Abstract
In rodent models of epilepsy, EEG implantation surgery is an essential modality to evaluate electrographic seizures. The inflammatory consequences of EEG electrode-implantation and their resultant effects on seizure susceptibility are unclear. We evaluated electrode-implantation in a two-hit model of epileptogenesis in C57BL/6 mice that included brief, recurrent febrile seizures (FS) at P14 and kainic acid induced seizures (KA-SZ) at P28. During KA-SZ, latencies to first electrographic and behavioral seizures, seizure severity, and KA dose sensitivity were measured. Mice that received subdural screw electrode implants at P25 for EEG monitoring at P28 had significantly shorter latencies to seizures than sham mice, regardless of early life seizure experience. Electrode-implanted mice were sensitive to low dose KA as shown by high mortality rate at KA doses above 10 mg/kg. We then directly compared electrode-implantation and KA-SZ in seizure naive CX3CR1GFP/+ transgenic C57BL/6 mice, wherein microglia express green fluorescent protein (GFP), to determine if microglia activation related to surgery was associated with the increased seizure susceptibility in electrode-implanted mice from the two-hit model. Hippocampal microglia activation, as demonstrated by percent area GFP signal and GFP positive cell counts, prior to seizures was indistinguishable between electrode-implanted mice and controls, but was significantly greater in electrode-implanted mice following seizures. Electrode-implantation had a confounding priming effect on the inflammatory response to subsequent seizures.
Keywords: Microglia, Kainic Acid, EEG, Inflammation, Epilepsy, Surgery
1. Introduction
Electroencephalographic (EEG) recordings are essential to verify that observed behavioral changes in rodents are seizures with electrographic correlates. Rodent EEG recordings are primarily performed with wire electrodes implanted in deep brain structures or screws implanted at the surface of the cortex. Despite precautions to minimize tissue injury, such as employing smaller and fewer electrodes, EEG electrode-implantation is invasive and reasonably expected to cause inflammation. EEG studies traditionally control for an effect of electrode-implantation, but an interaction between surgery-related inflammation and experimental treatments may be missed if inflammatory markers are excluded from outcome measures.
Inflammation related to electrode-implantation deserves attention in rodent models of epilepsy because inflammation has been implicated in epileptogenesis [19, 20]. The inflammatory cascade involving activation of microglia and astrocytes, blood-brain barrier (BBB) breakdown, and peripheral immune cell infiltration is a hallmark of both the inflammatory response to seizures and to traumatic brain injury (TBI) [13, 16, 17]. Microglia activation in particular is critical to the initial clearance of necrotic neurons [9] and to long term seizure susceptibility [1]. In rodent models of epilepsy, the immune response to electrode-implantation may alter the inflammatory consequences of seizure-induction.
We monitored a two-hit mouse model of epileptogenesis with EEG, and included behavioral and inflammatory outcome measures. The two-hit model included brief, recurrent febrile seizures (FS) at P14 followed by kainic acid induced seizures (KA-SZ) at P28, both of which are associated with immune activation. Febrile seizures often occur in the inflammatory context of infection [8] and have been associated with spontaneous seizures in adult rats [5]. KA-SZ cause microglia activation in the hippocampus which can be aggravated by early life seizure experience [1]. Microglia activation was quantified using transgenic CX3CR1GFP/+ mice that express green fluorescent protein (GFP) in microglia. We isolated a confounding effect of EEG electrode-implantation on seizure induction in the two-hit model, with implications for experimental designs, especially for surgical controls.
