Abstract
Objective
Our aim was to characterize the nutrient intake of children with chronic kidney disease (CKD) relative to recommended intake levels.
Methods
We conducted a cross-sectional study of dietary intake assessed by food frequency questionnaire (FFQ) in The North American Chronic Kidney Disease in Children (CKiD) prospective cohort study. Nutrient intake was analyzed to estimate the daily consumption levels of various nutrients and compared to national guidelines for intake.
Results
There were 658 FFQs available for analysis; 69.9% of respondents were boys, with a median age (Interquartile range [IQR]) of 11 years (8–15). Median daily sodium, potassium and phosphorus intake of the cohort was 3089 mg (2294–4243), 2384 mg (1804–3076) and 1206 mg (894–1612) respectively. Sodium and phosphorus consumptions were higher than recommended in all age groups. Caloric intake decreased with dropping glomerular filtration rate (p=0.003). Median daily caloric intakes were 1307 kcal in male children 2–3 years old, 1875 kcal in 4–8 year old, 1923 kcal in those 9–13 years old, and 2427 kcal in those 14–18 years old. Respective levels for girls were 1467 kcal, 1736 kcal, 1803 kcal, and 2281 kcal. Median protein intake exceeded recommended levels in all age groups, particularly among younger participants. Younger children were more likely than older children to exceed the recommended intakes for phosphorus (p<0.001) and the age-specific recommended caloric intake (p<0.001). Macronutrient distribution (carbohydrate: fat: protein) was consistent with recommendation.
Conclusions
Children in the CKiD cohort consumed more sodium, phosphorus, protein and calories than recommended. The gap between actual consumption and recommendations indicates a need for improved nutritional counseling and monitoring.
Keywords: Dietary assessment, Nutrient Intake, Children, Chronic Kidney Disease
Introduction
Achieving an optimal nutritional status is essential for managing pediatric chronic kidney disease (CKD) and dietary guidance is frequently provided in clinical practice to achieve a metabolic balance, which is vital for normal growth. Guidelines addressing optimal macro- and micronutrient intake for children with CKD are available [1], with intake of sodium, potassium, phosphorus, protein and total calories being common targets of nutritional monitoring.
The relationship between sodium intake and the development of hypertension has been described in many epidemiological studies [2]. In addition to hypertension, increased dietary sodium is associated with worsening of albuminuria [3] and blunting of the anti-proteinuric efficacy of an angiotensin-converting enzyme inhibitor (ACEI) [4]. In the U.S., a daily sodium intake <1500 mg for people with CKD has been recommended [5]. Both the Kidney Disease Outcomes Quality Initiative (KDOQI) [1] and Kidney Disease: Improving Global Outcomes (KDIGO) [6] guidelines have adopted the age-based recommended daily intake levels derived from healthy children for those with CKD. Although direct evidence relating dietary potassium and phosphorus intake to outcomes in pediatric CKD is scarce, nutritional monitoring and counseling such intake are still much emphasized in clinical practice due to the potential adverse outcome related to hyperkalaemia and the importance of controlling metabolic bone disease through prevention of hyperphosphatemia.
Malnutrition and protein energy wasting are common causes of poor growth in children with CKD [7]. Protein intake is recommended to be at least 100% of the dietary reference intakes (DRI) for ideal body weight in children with CKD stages 3–5 [1,7]. Although caloric intake is also an important determinant of growth [7], it is well known that children with compromised renal function often have reduced energy intake resulting in growth failure [8]. Intensive nutritional support and management is therefore particularly important for growth of children with CKD, especially during the first two years of life when nutritional intake has a strong direct relationship with growth velocity [9]. Conversely, the independent effect of obesity on progression of CKD is of growing concern [10,11]. Interestingly, in children with end-stage renal disease (ESRD), both low and high body mass index (BMI) are associated with an increased risk of mortality [12]. In addition, the composition of macronutrients is important. For example, a study in Taiwan demonstrated that among 599 adult stage 3–5 nondialysis CKD patients, overall energy intake was lower, whereas the dietary protein intake was higher than recommended, and both were associated with lower GFR [13]. In fact, the KDOQI guidelines provide recommended ranges for age-based macronutrient distribution for children with CKD stage 2-5D [1].
While national surveys of nutrient intake among the general population exist in the U.S. [14], dietary data specifically targeting pediatric CKD populations are limited. The Chronic Kidney Disease in Children (CKiD) study is a multi-center prospective cohort study of childhood CKD conducted in North America [15]. A Food Frequency Questionnaire (FFQ) was used to prospectively collect dietary intake data (Supplementary Table 1). In this analysis, we describe the baseline nutrient intake among CKiD participants. We also compare our results with those reported in the general population and the recommended targets from the KDOQI and KDIGO guidelines.
