Abstract
The Centers for Disease Control has declared opioid abuse to be an epidemic. Overdose deaths are largely assumed to be the result of excessive opioid consumption. In many of these cases, however, opioid abusers are often polydrug abusers. Benzodiazepines are one of the most commonly co-abused substances and pose a significant risk to opioid users. In 2016, the FDA required boxed warnings – the FDA’s strongest warning – for prescription opioid analgesics and benzodiazepines about the serious risks associated with using these medications at the same time. The point of our studies was to evaluate the interactions between these two classes of drugs. We investigated whether diazepam adds to the depressant effects of opioids or do they alter the levels of tolerance to opioids. In the present study, we have found that the antinociceptive tolerance that developed to repeated administration of oxycodone was reversed by an acute dose of diazepam. Antinociceptive tolerance to hydrocodone was also reversed by acute injection of diazepam; however, a four-fold higher dose of diazepam was required when compared to reversal of oxycodone-induced tolerance. These doses of diazepam did not potentiate the acute antinociceptive effect of either opioid. The same dose of diazepam that reversed oxycodone antinociceptive tolerance also reversed oxycodone locomotor tolerance while having no potentiating effects. These studies show that diazepam does not potentiate the acute effect of prescription opioids but reverses the tolerance developed after chronic administration of the drugs.
1. Introduction
Reducing opioid overdose deaths is an important public health and drug policy goal. Much of the current opioid epidemic can be attributed to the rise in use of prescription opioid pain analgesics such as oxycodone and hydrocodone. These drugs have become widely prescribed, with enough opioid analgesics sold in 2010 to medicate every American adult with a typical dose every 4 hours for 1 month. In the United States, at least half of all opioid overdose deaths involve a prescription opioid. (CDC, 2011).
The dangers of accidental opioid overdose are mainly due to respiratory depressive effects. Chronic use of opioids results in the development of tolerance (a decrease in pharmacologic response following repeated or prolonged drug administration) to the analgesic, euphoric, and respiratory depressive effects. This leads to addicts and patients taking higher doses in order to obtain the euphoric high or the analgesic effects, respectively. However, it has been shown that tolerance to different effects of opioids do not occur at the same rate or to the same extent (Hill et al., 2016). It has been suggested that, in man, tolerance to euphoria develops to a greater extent than to respiratory depression (White and Irvine, 1999).
Opioid overdose deaths are largely assumed to result from excessive opioid administration alone. However, opioid abusers are often polydrug users, consuming benzodiazepines, ethanol, cocaine and/or gabapentoids along with opioid drugs. Benzodiazepines and ethanol have been found to pose a significant risk to chronic opioid users, particularly in those taking methadone (National Treatment Agency for Substance Misuse [NTA], 2007). The CDC has reported that benzodiazepines were involved in 31% of opioid related drug poisoning deaths in recent years (Chen et al., 2014). Benzodiazepines, ethanol, and opioids are all considered central nervous system depressants and their effects may be additive or synergistic. Recently, our lab has published that low doses of ethanol and diazepam, which have no observable effect of their own, significantly and dose-dependently reduced the antinociceptive tolerance produced by morphine while not affecting the acute responses (Hull et al., 2013). Low doses of ethanol reversed morphine tolerance at the level of single brain neurons (Llorente et al., 2013) and in a rodent model of respiratory depression (Hill et al., 2016).
A major limitation of the previously described studies is that they have not investigated oxycodone and hydrocodone, two commonly prescribed opioid analgesics. Limited research has been done with these drugs when compared to the opioid standard, morphine, and the illicit compound heroin. Although all are considered opioids, these compounds may differently interact through the μ opioid receptor (MOR). They have been shown to have different pharmacokinetic properties, varying affinities for the μ opioid receptor, potentially interact with other opioid receptors and have different off-target effects (Nielsen et al., 2007). Therefore, it is important to investigate how commonly prescribed opioids compare in their effects to morphine.