2. Materials and Methods
2.1. Experimental Design
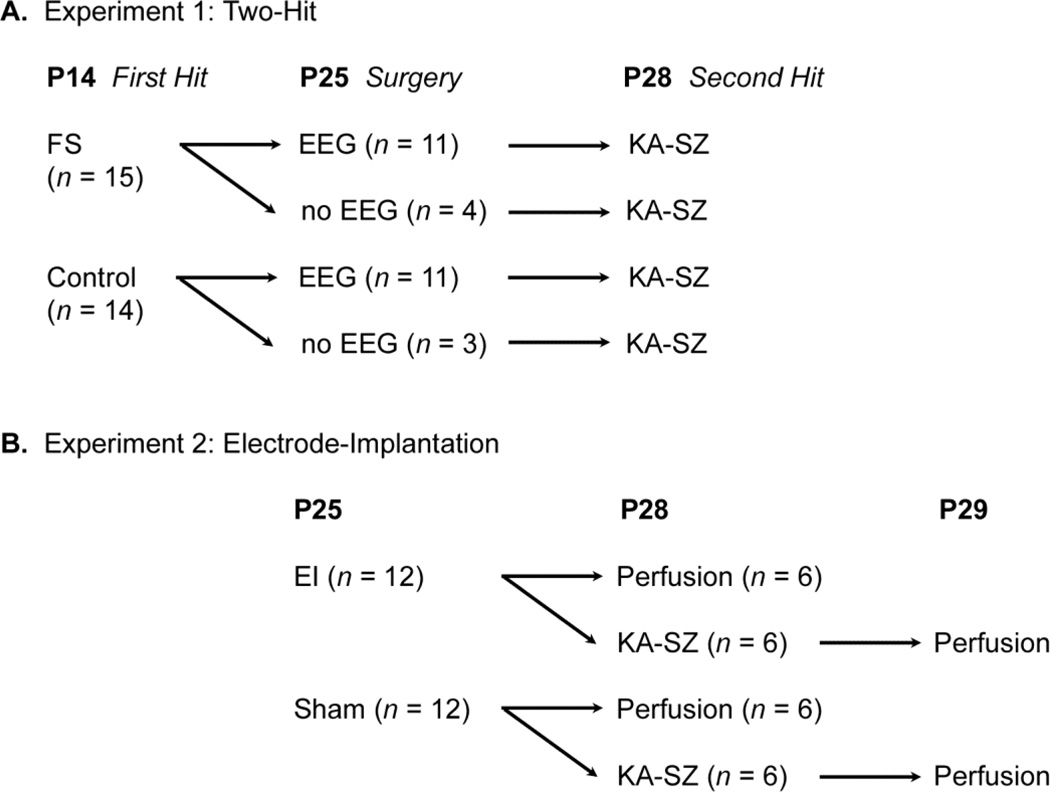
The experimental design is illustrated in Figure 1.
Fig. 1.
Experimental Design.
Experiment 1 (Two-Hit): At P14, C57BL/6 mice either experienced FS (lipopolysaccharide (LPS) + hyperthermia) (n = 15) or served as littermate controls (saline + normothermia) (n = 14). Subsets of the FS (n = 11) and control mice (n = 11) underwent EEG implantation surgery at P25, followed by KA-SZ at P28. The other FS (n = 4) and control (n = 3) mice received sham surgery (see below) at P25 followed by KA-SZ at P28. To visualize hippocampal inflammation, CX3CR1GFP/+ mice (n = 3) had EEG implantation at P25, KA-SZ at P28, and perfusion at P29; two were exposed to FS and one, no FS at P14.
Experiment 2 (Electrode-Implantation): To quantify microglia activation associated with electrode-implantation, CX3CR1GFP/+ mice were implanted with EEG electrodes (n = 6) or received sham surgery (n = 6) at P25. Mice were perfused and brain tissue was collected at P28. To examine the influence of screw implantation on seizure-induced microglia activation, another set of CX3CR1GFP/+ mice underwent electrode-implantation (n = 6) or sham surgery (n = 6) at P25 followed by KA-SZ at P28. Mice were perfused and brain tissue was collected at P29.
2.2. Animals
We used C57BL/6 mice and CX3CR1GFP/+ transgenic C57BL/6 mice (The Jackson Laboratory) in which the fractalkine chemokine receptor was replaced by a green fluorescent protein (GFP) reporter gene [10]. These mice expressed GFP in microglia, monocytes, dendritic cells, and a subset of natural killer cells [10]. Mice were housed on a 12-hour light cycle at an ambient temperature of 21°C with access to water and rodent chow ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Stanley Manne Children’s Research Center Institutional Animal Care and Use Committee.
2.3. Brief, recurrent febrile seizures
At P14, C57BL/6 mice (n = 19) received an i.p. injection of bacterial endotoxin lipopolysaccharide (LPS) (Sigma Aldrich, St. Louis, MO) at 10 µg/kg. Control mice received a volume adjusted injection of saline, were separated from the dam, and were placed in an incubator set to 30°C for the duration of FS induction. The low dose LPS induced systemic inflammation characteristic of a febrile illness [6]. Two hours after the LPS injection, mice were placed in an open Plexiglass box (13×24×12.6 cm) inside an incubator at an ambient temperature of 42.5°C. Core body temperature was measured rectally (Temp 10T Thermocouple Thermometer, Oakton Instruments, Vernon Hills, IL) at 5–10 minute intervals depending on seizure severity. If temperature exceeded 41.5°C, mice were temporarily removed from the incubator to receive a saline injection and allow their temperature to decrease to 38°C before being returned to the incubator. Behavioral seizures including facial automatism, limb clonus, clonic jerks, and generalized tonic-clonic convulsions (GTC), were recorded. After GTC, mice were removed from the incubator, received a saline injection, and began a 30 minute recovery period at room temperature. Mice experienced 3 rounds of hyperthermia over 2.5 hours, each lasting 15–30 minutes, separated by 30 minute recovery periods. The hyperthermic period lasted until the mouse demonstrated GTC, usually around 10–20 minutes, and was otherwise capped at 30 minutes. Mice that showed at least two GTC (15/19) were included for further analysis. All mice were returned to the dam after a total separation of approximately 3 hours.
