Abstract
Purpose
To review the most recent findings, characteristics, faults and future perspectives of optical coherence tomography angiography (OCTA) in age-related macular degeneration (AMD).
Recent Findings
In dry AMD, OCTA is useful on the evaluation of choriocapillaris perfusion and detection of naïve quiescent non-exudative choroidal neovascularization (CNV). In wet AMD, OCTA can provide detailed anatomic and morphologic information of CNVs, which may help to understand why and how they develop and become active. In other hand, the many artifacts present in OCTA images may lead to misinterpretation and misdiagnosis.
Summary
OCTA is a still developing technology that is able to provide a large amount of anatomic, functional and morphologic information in macular diseases and, particularly, AMD. As the technology evolves, the need of dye-based modalities tends to decrease.
Keywords: optical coherence tomography angiography (OCTA), age-related macular degeneration (AMD)
Introduction
Age-related macular degeneration (AMD) is the main cause of visual impairment in developed countries in individuals with more than 50 years of age.1, 2 Multimodal imaging has led to a remarkable improvement in our understanding of the physiopathology of macular diseases. Combining the different imaging modalities has allowed the retinal specialists not only to understand disease progression, but most importantly, to monitor treatment response. Fluorescein angiography (FA) is the method of choice for classifying choroidal neovascularization (CNV) as classic, occult, or combination subtypes, essential in the era of photodynamic therapy3, but continue to be useful for detection of “active” neovascularization. It is an irreplaceable method to analyze and monitor leaks and compare them with staining and window defects. In contrast to FA, indocyanine green angiography (ICGA) uses a dye that is 98% protein bound, providing more detailed images of the choroid and thus identifying the entire extension of the CNV. However, both modalities are invasive, requiring intravenous dye injection, which can cause some side effects as nausea and anaphylaxis.4, 5 Moreover, are time consuming in a routine use and can only capture early transit in one eye. Optical coherence tomography (OCT) has revolutionized the diagnostic approach and treatment of macular diseases. Particularly in AMD, OCT is able to detect the presence of drusen, retinal pigmentary epithelium (RPE) atrophy, fibrovascular complex, sub and intra-retinal fluid, among other features.6
Optical coherence tomography angiography (OCTA) has been studied for more than 10 years, but only recently became available to retinal specialists. Unlike traditional angiography, OCTA is safer and non-invasive, because it does not require the use of exogenous dyes. It is a novel imaging modality that allows direct visualization of the retinal and choroidal vasculature in vivo without dye leakage and staining that could possibly obscure the limits and anatomy of pathologies.7 OCTA obtains multiple B-scans at the same position and detects erythrocyte movement by analyzing the signal decorrelation between scans.8 It’s fast, which is an enormous advantage to other methods when evaluating patients that require frequent visits. It’s three dimensional, unlike the two-dimensional image of FA and ICGA, allowing visualization of the exact localization and the dimensions of a lesion.7 Structural en face OCT combines OCT and monochromatic or angiographic confocal scanning laser ophthalmoscope analysis. The en face acquisition areas range from 2 × 2 mm to 12 × 12 mm. The scan quality decreases with a widened field of view because of the limited number of OCT B-scans for all scanning areas (Figure 1). This range has been quite sufficient for analysis of AMD since the 3×3mm central scan encompasses most of the AMD disease. In other hand, this new technology is prone to several image artifacts, like shadow, projection and motion artifacts.9–13 OCTA can also miss areas of slow or turbulent blood flow below its minimum threshold of detectable flow.9, 10 Furthermore, there is a difference between the two main technologies of OCTA, the spectral domain (SD-OCTA) and the swept source OCTA (SS-OCTA). Most of the commercial devices are SD-OCTA, and uses a shorter wavelength, approximately 840 nm, which is substantially attenuated by the RPE. The sub-RPE tissue attenuation may be more important in drusen or RPE thickening, since the RPE is an important hyperreflective barrier, making it difficult for the light pass through it.14–16 The SS-OCTA uses a longer wavelength, approximately 1050 nm, thereby being able to penetrate deeper into the choroid. The different light source also contributes for the improved visualization of the sub-RPE structures.15
Figure 1.