The goals of this study were to determine if benzodiazepines potentiate the acute antinociceptive effects of commonly prescribed opioids as well as to determine if they act to reduce tolerance to these opioids. We characterized the development of antinociceptive tolerance to oxycodone and hydrocodone in mice and investigated whether diazepam could reverse tolerance as it does morphine-induced tolerance. Antinociception, as measured by the rodent warm water tail-immersion assay, was chosen for these studies because it has been shown to be a good predictor of antinociception and tolerance for a wide range of compounds in humans. Furthermore, our group has used this assay to extensively investigate the mechanisms of opioid tolerance (Hull et al., 2013, 2010; Smith et al., 2007).
2. Results
2.1. Effects of Acute Diazepam in Drug-Naïve Mice
Diazepam was administered at doses of 0.5, 1, and 2 mg/kg i.p. and mice were monitored over a 3-hour period for behavioral changes and assessed in the warm-water tail immersion test at 30-minute intervals over 2 hours. No antinociceptive effects or behavioral changes, including locomotor activity, were observed at any of these doses.
2.2. Tolerance Development to Oxycodone
Baseline latencies were taken prior to the beginning of the hourly subcutaneous injections. Mice were randomly assigned to either a chronic saline or chronic opioid schedule whereby seven hourly injections of isotonic saline or an ED80 dose of oxycodone were given s.c. After seven injections, mice were injected with a challenge dose of oxycodone (0.25, 0.5, 1, 2 or 4 mg/kg) at the 8-hour time point (Figure 1). Dose-response curves for oxycodone after chronic injections of saline generated similar ED50 values to those in acute dose response experiments (1.19 mg/kg (1.00–1.41, 95% CL)). The ED50 was significantly shifted to the right 1.6-fold, indicating tolerance was observed, in the animals chronically injected with oxycodone prior to receiving the challenge injections (1.84 mg/kg (1.58 – 2.14, 95% CL)). The sample size of each group was 20 animals [Acute Oxycodone, n =20; Chronic Oxycodone, n = 20].
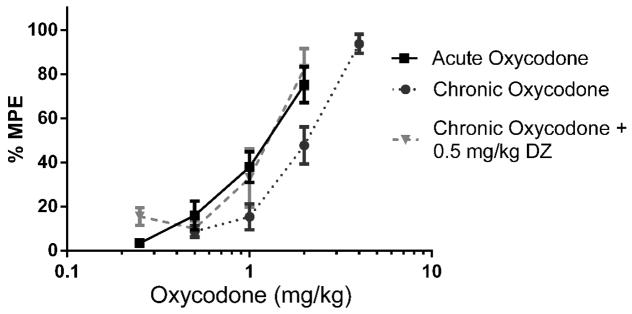
Figure 1.
Tolerance to oxycodone, developed using a single-day injection paradigm, was significantly reversed by 0.5 mg/kg diazepam (n = 10 – 20). Latency to tail withdrawal (% MPE ± SEM) among mice that were drug naïve, repeatedly treated with oxycodone, or repeatedly treated with oxycodone and diazepam pretreatment 30 minutes before testing. Various doses of the oxycodone were used for construction of dose-response curves for calculation of ED50 values.
2.3. Reversal of Oxycodone Antinociceptive Tolerance with Diazepam in Tail Immersion Assay
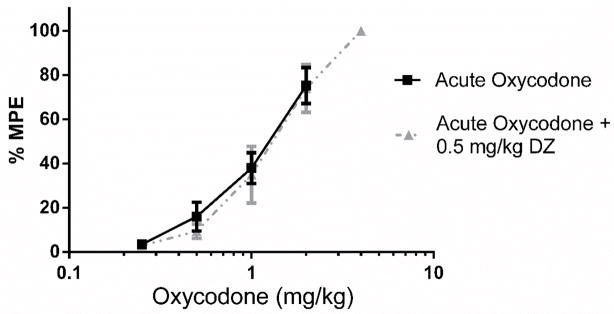
Baseline latencies were obtained in the tail immersion test in the morning before any injections. Following the development of tolerance (single day tolerance model), diazepam (0.5 mg/kg i.p.) was administered. Thirty minutes later, the mice were challenged with doses of oxycodone s.c. for construction of dose-response curves for calculation of the ED50 values (Figure 1; Table 1). Diazepam fully reversed the oxycodone-induced tolerance. The sample size of each group was between 10–20 animals [Acute Oxycodone, n =20; Chronic Oxycodone, n= 20; Chronic Oxycodone + 0.5 mg/kg DZ, n= 12]. The same dose of diazepam did not potentiate the antinociception produced by acute doses of oxycodone in naive mice (Figure 2) [Acute Oxycodone, n =20; Acute Oxycodone + 0.5 mg/kg DZ, n= 11].
