Abstract
Introduction: In recent years, low-power lasers have been widely used in medicine. With the introduction of affordable light emitted diode (LED), clinical application of LED light has become more and more popular. However, some researchers believe that due to lack of coherence of the LED light, it can be different in terms of biological effects, in comparison with laser. In this study, the biological effects of low-level laser (LLL) to those of LED light are compared and discussed.
Methods: Human skin fibroblast cell line Hu02 was irradiated with LLL and LED light with a wavelength of 660 nm, power output of 35 mW and in continuous mode and the control group was not irradiated. The biological effects were compared through analysis of cell proliferation, production of reactive oxygen species (ROS) within the cell and rate of cell division.
Results: Our findings showed that production of ROS within the cell was linearly increased both in the LED and laser light irradiated cells. However, laser light is more incremental in comparison to LED light. The MTT results showed that laser light at low energy density (less than 5 J/cm2) increased the rate of cell proliferation after 24 hours. Although, the rate of cell division was increased in energy density of 1 J/cm2 compared to the control group, this increase was not statistically significant.
Discussion: The findings indicated that the coherence properties of laser light provided more energy for the cells, and in a constant energy density, laser light created more oxidative stresses in comparison with LED light.
Keywords: Low level laser, LED light, Reactive oxygen species, fibroblast cell
Introduction
In recent decades, low-level laser (LLL), from visible to near-infrared (NIR) wavelength range, has been widely used for promoting tissue repair in humans or animals.1 It is broadly applied to reduce inflammation,2 promote tissue regeneration,3 relieve pain4 enhance healing process of wound5,6 and increase cell proliferation.7,8
The molecular mechanisms of biological responses after LLL exposure have not been fully demonstrated. It is believed that physiological effects induced by LLL depend on the absorption of energy by some molecular photoacceptors or chromophores in the target cells. Karu in 1989 reported that the components of electron transport chain systems in mitochondria including cytochrome c oxidase molecule can be used as the primary light receptors for the absorption of red-NIR light spectrum.9-11 This absorbed energy is transformed into ATP that increases the activity of the mitochondrial proton pump, increasing oxygen consumption and enhancing synthesis of nicotinamide adenine dinucleotide dehydrogenase (NADH), RNA and protein.12-14 Finally, these changes will lead to increased expression of growth factors and cell proliferation.18,19 Furthermore, the most important intracellular organelle that produces reactive oxygen species (ROS) is the mitochondria. ROS is a natural by-product of electron transport chain activity. LLL was reported to enhance the level of intracellular ROS generation19-22 and it has shown that the production of ROS could activate NF-kB pathway.23 Activation of NF-kB effects expression of some genes participating in cell survival and proliferation.23-25
In recent years, besides LLL therapy, another affordable light source was developed named light emitting diode (LED).15 There are some differences between laser and LED including LED light is non-coherent (radiating in all directions) and divergent that wastes energy and may require special optics to focus the available energy into the desired areas. However, LLL and LED have similarities in physical parameters including wavelength, output power, power and energy density, as well as radiation levels, and there is evidence that they have similar healing effects on cells and tissues.22,23,26
The goal of this study was to compare the effects of LLL and LED on generation of ROS in cells and their differences in absorption of energy. Also the effects of laser and LED on cell proliferation were searched in human fibroblast cell line.
Materials and Methods
Cell Culture
Human skin fibroblast cell line Hu02 (cell No: IBRC C10309) was purchased from cell bank of Iranian Biological Research Center of Iran. Cells were cultured in CO2 incubator at 37°C. Cell culture medium was RPMI-1640 supplemented with fetal bovine serum (FBS; 10%) and penicillin/streptomycin solution (1%). Confluent cells were trypsinized using 0.25% trypsin-EDTA and seeded at density of 2 000 000 cells/25 cm2 cell culture treated flasks (Nunc, Denmark). All cell culture medium and reagents were from Gibco, Germany. Trypan blue exclusion method was used to determine viable cell yield and cell proliferation. For this purpose, after culture and trypsinization, cells were stained with trypan blue (Sigma, USA) dye (0.4% solution) and then viable cells were counted using a hemocytometer.