2.4. Surgery
Mice were anesthetized via inhalation of 4% isoflurane in oxygen and maintained at 1.5%. Buprenorphine (0.1mg/kg) was administered subcutaneously at the outset of surgery. The mice were stabilized in a mouse stereotaxic apparatus (Stoelting, Wood Dale, IL). The head was shaved and disinfected with alcohol and betadine before a midline incision of 1cm was made in the scalp. The periosteum was wiped away with sterile swabs. For mice in the sham surgery control, the scalp was then sutured closed. In experiment 1, EEG-implanted mice received prefabricated EEG/EMG headmounts (8201, Pinnacle Technology, Lawrence, KS). The headmount was first fixed to the skull with cyanoacrylate. Four pilot holes were placed in the skull through openings in the headmount with a 23 gauge needle at the following coordinates relative to bregma: AP: +2 mm, ML: ±1.5 mm and AP: - 4mm, ML: ±1.5mm. Four stainless steel EEG screws (8209, Pinnacle) were then inserted through openings in the headmount and manually rotated into the pilot holes. Silver epoxy was applied to ensure electrical connectivity between the electrodes and the headmount. The complex was then stabilized and affixed to the skull with dental acrylic and the scalp was sutured closed behind the headmount. In experiment 2, mice had two small pilot holes placed in the skull with a 23 gauge needle at the following stereotactic coordinates relative to bregma: AP: −0.8 mm, ML: +1.2 mm, and AP: - 2.5 mm, ML: −1.2mm. Two EEG screws were then manually rotated into the pilot holes. The scalp was sutured closed over the screws. After surgery, all mice had antibiotic ointment and EMLA cream applied to the incision and were placed in a clean cage on a warm heating pad to recover.
2.5. Kainic acid-induced seizures
In experiment 1, mice received a single i.p. injection of 10mg/kg KA (AG Scientific, San Diego, CA). The KA dose was titrated in a preliminary study evaluating doses of 12.5 mg/kg, 10.5 mg/kg, and 10 mg/kg KA. At doses higher than 10 mg/kg, the mortality rate was unacceptably high (60–100%). Behavioral observations and electrographic recordings were used to determine latency from KA injection to the first behavioral or electrographic seizure. Seizures were recorded and scored on a scale from 0 to VI: 0 - no response; I - behavioral arrest; II - staring, pawing, head bobbing; III - clonic jerks, rearing, and falling; IV continuous grade III seizures lasting longer than 30 minutes (status epilepticus (SE)); V - generalized tonic-clonic convulsions (GTC); VI - death.
In experiment 2, mice received i.p. KA injections reaching a total dose of 10–25 mg/kg. An initial bolus of 10 mg/kg KA was administered, followed by 5 mg/kg injections every 30 minutes until the mice demonstrated grade IV seizures. Behavioral observations of seizure severity were based on a maximal response achieved on the scale used above.
2.6. EEG recording
EEG recordings were made with the PAL 8200 three-channel monitoring system (Pinnacle Technology, Lawrence, KS). Mice were tethered to the recording system and able to move freely about the monitoring cage. Each mouse was recorded for 1–2 hours after the KA injection. EEG recordings were reviewed on the Sirenia Seizure Pro software (Pinnacle Technology, Lawrence, KS) by researchers blinded to the experimental conditions.
2.7. Quantification of microglia
Mice were deeply anesthetized with CO2 and perfused transcardially with ice cold PBS, followed by 4% paraformaldehyde. Brains were removed, post-fixed in 4% paraformaldehyde for 24 hours, and placed in 30% sucrose for cryoprotection. Brains were cut into 40 µm horizontal sections with a freezing microtome. Every 6th section was collected and 6 sections spanning the septotemporal axis of the hippocampus were mounted with Prolong Gold Antifade Mountant (Thermo Fisher Scientific, Waltham, MA). The hippocampal sections were photographed using confocal microscopy. Images of hilus were captured at 20x magnification and the percent area of green fluorescence and total counts of green fluorescent cells were quantified using ImageJ (1.43u, Public Domain, NIH) by observers who were blinded to the treatment groups. Sum total spot count—defined as cell counts reflecting density of microglia—was quantified by including brightly fluorescent microglia with visible processes and distinct soma of at least 5 µm diameter while excluding cells out of focus with indistinct borders. A total of 144 hippocampal sections were analyzed from 24 animals (three sections per animal, two hippocampi per section).
