Abstract
Introduction: In recent years, the use of ceramic base zirconia is considered in dentistry for all ceramic restorations because of its chemical stability, biocompatibility, and good compressive as well as flexural strength. However, due to its chemical stability, there is a challenge with dental bonding. Several studies have been done to improve zirconia bonding but they are not reliable. The purpose of this research is to study the effect of plasma treatment on bonding strength of zirconia.
Methods: In this in vitro study, 180 zirconia discs’ (thickness was 0.85-0.9 mm) surfaces were processed with plasma of oxygen, argon, air and oxygen-argon combination with 90-10 and 80-20 ratio (n=30 for each group) after being polished by sandblast. Surface modifications were assessed by measuring the contact angle, surface roughness, and topographical evaluations. Cylindrical Panavia f2 resin-cement and Diafill were used for microshear strength bond measurements. The data analysis was performed by SPSS 20.0 software and one-way analysis of variance (ANOVA) and Tukey test as the post hoc.
Results: Plasma treatment in all groups significantly reduces contact angle compare with control (P=0.001). Topographic evaluations revealed coarseness promotion occurred in all plasma treated groups which was significant when compared to control (P<0.05), except argon plasma treated group that significantly decreased surface roughness (P<0.05). In all treated groups, microshear bond strength increased, except oxygen treated plasma group which decreased this strength. Air and argon-oxygen combination (both groups) significantly increased microshear bond strength (P<0.05).
Conclusion: According to this research, plasmatic processing with dielectric barrier method in atmospheric pressure can increase zirconia bonding strength.
Keywords: Plasma laser, Zirconia ceramics, Resin composite, Surface properties
Introduction
Nowadays, applications of ceramic based zirconia in restorative dentistry due to its esthetic and mechanical properties have been widespread.1,2 Martensitic transformation of yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) ceramics causes high flexural strength and toughness compared to other dental ceramic materials.3-6 Despite these advantages, it is hard to obtain a proper bonding to other substrates because of its hydrophobic surface.7,8
Several studies have shown that unmodified surfaces display low bond strength and eventually leads to adhesive failure,9-11 so there is a need to intensify surface properties of these ceramics to reach an effective bonding.12 These surface modifications are based on roughening the surface and increasing its wettability.13-16 Conventional adhesion techniques used for silica-based ceramic (such as etching by hydrofluoric acid) cannot be used for zirconia ceramics, because it fails to achieve adequate surface roughness and will lead to poor bonding strength.10,17-19
The airborne Al2O3 particle abrasion of zirconia ceramics surface has been found an effective method for achieving durable ceramic-resin bonding. This method increases the surface roughness and wettability, which leads to mechanical and chemical bonding between resin and ceramic surface.20-24 However, it has been reported that particle abrasion by alumina can create mechanical damage on the surface of ceramic, which could affect bonding performance in long term.25-27 To deal with this problem, it is recommended to use softer and rounder abrasive particles26 or omitting these particles.28
To avoid these problems, researchers tried to find alternative surface treatment for improving bonding performance of zirconia ceramics: selective infiltration etching,29 chlorosilane combined with water vapor,7 the fluorination vapor techniques,24 ion bombardment,15,30 and plasma-coating techniques.15,31,32
Recently, application of plasma in dentistry has thrived specially as an effective tool for surface enhancement.32 It has been suggested that plasma modification of surfaces can improve zirconia-resin bonding performance by changing functional groups on the surface creating reactive sites on surfaces.31,33 Valverde et al reported that application of non-thermal plasma for zirconia ceramic surface treatment significantly increases the micro tensile bond strength.31
The objectives of the present study were to (1) evaluate surface properties of Cercon® zirconia ceramics, (2) specify the impact of non-thermal plasma on zirconium bonding strength to resin composite cements.