Laser and LED Bio-stimulation
In this study, the cells were cultured into 24 and 96 well plates and then irradiated. 3 experimental groups were studied including (1) control group with no irradiation, (2) laser irradiated group and (3) LED light irradiated group. We used a red Laser (Lasotronic/ 660 nm, 50 mW,) and LED light (660 nm, 35 mW) in continuous manner. The width of the laser beam was 1 cm2 and the fiber tip was located exactly 1 cm in front of the cell layer. The laser power of 35 mW was used in all experiments. Cells were irradiated as following: single dose from 8 seconds up to 80 seconds, power density of 35 mW/cm2 and energy density of 1, 5 and 10 J/cm2. Cells were then incubated for 24 hours prior to analyses.
Measurement of Cell Viability
Cell viability was determined using the colorimetric 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), according to the manufacturer’s instructions. This method is based on the ability of living cells in converting soluble tetrazolium salt to insoluble formazan. Briefly, 24 hours after treatment of the cells with laser light or LED, 20 μL of MTT reagent (10 mg/mL; Sigma, USA) was added to 96 cell culture well plates (20 000 cells/ cm2). The final volume was 200 μL. The incubation time was 3 hours at 37°C. Then 200 μL of dimethyl sulfoxide (DMSO) was added, as the solvent reagent. ELISA plate reader (BioTech Company, USA), at 570 nm wavelength was used to quantitate the production of formazan from MTT cleavage. Untreated cells were used as control. The cell viability was calculated according to the following formulae:
The percentage of cytotoxicity was calculated according to the following formulae:
% Cytotoxicity = [(1−mean absorbance of toxicant treated cells)/ mean absorbance of negative control]* 100
%Viability =100– % cytotoxicity
Measurement of the Activity Of Intracellular Reactive Oxygen Species
2, 7 Dichlorodi Hydrofluoresceindiacetate (DCFH-DA) fluorescent probe (sigma, USA) was used to measure the production of ROS within the cell. DCFH-DA is a fluorogenic dye that determines the activity of ROS molecules within the cell. After release into the cell, deacetylation of DCFDA by cellular esterases turns it to a non-fluorescent substance. Afterwards, it is oxidized by reactive oxygen molecules into 2’, 7’ –dichlorofluorescein (DCF). DCF is a highly fluorescent substance which can be monitored by fluorescence spectroscopy. To perform the test, the cells were passaged and were then transferred to a 96-well plate (20 000 cells/cm2). 48 hours later, the cells were irradiated with the laser and LED light. Thirty minutes after the irradiation, cells were treated with 20 μM DCFH-DA and incubated at 37°C for 45 minutes. Afterwards, cells were washed with PBS and read in 530 nm by plate reader device (BioTek H4).
Measurement of the Doubling Time
Hu02 cells were seeded at a density of 2×105 cell/cm2 in 24 well plates in triplicate. Trypan blue dye exclusion method was used for cell counting in seven consecutive days. For each experiment group, 3 wells were counted. The culture medium was renewed every 3 days. The doubling time (Td) of each group was determined by the Patterson formula as follow25:
Td = T×log2/log (N2/N1)
N1: cell number on the first day of the exponential phase
N2: cell number at time from N1 to N2 after culture (end of exponential phase) T (h): the time from N1 to N2
Statistical Analysis
Data analysis was done using the SPSS software, version 17. All data were showed as the means ± standard error of the mean (SEM). Analysis of variance (ANOVA), Bonferroni post hoc test and t test were used for comparison of the medium between groups. All experiments were done in triplicate. P < 0.05 was considered as significant.
Results
In this work, MTT test was used to assay the effect of LLL and LED light on cell proliferation and survival ability of the human skin fibroblast cell line. MTT indicates cell death at later stages of apoptosis in which metabolization of tetrazolium salts (MTT) is reduced.27-29 Figure 1 shows the results of MTT test in Hu02 cell line that irradiated with red laser light (660 nm) after 24 hours against the control group. The number of viable cells significantly (P < 0.05) increased (up to 50%) following exposure to doses of 1 and 5 joules energy densities per cm2 of LLL (Figure 1). Our data revealed that increasing LLL doses up to 10 J had no significant influence on the viability of the cells. In contrast to LLL, LED light had no significant impact on the proliferation of Hu02 cells (Figure 2).