2.8. Statistical analysis
Student’s t-test or one-way analysis of variance (ANOVA) with Tukey post-hoc test were used to compare KA doses, percent area of green fluorescent signal, and cell counts between experimental groups (GraphPad Prism v. 5.0, GraphPad Software, Inc., La Jolla, CA). Values are expressed as mean ± standard error of the mean (SEM). Significance was defined as p < 0.05 for all tests.
3. Results
3.1. Experiment 1 (Two-Hit)
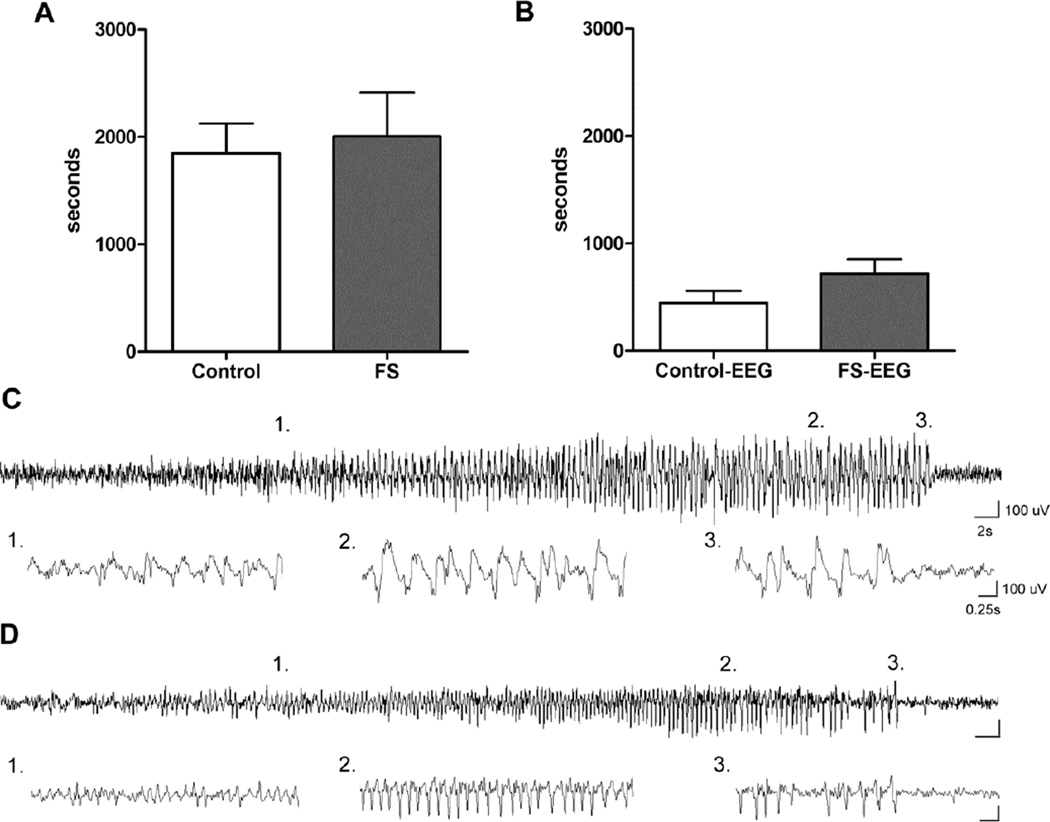
The majority of mice (15/19) experienced 2–3 GTCs during FS induction and all mice (19/19) presented behavioral seizures during KA-SZ. At second hit KA-SZ, there was no difference in latency to seizures between FS experienced and control mice as determined by behavioral observations and EEG recordings (Fig. 2A & B). In EEG-implanted mice, electrographic seizure severity and duration was similar between FS vs. control groups (% seizures/recording time 44.4 +/− 4.6 (n = 7) vs. 47.1 +/− 15.8 (n = 4), p = 0.84). The first electrographic seizures were detected within 10 minutes of the injection in both FS and control mice (Fig. 2B). Early electrographic seizures often had subtle behavioral correlates such as behavioral arrest or head nodding (Fig. 2C). Convulsive seizures (forelimb clonus, rearing and falling, or clonic jerks) occurred at around 15–20 minutes after KA injection and were always accompanied by clear electrographic changes.
Fig. 2.
Lack of effect of brief, recurrent FS on later life seizure susceptibility.
A. Latency to onset of behavioral seizures (GIII) during KA-SZ at P28 in mice with prior exposure to febrile seizures at P14 and their control littermates (n = 4; 3, p = 0.78). These mice had no EEG implants and underwent only sham surgery at P25.