Methods
Specimen Preparation
Nine blocks of Cercon® base colored 47 blocks (DeguDent, Hanau, Germany) were cut by low speed diamond saw (Secotom-50, Struers, Ballerup, Denmark) in 180 discs (thickness was 0.85-0.9 mm) and were polished in 3 sandblasting phases by 800 grit, 1500 grit, 3000 grit, respectively. According to the factory’s data, these ceramics have 5% yttrium oxide, below 2% hafnium oxide, and below 1% aluminium oxide and silicon oxide. The discs surfaces were washed for 5 minutes with distilled water in ultrasonic machine and sintered after one-day drying; the thickness was 0.75 mm in this phase. Sample coarseness increased after sintering. The surfaces were polished with 1000 grit emery to make a flat sample surface. Prior to surface modification treatments, samples were washed after polishing with acetone, iso-propanol, and distilled water in 3 phases for 5 minutes in ultrasonic set.
The specimens were placed in reactor in order to surface modification via plasma. Plasma treatment was performed based on their experimental groups, with a low density cold active inert argon, oxygen, and air gas plasma beam for 20 seconds over a distance of 10 millimeters. There were 6 study groups based on treatments:
1) ceramics treated by oxygen plasma
2) ceramics treated by argon plasma
3) ceramics treated by plasma of air
4) ceramics treated by 20% oxygen and 80% argon combination
5) ceramics treated by 10% oxygen and 90% argon combination
6) untreated ceramics (control).
Tektronix DPO 3012 oscilloscope (Beaverton, Oregon, USA), Tektronix TCP 202 flow probe (Beaverton, Oregon, USA) and Tektronix p6015 (Beaverton, Oregon, USA) high voltage probes were used to measure the input voltage for producing plasma.
Contact angle measurement
In order to measure contact angle in specimens (n = 60), the sessile drop technique was performed by a micrometer set (EasyDrop, Kruss, Germany) using 2 different liquids with different polarities (distilled water and diiodomethane). Before this test, the 60 specimens were randomly allocated into 6 groups, with 5 of them treated by plasma (n = 50) and 1 group untreated (n = 10). After 5 seconds, a CCD camera made digitalized standard photos. We used ImageJ software (National Institutes of Health, Bethesda, Maryland, USA) and a plugin (DropSnake) for image processing, which was previously described by Stalder et al.34
Topographical Evaluation and Surface Roughness Measurement
Phase imaging of the discs used was performed in the previous step (n = 30) via an atomic force microscopy (easyscan 2, Nanosurf AG, Liestal, Switzerland) to assess the topography and coarseness of discs’ surfaces. Each specimen’s surface was scanned in 2 points at the center, 2 points at the perimeter, and 2 points between the center and perimeter. 256 × 256 resolution and 1 Hz scan rate were utilized to obtain topography on a 90 μm × 90 μm area. The accuracy of this setting is lower than 0.5 nm in Z axis and was 1 nm in XY axis. Prior to the scanning, all surfaces were blown through with cold air and cleaned by alcohol.
Microshear Bond Strength Measurement
After surface processing, a cemented probe was applied to the sample to perform micro tensile and cutting bond strength tests in remaining disks (n = 120). PANAVIA F 2.0 resin composite cement (Kuraray, Okayama, Japan) was filled in custom made tygon silicone tubes with an inner diameter 1.14 mm and 1 mm height on top of each disc. After light curing (Flashsoft, CMS dental, Denmark) for 40 seconds, the resin blocks were incubated at 37 °C for 24 hours to achieve maximum chemical polymerization. Micro tensile tester (Bisco, Schaumburg, IL, USA) with tension velocity of 1 mm/min was used in this study. Micro-shear bond strengths were calculated by dividing peak load by the cross-sectional area of the composite cylinder. A stereomicroscope (Karl Zeiss, Germany) was used to evaluate bonding failure at ×10 magnification.
Statistical Analysis
Data analysis was performed by SPSS 20.0 software (IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) at a 0.05 confidence level was used to compare bonding strength data and Tukey test as the post hoc.
Results
Contact Angle
Table 1 shows descriptive statistics of treated groups and untreated group (control). Plasma treatment in all groups significantly reduces contact angle compare with control (P = 0.001). Also, the comparisons between groups showed significant difference (P < 0.05) except between air plasma and 10% argon-90% oxygen plasma (P = 0.094). Figure 1 shows the boxplots of contact angles in study groups.
Table 1. Means of Contact Angle in Different Plasma Treated Groups and Without Treatment Group (Control) .
| Study Groups | Mean | Standard Deviation |
| Control | 63.00 | 2.000 |
| Oxygen | 32.00 | 4.000 |
| Air | 27.00 | 1.000 |
| Argon | 33.00 | 3.000 |
| 10% Argon-90% Oxygen | 34.00 | 1.000 |
| 20% Argon-80% Oxygen | 23.00 | 2.000 |
Figure 1.
