Abstract
The animus toward placebos arises from the notion that the symptoms they alleviate must belong mostly to psychosomatic illnesses. But lately the mindset that underlies placebo disparagement has been fading. The author talks with experts about placebo research, including implications for provider–patient encounters.
Placebos. Compared to real drugs, they’re orphans, or at best very distant relatives who show up too often. The fact that the dummy pill’s outsized effects occur in good people, even in good scientists, has been viewed with discomfort and even embarrassment. Back in 1955, Henry K. Beecher, MD, listed the placebo’s common purposes in the Journal of the American Medical Association and included among them: “as a psychological instrument in the therapy of certain ailments arising out of mental illness, as a resource of the harassed doctor in dealing with the neurotic patient.”1 Two years ago, Professor Ted Kaptchuk, DOM, head of the Program in Placebo Studies at Beth Israel Deaconess Medical Center and Harvard Medical School—widely considered a “guru” of placebo research—made this comment about placebo effects in a New England Journal of Medicine article: “Placebo effects are often considered unworthy and illegitimate. They are thought to be unscientific and caused by bias and prejudice.” 2 In a recent interview with P&T, Dr. Kaptchuk went a step further: “Medicine has at best ignored the placebo effect, but at its worst it has been undervalued, marginalized, and often demonized.”

Ted Kaptchuk, DOM
The animus toward placebos arises from the notion that the symptoms they alleviate must belong to psychosomatic illnesses, ones believed to be actually just in your head, not truly, objectively real, and surely not justifiable—quite like bias and predjudice, those banes of rationality that spurred the progenitors of modern science to discriminate between what is objective and what is subjective.
But lately the mindset that underlies placebo disparagement has been fading. A shift in perspective evidenced by a change in terminology has slipped in quietly over a few decades—from psychosomatic to mind–body. An etymologist would find their roots to mean the same, but the distinctly pejorative scent of the first is absent from the second.
The Lowly Placebo’s Ascendance
What is dignifying the placebo and raising it above the status of a mere pickpocket that lifts imagined symptoms from neurotic individuals? What is making it a subject of in-depth study rather than an element that needs to be controlled for and subtracted from clinical trial results to identify the absolute effect of “real” drugs? Technological advances, especially brain imaging and advanced biochemical analyses, have empowered study of the specific substances and the locations of their transformations that occur during cognitive activity. Many psychic phenomena previously denigrated as out-of-bounds and subjective have been nudged into territories now accessible to sophisticated measurement and quantification. “By demonstrating a neurobiological substrate, neuroimaging has served to legitimize the placebo effect,” Dr. Kaptchuk stated.
Awareness in the medical community of the nuances of placebo research has rarely kept pace with the more dramatic findings, however. For example, few medical professionals know that some analgesic drugs work only in the presence of a placebo stimulus. If these pharmaceuticals are administered invisibly, with no medical professional telling patients that they are receiving a painkiller, nothing happens. But if patients are told that they are being given an analgesic, the degree of pain relief is far stronger than if they instead get a dummy pill. What has been demonstrated emphatically is that both drugs and cognitive messages activate specific neuronal pathways and biochemical processes.
Few also are aware that when given to patients who are told explicitly that the substance they are receiving is inert and of no intrinsic medical value, placebos can be effective anyway. Also, recent research shows that subliminal messages flashed before the eyes of subjects for intervals far shorter than register consciously can be used to condition (as with Pavlov’s dog) both positive and negative associations. Among the conditioning factors proven to be consequential around painful stimuli: the warmth and competence of the provider.
Key Placebo Studies Started With Pain
In a 1978 landmark study of post-dental extraction analgesia, neuroscientist Jon Levine showed that the release of endogenous opioids (endorphins) by placebos can be blocked by the opioid antagonist naloxone, confirming the biochemical context of placebo events.3 While advances since have been incremental and cumulative, the designation of the period from 1990 to 1999 as “The Decade of the Brain” by presidential proclamation (from George H. W. Bush) was a watershed leading to rapid progress. Among many studies, a few demarcate some main features. Research published in 1995 by Fabrizio Benedetti, MD, Professor of Physiology and Neuroscience at the University of Turin Medical School, in collaboration with his Turin colleagues Martina Amanzio and Giuliano Maggi, uncovered unexpected nuances in placebo responses. Remarkably, their research showed that a placebo model encompassing only two distinct neural mechanisms for alleviation of pain—one from the top down via expectation pathways, and another from the bottom up with a specific pharmocodynamic component—was not just simplistic but incomplete.4
The Turin researchers’ classic clinical trial involving post-operative pain demonstrated that with respect to pain relief, the cholecystokinin antagonist proglumide was better than placebo, and placebo was better than no treatment. The standard interpretation would have been, they point out, that proglumide is an effective painkiller that acts on pain pathways and that placebo works by inducing the expectation of analgesia. But a third arm of the trial showed otherwise. An infusion of proglumide that subjects were not aware of was completely ineffective, implying the unanticipated conclusion that proglumide acts not on pain pathways but on expectation pathways, enhancing placebo effects. It alleviates pain only in the presence of a placebo procedure. 4 So rather than being a painkiller, proglumide acts on placebo-activated opioid mechanisms. Cholecystokinin plays a part in complex environmental–social stimuli and interactions between safety cues and the endogenous opioid systems, underscoring the involvement of cholesyctokinin-opioid systems in cognitive processes.5 Typically, then, any analgesic treatment consists of both a specific pharmacodynamic component and a placebo component induced by the psychosocial context of the delivery of the treatment.