Materials and Methods
Study participants and Design
The CKiD study enrolled children aged 1–16 years with estimated GFR (eGFR) of 30–90 ml/min/1.73m2 (Cohort 1, enrolled between 2005 to 2009) using the Schwartz formula [16] or 45–90 ml/min/1.73m2 (Cohort 2, enrolled since 2011) using the updated Schwartz formula [17] across centers from North America. The study design has been previously reported [15] and details of the inclusion/exclusion criteria are shown in the Supplementary Table. Each participating site received institutional review board approval and all participants provided informed consent.
Patient demographic and biomedical data
Patient data collected at the baseline visit of interest for this analysis include demographic data and diagnosis (glomerular or non-glomerular disease). Diagnoses were classified into these two categories, as this may have potential impact on nutritional intake, particularly salt consumption. For the analysis of sodium intake, the diagnoses were split between salt losing (obstructive uropathy, aplastic/hypoplastic/dysplastic kidneys, syndrome of agenesis of abdominal musculature, and congenital urologic disease [bilateral hydronephrosis]) and nonsalt losing (all other diagnoses). Height and weight were determined by taking the average of two independent measures at each study visit. An early morning urine sample was collected for measurement of proteinuria. Baseline GFR of individual particants was measured by the disappearance curve of plasma iohexol [18], which may be different from the estimated GFR obtained at enrollment. When a measured GFR was unavailable, it was estimated using equations developed from the CKiD study [9].
Food Frequency Questionnaire (FFQ)
The FFQ used in CKiD was adopted and modified from the Harvard Services Food Frequency Questionnaire, which was originally developed as a 61-item questionnaire for assessing the nutrient intake of low-income women aged ≥16 years old [20]. It was subsequently modified as a 84-item FFQ and is validated as a dietary assessment tool in native American and Caucasian children 1–5 years of age [21]. In fact, FFQ is a widely adopted method for assessment of dietary intake in different populations, including both paediatric and adult subjects [22–25]. The FFQ used in CKiD consists of three different versions targeting children aged 1–5 years, children attending K-8th grade, and those attending 9th–12th grade, and contains either 86 or 87 food items to which respondents indicate the frequency of consuming a serving of each food item in the past 28 days. The FFQ was first collected six months after enrollment, then annually. It was completed either by the participant or their guardian(s) through self-reporting or assisted by a study coordinator. Study coordinators were trained in providing standard instructions to the participant/guardian.
Dietary data analysis and covariate definition
Only the FFQs obtained at the baseline were analyzed. The reported frequency of consuming any particular food item on the FFQ was first divided by 28 to obtain a daily consumption frequency. For each food item, a reference value was available for (i) standard portion size by age and (ii) average nutrients and energy level by review of several existing databases and references [14, 26–28]. All foods on the questionnaire were analyzed using the Nutrition Data System for Research (NDSR, version 2013). The specific daily nutrient or caloric intake for a particular food item could then be calculated by the following formula: daily consumption frequency * standard portion size * average nutritional value.
Total energy and all other nutrient levels were determined by summing the values obtained for each individual food item. Estimated energy requirement (EER) was calculated for each participant based on their height, weight, age, sex, and physical activity coefficient [29]. We conservatively assumed participants were at the low physical activity level as, only 13 % of children >12 years old in the CKiD cohort meet the American Academy of Pediatrics recommendation for physical activity in youth [30]. The percent EER (%EER) was then determined by: daily energy intake from FFQ / EER*100 %.
Total caloric intake was calculated from the intake of fat, protein and carbohydrate. The estimated daily protein intake was standardized as protein intake (g) divided by average body weight (kg). We categorized GFR as suggested by the KDIGO guideline [6]. Proteinuria was determined by urine protein/creatinine ratio (uP/C) and participants were categorized according to their proteinuria level: <0.5, 0.5–1.9, >2.0 mg/mg.