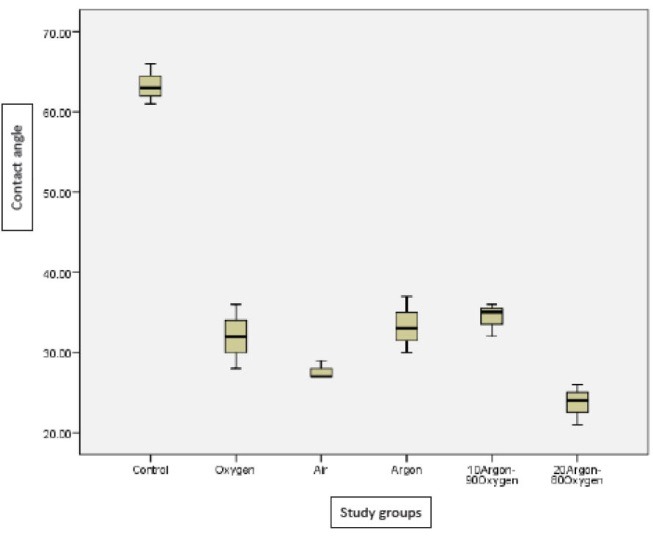
Boxplots for Contact Angles (Degree) of Different Plasma Treated Groups and Without Treatment Group (Control).
Surface Roughness
Descriptive statistics of study groups are presented in Table 2. Topographic evaluations of each group are showed in Figure 2 to 6. Coarseness promotion occurred in all plasma treated groups which was significant when compared to control (P < 0.05) except for the argon plasma treated group that significantly decreased surface roughness (P < 0.05). The boxplots of surface roughness are showed in Figure 7.
Table 2. Means of Surface Coarseness in Different Plasma Treated Groups and Without Treatment Group (Control) .
| Study Groups | Mean | Standard Deviation |
| Control | 8.00 | 0.000 |
| Oxygen | 23.03 | 1.000 |
| Air | 10.00 | 0.000 |
| Argon | 4.00 | 0.000 |
| 10% Argon-90% Oxygen | 14.00 | 0.000 |
| 20% Argon-80% Oxygen | 17.00 | 0.000 |
Figure 2.
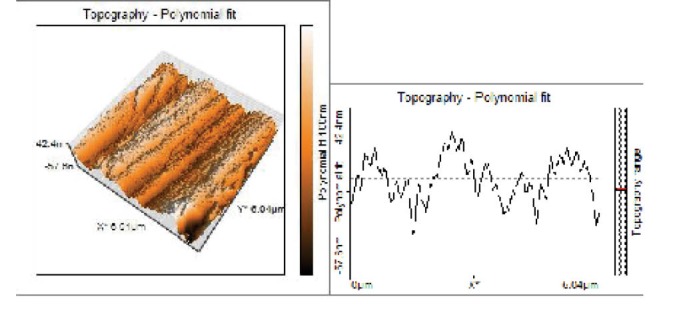
Topographical Evaluations of Specimens’ Surface of Untreated Group (Control).
Figure 6.
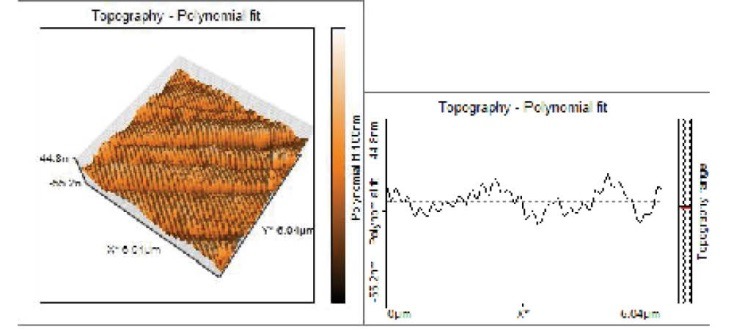
Topographical Evaluations of Specimens’ Surface of Combination of Argon and Oxygen Plasma Treated Group.
