Abstract
Despite the recent advances in the clinical management of melanoma, there remains a need for new pharmacological approaches to treat this cancer. 2-methoxyestradiol (2ME) is a metabolite of estrogen that has shown anti-tumor effects in many cancer types. In this study we show that 2ME treatment leads to growth inhibition in melanoma cells, an effect associated with entry into senescence, decreased pRb and CyclinB1 expression, increased p21/Cip1 expression and G2/M cell cycle arrest. 2ME treatment also inhibits melanoma cell growth in colony formation assays, including cell lines with acquired resistance to BRAF and BRAF+MEK inhibitors. We further show that 2ME is effective against melanoma with different BRAF and NRAS mutational status. Moreover, 2ME induced the retraction of cytoplasmic projections in a 3D spheroid model and significantly decreased cell proliferation in a 3D skin reconstruct model. Together our studies bring new insights into the mechanism of action of 2ME allowing melanoma targeted therapy to be further refined. Continued progress in this area is expected to lead to improved anti-cancer treatments and the development of new and more effective clinical analogues.
Graphical Abstract
Introduction
Melanoma incidence continues to increase year-on-year and is currently responsible for >80% of deaths from skin cancer (1). Melanomas are known to have the highest mutational load of all cancers. Among these, activating mutations in the serine-threonine kinase BRAF are the most prevalent and lead to the constitutive activation of the Ras–mitogen-activated protein kinase (MAPK) pathway (2). A number of small molecule inhibitors, including vemurafenib and dabrafenib, are successful at treating BRAF mutant melanoma, albeit for only limited periods of time (3–5). Indeed, long-term treatment has showed that the tumors often become resistant to such inhibitors (3, 4). Acquired BRAF inhibitor resistance is known to be mediated through several mechanisms with reactivation of the RAF-MEK-ERK signaling pathway being the most prevalent in >70% of cases. Increased RAF-MEK-ERK signaling can occur secondary to many events including the acquisition of NRAS mutations, mutations in MEK1/2, BRAF-splice mutants and through increased receptor tyrosine kinase signaling (6). Thus, despite the advances in the melanoma treatment, there is still an urgent need to identify new therapeutic strategies.
D’Amato and collaborators demonstrated the potential activity of 2-methoxyestradiol (2ME), a natural metabolite of the endogenous estrogen hormone 17βestradiol, to hinder angiogenesis through inhibition of tubulin polymerization (7). Moreover, Fotsis and collaborators revealed the high cytotoxicity of 2ME in vascular endothelial cells leading to inhibition of tumor angiogenesis and growth in vivo (8). Multiple in vitro and in vivo studies have shown the potential of 2ME to induce cell death and inhibit angiogenesis across many different tumor types (9–13). In melanoma, the antitumor effect of 2ME has been little explored. To date, one study has reported 2ME to induce G2/M phase cell cycle arrest and apoptosis in a human melanoma cell line (13). In another study, using eight different human melanoma cell lines, 2ME promoted apoptotic cell death by activation of the intrinsic pathway and blockage of cell cycle progression in vitro, as well as regression of the primary and metastatic tumors in vivo (14).
2ME has completed Phase I/II clinical trials for single-drug treatment of breast, renal, prostate, multiple myeloma and glioblastoma multiforme tumors (Clinicaltrials.gov - NCT00030095; NCT00444314; NCT00394810; NCT00592579; NCT00306618) and was associated with few side effects (15). In combination with other drugs, such as temozolomide (an alkylating agent) and sunitinib (a multi-targeted tyrosine kinase inhibitor), 2ME caused significant decrease in tumor growth (Clinicaltrials.gov - NCT00481455; NCT00444314). Overall, systemic toxicity was low and there was evidence of efficacy (16–18). In lights of these results, and the need for new drugs with anti-melanoma activity, we undertook testing of this compound in different melanoma cell lines harboring multiple mutation profiles, as well as in BRAF inhibitor (BRAFi) and BRAF+MEK inhibitor (BRAFi+MEKi) resistant cell lines. We demonstrated that 2ME is anti-proliferative and induces cell death in 2D and 3D models. Moreover, 2ME was able to inhibit colony formation and decrease the number of cellular projections in melanoma cells with acquired resistance to BRAFi and BRAFi+MEKi. Thus, 2ME has promising anti-melanoma activity that is worthy of further exploration.
MATERIAL AND METHODS
Cell culture
The melanoma cell lines SK-Mel-28, SK-Mel-103, SK-Mel-147, M245, WM1361A and WM1366 were kindly donated by Dr. Maria S. Soengas (Melanoma Group, CNIO, Spain). Primary cultures of fibroblasts were obtained from the foreskins of University Hospital (Hospital Universitário – HU-USP) patients, provided by Dr. Linda Maximiano. To this end, the project has undergone review and approval by the Ethics Committee of HU-USP (CEP Case 943/09). Melanoma cells and fibroblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin and 50 μg/ml streptomycin. All cells were maintained at 37°C under 5% CO2 atmosphere.
Proliferation assay
Cells were seeded in 96-well plates with 2.5 × 103 cells in 100 μL medium per well overnight before treatment with increasing concentrations of 2ME. Metabolic activity was assayed after incubation with 2ME for 72 hours, using Alamar Blue reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA) (19).
Clonogenic Assay
Cells were seeded on 6-well plates (5×103) and cultured for 24 h. After that, cells were treated with 0.0 or 0.5 μM of 2ME and maintained in culture for 14 days. The medium and treatment were replaced three times every week. Cells were further stained with crystal violet (0.5% w/v) for colonies counting.
Senescence-associated β-Galactosidase (SA-βgal)
Cells were seeded in six-well plates (5×104) and, after 24h, treated with 0.0 or 1.0 μM of 2ME. After fixation, SA-βgal cytochemical detection was performed according to Debacq-Chainiaux and collaborators (20). Plates were further incubated overnight at 37°C. Blue stained cells were detected under a bright-field microscope for senescence evaluation.