Dr. Benedetti’s further research papers published in 2001 and 2003 explored hidden injections of analgesics in healthy volunteers and in patients in clinical settings. The latter comprised pain and anxiety studies of postoperative patients and patients receiving treatment for Parkinson’s disease.6 Postoperative patients were told that they might be receiving a painkiller or nothing via an infusion machine, but that they would not know if or when treatment was being administered. Parkinsonian patients treated via monthly subthalamic stimulus had the intensity varied. Healthy volunteers were told they were being given an active drug (beta blocker or muscarinic antagonist) or nothing.6
Half of the 42 post-thoracotomy patients were given open infusions of morphine, with a medical professional telling them that they were receiving a potent painkiller. The other 21 were connected to a pre-programmed infusion machine that invisibly delivered the same dose. Also, stoppages of morphine were conducted in both open and hidden manners. Another 30 thoracotomized patients who had high levels of anxiety were randomized to open or hidden diazepam. Among the postoperative patients, pain decreases were significantly greater (P = 0.001) in those told openly they were receiving pain medication. With stoppage of morphine, pain intensity did not differ between the two groups at the time of the interruption, but afterward the pain increase was larger when the interruption had occurred openly (P = 0.001). More patients in the open group requested additional analgesia (P = 0.019).6
The pattern was similar with assessments of anxiety and diazepam via the State-Trait Anxiety Inventory (STAI)7 among postoperative patients who had above-normal scores, except that covertly administered diazepam was totally ineffective (P = 0.005).6 In Parkinson’s disease, open interruptions of subthalamic stimulation induced significantly larger reductions in movement velocity at 30 minutes than hidden ones (P = 0.009). Also, when stimulation was openly increased from 40% of optimal to optimal, efficacy greater than that for an identical hidden increase was significantly improved (P = 0.03). Healthy volunteers given propranolol openly had greater reductions in heart rate than those given propranolol covertly after 15 minutes (P = 0.05). Conversely, open injections of atropine induced higher heart rates than covert ones (P = 0.036).6
Dr. Benedetti pointed out that open interruptions of morphine, diazepam, and subthalamic stimulation produced greater worsening of symptoms compared to hidden interruptions. “Therefore, if the patient is told that a treatment is going to be stopped, a sort of nocebo phenomenon may occur.” Dr. Benedetti concluded that the reduced therapeutic effect after a hidden therapy shows that the patient’s knowledge about the treatment and/or the doctor–patient relationship are of crucial importance. He also noted that the research was not able to identify which of three factors predominated: awareness of the treatment, the presence of the therapist, or the patient’s expectation of the outcome.6
Is It Really a Placebo?
Dr. Benedetti emphasized that no actual placebos were given in his research. “It is probably wrong to call placebo effect the difference between open and hidden treatments, since no placebos are given. Meaning response is perhaps more appropriate, in order to make it clear that the crucial factor is not so much the inert treatment per se but rather the meaning around the medical treatment.” 6
With his Turin colleague Luana Colloca, MD, Dr. Benedetti pointed out in a 2005 review article on placebos that there is more than one type of placebo effect, with different mechanisms behind each of them.8 Placebo power has been shown to extend to immunosuppression, and that immunosuppression can be conditioned. Behaviorally conditioned immuno suppression, first described in rodents, was demonstrated in healthy humans in a randomized, double-blind, placebo-controlled study published in 2002 by Goebel et al. at the University of Essen in Germany. In four sessions over three days, subjects received cyclosporin A paired separately with a distinctly flavored drink. The following week, the drink plus placebo capsules induced immune-function suppression assessed through interleukin (IL)-2 and interferon-gamma (IFN-γ) mRNA expression and intracellular production and in vitro release of IL-2 and IFN-γ, as well as lymphocyte proliferation.9
“The mental events induced by placebo administration can activate mechanisms that are similar to those activated by drugs,” Drs. Colloca and Benedetti wrote, “which indicates a similarity between psychosocial and pharmacodynamic effects …” 8 In a further piece (“Placebo-induced improvements: how therapeutic rituals affect the patient’s brain”), Dr. Benedetti states, “The placebo effect has evolved from being thought of as a nuisance in clinical research to a biological phenomenon worthy of scientific investigation.” A further shift has occurred, though, as the focus becomes less on the sugar or starch pill and more on a broader placebo conception. Dr. Benedetti added, “The study of the placebo effect and of its evil twin, the nocebo effect, is basically the study of the therapeutic ritual around the patient, and it plays a crucial role in the therapeutic outcome.” 10
Do We Need to Reform Informed Consent?