Figure 7.
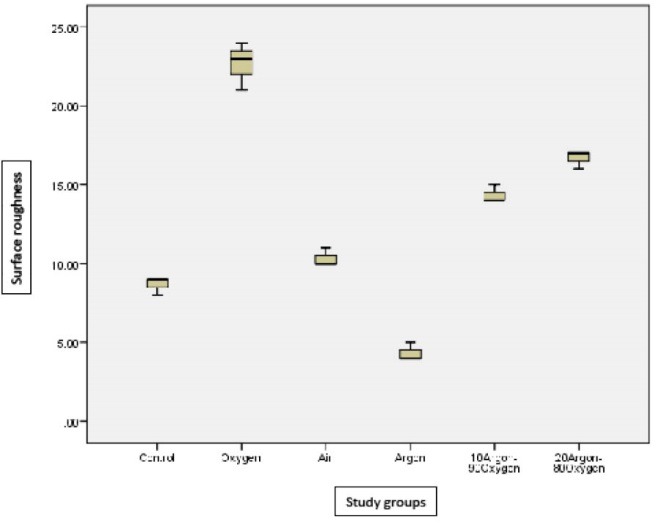
Boxplots for Surface Roughness (nm) of Different Plasma Treated Groups and Without Treatment Group (Control).
Figure 3.
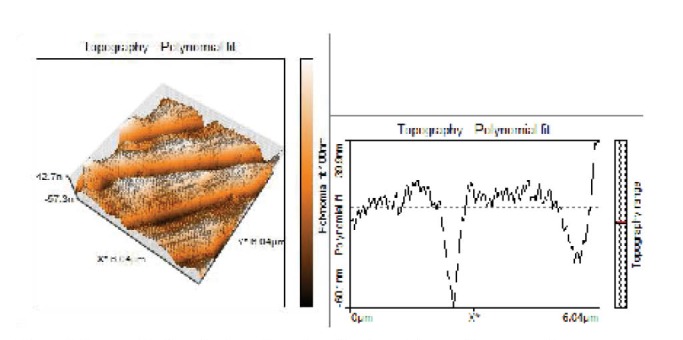
Topographical Evaluations of Specimens’ Surface of Argon Plasma Treated Group.
Figure 4.
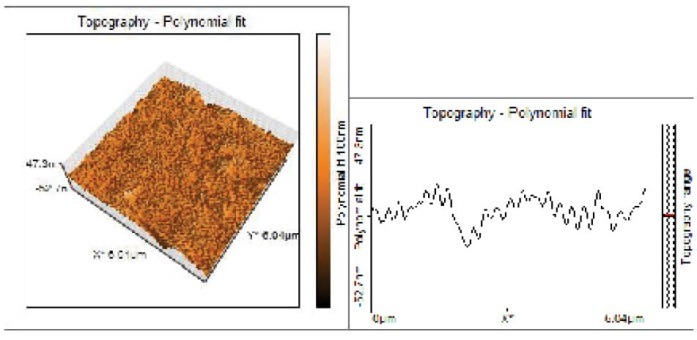
Topographical Evaluations of Specimens’ Surface of Air Plasma Treated Group.
Figure 5.
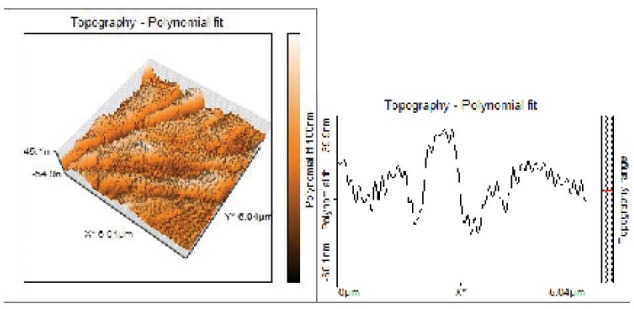
Topographical Evaluations of Specimens’ Surface of Oxygen Plasma Treated Group.
Microshear Bond Strength
In all treated groups, microshear bond strength increased, except for the oxygen treated plasma group which decreased this strength (Table 3). Air and argon-oxygen combination (both groups) significantly increased microshear bond strength (P < 0.05). Figure 8 shows the boxplots of means of microshear bond strength in different plasma treated groups and without treatment group.