B. Latency to first electrographic seizures was also similar between FS and control (n = 11/groups, p = 0.13).
C. Example of first electrographic seizure detected and accompanied by subtle behavioral changes: comparison between control mouse (top panel) and
D. FS mouse (bottom panel).
3.2. EEG implantation contributed to KA sensitivity
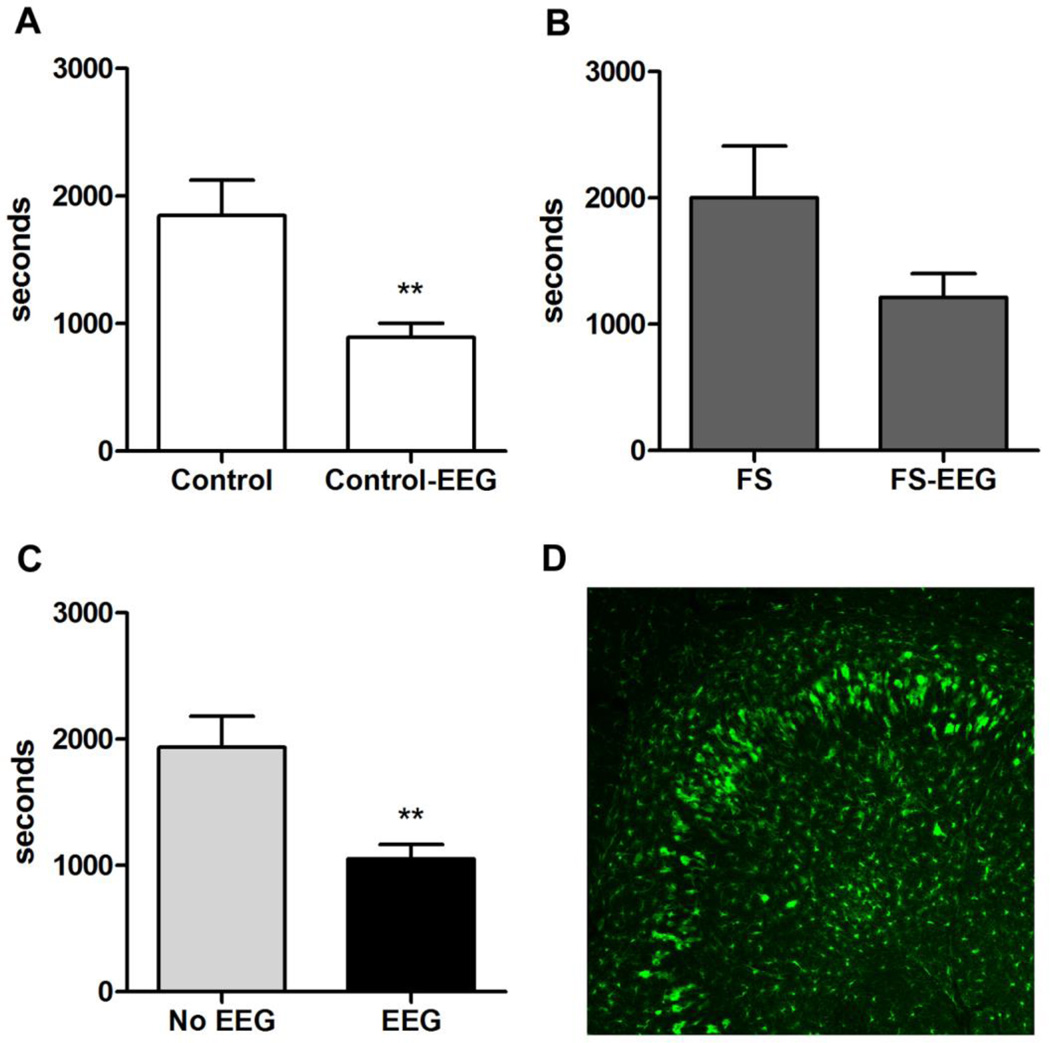
Mice implanted with EEG headmounts were sensitive to low dose KA. The mortality rate was 100% at 12.5 mg/kg (2/2), 60% (5/8) at 10.5 mg/kg, and 32% (7/22) at 10 mg/kg. We accepted the 70% survival rate at 10 mg/kg KA because sham mice showed perceptible seizure signs at this dose, permitting latency comparisons between groups. FS mice (n = 3) and control mice (n = 4) that died during KA-SZ at 10 mg/kg were included in our latency analysis. Behavioral seizure onset was significantly shortened by EEG implantation (Fig 3C). Pooling both FS (Fig. 3B) and control (Fig. 3A) mice across surgery, EEG-implanted mice demonstrated GIII behavioral seizures around 15 minutes before sham mice (Fig. 3C).
Fig. 3.
Significant increase in susceptibility to KA-SZ and marked seizure-induced microglia activation after EEG implantation.
A. Latency to first behavioral seizures (GIII) in sham-operated control mice (Control-sham) compared to EEG-implanted control mice (Control-EEG) (n = 3 vs n = 11, p = 0.0027). Neither group experienced early-life FS.