The association between informed consent and nocebo effects is particularly problematic. Dr. Kaptchuk, in “Placebo effects in medicine,” observed that among benign prostatic hypertrophy patients treated with finasteride, those informed of potential sexual side effects reported them at rates triple those found in patients not informed. “Finding a way to balance the need for full disclosure of potential adverse effects of drugs with the desire to avoid inducing nocebo effects is a pressing issue in health care,” he wrote. He cited a range in studies for discontinuation rates attributed to adverse side effects among patients receiving placebos from 4% to 26%. Many purported side effects of drugs that physicians treat, Dr. Kaptchuk said, are anticipatory nocebo effects.2
The nature of the therapeutic encounter gets a further stretching with Dr. Kaptchuk’s surprising study in 2010 in irritable bowel syndrome (IBS). In his study introduction, he observed that while it is generally held that placebo responses require concealment or deception, this understanding presents an ethical conundrum around principles of patient autonomy and informed consent.11 Few among 679 U.S. internists and rheumatologists polled reported giving inert placebos or injections, according to a 2008 national survey. About half, however, were often prescribing other substances they knew to be irrelevant to patients’ complaints, including over-the-counter analgesics (41%), vitamins (38%), antibiotics (13%), and sedatives (13%).12 Dr. Kaptchuk’s discomfort here is that giving a placebo surreptitiously can ultimately undermine the trust that underlies the therapeutic patient–physician relationship, and potentially can lead to medical harm. “Finding effective means of harnessing placebo responses in clinical practice without deception is a high priority,” he wrote.11
Dr. Kaptchuk and colleagues chose IBS as a subject of study because it is a top-10 reason for patients to access primary care and a condition with strong impacts on quality of life, work productivity, and consumption of health-related resources. In addition, significant placebo responses have been reported in IBS. Investigators tested the common belief that awareness that a placebo treatment is, indeed, a placebo treatment would undermine its efficacy.11
They launched a three-week, single-center, randomized controlled trial of open-label placebo versus no treatment among 80 patients meeting Rome III criteria for IBS (scores of at least 150 on the IBS Symptom Severity Scale [IBS-SSS]). Patients were allowed to continue IBS medications as long as they had been on stable doses for at least 30 days prior to entering the study. The provider clearly explained that those randomly selected to the placebo group would receive pills containing no medication, but that placebo effects have been shown to be powerful in many cases. All scheduled physician visits were in the context of a warm, supportive patient–practitioner relationship. Patients in both treatment arms experienced the same frequency and duration of contact time, and the content of the interactions was very similar. Outcome measures included the IBS Global Improvement Scale, the IBS-SSS, and the IBS–Adequate Relief, assessing symptom relief and quality of life.11