Table 3. Means of Microshear Bond Strength in Different Plasma Treated Groups and Without Treatment Group (Control) .
| Study Groups | Mean | Standard Deviation |
| Control | 25.05 | 3.00125 |
| Oxygen | 22.00 | 2.08986 |
| Air | 42.00 | 2.04158 |
| Argon | 27.00 | 3.00000 |
| 10% Argon-90% Oxygen | 30.00 | 1.05276 |
| 20% Argon-80% Oxygen | 44.00 | 3.00000 |
Figure 8.
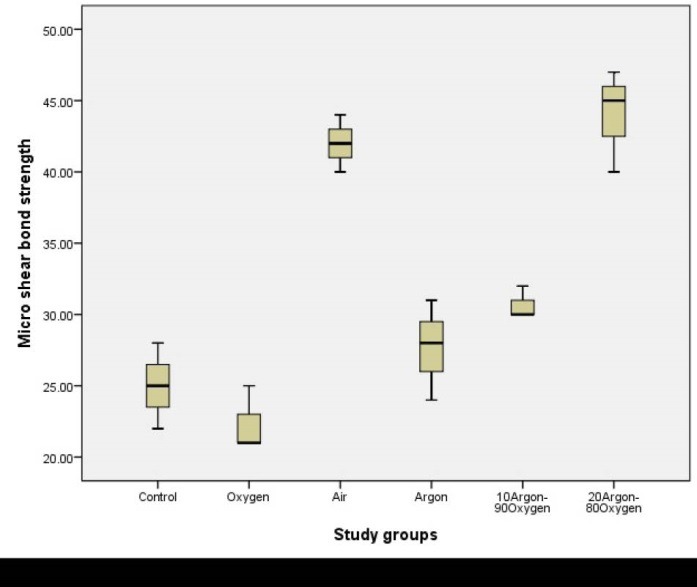
Boxplots for Microshear Bond Strength (MPa) of Different Plasma Treated Groups and Without Treatment Group (Control)
Discussion
Due to mechanical properties of ceramic based zirconia, obtaining appropriate bonding of Y-TZP in dental procedures is difficult.7,11,35 Several treatments have been used in studies to improve bonding properties of these materials.7,15,20,22,24,29,30,36,37 Recently, it has been suggested that the application of plasma can lead to enhanced zirconia-resin bonding by making changes on the surfaces of Y-TZP.31-33 In the present study, we assessed the effects of various types of non-thermal plasmas on Y-TZP in terms of surface properties and bonding strength.
Surface energy (SE) is a term that expresses the intermolecular forces on the surface of a material and it depends on the values of the polar and disperse components of surface.38 Greater value of the SE leads to better wettability of the surface and better bonding properties of the material.39 A common way for calculating the SE of a surface is contact angle measurements of liquids which amount has a reverse relationship with SE.38 The findings of the present study indicated that all kinds of plasma treatments reduced the contact angle of zirconia. These findings are consistent with Noro et al40 and Valverde et al31 studies. In Valverde et al31 study, it has been suggested that the reason for this phenomenon is that after plasma application, O elements in the surface will increase which leads to higher polarity. It also has been reported that gas of the plasma increases the formation of the active peroxide radicals and excessive functional groups (such as C-O and C-OH) on the treated surfaces of inert materials like Y-TZP, which cause higher SE.41,42 Formation of oxygen functional groups on zirconia after using plasma was confirmed by XPS analysis in Noro et al40 study. Due to higher polarity which could result in greater SE value and wettability, the adhesion properties and bonding strength of zirconia will be enhanced by the application of plasma.31,39,43
In the field of microshear bond strength, air and argon-oxygen groups increased microshear bond strength, but there were no significant differences between control group and other study groups. In Derand et al study,10 it has been reported that shear strength between zirconia surface and resin intensified by the RF plasma treatment. Also, Ito et al44 suggested that the application of atmospheric-pressure low temperature plasma enhance shear bonding strength of zirconia. As it has been mentioned before, application of plasma can enhance bonding strengths by increasing the SE value. Another mechanism for this improvement, is that gas plasma treatment removes organic contamination of zirconia by breaking C-H and C-C bonds.31,45
As previously mentioned, the airborne Al2O3 particle abrasion of zirconia ceramics surface has been reported as effective treatment in some literatures.20-24 Ito et al44 compared this treatment with gas plasma treatment. They reported that these treatments both increased the shear bond strength but there were no significant differences between them. They suggested that Al2O3 particle abrasion affect crystal structure of zirconia and generate micro-cracks which leads to higher SE and better wettability and bonding properties.