Protein expression by Western Blotting
Proteins were extracted and blotted (30 μg) as described by Faião-Flores and collaborators (2016) (21). After analysis, Western Blots were stripped once and reprobed for β-actin or GAPDH to show even protein loading. Antibodies to phosphorylated RB S807/811, total RB, Parp, Caspase-9, Caspase-7, Bcl-2, H3K9me3 and CyclinB1 were purchased from Cell Signaling Technology (Beverly, MA, USA). The monoclonal antibodies to β-actin and GAPDH were purchased from Sigma (St. Louis, MO, USA). Monoclonal antibody to p21 was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Cell cycle analysis by flow cytometry
Cells were seeded on 6-well plates (5×105) and cultured for 24 h. After that, cells were treated with 0.0, 0.5, 1.0 or 10.0 μM of 2ME for 24 or 48h. Then, cells were collected and pellets were washed with cold PBS. The cells were further incubated with propidium iodide (50 μg/mL) and 0.1% triton x-100 for 30 min followed by flow cytometry analysis (FACSCantoBeckton Dickson, CA, USA).
Apoptosis analysis by flow cytometry
After treatment with 2ME (0–10 μM) for 5 days, cells were washed once with Annexin binding buffer [10 mmol/L HEPES (pH 7.4), 140 mmol/L NaCl, 5 mmol/L CaCl2], resuspended in 100 μL binding buffer containing 1 μL APC-conjugated Annexin V (R&D Systems, Minneapolis, MN, USA), and incubated at 37°C for 15 min. Cells were then analyzed for Annexin V green fluorescence using flow cytometry.
Three-Dimensional (3D) Spheroid Growth
Melanoma spheroids were prepared using the liquid overlay method. Briefly, 200 μL of melanoma cells (25,000/mL) were added to a 96-well plate coated with 1.5% agar (Difco, Sparks, MD, USA). Plates were incubated for 72 hours, by which time cells had organized into 3D spheroids. Spheroids were then harvested and implanted into a bovine collagen I gel containing EMEM, L-glutamine, and 2% fetal bovine serum. Normal 2% melanoma medium was overlaid on top of the solidified collagen. Spheroids were treated with 5.0 μM of 2ME for 5 days. Spheroids were then washed twice in PBS before being treated with calcein-AM and ethidium bromide (Molecular Probes, Eugene, OR, USA) for 1 hour at 37°C according to the manufacturer’s instructions. After this time, pictures of the invading spheroids were taken using a Nikon-300 inverted fluorescence microscope. Calcein and ethidium bromide staining were used as a live/dead cell marker, respectively. The images were imported to Definiens Tissue Studio v4.1 software to analyze the fluorescence as described by Sandri et al., (2016) (22) and the ratio was determined by live fluorescence divided by dead fluorescence.
Human skin reconstructs containing melanoma cells
The skin model was reconstructed based on the previous description by Sandri and collaborators (2016) with minor changes (22). The SK-Mel-28 melanoma cells were incorporated into the epidermis at the same time as keratinocytes and melanocytes. After 24 hours of submersion, the skin samples were transferred to the air-liquid interface and maintained for two weeks in culture medium composed of DMEM/HAM’s F-12 supplemented with 10% FBS. Then, the reconstructed skin was treated with 5.0 μM of 2ME for 72h, followed by fixation with 4% paraformaldehyde solution and permeabilization with 0.2% Triton X-100 prior to blockage with 1% BSA/PBS. The samples were incubated with hematoxylin/eosin (HE) for histology or primary antibodies (Laminin and Filagrin) for immunofluorescence overnight at 4°C. On the next day, the immunohistochemistry samples were incubated with secondary antibodies for 1 hour at room temperature and washed with PBS. The coverslips were mounted with ProLong Gold antifade reagent with DAPI (Life Technologies, Carlsbad, CA, USA) and imaged using fluorescence microscopy.
Statistical analysis
Data show the mean of at least 3 independent experiments. GraphPad Prism 5 statistical software was used to perform the Student’s t test for comparison of treatment effects in the different culture conditions. Results were considered statistically significant when where (*) indicates p≤0.05, (**) indicates 0.05≤ P ≤ 0.01, (***) indicates P≤0.001.
RESULTS
2ME induces inhibition of proliferation in melanoma cells
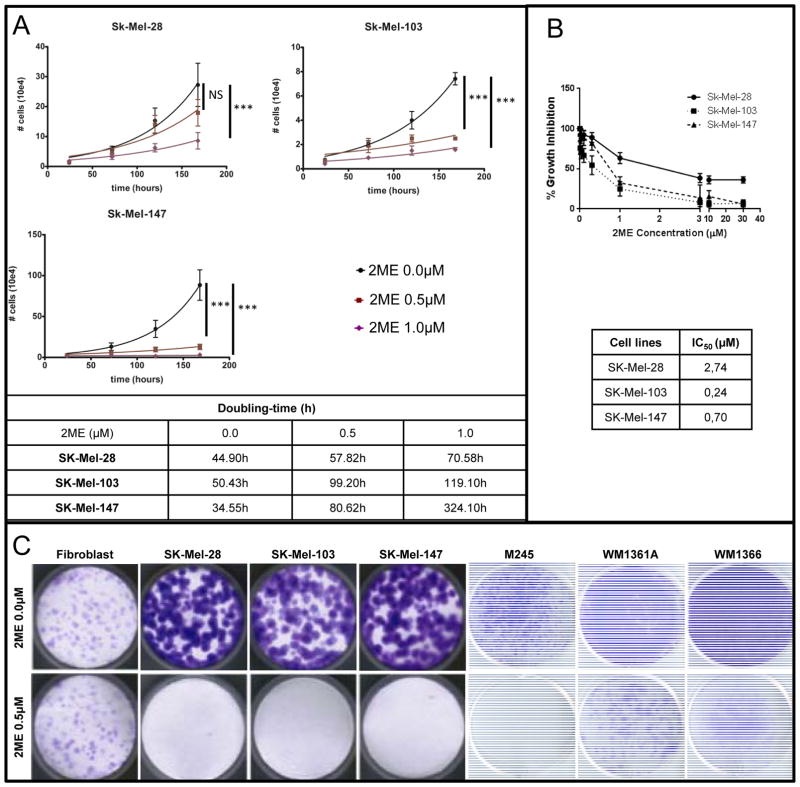
Experiments were carried out to compare the growth rate of melanoma cells under different concentrations of 2ME (0.0, 0.5 and 1.0 μM). As observed in Figure 1A, cell growth was significantly decreased starting from 0.5 μM for SK-Mel-103 and SK-Mel-147 cell lines (NRAS mutated), and starting from 1.0 μM for SK-Mel-28 cell line (BRAF mutated). To calculate the 50% inhibitory concentration (IC50) of 2ME, cells were incubated for 72h with 2ME at concentrations ranging from 0.01 to 30 μM, as shown in Figure 1B. Interestingly, NRAS mutated SK-Mel-103 and SK-Mel-147 cell lines were more sensitive to 2ME effects, and had IC50 values below 1 μM.
