Abstract
Objective
To determine the pharmacokinetics of orally administered rapamycin in healthy dogs.
Animals
5 healthy purpose-bred hounds.
Procedures
The study consisted of 2 experiments. In experiment 1, each dog received rapamycin (0.1 mg/kg, PO) once; blood samples were obtained immediately before and at 0.5, 1, 2, 4, 6, 12, 24, 48, and 72 hours after administration. In experiment 2, each dog received (0.1 mg/kg, PO) once daily for 5 days; blood samples were obtained immediately before and at 3, 6, 24, 27, 30, 48, 51, 54, 72, 75, 78, 96, 96.5, 97, 98, 100, 102, 108, 120, 144, and 168 hours after the first dose. Blood rapamycin concentration was determined by a validated liquid chromatography-tandem mass spectrometry assay. Pharmacokinetic parameters were determined by compartmental and non-compartmental analyses.
Results
Mean ± SD blood rapamycin terminal half-life, area under the concentration-time curve from 0 to 48 hours after dosing, and maximum concentration were 38.7 ± 12.7 h, 140 ± 23.9 ng•h/mL, and 8.39 ± 1.73 ng/mL, respectively, for experiment 1, and 99.5 ± 89.5 h, 126 ± 27.1 ng•h/mL, and 5.49 ± 1.99 ng/mL, respectively, for experiment 2. Pharmacokinetic parameters for rapamycin after administration of 5 daily doses differed significantly from those after administration of 1 dose.
Conclusions and Clinical Relevance
Results indicated that oral administration of low-dose (0.1 mg/kg) rapamycin to healthy dogs achieved blood concentrations measured in ng/mL. The optimal dose and administration frequency of rapamcyin required to achieve therapeutic effects in tumor-bearing dogs, as well as toxicity after chronic dosing, needs to be determined.
Rapamycin, also known as sirolimus, was first isolated from Streptomyces hygroscopicus on Easter Island in the mid-1970’s1 and was initially characterized as an antifungal and antimicrobial agent. Since then, its immunosuppressant and anti-cancer properties have been recognized.2 The mTOR acts as the nutrient-sensor of the cell and integrates signals to regulate cell growth and metabolism. Rapamycin inhibits mTOR, which blocks the pathways required for cell-cycle progression, proliferation, and angiogenesis.3
Because rapamycin has immunosuppressive properties, it is commonly administered to human patients after organ transplantation. Human organ transplant recipients who are administered rapamycin have a decreased risk of cancer development or recurrence, compared with those who are administered other immunosuppressive agents such as cyclosporine.4 In human oncology, rapamycin and its derivatives are being used as antineoplastic agents with increasing frequency, and have been associated with beneficial effects in patients with renal clear cell carcinoma, pancreatic neuroendocrine tumors, and mantle cell lymphoma.5 Clinical trials are currently underway to determine the efficacy of rapamycin for the treatment of human patients with various other types of cancer.6–8
The mTOR pathway is present and active in various immortalized canine cell lines such as osteosarcoma,9 hemangiosarcoma,10,11 and malignant melanoma.12 Exposure of those cell lines to rapamycin results in a significant, dose-dependent decrease in viable tumor cells.9,12 The results of those in vitro studies9,12 suggest that rapamycin might be efficacious for the treatment of common metastatic cancers in companion animals.