One of advantages of this study was performing the process in a simpler environment. The reactor used in this study was cheaper and easily made. This reactor works in atmosphere pressure and therefore do not need vacuity equipment. This study used direct plasma processing in which ions together with electrons have great role in making reactions, while indirect processing has lower efficiency. Using air as a plasma environment producing gas was the next benefit that results in isolated chamber deletion from reactor and this can lead to easier processing in shorter time.
Conclusion
Based on our findings, it could be concluded that; (1) plasma processing of Y-TZP with oxygen, argon, air and the combination of oxygen and argon decreases the contact angle and in other words increases the SE. (2) Plasma processing of Y-TZP with above gases decreases the coarseness of surface. (3) Plasma processing of Y-TZP increases the shear bond strength using air and the combination of oxygen and argon as plasma gases. Since we conducted bonding immediately after treatment and then assessed the bonding properties of Y-TZP surface, we suggest that further studies investigate long term effect of plasma treatment of Y-TZP because the active surface state changes over time.
Ethical Considerations
The proposal of study was approved by the Ethics Committee, Deputy of Research, Shahid Beheshti University of Medical Sciences.
Conflict of Interests
Authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank the Research Deputy of Shahid Beheshti University of Medical Sciences for financially supporting this study.
Please cite this article as follows: Tabari K, Hosseinpour S, Mohammad-Rahimi H. The impact of plasma treatment of Cercon® zirconia ceramics on adhesion to resin composite cements and surface properties. J Lasers Med Sci. 2017;8(Suppl 1):S56-SS61. doi:10.15171/jlms.2017.s11.
References
- 1.Little DA, Graham L. Zirconia: simplifying esthetic dentistry. Comp Cont Educ Dent. 2004;25(6):490. [PubMed] [Google Scholar]
- 2.Luthardt RG, Sandkuhl O, Reitz B. Zirconia-TZP and alumina--advanced technologies for the manufacturing of single crowns. Eur J Prosthodont Restor Dent. 1999;7(4):113–119. [PubMed] [Google Scholar]
- 3.Chevalier J, Gremillard L, Virkar AV, Clarke DR. The tetragonal-monoclinic transformation in zirconia: lessons learned and future trends. J Am Ceram Soc. 2009;92(9):1901–1920. doi: 10.1111/j.1551-2916.2009.03278.x. [DOI] [Google Scholar]
- 4.Kosmač T, Oblak C, Jevnikar P, Funduk N, Marion L. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic. Dent Mater. 1999;15(6):426–433. doi: 10.1016/s0109-5641(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 5.Denry IL, Holloway JA. Microstructural and crystallographic surface changes after grinding zirconia-based dental ceramics. J Biomed Mater Res B Appl Biomater. 2006;76B(2):440–448. doi: 10.1002/jbm.b.30382. [DOI] [PubMed] [Google Scholar]
- 6.Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008;24(3):299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Piascik J, Swift EJ, Thompson J, Grego S, Stoner B. Surface modification for enhanced silanation of zirconia ceramics. Dent Mater. 2009;25(9):1116–1121. doi: 10.1016/j.dental.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohbauer U, Zipperle M, Rischka K, Petschelt A, Müller FA. Hydroxylation of dental zirconia surfaces: characterization and bonding potential. J Biomed Mater Res B Appl Biomater. 2008;87(2):461–467. doi: 10.1002/jbm.b.31126. [DOI] [PubMed] [Google Scholar]
- 9.Valandro LF, Özcan M, Bottino MC, Bottino MA, Scotti R, Bona AD. Bond strength of a resin cement to high-alumina and zirconia-reinforced ceramics: the effect of surface conditioning. J Adhes Dent. 2006;8(3):175–181. [PubMed] [Google Scholar]