Figure 1.
Antiproliferative activity of 2ME in human melanoma cell lines. (A) Growth curves of Sk-Mel-28, Sk-Mel-103 and Sk-Mel-147 cell lines after 0.0, 0.5 or 1.0 μM of 2ME for 7 days. (B) Growth curves of Sk-Mel-28, Sk-Mel-103 and Sk-Mel-147 cell lines for IC50 estimation. (C) Clonogenic assay with normal fibroblasts and melanoma cell lines Sk-Mel-28, Sk-Mel-103, Sk-Mel-147, M245, WM1361A and WM1366 treated with 2ME for 14 days. Cells were stained with crystal violet. Experiments were performed in triplicates. Values are expressed as mean ± s.d. Significance is indicated by *P<0.05, **P<0.01 and ***P<0.001.
We then evaluated the effects of 2ME in the melanoma cell lines in long-term colony formation assays using skin fibroblasts as a control normal cell line. These results showed that 2ME treatment at 0.5 μM promoted a decrease in colony formation in all the melanoma cell lines studied, but not in the normal, non-transformed cells (Figure 1C). We also evaluated the effects of 2ME in BRAFi (vemurafenib) resistant (R) and BRAFi+MEKi (vemurafenib + trametinib) resistant (RT) melanoma cell lines, obtained by chronic exposure to the respective inhibitors and found that the resistant cell lines also showed sensitivity to 0.5 μM of 2ME (Supplementary Figure 1).
Senescence phenotype and cell cycle arrest are induced by 2ME in melanoma cell lines
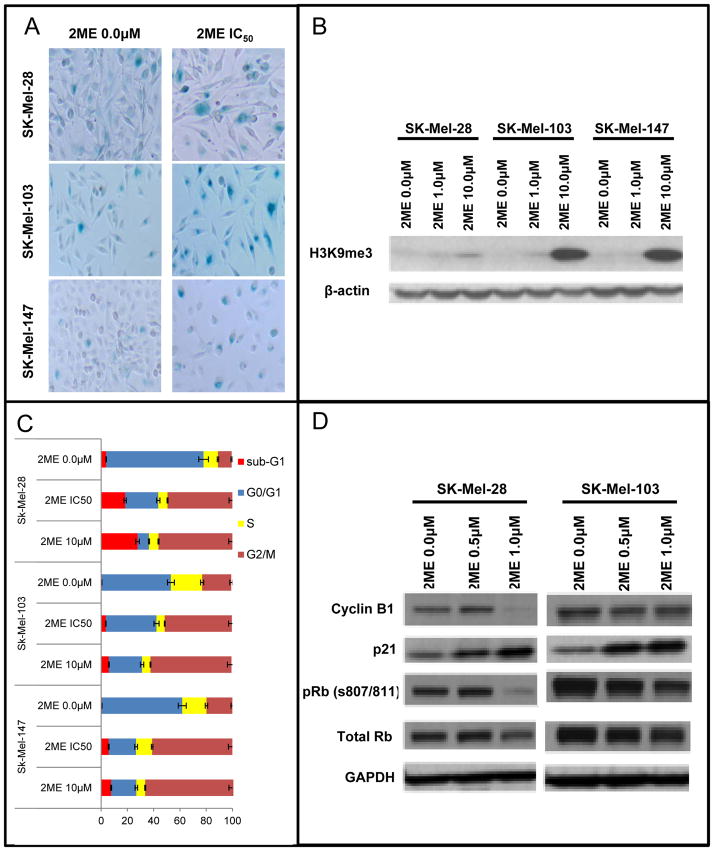
We next turned our attention to the mechanisms associated with 2-ME-mediated growth inhibition and began by evaluating potential entry into a senescence-like state (23). It was found that 2ME (1.0 μM) induced senescence as shown by increased senescence associated Beta-galactosidase (SA-βgal) blue staining after 5 days of culture (Figure 2A). Treatment of the SK-Mel-28, SK-Mel-103 and SK-Mel-147 with their IC50 value of 2ME showed a higher percentage of SA-βgal staining compared to the control group (Supplementary table 1). Expression of the senescence marker Histone H3 trimethylated in the Lysine 9 (H3K9Me3), also showed a slight increase following treatment of the cells with 10μM of 2ME for 72h (Figure 2B).
Figure 2.