Information regarding the use of rapamycin in dogs is limited. In dogs with experimental renal allografts, oral administration of rapamycin at a dose of 2 mg/kg resulted in severe toxicosis that was characterized by oral ulceration, anorexia, diarrhea, vasculitis, and death.13 Administration of rapamycin (0.5 mg/kg, IV) in combination with cyclosporine to dogs with experimental renal allografts resulted in similar marked toxicosis.14 In that study,14 administration of rapamycin (0.05 mg/kg, IV) was well-tolerated by the dogs, but it did not provide sufficient immunosuppression to prevent rejection of the renal allograft. Likewise, administration of rapamycin (0.1 mg/kg, IM q 48 h or twice weekly) in combination with cyclosporine and antilymphocyte serum, although well tolerated by dogs, was not sufficient to prevent renal allograft rejection.15 The effect of cyclosporine on the pharmacokinetics of rapamycin is unknown. In dogs with glycogen storage disease type III, oral administration of rapamycin at doses of 0.5 to 1.0 mg/kg/d for 8 to 14 months was well tolerated.16
To our knowledge, only 1 study17 has been performed to determine the pharmacokinetics of rapamycin in dogs with osteosarcoma. In that study,17 rapamycin was administered by IM injection at doses ranging from 0.01 to 0.08 mg/kg, and a maximally tolerated dose was not achieved, although modulation of the mTOR pathway was detected in peripheral blood mononuclear cells and tumor biopsy specimens obtained from dogs in each dose cohort. However, IM administration of rapamycin resulted in the formation of sterile abscesses at the injection site, and administration of the drug by a SC route likewise resulted in sterile abscesses at the injection site.17 Consequently, oral administration of rapamycin is generally preferred over parenteral administration in dogs. The pharmacokinetics of rapamycin following oral administration have not been characterized. The purpose of the study reported here was to determine the pharmacokinetics of orally administered rapamycin in healthy dogs.
Materials and Methods
Animals
Five purpose-bred hound dogs (3 sexually intact females, 1 spayed female, and 1 castrated male) with a median age of 11 years (range, 11 to 15 years) and weight of 21 kg (range, 18.2 to 33 kg) were used in the study. Each dog was determined to be healthy on the basis of results of a physical examination, CBC, serum biochemical analysis, and urinalysis. Dogs had ad libitum access to water and were fed a dry canine maintenance food once daily in the evening. All study procedures were approved by the Institutional Animal Care and Use Committee at the University of Tennessee.
Study design
The study consisted of 2 experiments with a 2-week washout period between experiments. In both experiments, dogs were administered rapamycin in the morning at least 12 hours after they were fed their daily allotment of food the previous evening. In experiment 1, each dog was administered rapamycina (0.1 mg/kg, PO) once. From each dog, a blood sample (2 mL) was collected by jugular venipuncture with a 20-gauge needle and evacuated blood collection system into blood collection tubes that contained heparin immediately before (0 hours) and at 0.5, 1, 2, 4, 6, 12, 24, 48, and 72 hours after rapamycin administration. In experiment 2, each dog was administered rapamycin (0.1 mg/kg, PO) once daily for 5 consecutive days. A blood sample (2 mL) was collected from each dog in the same manner as in experiment 1 immediately before (0 hours) and at 3, 6, 24 (immediately before administration of second dose of rapamycin), 27, 30, 48 (immediately before administration of third dose of rapamycin), 51, 54, 72 (immediately before administration of fourth dose of rapamycin), 75, 78, 96 (immediately before administration of fifth and final dose of rapamycin), 96.5, 97, 98, 100, 102, 108, 120, 144, and 168 hours after administration of first dose of rapamycin. Dogs were continuously monitored throughout the study period, and information regarding the daily food and water intake, energy level, defecation, and urination of each dog was recorded as were any adverse effects observed.
Blood sample processing
Following collection, blood samples were transferred to cryogenic vials and stored frozen at −80°C for 1 month until analysis. A validated method for extraction of a rapamycin analog was used as described18 to extract rapamycin from 100 μL of each blood sample by means of protein precipitation with 0.2M zinc sulfate followed by liquid-liquid extraction with 1 mL of ethyl acetate. The organic layer (900 μL) was dried and reconstituted in a solution that consisted of 50% methanol and 50% ultrapure water.
The rapamycin concentration in each sample was determined by use of liquid chromatography-tandem mass spectrometry with tacrolimus as an internal standard. The system consisted of a mass spectrometerb equipped with Ion Drive Turbo V ionspray sourcec that was interfaced with a liquid chromatograph system.d Chromatographic separation was obtained with a C8 2.5μM (4.6 × 50 mm) columne with a C18 (4.0 × 2.0 mm) guard columnf by use of a liquid chromatography gradient that consisted of mobile phase A (10mM ammonium formate with 0.1% formic acid) and mobile phase B (0.1% formic acid in acetonitrile). Beginning with 50% mobile phase B, chromatographic resolution was achieved by increasing mobile phase B linearly from 50% to 90% from 1 to 2 minutes, maintaining at 99% from 2 to 3 minutes, decreasing linearly from 99% to 50% from 3 to 4 minutes, and equilibrating at 50% from 4 to 5.5 minutes. The liquid chromatography flow rate was 1.5 mL/min, and the sample injection volume was 15 μL. The analysis run time was 5.5 minutes.