Table 1.
Diazepam Reversal of Oxycodone Tolerance
| Treatment | Oxycodone ED50 (mg/kg (95% C.L.)) |
|---|---|
| Acute Oxycodone + Vehicle | 1.19 (1.00–1.41) |
| Acute Oxycodone + Diazepam (0.5 mg/kg) | 1.25 (1.04–1.50) |
| Chronic Oxycodone + Vehicle | 1.84 (1.58–2.14)* |
| Chronic Oxycodone + Diazepam (0.5 mg/kg) | 1.12 (0.88–1.43) |
Significantly different than Acute Oxycodone + Vehicle group based on non-overlapping 95%
Figure 2.
0.5 mg/kg Diazepam did not potentiate the antinociception produced by oxycodone in the tail immersion test. (n = 10–20). Latency to tail withdrawal (% MPE ± SEM) among mice that were drug naïve or pretreated with diazepam 30 minutes before testing. Various doses of the oxycodone were used for construction of dose-response curves for calculation of ED50 values.
2.4. Tolerance Development to Hydrocodone
Baseline latencies were taken prior to the beginning of hourly subcutaneous injections. Mice were assigned randomly to either a chronic saline or chronic opioid schedule whereby seven hourly injections of isotonic saline or an ED80 dose of hydrocodone were given s.c. After seven injections, all mice were injected with final challenge doses of hydrocodone (1, 2, 4, 8, 16 or 32 mg/kg) at the 8-hour time point (Figure 3). Dose response curves of hydrocodone after chronic injections of saline generated similar ED50 values to those in acute dose response experiments (5.51 mg/kg (4.97–6.12, 95% CL)). The ED50 was significantly shifted 2.4-fold to the right in the animals chronically injected with hydrocodone prior to receiving the challenge injections (13.18 mg/kg (11.00–15.80, 95% CL)). The sample size of each group was between 6–12 animals [Acute Hydrocodone, n =6; Chronic Hydrocodone, n= 12]
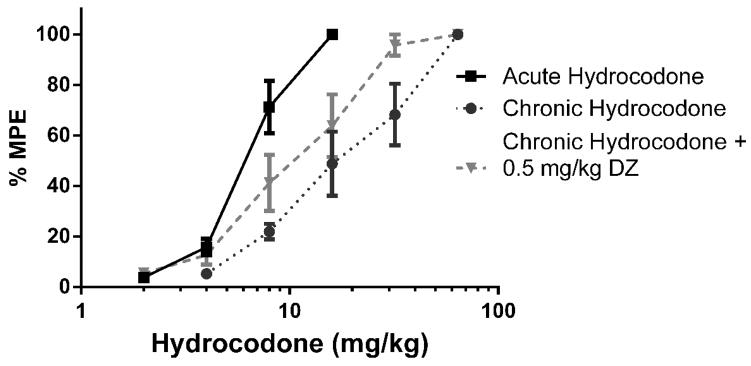
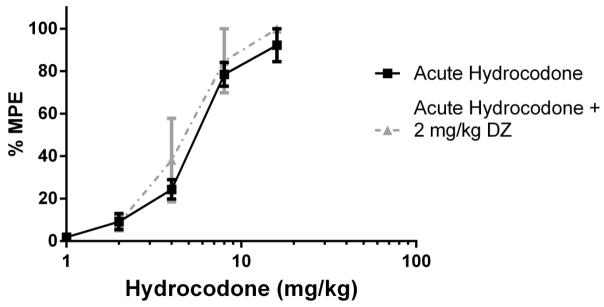
Figure 3.