2ME treatment induces senescence and cell cycle arrest in human melanoma cells. (A) Senescence-associated β-galactosidase cytochemical detection after treatment with 1.0 μM of 2ME for 5 days. (B) Immunoblotting for Histone H3 trimethyl Lysine 9 (H3K9ME3) after treatment with 1.0 and 10.0 μM of 2ME for 5 days. β-actin was used as a loading control. (C) Cell cycle progression evaluation by flow cytometry after 2ME treatment for 24 and 48h. (D) Western Blotting analysis of cell cycle-related proteins after treatment with 0.5 and 1.0 μM of 2ME for 5 days. GAPDH was used as a loading control. Experiments were performed in triplicates.
In cell cycle analysis studies, a 24h treatment with the IC50 and 10.0μM of 2ME, led to a G2/M phase cell cycle arrest in SK-Mel-28, SK-Mel-103 and SK-Mel-147 cell lines (Figure 2C). The same effect was not observed at 0.5μM and 1.0μM in this acute exposure. We next investigated the effects of 2ME upon the expression of a series of of important cell cycle proteins by Western Blotting. In these studies we treated SK-Mel-28 and SK-Mel-103 cell lines with 0.5 and 1.0μM of 2ME for 5 days (Figure 2D). It was found that phosphorylation of the Rb protein at serine 807 and 811 (pRb) were down-regulated in both cell lines. pRb is required for the exit of the G0 phase of the cell cycle, and its phosphorylation is enhanced by Cyclin C expression (24), hence, inhibition of pRb S807/811 is associated with cell cycle arrest. Additionally, p21Cip1, an inhibitor of the CDK/Cyclin complex, was also up-regulated in the presence of 2ME. Furthermore, Cyclin B1 also showed an up-regulation. The coordinated change in all of these cell cycle proteins is likely to underlie the effects of 2ME upon cell proliferation. To further investigate the molecular mechanisms underlying the inhibition of proliferation, we examined the potential effects of 2ME upon pERK signaling by Western Blotting. After treatment with 1.0μM of 2ME (1h -72h), no alteration of ERK phosphorylation status was observed. These results suggest that the inhibition of proliferation is not due to direct inhibition of the MAPK signaling (Supplementary figure 2). This led us to hypothesize that inhibition of the MAPK pathway in combination with 2ME would further inhibit cell growth and proliferation, leading to more effective tumor growth inhibition.
2ME induces the apoptotic pathway in melanoma cell lines
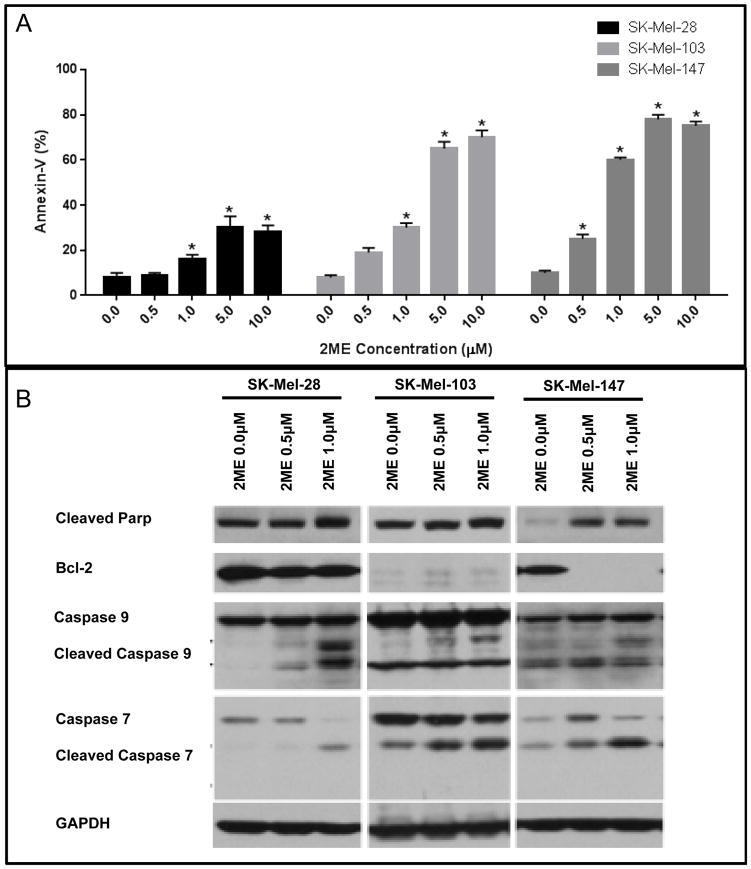
Next we assessed apoptosis induction through detection of Annexin V positive cells by flow cytometry. 2ME induced significant levels of apoptotic cell death starting at a concentration of 1.0 μM in SK-Mel-28 and SK-Mel-103 cells, and from 0.5 μM in SK-Mel-147 cell line (Figure 3A). It was additionally found that 2ME treatment increased expression of cleaved PARP, caspase-9 and caspase-7 in all of the cell lines after 5 days of treatment, especially at the higher concentration (1 μM). The anti-apoptotic protein Bcl-2 was downregulated after 2ME treatment only in the SK-Mel-147 cell line, showing that, although apoptosis is induced across cell lines, the mechanism of induction may be cell type-dependent (Figure 3B).
Figure 3.
Apoptotic cell death induced by 2ME treatment. (A) Flow cytometry analysis of apoptotic cell death (Annexin V positive cells) after treatment with 0.5, 1.0, 5.0 and 10.0 μM of 2ME for 5 days. (B) Western Blotting analysis of pro- and anti-apoptotic proteins after treatment with 2ME for 3 days. GAPDH was used as a loading control. Experiments were performed in triplicates. Values are expressed as mean ± s.d. Significance is indicated by *P<0.05, **P<0.01 and ***P<0.001.