The mass spectrometer settings were optimized for rapamycin and tacrolimus as follows: turbo ionspray temperature, 300°C; ionspray voltage, 5,500 V; declustering potential, 25 (rapamycin) and 60 (tacrolimus); entrance potential, 14 (rapamycin) and 24 (tacrolimus); collision energy 19 (rapamycin) and 26 (tacrolimus); collision cell exit potential, 18 (rapamycin) and 15 (tacrolimus); curtain gas (N2), 30 U; collision gas (N2), high; nebulizer gas (N2), 50 U; and auxiliary gas (N2), 50 U. Similar to the results of another study,19 the predominant product ion of the ammonium adduct of rapamycin ([M + NH(4)]+ precursor ion, m/z 931.7) was m/z 864.5. The mass spectrometer was run in multiple-reaction mode with positive electrospray ionization monitoring ion transition m/z of 931.7 to 864.5 for rapamycin and m/z of 821.7 to 768.5 for tacrolimus. The scan times for each ion transition were 50 milliseconds, and quadrupoles 1 and 3 were both operated in unit resolution mode.
Rapamycin concentrations were calculated by use of linear regression analysis of peak area ratios (ie, rapamycin area vs tacrolimus area) and back calculation from standard curves generated from blank canine whole blood that was spiked with rapamycin in increasing concentrations that ranged from 0.25 to 50 ng/mL. The standard curves were linear (r2 > 0.99) when 1/x2 weighting was used. The lower limit of quantification for rapamycin in canine blood was 0.25 ng/mL as determined by standard analytical method validation guidelines.20 For each day of analysis, a minimum of 3 quality control samples were prepared by spiking blank canine whole blood samples with each of 3 concentrations (0.5, 2.5, and 10 ng/mL) of rapamycin. Percentage accuracy of the quality control samples was calculated as (1 − [{actual value − measured value}/actual value]) × 100 and the percentage precision (or relative SD) was calculated as (SD for the measured mean value/measured mean value) × 100. For the quality control samples (rapamycin concentrations, 0.5, 2.5 and 10 ng/mL), the intra-run accuracy ranged from 93.8% to 97.9%, and the precision ranged from 4.6% to 8.0%. For the spectrum of rapamycin concentrations (0.25 to 50 ng/mL) used to generate the standard curves, the inter-run accuracy ranged from 96.4% to 100%, and the precision ranged from 1.9% to 6.8%. All accuracy and precision calculations were within the guidelines (accuracy, > 85%; precision, ± 20%) established for validation of an acceptable analytical method.20
Statistical analysis
Rapamycin concentration-time data were analyzed with noncompartmental methodsg and compartmental methods by the use of an NME approach.h For the compartmental analysis, data from both experiments were analyzed together, and the IOV between doses was evaluated as was the interindividual and residual variability and magnitude of change in pharmacokinetic parameters associated with multiple doses. The relevance of including IOV in the model was assessed on the basis of a decrease in both interindividual and residual variability for pharmacokinetic parameters and the likelihood ratio test, which estimated the significance of the decrease in the −2 log-likelihood function between nested models (significance was set at P < 0.01). Residual and SE plots and plots of predicted versus observed values were evaluated to assess how well the data fit the compartmental model.
The Cmax and Tmax were determined from a concentration-time plot of each dog’s data. The AUC0-48h after the first dose of rapamycin was calculated with the log-linear trapezoidal method. The terminal rate constant was derived from the slope of the terminal elimination phase of the semilogarithmic decay curve. The t1/2 was calculated by dividing 0.693 by the terminal rate constant.
Results
Dogs
None of the dogs had a substantial change in energy level, appetite, water intake, urination, or defecation during the study period. One dog vomited once between 3 and 5 hours after rapamycin administration on day 4 of experiment 2. No other adverse effects were observed. One dog was removed from the study on day 3 of experiment 2 because of an acutely ulcerated lipoma that required surgical removal. Thus, the pharmacokinetic parameters for experiment 1 represent the mean for 5 dogs and those for experiment 2 represent the mean for only 4 dogs.