0.5 mg/kg Diazepam did not fully reverse the antinociception produced by hydrocodone in the tail immersion test (n = 6 – 12). Latency to tail withdrawal (% MPE ± SEM) among mice that were drug naïve, repeatedly treated with hydrocodone, or repeatedly treated with hydrocodone and pretreated with diazepam 30 minutes before testing. Various doses of the hydrocodone were used for construction of dose-response curves for calculation of ED50 values.
2.5. Reversal of Hydrocodone Antinociceptive Tolerance with Diazepam in Tail Immersion Assay
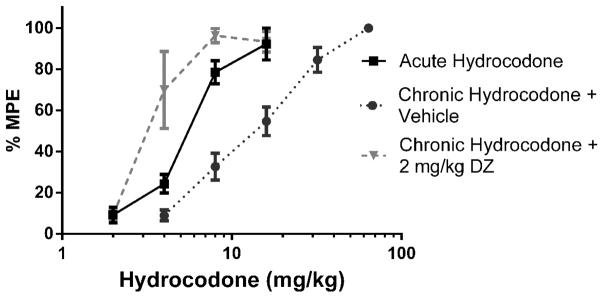
Baseline latencies were obtained in the tail immersion test in the morning before any injections. Following the development of tolerance (single day tolerance model), diazepam was administered i.p. Thirty minutes later, the mice were challenged with doses of hydrocodone s.c. for construction of dose-response curves for calculation of the ED50 values (Table 2). In contrast with oxycodone, 0.5 mg/kg diazepam did not fully reverse antinociceptive tolerance to hydrocodone (Figure 3). 2 mg/kg diazepam fully reversed hydrocodone tolerance and actually significantly potentiated the antinociceptive effect of hydrocodone after chronic administration (Figure 4). However, 2 mg/kg diazepam did not potentiate the antinociception produced by an acutely administered dose of hydrocodone (Figure 5). The sample size of each group was between 6–12 animals [Figure 3: Acute Hydrocodone, n =6; Chronic Hydrocodone, n= 12; Chronic Hydrocodone + 0.5 mg/kg DZ n = 8; Figure 4: Acute Hydrocodone, n =12; Chronic Hydrocodone, n= 12; Chronic Hydrocodone + 2 mg/kg DZ n = 6; Figure 5: Acute Hydrocodone, n =12; Acute Hydrocodone + 2 mg/kg DZ n = 6].
Table 2.
Diazepam Reversal of Hydrocodone Tolerance
| Treatment | Hydrocodone ED50 (mg/kg (95% C.L.)) |
|---|---|
| Acute Hydrocodone + Vehicle | 5.51 (4.97–6.12) |
| Acute Hydrocodone + Diazepam (2 mg/kg) | 4.48 (3.22–6.25) |
| Chronic Hydrocodone + Vehicle | 13.18 (11.00–15.80)* |
| Chronic Hydrocodone + Diazepam (0.5 mg/kg) | 9.92 (7.74–12.70)* |
| Chronic Hydrocodone + Diazepam (2 mg/kg) | 3.77 (2.83–5.04) |
Significantly different than Acute Hydrocodone + Vehicle group based on non-overlapping 95%
Figure 4.
2 mg/kg Diazepam fully reversed the antinociception produced by hydrocodone in the tail immersion test (n = 6 – 12). Latency to tail withdrawal (% MPE ± SEM) among mice that were drug naïve, repeatedly treated with hydrocodone, or repeatedly treated with hydrocodone and pretreated with diazepam 30 minutes before testing. Various doses of the hydrocodone were used for construction of dose-response curves for calculation of ED50 values.
Figure 5.
2 mg/kg Diazepam did not potentiate the antinociception produced by hydrocodone in the tail immersion test (n = 6 – 12). Latency to tail withdrawal (% MPE ± SEM) among mice that were drug naïve or pretreated with diazepam 30 minutes before testing. Various doses of the hydrocodone were used for construction of dose-response curves for calculation of ED50 values.