Patients receiving placebo pills openly versus those receiving no treatment had significantly higher scores in the primary outcome of global improvement at both the 11-day midpoint (P < 0.001) and the 21-day endpoint (P = 0.002). Symptom severity change (P = 0.03) and percent with adequate response (P = 0.03) were also significantly in favor of the open placebo at three weeks. A strong trend in favor of the placebo group was also reported in quality of life change (P = 0.08). Dr. Kaptchuk commented that the magnitude of improvement reported in the open-label placebo group was not only statistically significant but also clinically meaningful. He added that the percentage of patients reporting adequate relief during the seven preceding days at the study end (59%) was comparable to responder rates in recent trials of commonly used IBS drugs. But Dr. Kaptchuk underscored, as well, that the placebo response rate was higher than is commonly reported in double-blind pharmaceutical studies (30% to 40%). What might explain so counterintuitive a finding? “Patients in our study accepted that they were receiving an active treatment, albeit not a pharmacological one, whereas patients in double-blind trials understand that they have only a 50% chance of receiving active treatment,” he speculated.11
Dr. Kaptchuk concluded: “Our data suggest that harnessing placebo effects without deception is possible in the context of 1) an accurate description of what is known about placebo effects, 2) encouragement to suspend disbelief, 3) instructions that foster a positive but realistic expectancy, and 4) directions to adhere to the medical ritual of pill taking.” 11

Karin Jensen, PhD
The Role of Nonconscious Cues
Karin Jensen, PhD, a researcher at Dr. Kaptchuk’s Harvard Program in Placebo Studies and the Therapeutic Encounter, later moved to the Karolinska Institute in Stockholm, Sweden, where she heads a laboratory. Dr. Jensen tested whether conditioned placebo and nocebo responses could be activated by conscious or nonconscious cues. The essence of her study was that in a computer conditioning sequence, clearly visible images of two different male faces were flashed on a computer screen, with each face cue consistently paired with a rapid high-heat or low-heat pain stimulus on the subject’s arm. In experiment 1, the face cues were exposed for 100 ms, long enough for all subjects to recognize them clearly. In the test phase, subjects rated a moderate pain higher when the stimulus was accompanied by the image of the face conditioned with the high-pain stimulus and lower with the low-pain face.13
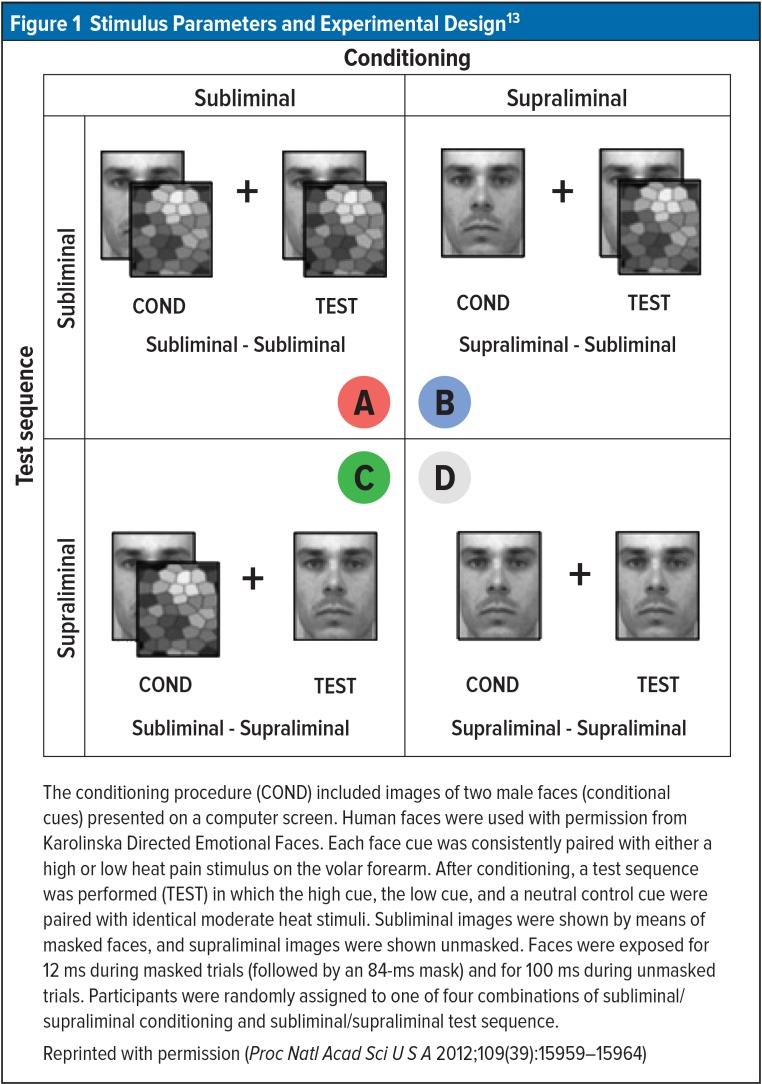
In experiment 2, the conditioning sequence was identical, with the same face cues paired with high- or low-pain stimulus exposed for 100 ms. For a test sequence using a moderate heat-pain stimulus, faces were flashed for only 12 ms (Figure 1). The face image in the figure represented the high cue for half the subjects and the low cue to the other half to prevent the chance that a certain face would have some inherent connotation that would affect the pain ratings. The image was flashed for too short a time for conscious recognition. Nevertheless, in the presence of the conditioned high-pain face, pain ratings were significantly higher than in the presence of the low-pain face, despite identical moderate temperature stimuli.13
Figure 1.
Stimulus Parameters and Experimental Design13
The conditioning procedure (COND) included images of two male faces (conditional cues) presented on a computer screen. Human faces were used with permission from Karolinska Directed Emotional Faces. Each face cue was consistently paired with either a high or low heat pain stimulus on the volar forearm. After conditioning, a test sequence was performed (TEST) in which the high cue, the low cue, and a neutral control cue were paired with identical moderate heat stimuli. Subliminal images were shown by means of masked faces, and supraliminal images were shown unmasked. Faces were exposed for 12 ms during masked trials (followed by an 84-ms mask) and for 100 ms during unmasked trials. Participants were randomly assigned to one of four combinations of subliminal/supraliminal conditioning and subliminal/supraliminal test sequence.