- 10.Derand T, Molin M, Kvam K. Bond strength of composite luting cement to zirconia ceramic surfaces. Dent Mater. 2005;21(12):1158–1162. doi: 10.1016/j.dental.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent. 2003;89(3):268–274. doi: 10.1067/mpr.2003.50. [DOI] [PubMed] [Google Scholar]
- 12.Attia A, Lehmann F, Kern M. Influence of surface conditioning and cleaning methods on resin bonding to zirconia ceramic. Dent Mater. 2011;27(3):207–213. doi: 10.1016/j.dental.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Osorio R, Ceballos L, Tay F, Cabrerizo Vilchez MA, Toledano M. Effect of sodium hypochlorite on dentin bonding with a polyalkenoic acid containing adhesive system. J Biomed Mater Res. 2002;60(2):316–324. doi: 10.1002/jbm.10074. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar Mendoza JA, Rosales Leal JI, Rodríguez Valverde MA, Cabrerizo Vílchez MA. Effect of acid etching on dentin wettability and roughness: self etching primers versus phosphoric acid. J Biomed Mater Res B Appl Biomater. 2008;84(1):277–85. doi: 10.1002/jbm.b.30871. [DOI] [PubMed] [Google Scholar]
- 15.Hallmann L, Ulmer P, Wille S. et al. Effect of surface treatments on the properties and morphological change of dental zirconia. J Prosthet Dent. 2016;115(3):341–349. doi: 10.1016/j.prosdent.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Hallmann L, Ulmer P, Lehmann F. et al. Effect of surface modifications on the bond strength of zirconia ceramic with resin cement resin. Dent Mater. 2016;32(5):631–639. doi: 10.1016/j.dental.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Kern M. Resin bonding to oxide ceramics for dental restorations. J Adhes Sci Technol. 2009;23(7-8):1097–1011. [Google Scholar]
- 18.Smith RL, Villanueva C, Rothrock JK. et al. Long-term microtensile bond strength of surface modified zirconia. Dent Mater. 2011;27(8):779–785. doi: 10.1016/j.dental.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Attia A, Kern M. Long-term resin bonding to zirconia ceramic with a new universal primer. J Prosthet Dent. 2011;106(5):319–327. doi: 10.1016/S0022-3913(11)60137-6. [DOI] [PubMed] [Google Scholar]
- 20.Lüthy H, Loeffel O, Hammerle CH. Effect of thermocycling on bond strength of luting cements to zirconia ceramic. Dent Mater. 2006;22(2):195–200. doi: 10.1016/j.dental.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Wegner SM, Kern M. Long-term resin bond strength to zirconia ceramic. J Adhes Dent. 2000;2(2):139–145. [PubMed] [Google Scholar]
- 22.Chintapalli RK, Rodriguez AM, Marro FG, Anglada M. Effect of sandblasting and residual stress on strength of zirconia for restorative dentistry applications. J Mech Behav Biomed Mater. 2014;29:126–137. doi: 10.1016/j.jmbbm.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 23.ÖZCAN M, Nijhuis H, Valandro LF. Effect of various surface conditioning methods on the adhesion of dual-cure resin cement with MDP functional monomer to zirconia after thermal aging. Dent Mater. 2008;27(1):99–104. [PubMed] [Google Scholar]
- 24.Piascik JR, Wolter SD, Stoner BR. Development of a novel surface modification for improved bonding to zirconia. Dent Mater. 2011;27(5):e99–e105. doi: 10.1016/j.dental.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Lawn BR, Malament KA, Thompson VP, Rekow ED. Damage accumulation and fatigue life of particle-abraded ceramics. Int J Prosthodont. 2006;19(5):442–448. [PubMed] [Google Scholar]
- 26.Zhang Y, Lawn BR, Rekow ED, Thompson VP. Effect of sandblasting on the long term performance of dental ceramics. J Biomed Mater Res B Appl Biomater. 2004;71(2):381–386. doi: 10.1002/jbm.b.30097. [DOI] [PubMed] [Google Scholar]