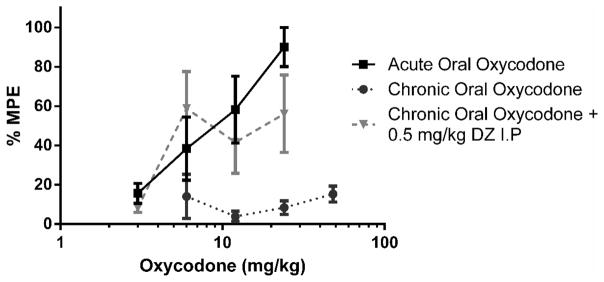
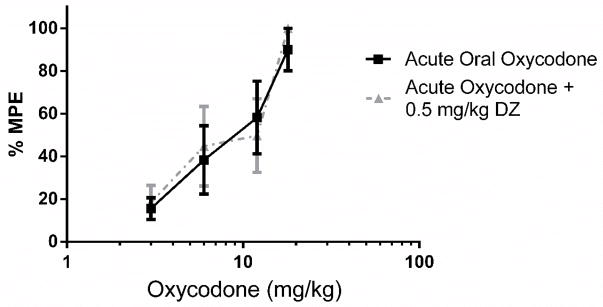
2.6. Reversal of Oral Oxycodone Antinociceptive Tolerance with Diazepam in Tail Immersion Assay
Following the development of maximum tolerance (4-day oral tolerance model), diazepam was administered i.p. Thirty minutes later, the mice were challenged with doses of oxycodone p.o. for construction of dose-response curves for calculation of the ED50 values. The ED50 of the acute oral oxycodone was 8.33 (5.77–12.03, 95% CL). There was marked tolerance to oral oxycodone following the 4-day oral tolerance model. The observation that 0.5 mg/kg diazepam was able to fully reverse the antinociceptive tolerance to oral oxycodone (Figure 6) was supported by the overlapping ED50: 13.24 mg/kg (4.00 – 43.93, 95% CL). Administering 0.5 mg/kg diazepam to animals given acute oral oxycodone generated the ED50 value of 7.27 mg/kg (5.01 – 10.54, 95% CL), indicating that it did not potentiate the antinociception produced by acute doses of oxycodone (Figure 7). The sample size of each group was between 6–7 animals [Figure 6: Acute Oral Oxycodone, n =6; Chronic Oral Oxycodone, n= 7; Chronic Oral Oxycodone + 0.5 mg/kg DZ n = 6; Figure 7: Acute Oral Oxycodone, n =6; Acute Oral Oxycodone + 2 mg/kg DZ n = 6].
Figure 6.
0.5 mg/kg Diazepam fully reversed the antinociception produced by multiple-day paradigm of oral oxycodone in the tail immersion test. (n = 6 – 7). Latency to tail withdrawal (% MPE ± SEM) among mice that were drug naïve, repeatedly gavaged with oxycodone over 4 days, or repeatedly gavaged with oxycodone over 4 days and pretreated with diazepam 30 minutes before testing. Various doses of the oxycodone were used for construction of dose-response curves for calculation of ED50 values.
Figure 7.
0.5 mg/kg Diazepam did not potentiate the antinociception produced by oxycodone (p.o.) in the tail immersion test (n = 6). Latency to tail withdrawal (% MPE ± SEM) among mice that were drug naïve or pretreated with diazepam 30 minutes before testing. Various doses of the oxycodone were used for construction of dose-response curves for calculation of ED50 values.
2.7. The Lack of the Development of Antinociceptive Tolerance to Oral Hydrocodone in Tail Immersion Assay
We attempted a model to produce tolerance to oral hydrocodone in the tail immersion assay. A dose response curve of acute oral hydrocodone was established and reliably repeated. However, we did not succeed in developing tolerance to oral gavages of hydrocodone (data not shown). Methods to establish such a model included a 4-day antinociceptive tolerance model using a twice a day dosing schedule of 32, 64,128, or 256 mg/kg.