Reprinted with permission (Proc Natl Acad Sci U S A 2012;109(39):15959–15964)
“Results from the present study demonstrate that placebo and nocebo mechanisms can be triggered by nonconscious cues, operating outside of conscious awareness,” Dr. Jensen concluded. She added that neuroimaging studies suggest that certain structures in the brain, such as the striatum and the amygdala, can process incoming stimuli before they reach conscious awareness, and thus they may mediate nonconscious effects on human cognition and behavior. Other studies that Dr. Jensen cited support such influence, with one even suggesting that the physician’s knowledge of likely active treatment influences placebo response in the patients. Another showed that in treatments with morphine or placebo by a human or machine that were both blinded and hidden, placebo responses were weaker in response to the machine. Nonconscious cues embedded in the patient–clinician interaction, she stated, may be inducing such effects.13
The influence of nonconscious cues was demonstrated in Dr. Jensen’s follow-up study in 2015. In the 2012 study, the face image had been flashed on the screen for 100 ms during the conditioning phase of experiment 2, with the result that a subliminal 12 ms exposure to the conditioned image influenced heat-pain perception significantly in the test phase. In the later study, exposure to the image of the face in the conditioning phase was limited to only 12 ms. Still, test phase findings showed that conditioning was achieved equally regardless of whether the conditioning image was subliminal or supraliminal (12 ms or 100 ms) for both analgesic and hyperalgesic pain responses. The authors’ conclusion: “We demonstrate that nonconscious associative learning can produce conditioned analgesic and hyperalgesic pain responses.”14
Implications for the Therapeutic Encounter
While the preceding merely scratches the surface of the trove of placebo research literature, it does sketch a progression that has led away from the discomfort around placebo effects and the sense that the main scientific task was to find ways to subtract them from drug studies in order to assess “real” drug results. The intensive 25-plus years of confirmatory laboratory and clinical findings are moving the inquiry beyond accepting the placebo’s power to its broader significance. Once the medical community recognizes the dummy pill as a stand-in for the vast web of relations woven into the therapeutic encounter, its goal then becomes to identify key factors and to favorably harness and maximize them for patients’ health and for individual and system-wide costs.
This year, Lauren C. Howe, PhD, and colleagues from Stanford University’s Mind & Body Lab took a direct look at how the elements of physician competency and warmth influence physiological outcomes. They recruited 164 healthy adults and told them that the study, about novel food preferences, required them to undergo initial health screening with a skin-prick test to assess allergic reactions. They each received a histamine stimulus and a cream with no active ingredients. They were then told either that the cream would reduce their reaction or increase it, and the skin reactions (wheal and flare) were measured.15
Investigators had trained a female health care provider to behave in one of four ways during the procedure: 1) high warmth/high competence, 2) high warmth/low competence, 3) low warmth/high competence, and 4) low warmth/low competence. The provider followed a detailed script to embody each condition. Warmth items pertained, for example, to eye contact, smiling, physical distance, name introductions, etc., and competence indicators pertained to the practitioner’s title, pressure-cuff skill, room appearance, etc.15
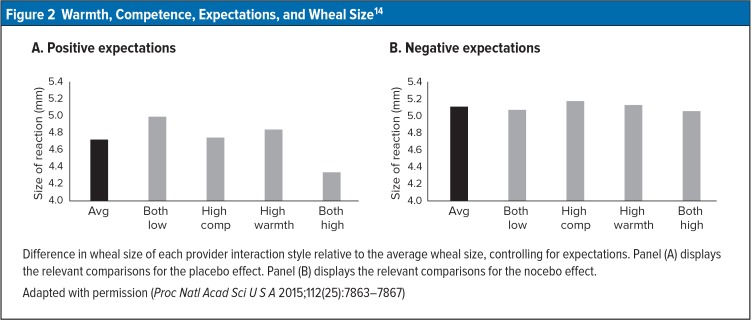
After nine minutes, the expectations as to the cream’s effects were reflected significantly in wheal/flare size. Furthermore, when the practitioner was neither warm nor competent, expectations did not influence wheal/flare size. When the provider was both warm and competent, on the other hand, wheal/flare size was smaller with positive expectations than it was with negative expectations. Intermediate effects were noted with hybrid conditions (high warmth/low competence; low warmth/high competence). However, when providers were both warm and competent, negative expectations did not increase wheal/flare size (Figure 2). Howe et al. concluded, “This study suggests that the placebo effect can be boosted or diminished by the social context, in this case marked by the warmth and competence of the health care provider.”15
Figure 2.
Warmth, Competence, Expectations, and Wheal Size14
Difference in wheal size of each provider interaction style relative to the average wheal size, controlling for expectations. Panel (A) displays the relevant comparisons for the placebo effect. Panel (B) displays the relevant comparisons for the nocebo effect.
Adapted with permission (Proc Natl Acad Sci U S A 2015;112(25):7863–7867)

Alia Crum, PhD
The investigators also noted an important task for future research: to explore the most effective ways that physicians may discuss negative expectations (e.g., side effects) with their patients while avoiding adverse consequences of those very discussions. Study coauthor Alia Crum, PhD, Assistant Professor of Psychology at Stanford and Director of the Stanford Mind & Body Lab, commented in an interview, “If you reinforce the efficacy of the drug by saying this drug may cause x, y, and z side effects because it works well and is so strong, it might lead to a different mindset than saying you may have to endure these problematic side effects of this drug.”
Still at Proof-of-Concept Stage?
Are the findings of placebo studies making waves in the health care universe? “We are still in the proof-of-concept phase,” Dr. Kaptchuk suggested, adding that the major impact of the findings to date has been inclusion of placebo courses in the curricula of many medical schools. “It’s a big shift to recognize that what goes on in the room between the patient and the physician or any allied health care provider is an important determinant of the outcome.”
Dr. Kaptchuk also observed that many drugs on the market are only marginally better than placebos. While placebos won’t cure cancer or replace surgery, they have a clear place in cancer-related fatigue, pain, and nausea. Depression symptoms and in some cases angina pain may be subject to huge placebo effects as well.