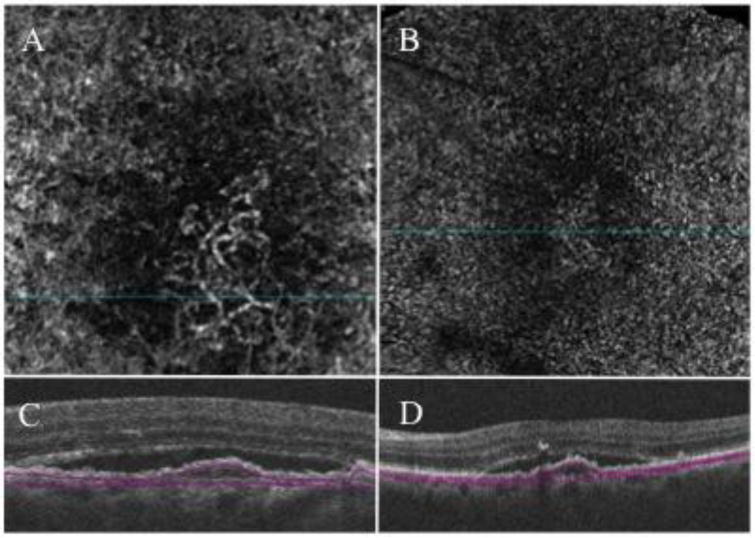
Spectral-domain (SD) optical coherence tomography angiography of a type 1 choroidal neovascularization from one eye of patient with exudative age-related macular degeneration. (A) 3×3 mm en face flow angiogram extracted from the slab shown on C revealing the neovascular network of a type 1 choroidal neovascularization (CNV); (B) 6×6 mm en face flow angiogram extracted from the slab shown on D. Differently from the 3×3 mm angiogram shown on A, the CNV is almost indistinguishable from the surrounding choriocapillaris due to lower resolution of the 6×6 angiogram. (C and D) B-scan from which the 3×3 and 6×6 mm angiogram on A and C, respectively, were extracted demonstrating the boundaries of the slab; top boundary under the retinal pigment epithelium and bottom boundary under the Bruch’s membrane.
OCTA of dry AMD
Dry AMD accounts for 85–90% of all cases of AMD.17 It is characterized by drusen, pigmentary changes, and photoreceptor and RPE loss. OCTA images in early dry AMD eyes suggest that there is a general reduction in choriocapillaris (CC) density in comparison to age-matched normal controls, interpreted as patches of CC loss or flow impairment.18 It is important to remind that OCTA may produce false positive images of flow impairment mostly in early AMD and under drusen when the RPE is healthy or thickened enough to block the signal from the CC. This is called shadow or masking artifact, and is more prone to happen in SD-OCTA devices. Therefore, one should be really careful when stating there is a reduction in the flow of sub-RPE layers, especially in SD-OCTA images.9, 10, 12, 15
With the progression of the dry AMD, the development of geographic atrophy (GA) occurs with increasing photoreceptor and RPE loss. In patients with GA, OCTA shows worsening of CC flow impairment under the regions of GA and this alteration may not be limited to the area of GA with evidence of flow impairment beyond that area.19 The changes underlying the area of GA are usually well visualized on both spectral domain and swept source OCTA since the hyperreflective barrier of the RPE is weakened in patients with GA and does not attenuate the OCTA signal.
Another important finding in dry AMD is the detection of quiescent, non-exudative, treatment-naïve CNVs.7, 20–22 The existence of these lesions was described before in histological specimens and with ICGA23, 24 and, with OCTA, they are quickly detected under unsuspicious RPE elevations and drusen. We emphasize that a proper analysis of OCTA images is mandatory to avoid false positives images. The projection artifact over drusen and RPE detachments can be easily misinterpreted as a CNV.16, 25 Until natural history data of quiescent, non-exudative, naïve CNVs is known, the recommendation is not to treat them. There is a possibility that these asymptomatic lesions may be supporting nutritionally the RPE and photoreceptors, thus anti-VEGF therapy could induce macular atrophy.26 Also, there is a lack of a goal for the therapy or how frequent the treatment should be performed.