2.8. Development of Locomotor Tolerance to Oral Oxycodone and Subsequent Reversal with Diazepam
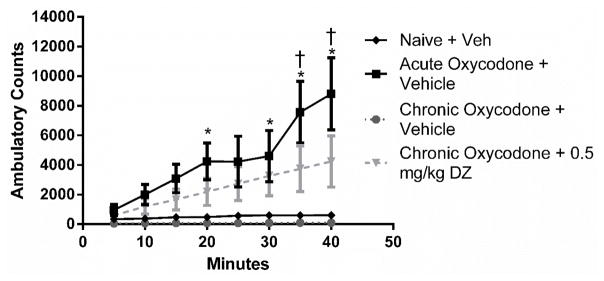
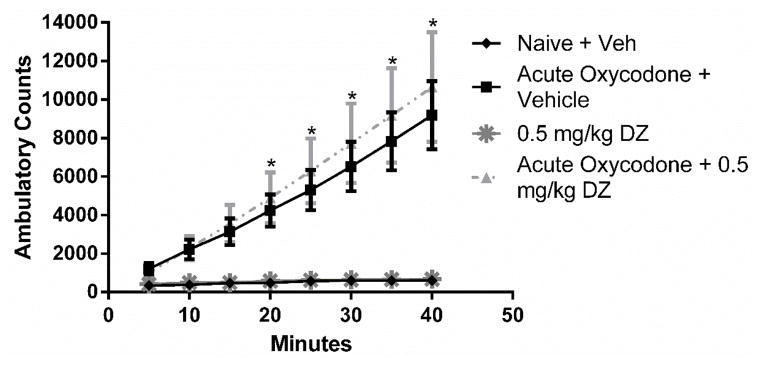
Tolerance was examined by assessing changes of locomotor activity after acute and repeated oxycodone gavages. A main effect was noted for treatment group [F (3,15) = 5.49, p < 0.001], time [F (7,105) = 10.89, p < 0.0001], and treatment × time interaction [F (21,105) = 4.29, p < 0.001]. Post hoc tests demonstrated acutely administered 64 mg/kg oral oxycodone produced significant increased ambulatory counts starting from 20 minutes (with the exception at the 25-minute time point) until the 40-minute endpoint (Figure 8). Using a modified 4-day oral tolerance model, tolerance to morphine’s stimulatory effects was observed. After the administration of 0.5 mg/kg diazepam to mice that were repeatedly treated with oral oxycodone, 64 mg/kg oral oxycodone produced significant increased ambulatory counts starting from the 35-minute time point until the 40-minute endpoint. In determining the effects of acute diazepam by itself or on acute oxycodone’s stimulatory effects, a two-way ANOVA revealed a main effect for treatment group [F(3,16) = 9.37, p < 0.001], time [F(7,112) = 34.62, p < 0.0001], and treatment × time interaction [F(21,112) = 10.42, p < 0.001]. Post hoc tests revealed that 0.5 mg/kg diazepam did not potentiate acute oxycodone’s stimulatory effects in the locomotor assay. This dose of diazepam did not show any stimulatory effects on its own (Figure 9). The sample size of each group was five animals with the exception of the chronic morphine group with four animals.
Figure 8.
Locomotor stimulation in response to vehicle or oxycodone (64 mg/kg p.o.) was assessed (n = 5). * indicates a significant effect of acute oxycodone increasing ambulatory counts as compared to mice naïve to the opioid (treated with vehicle). † indicates a significant effect of chronic oxycodone + 0.5 mg/kg diazepam increasing ambulatory counts as compared to mice naïve to the opioid (p < 0.05).
Figure 9.
Locomotor stimulation in response to vehicle, diazepam (0.5 mg/kg i.p.) or oxycodone (64 mg/kg p.o.) was assessed (n = 5). * indicates a significant effect of both acute oxycodone and acute oxycodone + 0.5 mg/kg diazepam increasing ambulatory counts as compared to mice naïve to the opioid (p < 0.05). 0.5 mg/kg diazepam does not potentiate acute oxycodone’s stimulatory effects. 0.5 mg/kg diazepam does not produce a difference in stimulatory counts when compared to naïve controls.
3. Discussion
Oxycodone and hydrocodone remain among the most commonly prescribed drugs for relief of acute and chronic pain. However, tolerance limits the long-term utility of these opioid agonists, leading to escalating doses of opioids to achieve the same analgesic effect while increasing the risks for abuse liability and death from respiratory depression. Additionally, people who become tolerant and then addicted to opioids usually are consuming other substances as well. Therefore, it is imperative to investigate the effects of these other substances on opioid tolerance. In this study, we investigated the effects of diazepam, a widely coabused substance, on oxycodone and hydrocodone antinociceptive tolerance and oxycodone locomotor stimulating tolerance in mice.