Pharmacokinetic parameters
None of the dogs had detectable rapamycin concentrations in their blood immediately prior to initiation of experiment 2. This suggested that the 2-week washout period between experiments 1 and 2 was sufficient for rapamycin elimination, and the results for experiment 2 were not affected by any residual blood concentrations of rapamycin carried over from experiment 1.
In experiment 1, the mean ± SD AUC0-48h was 140 ± 23.9 ng•h/mL (range, 116 to 168 ng•h/mL), t1/2 was 38.7 ± 12.7 hours (range, 27.2 to 59.6 hours), Tmax was 3.3 ± 2.5 hours (range, 0.5 to 6 hours), Cmax was 8.39 ± 1.73 ng/mL (range, 5.86 to 10.6 ng/mL), and concentration 48 hours after drug administration was 1.38 ± 0.45 ng/mL (range, 0.78 to 1.86 ng/mL). In experiment 2, the mean ± SD AUC0-48h was 126 ± 27.1 ng•h/mL (range, 95.9 to 160 ng•h/mL), t1/2 was 99.5 ± 89.5 hours (range, 39.1 to 231 hours), Tmax was 4.5 ± 1.0 hours (range, 4 to 6 hours), Cmax was 5.49 ± 1.99 ng/mL (range, 3.70 to 8.18 ng/mL), and concentration 48 hours after drug administration was 1.70 ± 0.40 ng/mL (range,1.45 to 2.30 ng/mL).
The structural model that provided the best fit for the data from experiment 2 included first-order absorption and bi-compartmental decay as follows:
where D represents the dose, tD represents the dosing time, and A, B, ka, α, and β represent the pharmacokinetic parameters of the model. The re-parameterized output included parameters for Cl/f, clearance of a drug after oral administration, Q/f, intercompartmental clearance of a drug after oral administration, V1/f, volume of the central compartment after oral administration, V2/f,volume in the peripheral compartment after oral administration, and ka.
A log-normal distribution provided the best fit for the pharmacokinetic parameters and was modeled as: θi = θμeηi,θ, where θi is the pharmacokinetic parameter for subject i, θμ is the mean pharmacokinetic parameter for the population, and ηi,θ is the deviation between subject i and the population mean, which was assumed to be normally distributed with a mean of 0 and constant variance of ω2θ.
The best residual model was proportional and was expressed as follows: Cpij = Cpμ,j × (1 + εij), where Cpij is the rapamycin concentration for subject i at time j, Cpμ,j is the mean rapamycin concentration for the study population at time j, and εij is the deviation between subject i and the population mean, which was assumed to be normally distributed with a mean of 0 and a constant variance of σ2.
The final compartmental NME model included a term for IOV that accounted for the apparent change in the pharmacokinetic parameters for rapamycin after daily oral administration for 5 consecutive days. The mean pharmacokinetic parameters for rapamycin on day 5 of experiment 2 were expressed as θμD5 = θμD1−4eβOCC2, where θμD1−4 is the mean parameter for days 1 through 4 and βOCC2 is the parameter for the mean change on day 5. The parameters for each subject were expressed as θi = θμe(ηi + η1OCC1 + η2OCC2), where OCC1 incorporated data from experiment 1 until rapamycin was expected to leave the system and also experiment 2 until day 5 and equaled 1 during days 1 to 4 of experiment 2 or 0 otherwise and OCC2 incorporated data beginning on day 5 of experiment 2 and equaled 1 on that day or 0 otherwise.
In experiment 1, the blood rapamycin concentration peaked twice at approximately 2 and 6 hours after administration (Figure 1). This finding was accommodated during modeling, and multiple peak rapamycin concentrations were not modeled for individual dogs. A preliminary model was created from the data for the overall study population (ie, data from experiments 1 and 2 were combined) that would allow for prediction of the duration that blood rapamycin concentration remains above a given target concentration. The multiple peak rapamycin concentrations for each dog in experiment 1 increased the model residual error; however, this did not prevent the NME compartmental model from adequately fitting the data.
Figure 1.