Statistical analysis
Patient baseline characteristics were described using median and interquartile range (IQR) for continuous variables and frequencies and percentages for categorical variables. Estimated nutrient intake per day and estimated energy distribution were analyzed as continuous variables and summarized using median and interquartile range. Differences in the proportions of categorical variables were tested using Pearson’s chi-squared or Fisher exact test. Trends of estimated nutrients intake by age or GFR categories were assessed nonparametrically using an extension of the Wilcoxon rank-sum test. Any FFQ with ≥2 5% missing values or inappropriate responses was discarded. For individual items in FFQ with <25 % missing, the median value was imputed. As done previously, FFQ with energy intake <500kcal or >5000kcal were regarded as implausible and excluded from analysis [31,32]. To further remove outliers, FFQ with %EER percentile <2.5 or >97.5 were also excluded. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
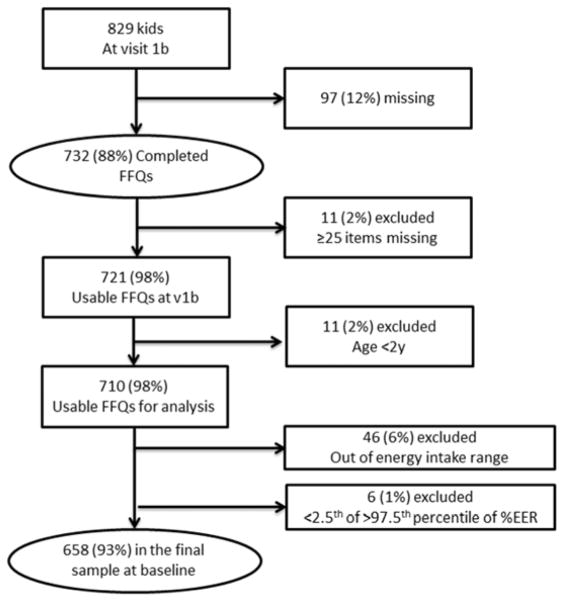
Of the 829 children with a baseline study visit, 732 completed FFQs. After applying the exclusion criteria mentioned above, 658 FFQs were available for analysis (Figure 1). Table 1 shows the baseline characteristics of the final sample. Most of the participants had a non-glomerular diagnosis (69.1%), 238 children (36.2%) were salt losers and 420 children (63.8%) were nonsalt losers. The median age (IQR) of the cohort was 11 years (8–15).
Figure 1.
Flow chart for data collection
Table 1.
Baseline characteristics of study participants expressed in either median (inter-quartile range) or number of participants (%).
| Characteristics | Median (IQR) or number (%) |
|---|---|
| Age (years) | 11.0 (8.0, 15.0) |
| Male sex | 394 (59.9) |
| African American race | 127 (19.3) |
| Diagnosis | |
| Glomerular cause | 203 (30.9) |
| Nonglomerular cause | 455 (69.1) |
| * GFR, ml/min/1.73m2 | |
| Stage 1(≥ 90) | 81 (12.3) |
| Stage 2 (60–89) | 196 (29.8) |
| Stage 3a (45–59) | 150 (22.8) |
| Stage 3b (30–44) | 155 (23.6) |
| Stage 4 (<30) | 75 (11.4) |
| Urine protein-creatinine ratio (mg/mg) | |
| < 0.5 | 379 (59.6) |
| 0.5 – 2.0 | 185 (29.1) |
| > 2.0 | 72 (11.3) |
Glomerular filtration rate (GFR) was wither measured by the disappearance curve of plasma iohexol or estimated using equations developed from the CKiD study [9].
Median sodium intake for the whole cohort was 3089 mg (2294–4243). Dietary sodium intake increased with age (Table 2). Sodium intake exceeded the recommended maximum daily intake in all age groups. Dietary sodium consumption was higher among salt losers than nonsalt losers, except in those aged 9–13 years old, but the difference was not statistically significant (p > 0.25 for all age groups).
Table 2.
Estimated daily sodium intake
| Age/years | #Recommended maximum total daily intake | All participants | Nonsalt losers | * Salt losers |
|---|---|---|---|---|
| 1–3 (N=39) | 1500 | 2180 (1690, 2805) | 2079 (1638, 2805) | 2180 (1719, 2702) |
| 4–8 (N=164) | 1900 | 2873 (2118, 4048) | 2623 (2039, 3946) | 3030 (2258, 4239) |
| 9–13 (N=235) | 2200 | 2937 (2244, 3678) | 2951 (2146, 3664) | 2921 (2321, 3690) |
| 14–18 (N=220) | 2300 | 3884 (2854, 5150) | 3847 (2803, 5051) | 4051 (2956, 5791) |
Intake amount was expressed in median (Interquartile range)
Adopted from Kidney Disease Outcomes Quality Initiative (KDOQI)1 and Kidney Disease: Improvig Global Outcomes (KDIGO)6 guidelines
Diagnoses for salt losers include obstructive uropathy, aplastic/hypoplastic/dysplastic kidneys, syndrome of agenesis of abdominal musculature, and congenital urologic disease (bilateral hydronephrosis)
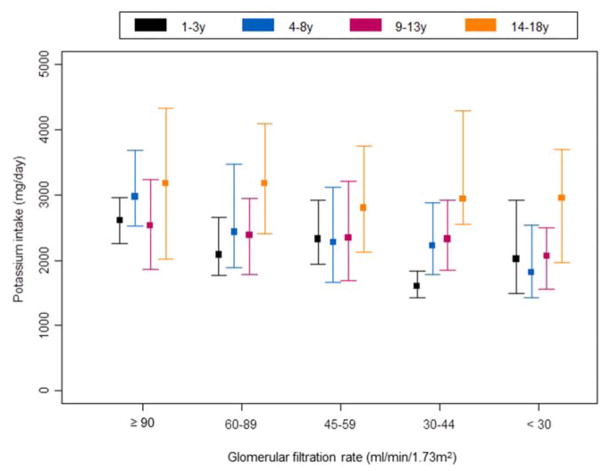
The distribution of potassium intake across different age groups and stages of CKD is depicted in Figure 2. Similar to sodium, potassium intake increased with age, and the median potassium intake in age groups 1–3 years, 4–8 years, 9–13 years, and 14–18 years were 2084 mg, 2377 mg, 2334 mg, and 2991 mg respectively. In general, the potassium intake levels increased significantly with age within most stages of CKD (p=0.001 for GFR 60–89ml/min/1.73m2; p=0.04 for GFR 45–59ml/min/1.73m2; p<0.001 for GFR 30–44ml/min/1.73m2 and p=0.003 for GFR <30ml/min/1.73m−2).