We found that diazepam reversed the antinociceptive tolerance that developed after repeated injections of subcutaneous oxycodone. The observation that an acute dose of diazepam, that was inactive alone, reversed tolerance to the antinociceptive effects of oxycodone is in agreement with our previous studies in which we demonstrated that diazepam reversed tolerance to the antinociceptive effects of morphine (Hull et al., 2013). We also demonstrated that the same dose of diazepam that reversed oxycodone tolerance did not significantly enhance the antinociceptive effect of acute oxycodone.
Patients are often administered oxycodone in tablets or solutions intended for oral use. Therefore, in a separate series of experiments, we investigated the ability of diazepam to reverse maximum tolerance of oral oxycodone. In these studies, to achieve that maximum tolerance, we administered 256 mg/kg oxycodone orally twice a day for four days. Testing on the fifth day, this protocol produced significant tolerance illustrated by the lack of effective doses as high as 64 mg/kg. The observation that 0.5 mg/kg diazepam was able to reverse this maximum tolerance further supports the phenomenon of oxycodone tolerance reversal by benzodiazepines.
Furthermore, we found that diazepam reversed the antinociceptive tolerance that developed after repeated subcutaneous injections of hydrocodone. However, the reversal of tolerance to hydrocodone required a larger dose of diazepam than needed to reverse oxycodone or morphine tolerance. It is clear that diazepam did not potentiate the acute antinociceptive effect of hydrocodone. Further investigation is needed into why diazepam reversed oxycodone and morphine antinociceptive tolerance at lower doses than it reversed hydrocodone tolerance. It is possible that this difference occurred because hydrocodone produced a greater amount of tolerance than oxycodone. However, this is unlikely because the greatest tolerance was achieved using a multiple day oral oxycodone model and this tolerance was reversed by 0.5 mg/kg diazepam. Hydrocodone is also commonly administered to patients in tablets or solutions intended for oral use. We were not able to achieve tolerance when hydrocodone was given orally. This can be due to the fact that hydrocodone has been found to be less potent in certain measures of opioid effects (Zacny and Gutierrez, 2009). However, it is uncertain as to why we observed a greater amount of tolerance to repeated subcutaneous injections of hydrocodone compared to oxycodone yet no significant tolerance to hydrocodone given repeatedly orally. Further studies are needed to examine whether there is an important pharmacokinetic difference between oral dosing of oxycodone and hydrocodone.
The interaction between benzodiazepines and opioids on antinociception has been reported in the literature but with disagreement. Doses of diazepam, up to 5 mg/kg, were inactive in our warm water tail-withdrawal assays. Several studies reported antinociception with diazepam (Jiménez-Velázquez et al., 2010, 2008; Sierralta and Miranda, 1992). This difference may lie in the different noxious stimuli used (chemical versus thermal) and route of administration (intracerebroventricular injection versus intraperitoneal). In our studies, there was no potentiation of oxycodone or hydrocodone induced antinociception with pretreatment doses of diazepam up to 0.5 mg/kg and 2 mg/kg, respectively. Whereas in other studies, intrathecal administration of benzodiazepines were found to enhance opioid antinociception (Bergman et al., 1988; Rattan et al., 1991). In agreement with our studies, others found neither an antinociceptive effect of benzodiazepines nor a potentiation effect of benzodiazepines on opioid induced antinociception (Rodgers and Randall, 1987; Mantegazza et al., 1982; Rosland et al., 1990). To our knowledge, benzodiazepines’ interaction with oxycodone or hydrocodone has not been previously investigated.