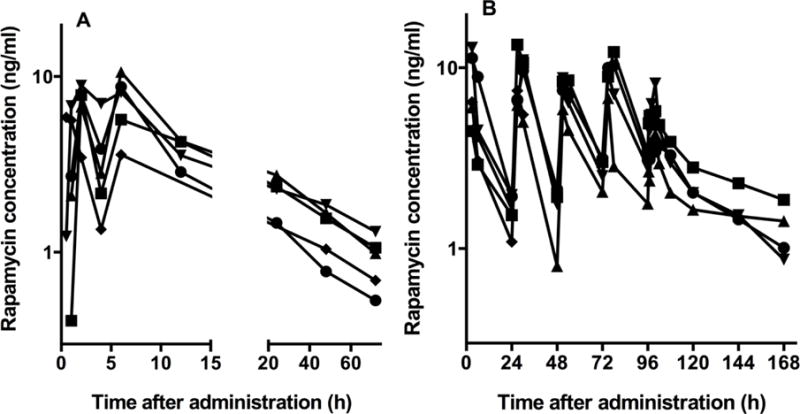
Blood rapamycin concentration over time for each of 5 healthy dogs following oral administration of rapamycin (0.1 mg/kg) once (experiment 1; A) and once daily for 5 consecutive days (experiment 2; B). The same dogs were used in both experiments, and there was a 14-day washout period between experiments 1 and 2. One dog was removed from the study during experiment 2 because of an acutely ulcerated lipoma that required surgical removal; therefore, only 4 dogs are represented in panel B.
The results of the compartmental NME analysis were summarized (Table 1). Inclusion of OCC2 in the model caused a decrease in the −2 log-likelihood test result from 501.30 to 422.85, and estimation of the IOV caused a decrease in the residual variability of the model from 45.1% to 33.1%. On the last day (day 5) of rapamycin administration in experiment 2, the mean ka increased from 0.294 to 2.17 h−1 and the mean V1/f increased from 4.53 to 16.8 L/kg, whereas the mean Cl/f decreased from 0.510 to 0.303 L•h/kg.
Table 1.
Pharmacokinetic parameters for rapamycin (0.1 mg/kg) following oral administration to 5 healthy dogs once (experiment 1) and, after a 2-week washout period, once daily for 5 consecutive days (experiment 2) as determined by compartmental NME analysis.
| Parameter | Experiment 1 | Experiment 2 | Parameter characterizing the change in a given parameter on the last day (day 5) of rapamycin administration in experiment 2 | ||||
|---|---|---|---|---|---|---|---|
| Mean estimate | IIV (%) | Mean estimate* | IIV (%) | IOV (%) | Parameter | P value | |
| ka (h−1) | 0.358 | 34.9 | 0.294 | 4.91 | 6.92 | 2.0 | 0.002 |
| Cl/f (L•h/kg) | 0.651 | 10.83 | 0.510 | 13.76 | 5.18 | −0.519 | 0.003 |
| V1/f (L/kg) | 6.74 | 71.42 | 4.53 | 41.20 | 5.70 | 1.31 | 0.002 |
| Q/f (L•h/kg) | 1.02 | 10.33 | 1.12 | 38.75 | — | — | — |
| V2/f (L/kg) | 18.1 | 7.55 | 22.1 | 8.85 | — | — | — |
One dog was removed from the study on day 3 of experiment 2 because of an acutely ulcerated lipoma that required surgical removal; thus, the estimates for experiment 2 represent the mean for 4 dogs. IIV = Inter-individual variability. V1/f = volume of the central compartment after oral administration, Q/f = Intercompartmental clearance of a drug after oral administration. V2/f = Volume of the peripheral compartment after oral administration. — = Not calculated.
Discussion
Results of the present study indicated that oral administration of low-dose (0.1 mg/kg) rapamycin to healthy dogs achieved blood concentrations of the drug that were measured in ng/mL. Oral administration of low-dose rapamycin for 5 consecutive days resulted in a significant increase in the ka and V1/f and decrease in the Cl/f, which may affect recommendations for long-term administration of this drug to dogs. Although the results of this study are preliminary and should be interpreted with caution given the small study population, it appeared that absorption and elimination of rapamycin in dogs was dependent on the dosing interval and changed soon after daily administration was initiated. That change might have been the result of an increase in the activities of intestinal cytochrome P450 3A4 (an enzyme that oxidizes drugs) or p-glycoprotein (a protein that pumps foreign substances out of cells) or otherwise altered metabolism. In an in vitro study,21 results following exposure of human and porcine small intestinal microsomes to rapamycin suggested that intestinal first-pass metabolism of rapamycin was associated with cytochrome P450 3A4. However, those findings are preliminary and further research is necessary to determine whether or when the dose of rapamycin should be adjusted following treatment initiation.