OCTA of exudative AMD
In 2014, Jia et al. first described the ability of a prototype SS-OCTA system to visualize and quantify CNV.27 Several studies followed Jia’s confirming OCTA’s capacity of detection of all three types of CNV.28–32 The sensitivity of the OCTA in detecting CNV lesions varies from 50% to 100%.27–29 Morphologically, a variety of CNV formats have been described. The medusa shape corresponding with vessels radiating from a central trunk vessel to all directions (Figure 2A); the seafan shape, with vessels branching to one side of the main trunk (Figure 2B); the glomerular shape, defined as globular structures with intertwined vessels (Figure 2C); and the “dead tree” shape, composed by a main pruned vascular trunk (Figure 2D).31, 33 These patterns still have questionable clinical relevance.
Figure 2.
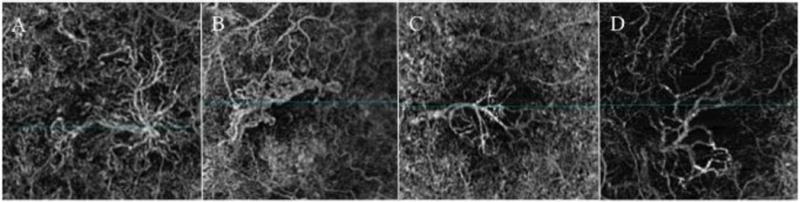
En face flow angiograms from diferente morphologic patterns of choroidal neovascularization (CNV). (A) Medusa shape, showing vessels radiating from the center to all directions; (B) seafan shape, presenting with vessels branching to one side of the main trunk; (C) glomerular shape, defined as globular structures with intertwined vessels; and (D) “dead tree” shape, composed by a main pruned vascular trunk.
In comparison to FA, OCTA proved to be a valuable tool for evaluation of CNV activity, even not recognizing leakage.34 Many studies reported decrease on the area of the CNV after treatment with anti-VEGF.35–38 Others found persistence of the neovascular network after therapy, even long time after injection in quiescent CNVs and macular fibrosis.38–41 Several artefactual pitfalls on OCTA can result in misinterpretation of CNV size change. First, the quality for detection of neovascular network between SD-OCTA and SS-OCTA instruments are significantly different.14, 15 The SS-OCTA technology is able to provide images deeper into the sub-RPE tissue, therefore is more reliable in the detection of the entire neovascular complex, including secondary smaller capillaries.14, 42 Measurements based on SD-OCTA images may be underestimating the size of the CNV. Second, when comparing images from the same CNV complex pre and post treatment, is imperative to utilize the exact same segmentation in order to ensure that the entire neovascular tissue was included in both images. However, the automatic segmentation is not reliably reproducible in diseases where the outer retina and RPE layers could be disorganized as in AMD.9, 10 Third, areas of atrophy, mainly when next to CNV, can acquire the appearance of a neovascular complex, allowing for inaccurate measurement, making precise evaluation challenging. This is called unmasking artifact.9, 10 Last is the questionable ability to differentiate between real absence of flow or merely reduced flow in treated lesions. If the flow in the vessels is too slow, beyond the threshold of detection, it will appear as absence of flow. So it’s possible that the CNV area may keep stable after treatment, but the flow within the neovascular network become slower, thus undetectable. New softwares, as the variable interscan time analysis, or VISTA, may help to detect sub-threshold flow signals and distinguish slow flow from flow absence.43
Conclusion
Fluorescein angiography, the gold standard for diagnosing CNV, is a dynamic exam able to show different patterns of dye transit and leakage,44, 45 however is invasive and time consuming. Moreover, it generates a two-dimensional image. In the last decade, OCT became essential in the diagnosis and management of AMD. Recently, OCTA with its cutting edge new technology allows for simultaneous evaluation of functional (OCT angiograms) and anatomical (OCT B-scans) information. Furthermore, it’s safe, fast and noninvasive. It is increasingly being tested in retina and research centers, not only remodeling our level of understanding and interpretation of the images but allowing for the identification of the limitations of the modality, such as artifacts and differences between commercially available devices. Careful designed prospective clinical trials are critically necessary to best determine the optimal role and use of OCTA. Developing companies are facing a technological run to improve the eye-tracking, the speed and number of scans, the segmentation, the quantification, among others software and hardware advances to provide reliable and reproducible quantitative analysis. OCTA may grant us the opportunity to decrease morbidity through early detection and intervention in macular diseases.