B. Latency to first behavioral seizures (GIII) in sham-operated FS mice (FS-sham) compared to EEG-implanted FS mice (FS-EEG) (n = 4 vs n = 11, p = 0.065)
C. Pooled behavioral seizure latency data comparing all mice (FS and controls) with versus without EEG implants (1054 ± 111.7 seconds vs 1937 ± 243.9 seconds; n = 22 vs n = 7, p = 0.001).
D. Example of activated microglia in hippocampus of EEG-implanted control CX3CR1GFP/+ mouse. The mouse experienced the first hit control condition, EEG implantation, and second hit KA-SZ. GFP positive cells were enlarged and abundant near the CA3 hippocampal subfields where prolonged KA-SZ are known to cause neuronal death.
Consistent with the significant difference in seizure susceptibility between EEG-implanted and sham mice, marked microglia activation was evident in the hippocampus in CX3CR1GFP/+ mice at P29, 24 hours after KA-SZ, whether or not the mice experienced FS at P14 (Fig. 3D). The marked inflammation in the EEG-implanted mice suggested that the increase in seizure susceptibility was related to electrode implantation.
3.3. Experiment 2 (Electrode-Implantation)
To quantify microglia activation related to EEG screw implantation, we used CX3CR1GFP/+ mice that underwent either screw-implantation or sham surgery at P25, followed by KA-SZ at P28. Electrode-implanted mice tended to require less total KA on average to reach SE compared to sham mice (15.8 ± 0.8 mg/kg vs 19.2 ± 1.5 mg/kg (n = 6/group, p = 0.086)). The tendency for electrode implanted mice to require less KA coincided with the increased sensitivity to KA by EEG-implanted mice observed in our two-hit experiment (Section 3.2).
3.4. Inflammatory effects of electrode implantation evident in hippocampus after status epilepticus
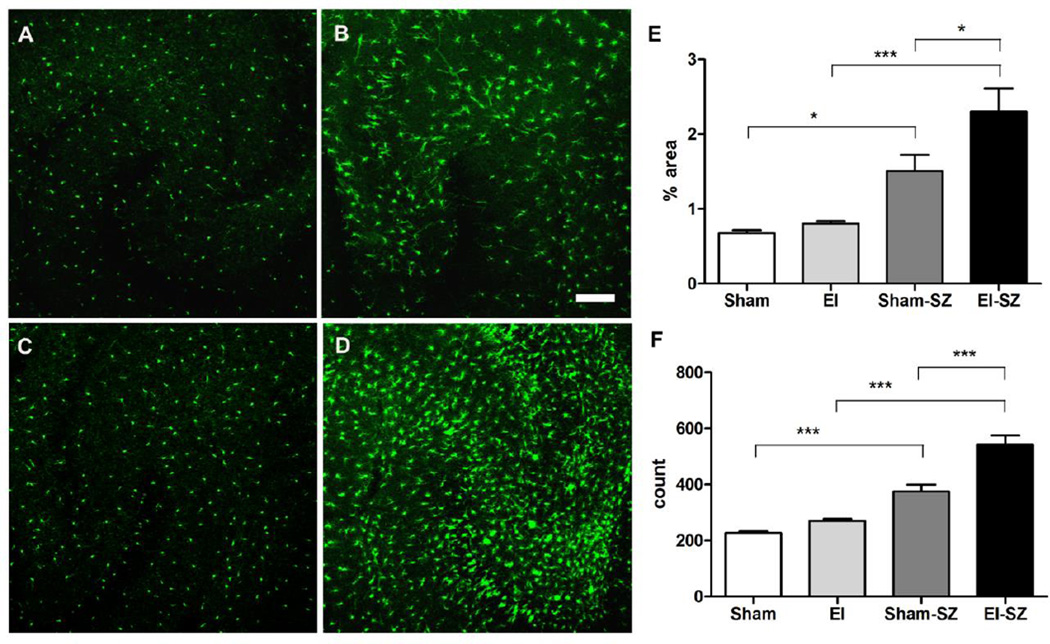
Electrode-implanted mice showed a significant increase in hippocampal microglia activation after KA-SZ. There was significant difference in percent area green fluorescent signal in the hippocampus (p < 0.0001) (Fig. 4E) and of GFP positive cell counts (p = 0.001) (Fig. 4F). While KA-SZ caused significantly increased microglia activation in both electrode-implanted and sham mice compared to their relative baselines, electrode-implanted mice had significantly more microglia activation than sham mice after KA-SZ (Fig. 4E & F, p < 0.001). In electrode-implanted mice, microglia showed marked activation after KA- SZ with larger cell bodies, extended processes, and increased cell counts.
Fig. 4.
Significant increase in inflammation after KA-SZ with electrode implantation.