Figure 1.
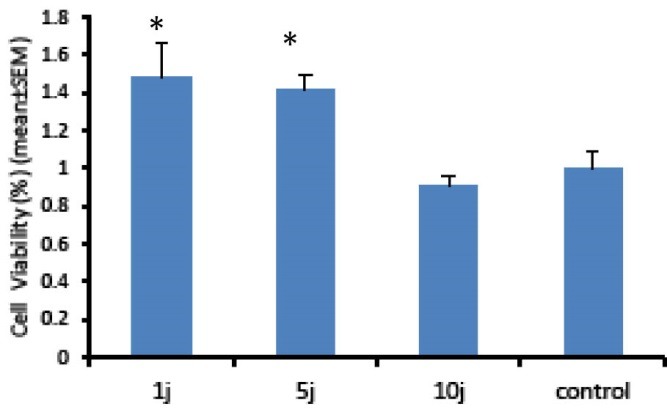
Evaluation of Low-Level Laser Light Effects With a Wavelength of 660 nm and Different Amounts of Energy Density on Cell Proliferation by the MTT Assay. As it is evident in the figure, low-energy laser radiation significantly increased the viability of Hu02 cells in doses of 1 and 5 J/cm2 (P < 0.05) and had no impact on viability of the cells in 10 J/cm2 doses. The histograms are the mean of 3 independent experiments; error bars represent SEM.
Figure 2.
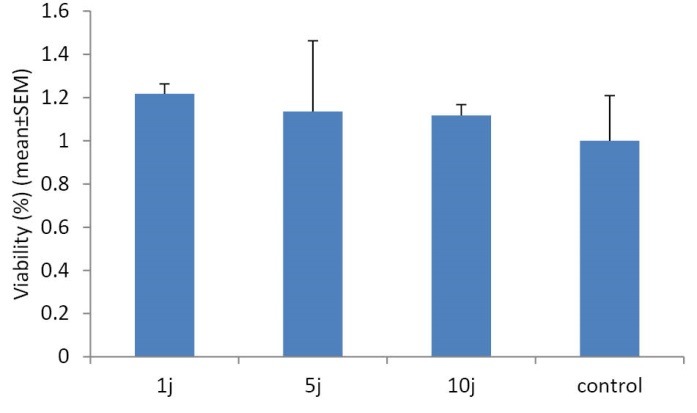
Evaluation of LED Light Effects With a Wavelength of 660 nm and Different Amounts of Energy Density on Cell Proliferation by the MTT Test. As it is evident in the figure, LED light had no impact on viability of the cells in comparison with the control group (P > 0.05). The values represent mean ± SEM and P < 0.05 considered as significant.
Measurement of Reactive Oxygen Species Activity
Here we hypothesized that LLL or LED light might change ROS levels in Hu02 cells. Therefore, the intracellular ROS level was analyzed by DCFH-DA probe. DCF analysis is a very sensitive, linear, and accurate test for tracing oxidative stress.30 Figures 3 and 4 show the levels of intracellular ROS, 30 minutes after the bio-stimulation of Hu02 cells with LLL and LED light, respectively. As it is evident in Figure 3, the level of ROS was significantly increased after the treatment of the cells with LLL light (P < 0.05) in all energy density of laser in a manner that is related to energy density (doses). In contrast to LLL, the levels of DCF fluorescence intensity were increased only by LED light with energy density of 10 J/cm2 in comparison with the non-irradiated group (Figure 4). These observations indicated that LLL and LED irradiation may induce the production of reactive oxygen molecules in a dose-dependent mode.
Figure 3.
