries. Biologics. 2015;9:1–6. doi: 10.2147/BTT.S68949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keyser FD. Choice of biologic therapy for patients with rheumatoid arthritis: The infection perspective. Curr Rheumatol Rev. 2011;7:77–87. doi: 10.2174/157339711794474620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok CC. Rituximab for the treatment of rheumatoid arthritis: An update. Drug Des Devel Ther. 2013;8:87–100. doi: 10.2147/DDDT.S41645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:871–83. doi: 10.1016/j.berh.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima A, Saito K, Kojima T, Amano K, Yoshio T, Fukuda W, et al. No increased mortality in patients with rheumatoid arthritis treated with biologics: Results from the biologics register of six rheumatology institutes in Japan. Mod Rheumatol. 2013;23:945–52. doi: 10.1007/s10165-012-0773-z. [DOI] [PubMed] [Google Scholar]