A-D: Representative confocal images of hippocampi and microglia morphology at P28
A. Hippocampus of sham mouse (Sham); B. Hippocampus of sham mouse 24 hours after KA-SZ (Sham-SZ); C. Hippocampus of electrode-implanted mouse 3 days after surgery (EI); D. Hippocampus of electrode-implanted mouse 3 days after surgery and 24 hours after KA-SZ (EI-SZ); E. Percent area green fluorescent signal in CA3 region of sham mice (Sham), electrode-implanted mice (EI), sham mice after KA (Sham-SZ), and electrode-implanted mice after KA (EISZ), n = 6/group, p < 0.0001, One-way ANOVA with post-hoc Tukey test (* p<0.05, ***p < 0.001).
F. GFP positive cell count in CA3 region, n = 6/group, p = 0.001, One-way ANOVA with post-hoc Tukey test (***p < 0.001).
3.5. No apparent inflammatory effects of electrode implantation in hippocampus prior to seizures
The hippocampi of electrode-implanted mice and sham mice sacrificed at P28, 3 days after surgery and without KA exposure, were indistinguishable. (Fig. 4A & C; 4E & F). There was no significant difference in the percent area of green fluorescent signal, or total number of microglia between electrode-implanted mice and sham mice (p > 0.05).
4. Discussion
We observed an increase in seizure susceptibility in all electrode-implanted mice, regardless of early life seizure experience. EEG-implanted mice experienced short latency to seizure onset, severe convulsions, and 32% mortality at KA doses (10mg/kg) less than half of the usual dose to induce SE, while 100% of sham mice littermates survived with minimal motor seizures and long latency to seizure onset. The reason for such a marked alteration in seizure susceptibility after electrode-implantation is unclear. The stress of the surgical procedure combined with implantation related brain inflammation may have contributed to increased excitability. It is also possible that electrode-implantation impacted KA bioavailability in the brain. No long-term effect of brief, recurrent FS at P14 could be demonstrated on susceptibility to KA-SZ at P25 in both sham and electrode-implanted mice. Our finding is consistent with the only moderate increase in epilepsy risk for children with non-complex FS and [3] and reflects the vast majority of children who experience FS and do not develop epilepsy [18]. Our results did not reflect the established association between early life prolonged FS and increased seizure susceptibility in adulthood [5]. Brief FS recurrent over 3 hours may not have been severe enough to have lasting consequences, but the EEG-implantation related increase in KA sensitivity likely masked any subtle differences between groups.
The priming effect of electrode-implantation on the inflammatory response to subsequent seizures highlighted concerns for designing control experiments. Electrode-implanted mice had significantly more hippocampal microglia activation than sham mice after KA-SZ, but prior to KA-SZ, microglia activation in electrode-implanted mice was indistinguishable from that of shams. Electrode-implantation had no apparent effect on baseline microglia activation, but had a priming effect on the microglial response to the subsequent epileptic insult. Basic surgical controls comparing the electrode-implanted and sham mice failed to reveal the impact of electrode-implantation on the inflammatory milieu, thus were inadequate controls.
The striking increase in hippocampal microglia counts after electrode-implantation and KA-SZ may be attributable to aggravated seizures and increased seizure-induced cell injury or to a widespread microglia response extending beyond the site of implantation related tissue injury. Three days after fluid percussive injury (mild TBI) in rats, microglia OX42 staining has been shown to increase in the hippocampus, thalamus, external capsule, and lateral and medial geniculate nuclei [2]. Seven days after TBI, diffuse microglia staining increases throughout the cortex [2]. The electrode-implantation explored in our study presumably caused more damage than fluid percussive injury because the screw electrodes of 0.6mm in diameter reached the cortex in multiple locations. Nonetheless, these results indicated global inflammatory consequences for focal trauma.
The potential for electrode-implantation to prime the immune system highlights necessary temporal considerations in experimental designs. Microglia number and functionality change throughout development and throughout the immune response to injury. Microglia counts are highest around P14, begin to decline around P21, and reach adult levels around 6 weeks [11, 15]. The P14 peak has been associated with increased seizure susceptibility [11]. In our study, FS and KA-SZ coincided with high microglia levels, therefore a similar experiment with adult rodents may find a weaker microglia response to electrode-implantation. The recovery period between P25 and P28 was arguably short and meant that seizures occurred during the acute inflammatory response to electrode-implantation. After CNS injury, microglia produce and respond to a variety of pro-inflammatory and anti-inflammatory signals as the immune response evolves [4, 14]. Prolonged seizures may have variable consequences if they coincide with different phases of the immune response to tissue injury and shifting microenvironments and immune cell populations.