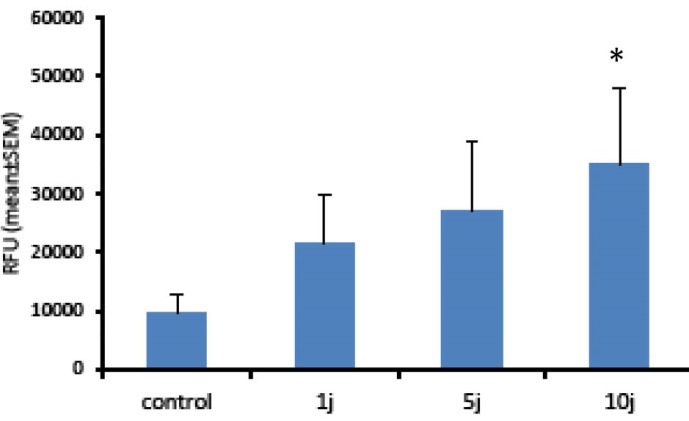
ROS Production Induced By Low Level Laser Light. Hu02 cells were stimulated with red light laser (660 nm) and the levels of intracellular ROS were measured using DCFH-DA after 30 minutes. All values are mean ± SEM; Differences were considered significant at P < 0.05.
Figure 4.
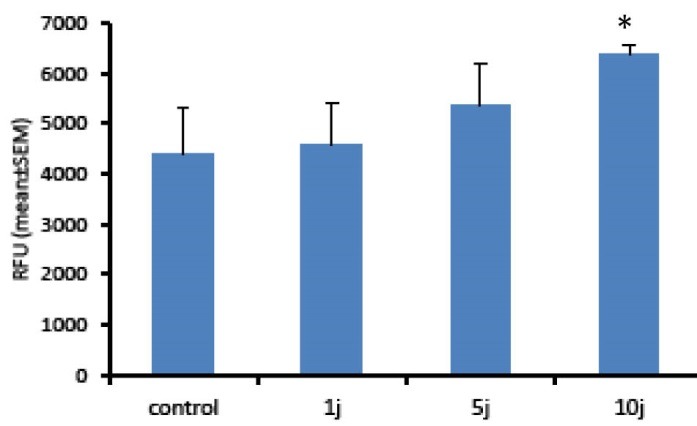
ROS Production Induced by LED Light. Hu02 cells were stimulated with LED light (660 nm) and the levels of intracellular ROS were measured using DCFH-DA after 30 minutes. All values are mean ± SEM; Differences were considered significant at P < 0.05.
Measurement of Cell Doubling Time
The measurement of cell doubling time is a good criterion for estimation of cell proliferation rate and formation of cell colony. It is the time that cells consumed in the exponential phase to double cell growth/count. Figure 5 shows the doubling time of Hu02 cells in control group, LLL and LED light irradiated groups. According to Peterson equation, doubling time was 31 hours in the control group. This time was 25 and 22 hours for LLL and LED light with 1 J/cm2 energy density, respectively. It means that LLL and LED light irradiation significantly increased the proliferation rate of Hu02 cells in comparison with the non-irradiated group (P < 0.05).
Figure 5.
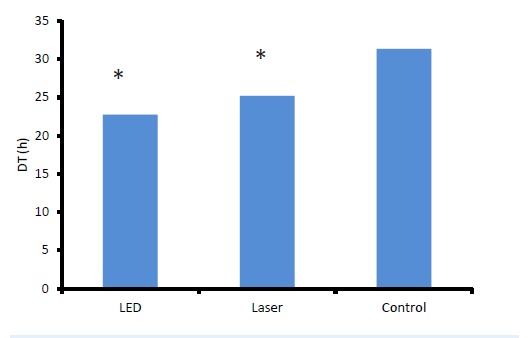
Doubling Time of Hu02 Cells in Control Group and LLL and LED Light Irradiated Groups. All values are mean ± SEM; Differences were considered significant at P < 0.05.