During experiment 1, blood rapamycin concentration peaked twice at approximately 2 and 6 hours after drug administration in all 5 dogs. Multiple peaks in the blood concentration of a drug following administration are most commonly caused by enterohepatic recirculation of the drug, but can also be the result of variable gastric emptying and intestinal transit time, multiple sites of drug absorption within the gastrointestinal tract, or solubility-limited absorption of the drug.22 To our knowledge, enterohepatic recirculation of rapamycin has not been described in any species. The cause of the multiple peaks in blood rapamycin concentration during experiment 1 is unknown, and multiple peaks in blood rapamycin concentration following drug administration were not observed in experiment 2.
Blood rapamycin concentrations similar to those achieved in the healthy dogs of the present study have been associated with antitumor activity in in vitro and in vivo murine cancer models.4,23 Rapamycin appears to have either cytostatic or cytotoxic properties that are dependent on the dose administered and the subsequent blood concentrations of the drug achieved. Low blood rapamycin concentrations (< 10 ng/mL) are associated with cytostatic or antiangiogenic activity, which halts tumor progression,4 and do not appear to be cytotoxic or immunosuppressive in mice.23 In tumor-bearing mice, a mean ± SD steady-state blood rapamycin concentration of 15 ± 1 ng/mL resulted in maximal antiangiogenic effects, and higher blood rapamycin concentrations did not improve the inhibitory effect on tumor progression.23 In that study,23 in vitro tumor angiogenesis was inhibited at rapamycin concentrations as low as 1 ng/mL. On the basis of the results of those studies,4,23 the rapamycin Cmax achieved in experiments 1 (8.39 ng/mL) and 2 (5.49 ng/mL) following administration of a low-dose (0.1 mg/kg/d) of the drug to the dogs of the present study should have been sufficient to cause cytostatic, antiangiogenic activity in rapamycin-sensitive tumors. In dogs, administration of low-dose rapamycin might be optimal because of the drug’s high cost and the fact that administration of higher doses frequently results in toxicosis in that species.
The present study was not without limitations. Blood samples were frozen at −80°C for 1 month prior to analysis, which might have adversely affected measurement of the rapamycin concentration in those samples. Results of other studies24,25 indicate that the rapamycin concentration is not substantially affected in blood samples stored frozen at −80°C for 90 days and subsequently thawed. Therefore, we do not believe that blood sample storage prior to analysis significantly affected measurement of blood rapamycin concentration in the present study. The number of dogs used in experiments 1 (n = 5) and 2 (4) was small, which might have affected the accuracy of the pharmacokinetic parameter estimates. However, the purpose of the present study was to determine whether oral administration of a low dose (0.1 mg/kg) of rapamycin to dogs would result in detectable blood concentrations of the drug and whether the pharmacokinetic parameters for rapamycin changed following administration of multiple doses. In human patients, administration of rapamycin results in substantial intra- and interindividual variability in the blood concentration of the drug, and serial monitoring of blood rapamycin concentration is recommended throughout the duration of treatment with the drug.26 The blood rapamycin concentrations varied substantially within and among the dogs of the present study, and serial monitoring of blood rapamycin concentration may also be necessary in dogs. Also, the dogs of the present study were healthy, and although the mean age of the study dogs was typical for dogs that develop tumors, the findings of this study might not be externally valid. The pharmacokinetic variables for rapamycin may differ in dogs with tumors because it is unknown whether the presence of a tumor affects the pharmacokinetics of rapamycin or similar drugs. Other limitations of the present study include the short treatment duration and the lack of pharmacodynamic monitoring. A steady-state blood rapamycin concentration was not achieved in dogs after oral administration of 0.1 mg of rapamycin/kg daily for 5 days. Results of another study17 indicate that it takes 12.5 days for rapamycin to reach a steady-state concentration in the blood of dogs.