Invasion decreases after 2ME treatment in 3D models
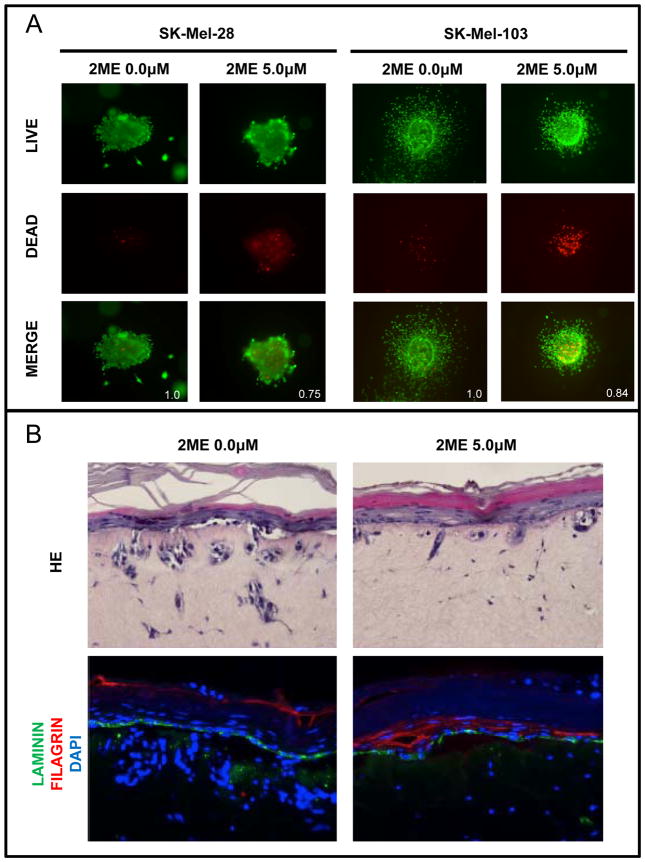
To test the effects of 2ME in a model more analogous to the in vivo 3D tumor environment, we next performed a series of 3D spheroid assays. The results showed that 2ME induced cell death as indicated by an increase in red fluorescence showing 25% and 16% of decrease in live/dead ratio in SK-Mel-28 and SK-Mel-103 melanoma cell lines (Figure 4A). In addition, we noted a retraction of melanoma cell cytoplasmic projections from the spheroids after 2ME treatment, possibly indicating anti-metastatic activity. The growth inhibition and the increase in cell death were also observed in SK-Mel-28RT cells, which are resistant to BRAFi+MEKi therapy (Supplementary Figure 3), showing that, even in a 3D model, 2ME was able to overcome acquired resistance to targeted kinase inhibitors.
Figure 4.
Decreased invasive potential induced by 2ME treatment. (A) 3D spheroids showing live (green – Calcein stained) and dead (red – Propidium iodide stained) Sk-Mel-28 and Sk-Mel-103 cells after treatment with 5.0 μM of 2ME for 5 days (400x magnification). (B) Reconstructed skin with Sk-Mel-28 melanoma cells after treatment with 5.0 μM of 2ME for 5 days. Top slides show HE staining and bottom slides show immunofluorescence staining for laminin (green), filagrin (red) and dapi (blue) (200x magnification).
These effects were further confirmed in the 3D artificial skin model with melanoma cells, as previously described (21, 22, 25). Untreated skin reconstructs were marked by melanoma invasion and/or migration through the basal membrane into the dermis, which is visible in both HE staining and fluorescence microscopy; invading melanoma cells nuclei are stained in blue by DAPI, below the basal membrane, and are shown in green by Laminin immunofluorescence. After 2ME treatment, the melanoma cells in this model showed significant decreases in proliferation as indicated by the lower DAPI staining. Furthermore, treated cells were scarcely detected in the dermis, indicating lower invasion/migration (Figure 4B).
The combination of BRAFi, MEKi and 2ME shows additive effect on naïve cells
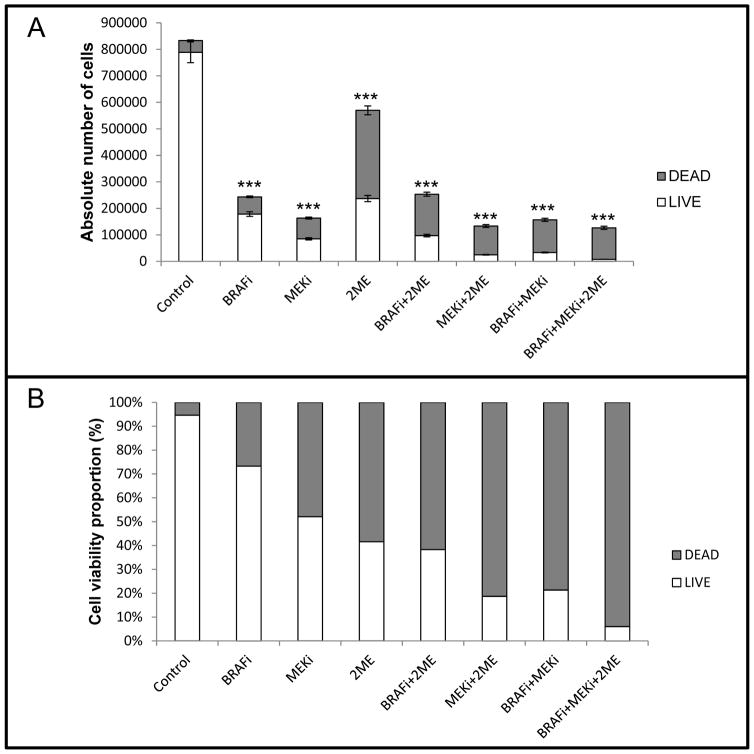
When used as a monotherapy, BRAFi, MEKi and 2ME showed 70.8, 80.4 and 31.6% of cell proliferation, respectively, after 72h (Figure 5A). While 2ME showed relatively lower potential in inhibition of cell proliferation, it induced more cell death (Figure 5B). Moreover, MEKi treatment combined with either BRAFi or 2ME was associated with a significantly higher cell death ratio (81.3 and 78.7%, respectively) in comparison to BRAFi, MEKi and 2ME monotherapies (26.7, 47.9 and 58.4%, respectively). Together, these three agents inhibited cell proliferation by >80% and led to an induction of cell death in >94% of the cells.