- 27.Hallmann L, Ulmer P, Reusser E, Hämmerle CH. Surface characterization of dental Y-TZP ceramic after air abrasion treatment. J Dent. 2012;40(9):723–735. doi: 10.1016/j.jdent.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Guess P, Zhang Y, Kim J-W, Rekow E, Thompson V. Damage and reliability of Y-TZP after cementation surface treatment. J Dent Res. 2010;89(6):592–596. doi: 10.1177/0022034510363253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboushelib MN, Feilzer AJ, Kleverlaan CJ. Bonding to zirconia using a new surface treatment. J Prosthodont. 2010;19(5):340–346. doi: 10.1111/j.1532-849X.2010.00575.x. [DOI] [PubMed] [Google Scholar]
- 30.Sá J, de Brito R, Moura C, Silva N, Alves MB, Alves Junior C. Influence of argon-ion bombardment of titanium surfaces on the cell behavior. Surf Coatings Technol. 2009;203(13):1765–1770. [Google Scholar]
- 31.Valverde GB, Coelho PG, Janal MN. et al. Surface characterisation and bonding of Y-TZP following non-thermal plasma treatment. J Dent. 2013;41(1):51–59. doi: 10.1016/j.jdent.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Cha S, Park YS. Plasma in dentistry. Clin Plasma Med. 2014;2(1):4–10. doi: 10.1016/j.cpme.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S, Chandra R, Tripathi S. et al. The bright future of dentistry with cold plasma—review. J Dent Med Sci. 2014;13:6–13. [Google Scholar]
- 34.Stalder A, Kulik G, Sage D, Barbieri L, Hoffmann P. A snake-based approach to accurate determination of both contact points and contact angles. Colloids Surf A Physicochem Engineer Aspects. 2006;286(1):92–103. [Google Scholar]
- 35.Komine F, Blatz MB, Matsumura H. Current status of zirconia-based fixed restorations. J Oral Sci. 2010;52(4):531–539. doi: 10.2334/josnusd.52.531. [DOI] [PubMed] [Google Scholar]
- 36.Piascik JR, Wolter SD, Stoner BR. Enhanced bonding between YSZ surfaces using a gas-phase fluorination pretreatment. J Biomed Mater Res B Appl Biomater. 2011;98(1):114–119. doi: 10.1002/jbm.b.31840. [DOI] [PubMed] [Google Scholar]
- 37.Valandro LF, Della Bona A, Bottino MA, Neisser MP. The effect of ceramic surface treatment on bonding to densely sintered alumina ceramic. J Prosthet Dent. 2005;93(3):253–259. doi: 10.1016/j.prosdent.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Janssen D, De Palma R, Verlaak S, Heremans P, Dehaen W. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Films. 2006;515(4):1433–1438. [Google Scholar]
- 39.Pashley DH, Carvalho RM, Sano H, Nakajima M, Yoshiyama M, Shono Y. et al. The microtensile bond test: a review. J Adhes Dent. 1999;1(4):299–309. [PubMed] [Google Scholar]
- 40.Noro A, Kaneko M, Murata I, Yoshinari M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal) J Biomed Mater Res B Appl Biomater. 2013;101(2):355–363. doi: 10.1002/jbm.b.32846. [DOI] [PubMed] [Google Scholar]
- 41.Silva NR, Coelho PG, Valverde GB. et al. Surface characterization of Ti and Y TZP following non thermal plasma exposure. J Biomed Mater Res B Appl Biomater. 2011;99(1):199–206. doi: 10.1002/jbm.b.31887. [DOI] [PubMed] [Google Scholar]
- 42.Chu PK, Chen J, Wang L, Huang N. Plasma-surface modification of biomaterials. Mater Sci Engineer R Rep. 2002;36(5):143–206. [Google Scholar]
- 43. Fridman A. Plasma Chemistry. Cambridge: Cambridge University Press; 2008.
- 44.Ito Y, Okawa T, Fukumoto T. et al. Influence of atmospheric pressure low-temperature plasma treatment on the shear bond strength between zirconia and resin cement. J Prosthodont Res. 2016;60(4):289–293. doi: 10.1016/j.jpor.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Wu C-C, Wei C-K, Ho C-C, Ding S-J. Enhanced hydrophilicity and biocompatibility of dental zirconia ceramics by oxygen plasma treatment. Materials. 2015;8(2):684–699. doi: 10.3390/ma8020684. [DOI] [PMC free article] [PubMed] [Google Scholar]