OCTA is still an unfolding technology with several limitations, but it won’t be surprising if OCTA becomes the gold standard for diagnosis and follow-up of macular diseases, particularly AMD.
Acknowledgments
Thanks to Eduardo Novais, MD (Boston Children’s Hospital) for help with reviewing this manuscript.
Footnotes
Conflict of Interest
Raquel Goldhardt and Luiz Roisman declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–26. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 2.NM B. Age-related macular degeneration is the leading cause of blindness. JAMA ophthalmology. 2004;291:1900–1. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 3.Cruess AF, Zlateva G, Pleil AM, Wirostko B. Photodynamic therapy with verteporfin in age-related macular degeneration: a systematic review of efficacy, safety, treatment modifications and pharmacoeconomic properties. Acta ophthalmologica. 2009;87:118–32. doi: 10.1111/j.1755-3768.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- 4.Su Z, Ye P, Teng Y, Zhang L, Shu X. Adverse reaction in patients with drug allergy history after simultaneous intravenous fundus fluorescein angiography and indocyanine green angiography. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2012;28:410–3. doi: 10.1089/jop.2011.0221. [DOI] [PubMed] [Google Scholar]
- 5.Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93:611–7. doi: 10.1016/s0161-6420(86)33697-2. [DOI] [PubMed] [Google Scholar]
- 6.Regatieri CV, Branchini L, Duker JS. The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthalmic surgery, lasers & imaging: the official journal of the International Society for Imaging in the Eye. 2011;42(Suppl):S56–66. doi: 10.3928/15428877-20110627-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roisman L, Zhang Q, Wang RK, Gregori G, Zhang A, Chen CL, et al. Optical Coherence Tomography Angiography of Asymptomatic Neovascularization in Intermediate Age-Related Macular Degeneration. Ophthalmology. 2016;123:1309–19. doi: 10.1016/j.ophtha.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Optics express. 2012;20:4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Ghasemi Falavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. The British journal of ophthalmology. 2016 doi: 10.1136/bjophthalmol-2016-309104. This study provides a review of the most common image artifacts of OCTA. All the understanding and interpretation of OCTA initiates by identifying its a. [DOI] [PubMed] [Google Scholar]
- *10.Chen FK, Viljoen RD, Bukowska DM. Classification of image artefacts in optical coherence tomography angiography of the choroid in macular diseases. Clin Exp Ophthalmol. 2016;44:388–99. doi: 10.1111/ceo.12683. For the same reason of Ghasemi’s paper, this study brings complementary information of image artifacts, focusing in the choroid. [DOI] [PubMed] [Google Scholar]
- 11.Shahlaee A, Samara WA, Sridhar J, Kasi SK, Hsu J, Ho AC. Accentuation of optical coherence tomography angiography projection artefacts on hyper-reflective retinal layers. Acta ophthalmologica. 2016 doi: 10.1111/aos.13164. [DOI] [PubMed] [Google Scholar]
- 12.Spaide RF, Fujimoto JG, Waheed NK. Image Artifacts in Optical Coherence Tomography Angiography. Retina. 2015;35:2163–80. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng F, Gregori G, Schaal KB, Legarreta AD, Miller AR, Roisman L, et al. Choroidal Thickness and Choroidal Vessel Density in Nonexudative Age-Related Macular Degeneration Using Swept-Source Optical Coherence Tomography Imaging. Investigative ophthalmology & visual science. 2016;57:6256–64. doi: 10.1167/iovs.16-20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novais EA, Adhi M, Moult EM, Louzada RN, Cole ED, Husvogt L, et al. Choroidal Neovascularization Analyzed on Ultrahigh-Speed Swept-Source Optical Coherence Tomography Angiography Compared to Spectral-Domain Optical Coherence Tomography Angiography. American journal of ophthalmology. 2016;164:80–8. doi: 10.1016/j.ajo.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane M, Moult EM, Novais EA, Louzada RN, Cole ED, Lee B, et al. Visualizing the Choriocapillaris Under Drusen: Comparing 1050-nm Swept-Source Versus 840-nm Spectral-Domain Optical Coherence Tomography Angiography. Investigative ophthalmology & visual science. 2016;57:OCT585–90. doi: 10.1167/iovs.15-18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng F, Roisman L, Schaal KB, Miller AR, Robbins G, Gregori G, et al. Artifactual Flow Signals Within Drusen Detected by OCT Angiography. Ophthalmic surgery, lasers & imaging retina. 2016;47:517–22. doi: 10.3928/23258160-20160601-02. [DOI] [PubMed] [Google Scholar]