Figure 5.
Combinatory therapy with BRAFi+MEKi+2ME induced cell proliferation inhibition and increase of cell death in naïve SK-Mel-28 melanoma cells. (A) Absolute number of cells after treatment with BRAFi (250nM), MEKi (2.5nM) and/or 2ME (500nM) for 72h. (B) Cell viability proportion normalized to 100% for each condition after treatment with BRAFi (250nM), MEKi (2.5nM) and/or 2ME (500nM) for 72h. Experiments were performed in triplicates. Values are expressed as mean ± s.d. Significance is indicated by *P<0.05, **P<0.01 and ***P<0.001.
Discussion
In the present work, we showed 2ME to have antiproliferative and cytotoxic effects, in 2D and 3D human melanoma cell culture models. 2ME is a metabolite of estrogen and has antitumoral activity against many cancer cells, such as lung cancer (9), ovarian cancer (10), hepatocellular carcinoma (11), colon cancer (12) and melanoma (13). Herein, 2ME affected cell lines with different genetic backgrounds, confirming that its effects are independent of BRAF and NRAS mutational status. It was shown for the first time that this drug has anti-mitotic and cytotoxic effects on BRAFV600E mutant melanoma cell lines that are resistant not only to BRAFi (Vemurafenib) but also to the BRAFi+MEKi combination.
Previous work in the field has shown that 2ME presents anti-proliferative effects in melanoma, but the mechanisms involved remain poorly understood. The first related study published by Ghosh and collaborators revealed the selective antiproliferative effect of 2ME against melanoma cell lines, while but not against non-tumorigenic fibroblasts (13). In addition, Dobos and collaborators showed proliferation inhibition, induction of apoptosis and alterations in tubulin polymerization in human melanoma (14). Different reviews on microtubule-targeting agents (MAT) cite 2ME as an anti-cancer agent that acts through impairment of tubulin polymerization by interaction with the colchicine site on β-tubulin subunit (26, 27).
In our study, we found that, in addition to the effects of 2ME upon tubulin polymerization (data not shown), there were other effects that led to the inhibition of proliferation. Of note, 2ME was found to induce senescence in all the melanoma cell lines tested. The senescence phenotype was more pronounced in the SK-Mel-103 and SK-Mel-147 cell lines, an effect associated with enhanced SA-βgal staining and a higher level of H3K9me3 expression. The expression of SA-βgal is the most widely used readout of senescence induction (28). Moreover, H3K9me3 expression is related to cell cycle arrest by local transcription repression of E2F-complexed retinoblastoma protein (29).
Despite showing anti-mitotic effect, the specific mechanisms of 2ME upon the cell cycle have not been previously reported in melanoma. 2ME is known to induce cell cycle arrest in G2/M phase in different tumors, i.e., osteosarcoma (30), esophageal carcinoma (31), prostate cancer (32), human endometrial cells (33) and melanoma (14). Correspondingly, we found that 2ME blocked cell cycle progression in melanoma cells, which was associated with dose-dependent upregulation of p21Cip1, inhibition of pRB phosphorylation (S807/S811) and inhibition of Cyclin B1 expression. It is likely that the combined modulation of all of these proteins leads to cell cycle arrest and inhibition of proliferation in these cells.
As previously described by other authors investigating gastric carcinoma (34), prostate cancer (35) and melanoma cells (13, 14), 2ME induced apoptosis in all melanoma cell lines evaluated herein, which was demonstrated by flow cytometric analysis of Annexin V staining and confirmed by Western Blot analysis of caspase and PARP cleavage. The induction of cell death was also observed in a 3D spheroid melanoma model after treatment with 2ME. Moreover, in this 3D model, we could observe inhibition of spheroid size and a decrease of invasion/migration potential by reduction of cellular projections. Likewise, invasive potential was modulated in a 3D reconstructed skin model as noted by the decrease in invasion/migration of melanoma cells into the dermis. Such modulation might be explained by the known antimitogenic and antimetastatic effects of 2ME (36).
Here we demonstrated that 2ME has multiple anti-tumorigenic properties in melanoma cells including the inhibition of proliferative capability, induction of cell cycle arrest and inhibition of invasive potential. Despite research advances, 2ME has limited biological accessibility and fast metabolic breakdown (37, 38). For this reason, many 2ME analogues have been developed and are currently undergoing preclinical evaluation (26, 38–42). Satisfactorily, these analogues have shown an increased bioavailability (43), while retaining their important mechanisms of action such as microtubule targeting, leading to proliferation inhibition and apoptosis induction of tumor cells (26).
In conclusion, we have shown for the first time that 2ME modulates cell cycle proteins leading to cell cycle arrest and induces senescence in melanoma cells. Interestingly, it was shown that such mechanisms were independent of BRAF or NRAS mutational status, since 2ME led to the same effects on colony inhibition in wild-type and mutant cells. Furthermore, inhibition of colonies formation and reduction of spheroids in resistant cells demonstrate that 2ME treatment can overcome BRAFi and BRAFi+MEKi resistance in melanomas. In view of its promising results in melanoma cells, it is of major importance to further understand the 2ME basic mechanisms of action. Progress in this area should allow us to improve melanoma treatment, through the development of new drug combinations that lead to improved clinical outcomes.