Our findings may be more relevant to models of acquired epilepsies than to genetic epilepsies, but the immune response to electrode-implantation is likely to interact with immune responses to seizures, regardless of underlying etiology. In models of acquired epilepsy, timing matters. The long-term consequences of KA-SZ can vary with seizure severity as well as age of induction. KA-SZ in rats at P6 and P9 protects against seizure-induced hippocampal injury at P30 [7], whereas KA-SZ in rats at P15 leads to increased seizure-induced neuronal death at P45 [12]. Electrode-implantation alters the severity of KA-SZ, confounding multi-hit models.
5. Conclusion
EEG electrode-implantation increased seizure susceptibility and seizure-related microglia activation in the hippocampus, interfering with a two-hit FS and KA-SZ model of epileptogenesis. The priming effect of electrode-implantation on hippocampal microglia activation became evident only after second seizure induction. EEG electrode-implantation affected the brain’s inflammatory milieu, altering the immune response to the subsequent stress of seizures. To avoid unintentionally creating a multi-hit model with implantation-related and seizure-related immune challenges, deliberate scheduling and careful control selection are essential to experimental designs using EEG monitoring in rodents.
Highlights.
Cortical EEG screw electrode implantation increases susceptibility to kainic acid induced seizures.
Electrode-implantation elicits non-negligible neuroinflammation that primes the immune response to future insults.
Microglia activation by EEG implantation may only be uncovered after subsequent seizures.
Acknowledgments
The authors thank H. Chung, J. Qi and S. Kienzle for their invaluable assistance with this work. This research was funded by NIH/NINDS R01NS073768.
Abbreviations
- ANOVA
one-way analysis of variance
- BBB
blood-brain barrier
- EEG
electroencephalogram
- EI
electrode-implanted
- FS
febrile seizures
- GFP
green fluorescent protein
- GTC
generalized tonic-clonic convulsions
- KA
kainic acid
- KA-SZ
kainic acid-induced seizures
- LPS
lipopolysaccharide
- SE
status epilepticus
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
I.B. wrote the first draft of the manuscript. I.B. and J.W. were equally responsible for the experimental design, experiments, and surgeries. J.H. performed surgeries and provided invaluable support. As principal investigator, S.K. contributed to the experimental design and to the writing of the manuscript.
References
- 1.Abraham J, Fox PD, Condello C, Bartolini A, Koh S. Minocycline attenuates microglia activation and blocks the long-term epileptogenic effects of early-life seizures. Neurobiol Dis. 2012;46:425–430. doi: 10.1016/j.nbd.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aihara N, Hall JJ, Pitts LH, Fukuda K, Noble LJ. Altered immunoexpression of microglia and macrophages after mild head injury. J Neurotrauma. 1995;12:53–63. doi: 10.1089/neu.1995.12.53. [DOI] [PubMed] [Google Scholar]
- 3.Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- 4.Benson MJ, Manzanero S, Borges K. Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia. 2015;56:895–905. doi: 10.1111/epi.12960. [DOI] [PubMed] [Google Scholar]
- 5.Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eun BL, Abraham J, Mlsna L, Kim MJ, Koh S. Lipopolysaccharide potentiates hyperthermia-induced seizures. Brain Behav. 2015;5:e00348. doi: 10.1002/brb3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman L, Hu S. Early-life seizures in predisposing neuronal preconditioning: a critical review. Life Sci. 2014;94:92–98. doi: 10.1016/j.lfs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S, Insel RA, Epstein LG. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331:432–438. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- 9.Hirt UA, Gantner F, Leist M. Phagocytosis of nonapoptotic cells dying by caspaseindependent mechanisms. J Immunol. 2000;164:6520–6529. doi: 10.4049/jimmunol.164.12.6520. [DOI] [PubMed] [Google Scholar]
- 10.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim I, Mlsna LM, Yoon S, Le B, Yu S, Xu D, Koh S. A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain Behav. 2015;5:e00403. doi: 10.1002/brb3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh S, Storey TW, Santos TC, Mian AY, Cole AJ. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adulthood. Neurology. 1999;53:915–921. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- 13.Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morganti JM, Riparip LK, Rosi S. Call Off the Dog(ma): M1/M2 Polarization Is Concurrent following Traumatic Brain Injury. PLoS One. 2016;11:e0148001. doi: 10.1371/journal.pone.0148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikodemova M, Kimyon RS, De I, Small AL, Collier LS, Watters JJ. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J Neuroimmunol. 2015;278:280–288. doi: 10.1016/j.jneuroim.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 18.Vestergaard M, Pedersen CB, Sidenius P, Olsen J, Christensen J. The long-term risk of epilepsy after febrile seizures in susceptible subgroups. Am J Epidemiol. 2007;165:911–918. doi: 10.1093/aje/kwk086. [DOI] [PubMed] [Google Scholar]
- 19.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu D, Miller SD, Koh S. Immune mechanisms in epileptogenesis. Front Cell Neurosci. 2013;7:195. doi: 10.3389/fncel.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]