Figure 2. Distribution of estimated daily potassium intake by age and glomerular filtration rate (GFR).
Lower and upper bound denote 25th and 75th percentiles respectively. Square denotes median.
Recommended daily intake for a normal child [33]: 1–3 years: 3000mg / 4–8 years: 3800mg / 9–13 years: 4500mg / 14–18 years: 4700mg
Median phosphorus intake in age group 1–3 years, 4–8 years, 9–13 years, and 14–18 years were 1004 mg, 1135 mg, 1181 mg, and 1387 mg respectively. Younger children were more likely than older children to exceed the age-specific DRI for phosphorus (p<0.001) (Table 3). The 2008 KDOQI guideline which suggests that children with CKD stages 3–5 or 5D should have dietary phosphorus intake reduced to 80% of DRI for age when the serum parathyroid hormone (PTH) level is above the target range and serum phosphorus level exceeds the normal reference for age [1]. Only 30.1 % (n=73) of those with elevated serum PTH met the recommendation and all of whom were 9–18 years old. All kids < 9 years old failed to meet the recommendation.
Table 3.
Estimated daily phosphorus intake
| Daily phosphorus intake (mg/day) | |||
|---|---|---|---|
|
| |||
| Age/years | #DRI | Phosphorus intake | % with intake exceeding DRI for age |
| 2–3 (N=39) | |||
| Female (N=12) | 460 | 1115 (803, 1327) | 100% |
| Male (N=27) | 460 | 986 (750, 1099) | 100% |
| 4–8 (N=164) | |||
| Female (N=64) | 500 | 1065 (764, 1375) | 94% |
| Male (N=100) | 500 | 1200 (867, 1678) | 94% |
| 9–13 (N=235) | |||
| Female (N=90) | 1250 | 1060 (802, 1488) | 38% |
| Male (N=145) | 1250 | 1238 (899, 1454) | 48% |
| 14–18 (N=220) | |||
| Female (N=98) | 1250 | 1274 (938, 1817) | 51% |
| Male (N=122) | 1250 | 1489 (1101, 1940) | 66% |
Intake amount was expressed In median (Interquartile range)
DRI: dietary reference intakes
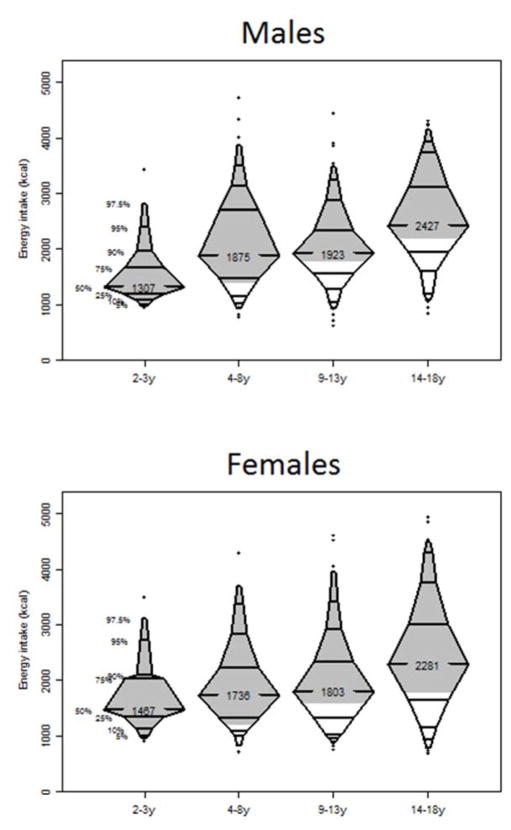
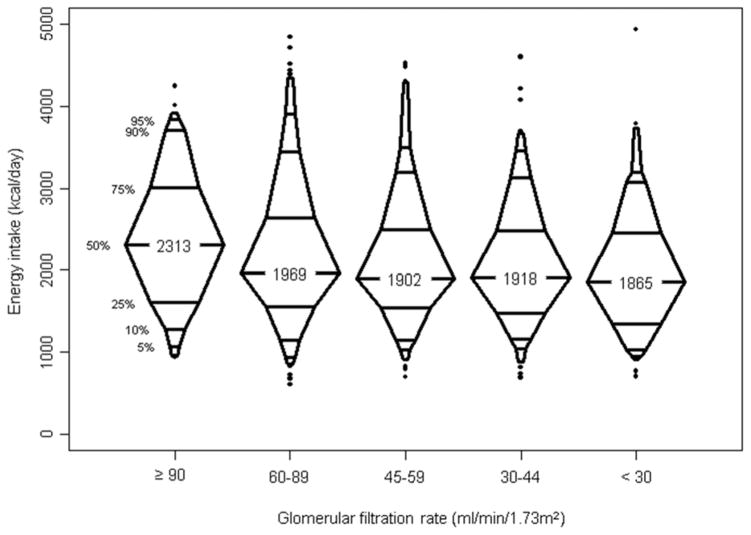
Figures 3 and 4 show the distribution of energy intake: 4% of the were using a nutritional supplement (formula supplement) 6 months prior to FFQ assessment, and half of them were still using the supplement 6 months after the FFQs were evaluated. Median daily energy intake for the whole cohort was 1968 kcal (1523–2574). As expected, energy consumption increased with age. We also found a decreasing trend of energy consumption with dropping GFR (p=0.003). Median energy consumption levels were higher than the recommended values in all age groups, implying at least half of the kids in each age groups, implying at least half of the kids consumed more than recommended. Younger children were more likely to exceed the recommended caloric intake than their older counterparts (p<0.001). Among children aged 2–3 years, 92.6% of girls and 91.7% of boys found to have excessive recommended energy intake, and the proportion decreased to 56.7% of girls and 58.6% of boys in those aged 9–13 years.
Figure 3. Distribution of energy intake (kcal) by age and sex.