Supplementary Material
Supplementary Table 1 – Senescent cells (%)
Acknowledgments
This work was supported by Fapesp (grant numbers 2010/50300-2, 2012/05732-7, 2013/05172-4, 2014/24400-0 and 2015/10821-7), and CNPq (grant numbers 150447/2013-2 and 471512/2013-3).
References
- 1.Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: from mutations to medicine. Gene Dev. 2012;26:1131–1155. doi: 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Smalley KS, Sondak VK. Melanoma--an unlikely poster child for personalized cancer therapy. The New England journal of medicine. 2010;363:876–878. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- 4.Aplin AE, Kaplan FM, Shao Y. Mechanisms of resistance to RAF inhibitors in melanoma. J Invest Dermatol. 2011;131:1817–1820. doi: 10.1038/jid.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smalley KS. Understanding melanoma signaling networks as the basis for molecular targeted therapy. J Invest Dermatol. 2010;130:28–37. doi: 10.1038/jid.2009.177. [DOI] [PubMed] [Google Scholar]
- 6.Nikolaou VA, Stratigos AJ, Flaherty KT, Tsao H. Melanoma: New Insights and New Therapies. J Invest Dermatol. 2012;132:854–863. doi: 10.1038/jid.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damato RJ, Lin CM, Flynn E, Folkman J, Hamel E. 2-Methoxyestradiol, an Endogenous Mammalian Metabolite, Inhibits Tubulin Polymerization by Interacting at the Colchicine Site. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3964–3968. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotsis T, Zhang YM, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigerer L. The Endogenous Estrogen Metabolite 2-Methoxyoestradiol Inhibits Angiogenesis and Suppresses Tumor-Growth. Nature. 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 9.Aquino-Galvez A, Gonzalez-Avila G, Delgado-Tello J, Castillejos-Lopez M, Mendoza-Milla C, Zuniga J, Checa M, Maldonado-Martinez HA, Trinidad-Lopez A, Cisneros J, Torres-Espindola LM, Hernandez-Jimenez C, Sommer B, Cabello-Gutierrez C, Gutierrez-Gonzalez LH. Effects of 2-methoxyestradiol on apoptosis and HIF-1 alpha and HIF-2 alpha expression in lung cancer cells under normoxia and hypoxia. Oncol Rep. 2016;35:577–583. doi: 10.3892/or.2015.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding L, Wang XQ, Zhang J, Mu ZL, Zhou XX, Liu PS. Underlying mechanism of 2-methoxyestradiol-induced apoptosis and growth arrest in SKOV3 human ovarian cancer cells. Eur Rev Med Pharmaco. 2015;19:2084–2090. [PubMed] [Google Scholar]
- 11.Ma L, Li GX, Zhu HQ, Dong XS, Zhao DL, Jiang X, Li J, Qiao HQ, Ni SB, Sun XY. 2-Methoxyestradiol synergizes with sorafenib to suppress hepatocellular carcinoma by simultaneously dysregulating hypoxia-inducible factor-1 and-2. Cancer letters. 2014;355:96–105. doi: 10.1016/j.canlet.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Jee SB, Park WY, Choi YJ, Kim B, Kim YH, Jun DY, Kim YH. Tumor Suppressor Protein p53 Promotes 2-Methoxyestradiol-Induced Activation of Bak and Bax, Leading to Mitochondria-Dependent Apoptosis in Human Colon Cancer HCT116 Cells. J Microbiol Biotechn. 2014;24:1654–1663. doi: 10.4014/jmb.1405.05062. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh R, Ott AM, Seetharam D, Slaga TJ, Kumar AP. Cell cycle block and apoptosis induction in a human melanoma cell line following treatment with 2-methoxyoestradiol: therapeutic implications? Melanoma Res. 2003;13:119–127. doi: 10.1097/00008390-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Dobos J, Timar J, Bocsi J, Burian Z, Nagy K, Barna G, Petak I, Ladanyi A. In vitro and in vivo antitumor effect of 2-methoxyestradiol on human melanoma. Int J Cancer. 2004;112:771–776. doi: 10.1002/ijc.20473. [DOI] [PubMed] [Google Scholar]
- 15.Kumar BS, Raghuvanshi DS, Hasanain M, Alam S, Sarkar J, Mitra K, Khan F, Negi AS. Recent Advances in chemistry and pharmacology of 2-methoxyestradiol: An anticancer investigational drug. Steroids. 2016;110:9–34. doi: 10.1016/j.steroids.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Muh CR, Joshi S, Singh AR, Kesari S, Durden DL, Makale MT. PTEN status mediates 2ME2 anti-tumor efficacy in preclinical glioblastoma models: role of HIF1 alpha suppression. J Neuro-Oncol. 2014;116:89–97. doi: 10.1007/s11060-013-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahut WL, Lakhani NJ, Gulley JL, Arlen PM, Kohn EC, Kotz H, McNally D, Parr A, Nguyen D, Yang SX, Steinberg SM, Venitz J, Sparreboom A, Figg WD. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006;5:22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 18.Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Update. 2003;6:355–361. doi: 10.1016/j.drup.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod. 2007;22:1304–1309. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 20.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-beta gal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 21.Faiao-Flores F, Alves-Fernandes DK, Pennacchi PC, Sandri S, Vicente AL, Scapulatempo-Neto C, Vazquez VL, Reis RM, Chauhan J, Goding CR, Smalley KS, Maria-Engler SS. Targeting the hedgehog transcription factors GLI1 and GLI2 restores sensitivity to vemurafenib-resistant human melanoma cells. Oncogene. 2016 doi: 10.1038/onc.2016.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandri S, Faiao-Flores F, Tiago M, Pennacchi PC, Massaro RR, Alves-Fernandes DK, Berardinelli GN, Evangelista AF, de Lima Vazquez V, Reis RM, Maria-Engler SS. Vemurafenib resistance increases melanoma invasiveness and modulates the tumor microenvironment by MMP-2 upregulation. Pharmacol Res. 2016;111:523–533. doi: 10.1016/j.phrs.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- 24.Ren S, Rollins BJ. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell. 2004;117:239–251. doi: 10.1016/s0092-8674(04)00300-9. [DOI] [PubMed] [Google Scholar]