Discussion and Conclusion
Today, phototherapy with red or infrared (600–1000 nm) coherent light (laser) and non-coherent (LED) light have been introduced as a valuable tool in medicine and the positive effect of phototherapy on growth of different cells is well known.31,32 It was demonstrated that laser and LED light have similar improvement impact on cells and tissues. Studies have shown that the healing influences of photomedicine are due to transformation of absorbed energy into ATP.33,34
In this study, we compared and evaluated the biological effects of low-power laser light with LED light. The main important difference between low power lasers and LED light is the coherence parameter. Studies have demonstrated that the levels of ROS production by cells can be used as a marker of energy absorption by the intracellular receptors following phototherapy. The levels of reactive oxygen reagents in Hu02 cells were significantly enhanced after treatment with laser or LED light. Our data revealed that increasing energy density up to 10 J/cm2 at a wavelength of 660 nm is in accordance with an enhancement in generation of ROS.
ROS species are very small molecules that have dual nature. They may act as a beneficial signaling molecule at low concentrations and a harmful cytotoxic molecule at high concentrations. They may hurt key vital molecules including DNA, proteins and lipids. There is evidence for participation of ROS molecules in the cell pathways especially cell signaling. Therefore, homeostasis of these molecules in the cells and tissues may have a critical role in signaling pathways.35 Our data showed that LED light increased ROS levels very gently in comparison to laser light and in a linear manner. This may suggest that there would be more limited impact of LED light compared with laser light.
In conclusion, low-power laser light radiation increased the production of ROS in human fibroblast cell line in comparison to LED light and caused more oxidative stress. Also, laser light may be more effective in increasing the viability and proliferation of the cells.
Ethical Considerations
The present study was approved by ethical committee of institutional review board.
Conflict of Interests
The authors declare that there is no conflict of interest.
Please cite this article as follows: Naderi MS, Razzaghi M, Esmaeeli Djavid G, Hajebrahimi Z. A comparative study of 660 nm low-level laser and light emitted diode in proliferative effects of fibroblast cells. J Lasers Med Sci. 2017;8(Suppl 1):S46-S50. doi:10.15171/jlms.2017.s9.
References
- 1.Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy-an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lizarelli RFZ, Lamano-Carvalho TL, Brentegani LG. Histometrical evaluation of the healing of the dental alveolus in rats after irradiation with a low-powered GaAlAs laser. SPIE. 1999;3593:49–55. [Google Scholar]
- 3.Djavid GE, Erfani R, Amoohashemi N, Pazouki M, Aghaee S, Pazokitroudi H. Effect of low level He-Ne laser on acute mucosal ulceration induced by indomethacine in rat. Proc SPIE. 2002;4903:189–92. [Google Scholar]
- 4.Djavid GE, Mehrdad R, Ghasemi M, Hasan-Zadeh H, Sotoodeh-Manesh A, Pouryaghoub G. In chronic low back pain, low level laser therapy combined with exercise is more beneficial than exercise alone in the long term: a randomised trial. Aust J Physiother. 2007;53(3):155–160. doi: 10.1016/s0004-9514(07)70022-3. [DOI] [PubMed] [Google Scholar]
- 5.Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985;5:31–39. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 6.Rochkind S, Rousso M, Nissan M, Villarreal M, Barr-Nea L, Rees DG. Systemic effects of low-power laser irradiation on the peripheral and central nervous system, cutaneous wounds and burns. Lasers Surg Med. 1989;9:174–182. doi: 10.1002/lsm.1900090214. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins D, Abrakamse H. Biological effects of helium-neon laser irradiation on normal and wounded human skin fibroblasts. Photomed Laser Sur. 2005;23:251–259. doi: 10.1089/pho.2005.23.251. [DOI] [PubMed] [Google Scholar]