“We already have a lot more knowledge than is being taken into account,” Dr. Crum observed. In “Making mindset matter,” published this year in BMJ, Dr. Crum argued that the time to act on the accumulating evidence that patient mindset affects outcomes is already here. She pointed out that medical diagnoses and treatments “are never isolated from patient mindsets and social context,” and those mindsets and contexts have widespread physiological consequences. Dr. Crum cited evidence that an individual’s beliefs affect nutrients’ physiological effects, affect individual’s benefits from exercise, influence whether stress is strengthening or debilitating—even to the extent of increasing likelihood of premature death.16 “We have been limited in our thinking about placebo effects because we view them as some mysterious response to an inert substance, but the effects are neither inert nor mysterious,” she said in an interview. The body’s natural ability to heal itself can be activated more or less by expectations and hopes and a myriad of other factors. The physician’s behaviors and appearance (white coats, race, gender), drugs (branding, advertising, pricing), even the hospital name can consequentially shape mindsets. “All of these are complicated, but that doesn’t mean we can’t systematically vary them and figure them out,” she added. She mentioned courses in planning stages at Stanford Medical School encompassing communication skills, empathy, and social contexts, but noted that the significance of these factors warrants a far greater place in physician training than they now generally receive. “That needs to be balanced out,” she said.
Dr. Kaptchuk, in “Placebo effects in medicine,” asked for continuing research to define, more minutely, “What are the relationships among attention, gaze, touch, trust, openness, confidence, thoughtful words, and manner of speaking that can together reduce perceived discomfort, disability, and disfigurement?” 2 Ever-deepening scientific study of these aspects of health professional–patient relationships often thought of as “soft” and subjective will likely continue to move the divide between objective and subjective in very substantial ways—such that the former grows larger at the expense of the latter. Dr. Kaptchuk’s take: “The whole idea of a placebo effect—the effect of something that has no effect—is crazy. We’re talking about the ocean that all of medicine swims in. My work is trying to change the art of medicine into the science of care.”

John Kugler, MD
Can the System Adapt Proportionally?
Is the momentum of ever-more technologically focused health care systems at odds with what placebo studies are revealing? Whether or not, for example, the electronic consolidation of vital patient information and treatment details will free up providers to better attend to Dr. Kaptchuk’s “ocean” factors of care is not certain. Thought leaders have long been raising concerns with regard to the widespread use of electronic health records (EHRs), sometimes called electronic medical records (EMRs). EHR use was given strong support by the 2009 Health Information Technology for Economic and Clinical Health Act’s $30 billion in funding. By 2011, medication errors were being reduced and guidelines-based treatment was increasing, but without evidence of better outcomes and greater efficiency.17 In 2010, internal medicine practitioners John Kugler, MD, and Abraham Verghese, MD, of the Stanford School of Medicine stated in an editorial (“The physical exam and other forms of fiction”) that the three-dimensional patient is being shrunken into a two-dimensional EMR caricature—the iPatient. “The fact that there is a drop-down box on the EMR that allows one to click to say that reflexes were normal or the cranial nerves are intact is no guarantee of the truth of these observations; indeed the ‘physical exam’ section of the EMR reads at times like a form of fiction,” they wrote.18
Why fiction? Because of what are called “note templates,” Dr. Kugler clarified in an interview. Report blanks are autofilled with data from the patient’s chart and then that information is carried forward. “We call it ‘the copy-and-paste problem.’ It’s supposed to be a time saver, but it shows details suggesting exams that really have not taken place. Because you can’t leave entries blank, you insert ‘normal’ and end up with a really long note,” Dr. Kugler said. “But you have actually typed very little of it. Billing and reimbursement are driving most of it.”
The EMR correlates poorly with what actually happened at the beside, Dr. Verghese said, elaborating on these concerns in a 2011 article in Annals of Internal Medicine. “Physicians often bypass the bedside evaluation for immediate testing and therefore encounter an image of the patient before seeing the patient in the flesh. In addition to risking delayed or missed diagnosis of readily recognizable disease, physicians who forgo or circumvent the bedside evaluation risk the loss of an important ritual that can enhance the physician–patient relationship.” He goes on to describe the bedside exam as part of a “rite of passage,” with the passage being the first step in the transition from sickness to health, and the rite being “the skilled examination of the body.” Essential to this ritual/process is “hands-on” expertise learned through observing teachers who have mastered both it and the art of keeping “the actual patient, as opposed to the iPatient, at the center of attention.” The patient’s permission to be examined “affirms the physician’s connection with and commitment to the patient.” When carried out poorly or perfunctorily the physical exam can be dehumanizing. When done well it allows the transfer of knowledge while preserving the patient’s identity and humanity. “In contrast, imaging and laboratory tests strip away external markers of personhood.”19
Dr. Kugler emphasized that the group focus on promoting bedside exam skills at Stanford Medical School is not just a longing look at the past of medicine through rose-colored glasses. He himself employs a portable point-of-care ultrasound unit in his bedside exams that enables ruling out fluid overload in the lungs or chest. Beyond reducing further unnecessary testing, Dr. Kugler said, “there is value in the way it creates a relationship with my patient. We look at the ultrasound images together, and my pointing out and discussing where a problem lies with the patient builds a trust that a generated report does not.”