Shaded regions show intake above the recommended daily caloric values in the respective age groups (1000kcal, 1400kcal, 1800kcal and 2200kcal for male and 1000kcal, 1200kcal, 1600kcal and 1800kcal for girls [33]).
Figure 4.
Distribution of energy intake (kcal) by glomerular filtration rate (GFR)
The highest daily protein intake (g/kg) was observed among those aged 1–3 years old and the intake level decreased in older participants (Table 4). When stratified by age and GFR, median protein intake was higher than recommended in all age groups. Among those with GFR 30–59 ml/min/1.73m2, the proportion of children with protein intake exceeding recommended levels was significantly higher in those aged 1–3 years (100%) than those aged 14–18 years (62%) (p<0.001).
Table 4.
Estimated daily protein intake (g/kg)
| Estimated daily protein intake (g/kg) | |||
|---|---|---|---|
| Age/years | GFR (ml/min/1.73m2) | CKiD | * Recommended intake |
| 1–3 (N=22) | 30–59 (N=19) | 3.30 (2.95, 5.65) | 1.05–1.50 |
| 15–29 (N=3) | 3.49–8.02^ | 1.05–1.25 | |
| 4–13 (N=249) | 30–59 (N=207) | 2.07 (1.42, 2.97) | 0.95–1.35 |
| 15–29 (N=42) | 1.77 (1.43, 2.48) | 0.95–1.15 | |
| 14–18 (N=109) | 30–59 (N=79) | 1.29 (1.02, 1.88) | 0.85–1.20 |
| 15–29 (N=30) | 1.50 (0.97, 2.04) | 0.85–1.05 | |
Intake amount is expressed as median (interquartile range)
GFR, glomerular filtration rate; CKiD, Chronic Kidney Disease in Children
Adopted from Kidney Disease Outcomes Quality Initiative (KDOQI) guideline
Min – Max
We also explored the distribution of macronutrients (Table 5). The major energy source was carbohydrates in both younger and older children, followed by fat and protein. Median percentages were similar at different ages, and energy distribution was similar to the KDOQI guidelines recommendation [1].
Table 5.
Estimated energy distribution (carbohydrate, fat and protein)
| Estimated energy distribution (%) | |||
|---|---|---|---|
|
| |||
| Age/years | Macronutrient | CKiD | * Recommended percentage |
| 1–3 (N=39) | Carbohydrate | 54 (50, 60) | 45–65 |
| Fat | 34 (30, 38) | 30–40 | |
| Protein | 13 (12, 15) | 5–20 | |
|
| |||
| 4–18 (N=619) | Carbohydrate | 53 (49, 58) | 45–65 |
| Fat | 35 (31, 38) | 25–35 | |
| Protein | 14 (12, 16) | 10–30 | |
Intake amount is expressed as median (interquartile range)
Adopted from Kidney Disease Outcomes Quality Initiative (KDOQI) guideline
Discussion
It is well recognized that nutritional monitoring and intervention is indispensable in pediatric CKD management, yet prospectively collected data in this area are scarce. CKiD is one of the largest cohorts of pediatric CKD patients and the prospectively collected dietary data permits an analysis of the impact of dietary intake on CKD progression and the development of comorbidities. Here we describe our baseline data and compare them with the recommended nutritional levels from the KDOQI and KDIGO guidelines.