- 8.Djavid GE, Goliaie B, Nikoofar A. Analysis of radiomodulatory effect of low-level laser irradiation by clonogenic survival assay. Photomed Laser Surg. 2015;33(9):452–9. doi: 10.1089/pho.2015.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chikarakara E, Fitzpatrick P, Moore E. et al. In vitro fibroblast and pre-osteoblastic cellular responses on laser surface modified Ti–6Al–4V. Biomed Mater. 2014;10(1):015007. doi: 10.1088/1748-6041/10/1/015007. [DOI] [PubMed] [Google Scholar]
- 10.Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Karu TI. Laser biostimulation: a photobiological phenomenon. J Photochem Photobiol. 1989;3(4):638–640. doi: 10.1016/1011-1344(89)80088-0. [DOI] [PubMed] [Google Scholar]
- 12.Greco M, Guida G, Perlino E, Marra E, Quagliariello E. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163(3):1428–134. doi: 10.1016/0006-291x(89)91138-8. [DOI] [PubMed] [Google Scholar]
- 13.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23(4):355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 14.Passarella S, Casamassima E, Molinari S. et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175(1):95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 15.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 16.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins D, Abrahamse H. Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg. 2006;24:705–714. doi: 10.1089/pho.2006.24.705. [DOI] [PubMed] [Google Scholar]
- 19.Hu WP, Wang JJ, Yu CL, Lan CCE, Chen GS, Yu HS. Helium-Neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J Invest Dermatol. 2007;127:2048–2057. doi: 10.1038/sj.jid.5700826. [DOI] [PubMed] [Google Scholar]
- 20.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 21.Yueh-Feng H, Jui-Hsiang H, Eng-Kean Y, Wen-Tyng L, Yu-Chi C, Ruoh-Chyu R. Effects of LED light irradiation on human foreskin fibroblasts and its implication to wound healing. J Med Biol Eng. 2012;33(2):155–162. [Google Scholar]
- 22.Spitler R, Berns MW. Comparison of laser and diode sources for acceleration of in vitro wound healing by low-level light therapy. J Biomed Opt. 2014;19(3):038001. doi: 10.1117/1.JBO.19.3.038001. [DOI] [PubMed] [Google Scholar]
- 23.Chen ACH, Praveen R, Arany PR, Huang YY, Tomkinson EM, Saleem T. et al. Low level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. Proc SPIE. 2009;7165:71650B. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karin M, Lin A. NF-kappa B at the crossroads of life and death. Nat Immunol. 2002;3(3):221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 25.Whelan HT, Houle JM, Whelan NT, Donohoe DL, Cwiklinski J, Schmidt MH. et al. The NASA Light-Emitting Diode Medical Program-Progress in Space Flight and Terrestrial Applications Space Tech & App. Int’l Forum. 2000;504:37–43. [Google Scholar]
- 26.Smith KC. Laser and LED photobiology. Laser Therapy, 2010;19:72–8. [Google Scholar]
- 27.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB. et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6(7):e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng CQ, Ma WL, Song YB, Guo QY, Wu QH, Zheng WL. Detection of cell apoptosis by MTT assay. Di Yi Jun Yi Da Xue Xue Bao. 2002;22(3):262–3. [PubMed] [Google Scholar]
- 29. Apoptosis, Cytotoxicity and Cell Proliferation. 4th ed. Germany: Roche Diagnostics GmbH; 2008.
- 30.Wan XS, Zhou Z, Kennedy AR. Adaptation of the dichlorofluorescein assay for detection of radiation-induced oxidative stress in cultured cells. Radiat Res. 2003;160:622–630. doi: 10.1667/3099. [DOI] [PubMed] [Google Scholar]
- 31.Jahangiri NY, Shabani M, Vatankhah N, Hashemian SJ, Akbari K. A combination of 670 and 810nm diode lasers for wound healing acceleration in diabetic rats. Photomed Laser Surg. 2010;28:621–627. doi: 10.1089/pho.2009.2634. [DOI] [PubMed] [Google Scholar]
- 32.Prindeze NJ, Moffatt LT, Shupp JW. Mechanisms of action for light therapy: a review of molecular interactions. Exp Biol Med (Maywood) 2012;237(11):1241–1248. doi: 10.1258/ebm.2012.012180. [DOI] [PubMed] [Google Scholar]
- 33.Bhide SA, Nutting CM. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Laser and light therapy for onychomycosis: a systematic review. Lasers Med Sci. 2014;29:823–829. doi: 10.1007/s10103-012-1232-y. [DOI] [PubMed] [Google Scholar]
- 35.Miura Y. Oxidative stress, radiation-adaptive responses, and aging. J Radiat Res. 2004;45:357–372. doi: 10.1269/jrr.45.357. [DOI] [PubMed] [Google Scholar]