When asked in 2017 if progress since his 2010 article would cause him to modify any of his earlier statements, Dr. Kugler replied, “It is as big a problem today as it was then.” Since then, however, increased curricular time at Stanford Medical School is devoted to teaching medical students how to interact honestly with the EMR (which he called “a powerful tool” that is here to stay) and “then get back to the patient’s bedside.” Dr. Kugler’s 2014 research into medical student clerkship EMR interactions showed high usage but no correlation between computer time and outcomes. The authors inferred that “excess [EMR] use comes at the expense of direct patient care.” 20 Who today has not heard complaints to this effect?
Implications for Care in the Long Term
The “hands-on” encounter with the patient for diagnosis and care may be especially central to internal medicine and primary care, where patient–provider relationships may be cultivated over the longer term. “We devote vast resources to intensive, one-off procedures, while starving the kind of steady, intimate care that often helps people more,” surgeon Atal Gawande wrote in a recent New Yorker piece.21 Research into Medicare spending has shown regions with higher concentrations of primary care practitioners to be associated with lower costs and better outcomes, compared with higher costs and worse outcomes in regions where specialist densities are higher.22 Dr. Gawande, both a dispassionate witness to the realities of medical practice and a passionate advocate for optimizing it, believes that “a battle for the soul of American medicine” is taking place. He observes that in the U.S., “the financial burden [of health care] has damaged the global competitiveness of American businesses and bankrupted millions of families, even those with insurance.” Dr. Gawande describes “enviably higher quality” care at lower prices in settings where collaborative physician teams focus on the totality of care, and the emergence of centers built “to treat patients the way subprime-mortgage lenders treated home buyers: as profit centers.” 23
In one of his New Yorker articles, Dr. Gawande relates his encounter with Harvard’s Asaf Bitton, MD, MPH, an internist and expert on the delivery of primary health care around the world. Dr. Bitton is a senior adviser at the Center for Medicare and Medicaid Innovation for Comprehensive Primary Care Plus, a multistate, multipayer effort matching payment reform to sustainable primary care transformation. His research has shown unequivocally that emphasizing and incentivizing primary care leads to reduced mortality and hospitalization rates. One proven factor is that those who have a doctor they see regularly are more likely to seek care for severe symptoms, which by itself contributes to lower death rates.21

Asaf Bitton, MD, MPH
Dr. Bitton’s Boston neighborhood clinic attracts 14,000 patient visits annually; it has three full-time and several part-time physicians, three physician assistants, three social workers, a nurse, a pharmacist, and a nutritionist. “It didn’t matter if patients had psoriasis or psychosis, the clinic had to have something useful to offer them,” Dr. Gawande observed after his visit. He marveled at how patients there whose main source of care is a primary care physician, virtually certain to have no knowledge advantage over specialists for any given condition, somehow manage to receive better health care—and he wondered what secret ingredient enables the success of such “medical general stores.” So Dr. Gawande asked staff, nurses, and doctors. They generally agreed: “It’s the relationship,” and even more completely, the relationship over time, the incrementally growing familiarity with the patient and the patient’s life in which health and illness are interwoven.21
Of course, this is beginning to sound a lot like Dr. Kaptchuk’s health care ocean, where drugs, devices, and procedures are the most highly visible but far from the only elements afloat. General principles behind solutions to our health care system’s large imbalances are known, Dr. Bitton said in an interview. But investing in incremental care means investing in benefits down the road, which rubs against the grain of quick-fix rescue mentality.
Dr. Gawande wrote in a hopeful vein that because “the patterns are becoming more susceptible to empiricism … The incrementalists are overtaking the rescuers.” But he concluded that unless the “antiquated priorities” of our age, one that has focused on heroic interventions and those who specialize in short-term, urgent repair, give way to strengthened valuation for incrementalists and strategies that pay off over time, millions will continue to “die from conditions that, increasingly, can be predicted and managed.” 21 To Dr. Bitton it means establishing teams of primary care providers who are not saddled with mountainous medical school debt (which pushes them toward specialty practices) and who have time to spend with patients “to figure out what the best treatments are according to their own individual life course, not according to studies conducted in overly controlled environments.” The outrageous differential in earnings between “rescuers” and “incrementalists” is an obstacle needing remedy, he said.
The expanded empiricism mentioned by Dr. Gawande may be an important factor that can shift the balance back toward the human aspects of care embodied in the placebo. These human aspects were evicted at the birth of the scientific method as a necessary sacrifice for the development of disciplined thinking and discovery. Francis Bacon (1561–1626), in his Novum Organum Scientiarum, urged a “dry light” for science as an antidote to human understanding’s vulnerability to error. Bacon prescribed a set of mental blinders like those that keep a horse from being distracted, to filter out unreliable input and let in reliable input garnered through observation, reason and “dry” intelligence. In medicine that meant reducing the field of study to that which can be measured and counted.