In the present study, oral administration of 0.1 mg of rapamycin/kg to healthy dogs resulted in blood concentrations of the drug that were measured in ng/mL. The pharmacokinetic parameters for rapamycin changed significantly following daily administration of the drug for 5 consecutive days. This should be considered when long-term treatment of dogs with rapamycin is considered; however the findings of this study are preliminary. Additional research is necessary to determine the optimal dose and administration frequency of rapamycin required to achieve therapeutic effects in tumor-bearing dogs as well as toxicity with chronic dosing.
Acknowledgments
Supported in part by the University of Tennessee Center of Excellence in Livestock Disease and Human Health and the Department of Small Animal Clinical Sciences Companion Animal and Oncology Funds. Pharmacokinetic analysis was performed in the Pharmacology Shared Resource with support from a University of Colorado Cancer Center Support Grant.
ABBREVIATIONS
- AUC0-48h
Area under the concentration-time curve from 0 to 48 hours after dosing
- Cmax
Maximum concentration
- Cl/f
Apparent blood clearance of drug after oral administration
- IOV
Interoccasion variability
- ka
Absorption rate constant
- mTOR
Mechanistic target of rapamycin
- NME
Nonlinear mixed effects
- t1/2
Terminal half-life
- Tmax
Time to maximum concentration
- V1/f
Volume of drug in the central compartment after oral administration
Footnotes
Presented as a poster at the 34th Annual Veterinary Cancer Society Conference, St. Louis, Missouri, October 2014.
Drs. LeBlanc and Martin-Jimenez contributed equally to the study.
Rapamune 1 mg tablets, Wyeth, Dallas, Tex.
AB SCIEX QTRAP 6500 triple quadrapole mass spectrometer, AB Sciex, Framingham, Mass.
AB Sciex, Framingham, Mass.
Nexera X2 ultra high performance liquid chromatograph system, Shimadzu Scientific Instruments, Inc, Columbia, Md.
XBridge Phenyl column, Water Corporation, Milford, Mass.
C18 guard cartridge, Phenomenex, Torrance, Calif.
Phoenix WinNonlin 6.3.0.395, Certara USA, Inc, Princeton, NJ.
Monolix 4.3.3, Lixoft, S.A.S, Orsay, France.
References
- 1.Baker H, Sidorowicz A, Sehgal SN, et al. Rapamycin (AY-22,989), a new antifungal antibiotic III. In vitro and in vivo evaluation. J Antibiot. 1978;31:539–545. doi: 10.7164/antibiotics.31.539. [DOI] [PubMed] [Google Scholar]
- 2.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 3.Dancey JE. Inhibitors of the mammalian target of rapamycin. Expert Opin Investig Drugs. 2005;14:313–328. doi: 10.1517/13543784.14.3.313. [DOI] [PubMed] [Google Scholar]
- 4.Guba M, Von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 5.Fasolo A, Sessa C. Targeting mTOR pathways in human malignancies. Curr Pharm Des. 2012;18:2766–2777. doi: 10.2174/138161212800626210. [DOI] [PubMed] [Google Scholar]
- 6.Beck JT, Ismail A, Tolomeo C. Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: an emerging treatment strategy for squamous cell lung carcinoma. Cancer Treat Rev. 2014;40:980–989. doi: 10.1016/j.ctrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Fumarola C, Bonelli MA, Petronini PG, et al. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol. 2014;90:197–207. doi: 10.1016/j.bcp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Liberal J, Gil-Martin M, Sáinz-Jaspeado M, et al. Phase I study and preclinical efficacy evaluation of the mTOR inhibitor sirolimus plus gemcitabine in patients with advanced solid tumours. Br J Cancer. 2014;111:858–865. doi: 10.1038/bjc.2014.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon IK, Ye F, Kent MS. Evaluation of the mammalian target of rapamycin pathway and the effect of rapamycin on target expression and cellular proliferation in osteosarcoma cells from dogs. Am J Vet Res. 2008;69:1079–1084. doi: 10.2460/ajvr.69.8.1079. [DOI] [PubMed] [Google Scholar]