- 17.Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Quiroz-Mercado H, Mandava N. Current knowledge and trends in age-related macular degeneration: genetics, epidemiology, and prevention. Retina. 2014;34:423–41. doi: 10.1097/IAE.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 18.Waheed NK, Moult EM, Fujimoto JG, Rosenfeld PJ. Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration. Developments in ophthalmology. 2016;56:91–100. doi: 10.1159/000442784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W, Moult EM, Waheed NK, Adhi M, Lee B, Lu CD, et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology. 2015;122:2532–44. doi: 10.1016/j.ophtha.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carnevali A, Cicinelli MV, Capuano V, Corvi F, Mazzaferro A, Querques L, et al. Optical Coherence Tomography Angiography: A Useful Tool for Diagnosis of Treatment-Naive Quiescent Choroidal Neovascularization. American journal of ophthalmology. 2016;169:189–98. doi: 10.1016/j.ajo.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 21.Nehemy MB, Brocchi DN, Veloso CE. Optical Coherence Tomography Angiography Imaging of Quiescent Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmic surgery, lasers & imaging retina. 2015;46:1056–7. doi: 10.3928/23258160-20151027-13. [DOI] [PubMed] [Google Scholar]
- 22.Palejwala NV, Jia Y, Gao SS, Liu L, Flaxel CJ, Hwang TS, et al. Detection of Nonexudative Choroidal Neovascularization in Age-Related Macular Degeneration with Optical Coherence Tomography Angiography. Retina. 2015;35:2204–11. doi: 10.1097/IAE.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92:615–27. [PubMed] [Google Scholar]
- 24.Hanutsaha P, Guyer DR, Yannuzzi LA, Naing A, Slakter JS, Sorenson JS, et al. Indocyanine-green videoangiography of drusen as a possible predictive indicator of exudative maculopathy. Ophthalmology. 1998;105:1632–6. doi: 10.1016/S0161-6420(98)99030-3. [DOI] [PubMed] [Google Scholar]
- 25.Coscas G, Lupidi M, Cagini C, Coscas F. ‘False-friend’ images on optical coherence tomography angiography: early choroidal neovascularization or artefact? Acta ophthalmologica. 2016 doi: 10.1111/aos.13078. [DOI] [PubMed] [Google Scholar]
- 26.Dhrami-Gavazi E, Balaratnasingam C, Lee W, Freund KB. Type 1 neovascularization may confer resistance to geographic atrophy amongst eyes treated for neovascular age-related macular degeneration. International journal of retina and vitreous. 2015;1:15. doi: 10.1186/s40942-015-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121:1435–44. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moult E, Choi W, Waheed NK, Adhi M, Lee B, Lu CD, et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic surgery, lasers & imaging retina. 2014;45:496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122:1228–38. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Kuehlewein L, Dansingani KK, de Carlo TE, Bonini Filho MA, Iafe NA, Lenis TL, et al. Optical Coherence Tomography Angiography of Type 3 Neovascularization Secondary to Age-Related Macular Degeneration. Retina. 2015;35:2229–35. doi: 10.1097/IAE.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 31.El Ameen A, Cohen SY, Semoun O, Miere A, Srour M, Quaranta-El Maftouhi M, et al. Type 2 Neovascularization Secondary to Age-Related Macular Degeneration Imaged by Optical Coherence Tomography Angiography. Retina. 2015;35:2212–8. doi: 10.1097/IAE.