CKiD participants consumed much more dietary sodium than the recommended limits. The excessive intake of sodium was most dramatic in the oldest age group. More than 25 % of adolescents aged 14–18 years old consumed >5150 mg of sodium daily, which is more than double the top end of the recommended range. Interestingly, sodium intake of the cohort was similar to the data obtained from the general population. Median daily sodium intake reported in the National Health and Nutrition Examination Survey (NHANES) 2011–2012 among those aged 2–5 years, 6–11 years, and 12–19 years were 2295 mg, 3081 mg, and 3593 mg respectively [14], which is similar to the corresponding levels of 2222 mg, 3028 mg, and 3577 mg among CKiD participants in these age ranges. A significant portion of pediatric CKD diagnoses comprise conditions under the category of congenital anomalies of kidney and urinary tract, which are commonly salt wasting and frequently require sodium supplementation. We found that those children with salt-wasting conditions consumed slightly more salt than those without salt-wasting conditions, although this difference was not significant statistically. An analysis of dietary sodium consumption data and its relationship with blood pressure in the CKiD cohort is currently underway. Our results indicate that greater effort is required to reduce dietary sodium consumption in subgroups of children with CKD–such as those with the non-salt-wasting conditions or with hypertension – given its potential adverse effects. Most dietary sodium comes from added salt during food processing or cooking [3], therefore behavioural strategies like avoiding processed food and reading food labels should be encouraged. In fact, the National Kidney Foundation provides practical suggestions on how to reduce dietary sodium intake [34].
Dietary potassium consumption in our cohort was associated with both GFR and age. That daily potassium intake decreased as CKD advanced was not surprising as children with lower GFR may have reduced appetite and may have received more intense nutritional counseling and intervention, both of which can lead to a decrease of potassium intake. Unlike sodium, data relating dietary potassium intake and outcome among CKD populations is limited. A study investigating dietary potassium intake among adult hemodialysis patients demonstrated that patients in the highest quartile of potassium intake have the highest risk of mortality (adjusted hazard ratio 2.4 with 95% confidence interval 1.1 – 7.5) compared with those in the lowest quartile [34]. Although restriction of potassium intake is recommended for children with CKD stage 2–5 and 5D who are at risk of hyperkalaemia [1], there is no specific guideline to suggest potassium intake levels in children of various ages and CKD staging who require restriction. Fruits and vegetables are the main sources of dietary potassium [35], and cautious consumption of these food items is recommended for those who require potassium restriction.
Hyperphosphatemia is known to associate with significant morbidity and mortality in CKD patients [36,37]. Although a clear association between dietary phosphorus intake and serum phosphorus level has not yet been demonstrated among early-stage CKD patients [38], dietary phosphorus limitation is still commoly advised among selected subgroups of CKD patients. Our result of high phosphorus intake may be partly explained by the high protein intake in our cohort, especially among younger kids, as the daily protein intake level far exceeded the recommended level in all age groups, with the most dramatic difference observed among the youngest participants. This can also be reflected by the finding that no patients in the younger age groups met the suggested phosphorus consumption levels. Nevertheless, the protein levels were still lower than the general population [39]. This also points out the difficulty of achieving a nutritional balance in clinical management of young children with CKD. Food items rich in protein contain high phosphorus load, and protein restriction is usually not recommended for children with CKD for promotion of growth. While on one hand, avoiding protein energy wasting is advocated, on the other hand, excessive dietary protein intake may contrinute to CKD progression and impact the control of metabolic bone disease [36].
Children in the CKiD cohort consumed more than the recommended caloric intake. Of note, young children with CKD, especially those younger than 2 years, are vulnerable to malnutrition and often require supplementary enteral feeding (such as tube feeding or gastrotomy feeding) for preservation of normal growth [1,7]. Yet, excessive caloric consumption occurred in all age groups. When we calculated and compared our data to figures obtained from the general population, we found that the daily caloric intake levels were similar to their peers in the US as reported in NHANES [14]. NHANES data was obtained from the community-based general population, and do not represent children with CKD. Therefore, our report represents a new finding in the CKD population. NHANES used one-day dietary recall interviews conducted by trained interviewers to derive the nutritional intake data [14], which differs from data we obtained from FFQ in CKiD. Therefore, results may not be directly comparable. Despite that, our study emphasizes the common problem of excessive dietary caloric intake and obesity in the pediatric with CKD, and the risk factors of obesity compounds the increased risk of accelerated CKD progression.
Macronutrient distribution in this study was consistent with the recommended levels in all age groups, in which carbohydrates were the main source of energy, followed by fat and protein. Although we did not specifically analyze the components of each macronutrient, such as distribution of dietary cholesterol, trans-fatty acids, saturated fatty acids, or types of carbohydrates, results still indicate a reassuring picture in terms of energy distribution.