- 10.Murai A, Asa SA, Kodama A, et al. Immunohistochemical analysis of the Akt/mTOR/4E-BP1 signaling pathway in canine hemangiomas and hemangiosarcomas. J Comp Path. 2012;147:430–440. doi: 10.1016/j.jcpa.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Murai A, Asa SA, Kodama A, et al. Constitutive phosphorylation of the mTORC2/Akt/4E-BP1 pathway in newly derived canine hemangiosarcoma cell lines. BMC Vet Res. 2012;8:128. doi: 10.1186/1746-6148-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent MS, Collins CJ, Ye F. Activation of the AKT and mammalian target of rapamycin pathways and the inhibitory effects of rapamycin on those pathways in canine malignant melanoma cell lines. Am J Vet Res. 2009;70:263–269. doi: 10.2460/ajvr.70.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collier DS, Calne R, Thiru S, et al. Rapamycin in experimental renal allografts in dogs and pigs. Transplant Proc. 1990;22:1674–1675. [PubMed] [Google Scholar]
- 14.Knight R, Ferraresso M, Serino F, et al. Low-dose rapamycin potentiates the effects of subtherapeutic doses of cyclosporine to prolong renal allograft survival in the mongrel canine model. Transplantation. 1993;55:947–949. [PubMed] [Google Scholar]
- 15.Hartner WC, Van der Werf WJ, Lodge PA, et al. Effect of rapamycin on renal allograft survival in canine recipients treated with antilymphocyte serum, donor bone marrow, and cyclosporine. Transplantation. 1995;60:1347–1350. [PubMed] [Google Scholar]
- 16.Yi H, Brooks ED, Thurberg BL. Correction of glycogen storage disease type III with rapamycin in a canine model. J Mol Med. 2014;92:641–650. doi: 10.1007/s00109-014-1127-4. [DOI] [PubMed] [Google Scholar]
- 17.Paoloni MC, Mazcko C, Fox E, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One. 2010;5:e11013. doi: 10.1371/journal.pone.0011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clavijo C, Strom T, Moll V, et al. Development and validation of a semi-automated assay for the highly sensitive quantification of Biolumus A9 in human whole blood using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3506–3514. doi: 10.1016/j.jchromb.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deters M, Kirchner G, Resch K, et al. Simultaneous quantification of sirolimus, everolimus, tacrolimus, and cyclosporine by liquid chromatography-mass spectrometry (LC-MS) Clin Chem Lab Med. 2002;40:285–292. doi: 10.1515/CCLM.2002.045. [DOI] [PubMed] [Google Scholar]
- 20.Bansel S, DeStefano A. Key elements of bioanalytical method validation for small molecules. AAPS J. 2007;9:E109–E114. doi: 10.1208/aapsj0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampen A, Zhang Y, Hackbarth I, et al. Metabolism and transport of the macrolide immunosuppressant sirolimus in the small intestine. J Pharmacol Exp Ther. 1998;285:1104–1112. [PubMed] [Google Scholar]
- 22.Davis NM, Takemoto JK, Brocks DR, et al. Multiple peaking phenomena in pharmacokinetic disposition. Clin Pharmacokinet. 2010;49:351–377. doi: 10.2165/11319320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Guba M, Koehl GE, Neppl E, et al. Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int. 2005;18:89–94. doi: 10.1111/j.1432-2277.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- 24.Salm P, Tresillian MJ, Taylor PJ, et al. Stability of sirolimus (rapamycin) in whole blood. Ther Drug Monit. 2000;22:423–426. doi: 10.1097/00007691-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Capone D, Gentile A, Polichetti G, et al. Stability of sirolimus and everolimus measured by immunoassay techniques in whole blood samples from kidney transplant patients. Int J Immunopathol Pharmacol. 2008;21:297–307. doi: 10.1177/039463200802100206. [DOI] [PubMed] [Google Scholar]
- 26.Kahan BD, Napoli KL, Kelly PA, et al. Therapeutic drug monitoring of sirolimus: correlations with efficacy and toxicity. Clin Transplant. 2000;14:97–109. doi: 10.1034/j.1399-0012.2000.140201.x. [DOI] [PubMed] [Google Scholar]