John Locke (1632–1704) further defined the discipline of scientific method by saying that the properties of objects reliable for study are those considered independent of the observer. In this category he placed measurable aspects such as solidity, extension, motion, number, and shape, which he called “primary qualities.” Those that produce sensations in the observer such as color, taste, temperature, smell, and sound he called “secondary qualities,” and he designated knowledge derived from them as subjective as opposed to the objective knowledge gained from studying primary qualities. Medical science joined the exclusionary movement that raised the quantitative above the qualitative.
In our age of refined instrumentation, imaging, and statistical analysis, the distinction between what Locke called primary objective qualities and secondary subjective ones is dissolving. Today we can question whether elevating solidity over temperature or over sound is justifiable—now that all of them can be quantified through means not yet devised in Locke’s day. When you add to this understanding the many realms of animal and human behavior—even qualitative ones now subject to rigorous testing—Bacon’s “knowledge is power” formula begins to work in favor of rescuing the modest placebo, that stand-in for medicine’s nexus of subtle, complex, mind–body–environment interrelations. While we still need ever-more-refined understanding, experts are stating that the time to act more forcefully on what we already know has arrived.
REFERENCES
- 1.Beecher HK. The powerful placebo. JAMA. 1955;159(17):1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 2.Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med. 2015;373(1):8–9. doi: 10.1056/NEJMp1504023. [DOI] [PubMed] [Google Scholar]
- 3.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet. 1995;346(8984):1231. doi: 10.1016/s0140-6736(95)92938-x. [DOI] [PubMed] [Google Scholar]
- 5.Wiertelak EP, Maier SF, Watkins LR. Cholecystokinin anti-analgesia: safety cues abolish morphine analgesia. Science. 1992;256(5058):830–833. doi: 10.1126/science.1589765. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F, Maggi G, Lopiano L, et al. Open versus hidden medical treatments: the patient’s knowledge about a therapy affects the therapy outcome. Prevention & Treatment. 2003;6 Article 1. [Google Scholar]
- 7.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists Press; 1983. [Google Scholar]
- 8.Colloca L, Benedetti F. Placebos and painkillers: Is mind as real as matter? Nat Rev Neurosci. 2005;6(7):545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- 9.Goebel MU, Trebst AE, Steiner J, et al. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16(14):1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti F. Placebo-induced improvements: how therapeutic rituals affect the patient’s brain. J Acupunct Meridian Stud. 2012;5(3):97–103. doi: 10.1016/j.jams.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilburt JC, Emanuel EJ, Kaptchuk TJ, et al. Prescribing “placebo treatments”: results of national survey of U.S. internists and rheumatologists. BMJ. 2008;337:a1938. doi: 10.1136/bmj.a1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen KB, Kaptchuk TJ, Kirsch I, et al. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A. 2012;109(39):15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen K, Kirsch I, Odmalm S, et al. Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc Natl Acad Sci U S A. 2015;112(25):7863–7867. doi: 10.1073/pnas.1504567112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe LC, Goyer JP, Crum AJ. Harnessing the placebo effect: exploring the influence of physician characteristics on placebo response [published online March 9, 2017] Health Psychol. doi: 10.1037/hea0000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crum AJ, Leibowitz KA, Verghese A. Making mindset matter. BMJ. 2017 Feb 15;356:j674. doi: 10.1136/bmj.j674. [DOI] [PubMed] [Google Scholar]
- 17.Jha AK. The promise of electronic records: around the corner or down the road? JAMA. 2011;306(8):880–881. doi: 10.1001/jama.2011.1219. [DOI] [PubMed] [Google Scholar]
- 18.Kugler J, Verghese A. The physical exam and other forms of fiction. J Gen Intern Med. 2010;25(8):756–757. doi: 10.1007/s11606-010-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verghese A, Brady E, Kapur CC, Horwitz RI. The bedside evaluation: ritual and reason. Ann Intern Med. 2011;155(8):550–553. doi: 10.7326/0003-4819-155-8-201110180-00013. [DOI] [PubMed] [Google Scholar]
- 20.Chi J, Kugler J, Chu IM, et al. Medical students and the electronic health record: ‘an epic use of time’. Am J Med. 2014;127(9):891–895. doi: 10.1016/j.amjmed.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Gawande A. The heroism of incremental care. The New Yorker. 2017 Jan 23; [Google Scholar]
- 22.Baicker K, Chandra A. Medicare spending, the physician workforce, and beneficiaries’ quality of care. Health Aff (Millwood) 2004 Jan-Jun;(Suppl Web Exclusives):W4-184–197. doi: 10.1377/hlthaff.w4.184. [DOI] [PubMed] [Google Scholar]
- 23.Gawande A. The cost conundrum: What a Texas town can teach us about health care. The New Yorker. 2009 Jun 1; [Google Scholar]