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 32.Miere A, Querques G, Semoun O, El Ameen A, Capuano V, Souied EH. Optical Coherence Tomography Angiography in Early Type 3 Neovascularization. Retina. 2015;35:2236–41. doi: 10.1097/IAE.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 33.Kuehlewein L, Bansal M, Lenis TL, Iafe NA, Sadda SR, Bonini Filho MA, et al. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. American journal of ophthalmology. 2015;160:739–48e2. doi: 10.1016/j.ajo.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 34.Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY VERSUS TRADITIONAL MULTIMODAL IMAGING IN ASSESSING THE ACTIVITY OF EXUDATIVE AGE-RELATED MACULAR DEGENERATION: A New Diagnostic Challenge. Retina. 2015;35:2219–28. doi: 10.1097/IAE.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 35.Kuehlewein L, Sadda SR, Sarraf D. OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye. 2015;29:932–5. doi: 10.1038/eye.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muakkassa NW, Chin AT, de Carlo T, Klein KA, Baumal CR, Witkin AJ, et al. Characterizing the Effect of Anti-Vascular Endothelial Growth Factor Therapy on Treatment-Naive Choroidal Neovascularization Using Optical Coherence Tomography Angiography. Retina. 2015;35:2252–9. doi: 10.1097/IAE.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 37.Phasukkijwatana N, Tan AC, Chen X, Freund KB, Sarraf D. Optical coherence tomography angiography of type 3 neovascularisation in age-related macular degeneration after antiangiogenic therapy. The British journal of ophthalmology. 2016 doi: 10.1136/bjophthalmol-2016-308815. [DOI] [PubMed] [Google Scholar]
- 38.Mastropasqua L, Toto L, Borrelli E, Carpineto P, Di Antonio L, Mastropasqua R. Optical Coherence Tomography Angiography Assessment of Vascular Effects Occurring after Aflibercept Intravitreal Injections in Treatment-Naive Patients with Wet Amd. Retina. 2016 doi: 10.1097/IAE.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 39.Wirth MA, Freiberg F, Pfau M, Wons J, Becker MD, Michels S. Optical coherence tomography angiography in age-related macular degeneration: persistence of vascular network in quiescent choroidal neovascularization. Acta ophthalmologica. 2016 doi: 10.1111/aos.13226. [DOI] [PubMed] [Google Scholar]
- 40.Souied EH, Miere A, Cohen SY, Semoun O, Querques G. Optical Coherence Tomography Angiography of Fibrosis in Age-Related Macular Degeneration. Developments in ophthalmology. 2016;56:86–90. doi: 10.1159/000442783. [DOI] [PubMed] [Google Scholar]
- 41.Miere A, Semoun O, Cohen SY, El Ameen A, Srour M, Jung C, et al. Optical Coherence Tomography Angiography Features of Subretinal Fibrosis in Age-Related Macular Degeneration. Retina. 2015;35:2275–84. doi: 10.1097/IAE.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 42.Lane M, Ferrara D, Louzada RN, Fujimoto JG, Seddon JM. Diagnosis and Follow-Up of Nonexudative Choroidal Neovascularization With Multiple Optical Coherence Tomography Angiography Devices: A Case Report. Ophthalmic surgery, lasers & imaging retina. 2016;47:778–81. doi: 10.3928/23258160-20160808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Ploner SB, Moult EM, Choi W, Waheed NK, Lee B, Novais EA, et al. TOWARD QUANTITATIVE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY: Visualizing Blood Flow Speeds in Ocular Pathology Using Variable Interscan Time Analysis. Retina. 2016 doi: 10.1097/IAE.0000000000001328. The variable interscan time analysis, or VISTA, is a promissing software to differenciate flow reduction from flow abscence. If successful, it can help to understand CC and CNV changes in AMD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gess AJ, Fung AE, Rodriguez JG. Imaging in neovascular age-related macular degeneration. Seminars in ophthalmology. 2011;26:225–33. doi: 10.3109/08820538.2011.582533. [DOI] [PubMed] [Google Scholar]
- 45.Tomi A, Marin I. Angiofluorographic aspects in age-related macular degeneration. Journal of medicine and life. 2014;7(Spec No. 4):4–17. [PMC free article] [PubMed] [Google Scholar]