There are several limitations of our study. First, we recognized there are inherent drawbacks of employing an FFQ in assessing nutrient intake, which include the lack of direct and quantitative method to assess nutrient consumption of individuals, inadequate coverage of all food items that an individual may consume, and an imprecise categorization of food items causing under- or over-estimation of nutrients consumed [40]. Second, the FFQ utilized in this study was not validated in every age or ethnic group. Despite these issues, the large number of systemically collected FFQ with the standardized method of analysis employed in the CKiD cohort provides valuable information. Although misclassication is possible, it is likely to occur randomly, though systematic under- or over-reporting of some food items is possible. Third, we used observed body weight to determine our estimated protein intake, whereas the reference values adopted from KDOQI guidelines suggest using ideal body weight instead [1]. While the use of ideal body weight can overcome the problem of overestimating protein intake in obese children and will improve the accuracy of protein intake estimation, there is currently no consensus on how to dertermine ideal body weight [41]. Prior to this report, few rigorous assessments of nutritional intake in a large nationally representative cohort of children with CKD have been published. We tried to minimize the inclusion of FFQs with implausible responses by setting strict exclusion criteria. Fourth, as supplementation may have significant impact on nutritional intake in this group of participants, we performed a sensitivity analysis by excluding the 27 patients who consumed nutritional supplements. Results were essentially the same. Finally, our study is a cross-sectional study that characterize nutritional intake at study entry. We cannot infer that decline in GFR or increase in age causes the changes we observed in nutritional intake. We did not assess how nutritional intake changes over time as kidney disease progressed or how nutritional intake impacted disease progression and the development of various comorbidities. Those issues will require further analysis of longitudinal data from follow-up in the CKiD. Regardless, these data provide a unique insight nutritional intake and potential points of modification to improve health outcomes for children with CKD.
Conclusion
Our analysis showed that children with CKD consumed more dietary sodium, phosphorus, protein, and calories than recommended by national guidelines, emphasizing the need for effective nutritional intervention strategies in this patient population.
Supplementary Material
Acknowledgments
Funding Source: National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung and Blood Institute (grants U01-DK-66143, U01-DK-66174, U01DK-082194, and U01-DK-66116).
Data in this manuscript were collected by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, Ph.D.), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.). The CKiD Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-82194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
Support for the nutritional analysis of the FFQ was provided by the National Center for Research Resources, Grant UL1RR024134, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ACEI
Angiotensin-converting enzyme inhibitor
- BMI
Body mass index
- CKD
Chronic kidney disease
- CKiD
Chronic Kidney Disease in Children
- EER
Estimated energy requirement
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- FFQ
Food frequency questionnaire
- IQR
Interquartile range
- NHANES
National Health and Nutrition Examination Survey
- uP/C
Urine protein/creatinine ratio
Footnotes
Conflict of Interest: The authors have indicated they have no potential conflicts of interest to disclose.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent: Informed consent was obtained from all individual participants included in the study.”
References
- 1.National Kidney Foundation. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD. Update. Am J Kidney Dis. 2008;53(Suppl 2):S1–S124. doi: 10.1053/j.ajkd.2008.11.017. 2009. [DOI] [PubMed] [Google Scholar]
- 2.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 3.Jones-Burton C, Mishra SI, Fink JC, Brown J, Gossa W, Bakris GL, Weir MR. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol. 2006;26:268–275. doi: 10.1159/000093833. [DOI] [PubMed] [Google Scholar]
- 4.Buter H, Hemmelder MH, Navis G, de Jong PE, de Zeeuw D. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant. 1998;13:1682–1685. doi: 10.1093/ndt/13.7.1682. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. U.S. Government Printing Office; Washington, DC: 2010. [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 7.Rees L, Mak RH. Nutrition and growth in children with chronic kidney disease. Nat Rev Nephrol. 2011;7:615–623. doi: 10.1038/nrneph.2011.137. [DOI] [PubMed] [Google Scholar]
- 8.Betts PR, Magrath G. Growth pattern and dietary intake of children with chronic renal insufficiency. BMJ. 1974;2:189–193. doi: 10.1136/bmj.2.5912.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees L, Jones H. Nutritional management and growth in children with chronic kidney disease. Pediatr Nephrol. 2013;28:527–536. doi: 10.1007/s00467-012-2258-x. [DOI] [PubMed] [Google Scholar]
- 10.Eknoyan G. Obesity and chronic kidney disease. Nefrologia. 2011;31:397–403. doi: 10.3265/Nefrologia.pre2011.May.10963. [DOI] [PubMed] [Google Scholar]
- 11.Kramer H. Dietary patterns, calories, and kidney disease. Adv Chronic Kidney Dis. 2013;20:135–140. doi: 10.1053/j.ackd.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C. Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis. 2000;36:811–819. doi: 10.1053/ajkd.2000.17674. [DOI] [PubMed] [Google Scholar]
- 13.Huang MC, Chen ME, Hung HC, Chen HC, Chang WT, Lee CH, Wu YY, Chiang HC, Hwang SJ. Inadequate energy and excess protein intakes may be associated with worsening renal function in chronic kidney disease. J Ren Nutr. 2008;18:187–194. doi: 10.1053/j.jrn.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Agriculture, Agricultural Research Service What we eat in America. [Accessed June 30, 2015]; Available at: http://www.ars.usda.gov/Services/services.htm?modecode=80-40-05-30#.