- 25.Brohem CA, Cardeal LB, Tiago M, Soengas MS, Barros SB, Maria-Engler SS. Artificial skin in perspective: concepts and applications. Pigment cell & melanoma research. 2011;24:35–50. doi: 10.1111/j.1755-148X.2010.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chua YS, Chua YL, Hagen T. Structure Activity Analysis of 2-Methoxyestradiol Analogues Reveals Targeting of Microtubules as the Major Mechanism of Antiproliferative and Proapoptotic Activity. Mol Cancer Ther. 2010;9:224–235. doi: 10.1158/1535-7163.MCT-09-1003. [DOI] [PubMed] [Google Scholar]
- 27.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 28.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 29.Fan DN, Schmitt CA. Detecting Markers of Therapy-Induced Senescence in Cancer Cells. Methods in molecular biology. 2017;1534:41–52. doi: 10.1007/978-1-4939-6670-7_4. [DOI] [PubMed] [Google Scholar]
- 30.Gorska M, Wyszkowska RM, Kuban-Jankowska A, Wozniak M. Impact of Apparent Antagonism of Estrogen Receptor beta by Fulvestrant on Anticancer Activity of 2-Methoxyestradiol. Anticancer research. 2016;36:2217–2226. [PubMed] [Google Scholar]
- 31.Du B, Zhao Z, Sun H, Ma S, Jin J, Zhang Z. Effects of 2-methoxyestradiol on proliferation, apoptosis and gene expression of cyclin B1 and c-Myc in esophageal carcinoma EC9706 cells. Cell biochemistry and function. 2012;30:158–165. doi: 10.1002/cbf.1830. [DOI] [PubMed] [Google Scholar]
- 32.Ray G, Dhar G, Van Veldhuizen PJ, Banerjee S, Saxena NK, Sengupta K, Banerjee SK. Modulation of cell-cycle regulatory signaling network by 2-methoxyestradiol in prostate cancer cells is mediated through multiple signal transduction pathways. Biochemistry. 2006;45:3703–3713. doi: 10.1021/bi051570k. [DOI] [PubMed] [Google Scholar]
- 33.Gong QF, Liu EH, Xin R, Huang XN, Gao N. 2ME and 2OHE2 exhibit growth inhibitory effects and cell cycle arrest at G2/M in RL95-2 human endometrial cancer cells through activation of p53 and Chk1. Mol Cell Biochem. 2011;352:221–230. doi: 10.1007/s11010-011-0757-x. [DOI] [PubMed] [Google Scholar]
- 34.Lin HL, Liu TY, Wu CW, Chi CW. 2-Methoxyestradiol-induced caspase-3 activation and apoptosis occurs through G(2)/M arrest dependent and independent pathways in gastric carcinoma cells. Cancer. 2001;92:500–509. doi: 10.1002/1097-0142(20010801)92:3<500::aid-cncr1348>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Bu SZ, Blaukat A, Fu X, Heldin NE, Landstrom M. Mechanisms for 2-methoxyestradiol-induced apoptosis of prostate cancer cells. Febs Lett. 2002;531:141–151. doi: 10.1016/s0014-5793(02)03478-6. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher G, Kataoka M, Roth JA, Mukhopadhyay T. Potent antitumor activity of 2-methoxyestradiol in human pancreatic cancer cell lines. Clinical Cancer Research. 1999;5:493–499. [PubMed] [Google Scholar]
- 37.Foster PA, Stengel C, Ali T, Leese MP, Potter BVL, Reed MJ, Purohit A, Newman SP. A comparison of two orally bioavailable anti-cancer agents, IRC-110160 and STX140. Anticancer research. 2008;28:1483–1491. [PubMed] [Google Scholar]
- 38.Visagie M, Mqoco T, Joubert A. Sulphamoylated estradiol analogue induces antiproliferative activity and apoptosis in breast cell lines. Cell Mol Biol Lett. 2012;17:549–558. doi: 10.2478/s11658-012-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theron AE, Nolte EM, Lafanechere L, Joubert AM. Molecular crosstalk between apoptosis and autophagy induced by a novel 2-methoxyestradiol analogue in cervical adenocarcinoma cells. Cancer Cell Int. 2013;13 doi: 10.1186/1475-2867-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kambhampati S, Rajewski RA, Tanol M, Haque I, Das A, Banerjee S, Jha S, Burns D, Borrego-Diaz E, Van Veldhuizen PJ, Banerjee SK. A Second-Generation 2-Methoxyestradiol Prodrug Is Effective against Barrett’s Adenocarcinoma in a Mouse Xenograft Model. Mol Cancer Ther. 2013;12:255–263. doi: 10.1158/1535-7163.MCT-12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visagie MH, Birkholtz LM, Joubert AM. 17-Beta-Estradiol Analog Inhibits Cell Proliferation by Induction of Apoptosis in Breast Cell Lines. Microsc Res Techniq. 2014;77:236–242. doi: 10.1002/jemt.22334. [DOI] [PubMed] [Google Scholar]
- 42.Visagie MH, Birkholtz LM, Joubert AM. A 2-methoxyestradiol bis-sulphamoylated derivative induces apoptosis in breast cell lines. Cell Biosci. 2015;5 doi: 10.1186/s13578-015-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stander A, Joubert F, Joubert A. Docking, Synthesis, and in vitro Evaluation of Antimitotic Estrone Analogs. Chem Biol Drug Des. 2011;77:173–181. doi: 10.1111/j.1747-0285.2010.01064.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 – Senescent cells (%)