- 15.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Abraham AG, Furth SL, Warady BA, Munoz A. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77:65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FC. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, Colditz GA. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J. 1999;3:167–172. doi: 10.1023/a:1022350023163. [DOI] [PubMed] [Google Scholar]
- 22.Newby PK, Perterson KE, Berkey CS, Leppert J, Willett WC, Colditz GA. Dietary composition and weight change among low-income preschool children. Arch Pediatr Adolesc Med. 2003;157:759–764. doi: 10.1001/archpedi.157.8.759. [DOI] [PubMed] [Google Scholar]
- 23.Newby PK, Peterson KE, Berkey CS, Leppert J, Willett WC, Colditz GA. Beverage Consumption Is Not Associated with Changes in Weight and Body Mass Index Among Low-Income Preschool Children in SD. Journal of the American Dietetic Association. 2005;104(7):1086–1094. doi: 10.1016/j.jada.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:683–692. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh DTT, Dibley MJ, Sibbritt DW, Tran HTM. Energy and macronutrient intakes in preschool children in urban areas of Ho Chi Minh City, Vietnam. BMC Pediatrics. 2008;8:44. doi: 10.1186/1471-2431-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee to Review Child and Adult Care Food Program Meal Requirements, Food and Nutrition Board. Child and Adult Care Food Program: Aligning Dietary Guidance for All. National Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- 27.Lowenburg M. Development of food patterns in young children. In: Pipes P, Trahms C, editors. Nutrition in Infancy and Childhood. 5. Mosby; St. Louis, MO: 1993. pp. 168–169. [Google Scholar]
- 28.Rockett H, Colditz GA. Assessing diets of children and adolescents. Am J Clin Nutr. 1997;65:S1116–S1122. doi: 10.1093/ajcn/65.4.1116S. [DOI] [PubMed] [Google Scholar]
- 29.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) National Academies Press; Washington DC: 2002. [DOI] [PubMed] [Google Scholar]
- 30.Clark SL, Denburg MR, Furth SL. Physical activity and screen time in adolescents in the chronic kidney disease in children (CKiD) cohort. Pediatr Nephrol. 2015 doi: 10.1007/s00467-015-3287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. The Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 32.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. Journal of the American Dietetic Association. 1995;95:336–340. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 33.American Heart Association. Gidding SS, Dennison BA, Birch LL, Daniels SR, Gillman MW, et al. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117(2):544–59. doi: 10.1542/peds.2005-2374. [DOI] [PubMed] [Google Scholar]
- 33.Hoy MK, Goldman JD, Murayi T, Rhodes DG, Moshfegh AJ. Sodium Intake of the U.S. Population: What We Eat In America, NHANES 2007–2008. [Accessed June 30, 2015];Food Surveys Research Group Dietary Data Brief No. 8. 2011 Oct; Available at: http://ars.usda.gov/Services/docs.htm?docid=19476.
- 34.Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, Kopple JD. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoy MK, Goldman JD. Potassium Intake of the U.S. Population: What We Eat In America, NHANES 2009–2010. [Accessed June 30, 2015];Food Surveys Research Group Dietary Data Brief No. 10. 2012 Sep; Available at: http://ars.usda.gov/Services/docs.htm?docid=19476.
- 36.Uribarri J. Dietary phosphorus and kidney disease. Ann NY Acad Sci. 2013;1301:11–19. doi: 10.1111/nyas.12201. [DOI] [PubMed] [Google Scholar]
- 37.Menon MC, Ix JH. Dietary phosphorus, serum phosphorus and cardiovascular disease. Ann NY Acad Sci. 2013;1301:21–26. doi: 10.1111/nyas.12283. [DOI] [PubMed] [Google Scholar]
- 38.Murtaugh MA, Filipowicz R, Baird BC, Wei G, Greene T, Beddhu S. Dietary phosphorus intake and mortality in moderate chronic kidney disease: NHANES III. Nephrol Dial Transplant. 2012;27:990–996. doi: 10.1093/ndt/gfr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulgoni VL., III Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:S1554–S1557. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]
- 40.Bross R, Noori N, Kovesdy CP, Murali SB, Benner D, Block G, Kopple JD, Kalantar-Zadeh K. Dietary assessment of individuals with chronic kidney disease. Semin Dial. 2010;23:359–364. doi: 10.1111/j.1525-139X.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillps S, Edlbeck A, Kirby M, Goday P. Ideal body weight in children. Nutrition in Clinical Practice. 2007;22:240–245. doi: 10.1177/0115426507022002240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.