In light of these results on antinociceptive tolerance, we tested the hypothesis that diazepam will have a similar effect in another paradigm of tolerance. We observed that oxycodone increased stimulatory activity in the locomotor activity assay. Marked tolerance was observed after repeated exposure to oxycodone over four days. Diazepam significantly reversed this marked tolerance. To rule out potentiation, we tested whether diazepam simply had an additive effect when combined with oxycodone. Diazepam did not potentiate acute oxycodone’s stimulatory effects nor did it have any stimulatory effects on its own. At this dose of diazepam, we did not detect either depressing or stimulatory effects. Diazepam is classified as a CNS depressant and has been shown to decrease locomotor activity (Spyraki and Papadopoulou, 1980). In certain cases (such as prolonged social isolation), low doses of diazepam have been shown either to be inactive or have a stimulatory effect, resulting in a bimodal dose response curve (Pinna et al., 2006). The similar reversal of tolerance seen in both antinociception and in locomotor activity suggest that diazepam acts on a mechanism of tolerance that is shared by both opioid-induced effects.
The precise mechanisms and neuronal circuitry involved remain to be elucidated. This tolerance-reversal effect could be responsible, in part, for the high incidence of polydrug use among opioid abusers. It has been suggested that opioid abusers occasionally combine the use of benzodiazepines with opioids to achieve a greater high (Jones et al., 2012). Whether this is simply an additive or synergistic effect is not clear and in a previous publication, we have suggested that compounds that act on GABA receptors increase the rewarding effects of opioid abuse by reducing tolerance (Hull et al., 2013). It has been theorized that both benzodiazepines and opioids produce a hyperpolarization of GABA interneurons which causes a reduction in the release of GABA which results in the disinhibition of dopaminergic neurons and an increase in extracellular dopamine in areas such as the striatum (Tan et al., 2011). The phenomenon of opioid-induced locomotion is due to mu opioid receptor-mediated increases in striatal dopamine (DA) release (Johnson and Glick, 1993; Kalivas and Duffy, 1987; Piepponen et al., 1999). GABAergic interneurons also play an important role in opioid antinociception (Lau et al., 2014). This suggests a potential site of action that should be further examined.
Our observations suggest this phenomenon is not due to the additive or synergistic effects of these CNS depressants but instead results from a reversal of tolerance. The result may be the same but the mechanism should be considered as new understandings of the tolerance mechanism will lead to new drug therapies such as tolerance-resistant analgesics. Similar reversal of opioid tolerance has been observed in models of respiratory depression and at the single cell level (Hill et al., 2016; Llorente et al., 2013). The reversant (reversing agent) in their studies was ethanol, which along with benzodiazepines, interacts with GABAA receptors.
The neurochemical mechanism of this reversal is likely due to diazepam’s action on GABAA receptors. Our laboratory demonstrated that the diazepam reversal of morphine tolerance was fully inhibited by bicuculline, a GABAA antagonist, but not by phaclofen, a GABAB antagonist. This was in contrast to ethanol as neither inhibitor fully reversed ethanol’s reversal or morphine tolerance until they were administered in combination (Hull et al., 2013). Future studies should include determining whether the reversal of oxycodone and hydrocodone tolerance is due to diazepam’s effects on GABAA receptors, if any subtypes are significant for the effect, and the necessary intracellular pathways for this phenomenon.
4. Conclusion
Collectively, our findings demonstrate that the administration of diazepam, at doses that are not antinociceptive or have any motor effects, reverses both antinociceptive and locomotor tolerance to orally active opioids. These doses of diazepam did not potentiate the acute effects of these prescription opioids. The findings reported here suggest that individuals who are taking oral opioids for chronic pain relief and an anxiety agent such as diazepam need be cognizant of the risk of reversal of tolerance to opioids that could lead to unintentional overdose deaths.
5. Experimental Procedure
5.1. Animals
Male Swiss Webster mice (Harlan Laboratories, Indianapolis, IN) weighing 25–30 g were housed five to a cage in animal care quarters and maintained at 22 +/− 2°C on a 12-hour light-dark cycle. Food and water were available ad libitum. The mice were brought to the test room (22 +/− 2°C, 12-hour light-dark cycle), marked for identification, and allowed 18 hours to recover from transport and handling. Protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Commonwealth University Medical Center and comply with the recommendations of the International Association for the Study of Pain (IASP).
5.2. Drugs and Chemicals
Morphine sulfate, oxycodone HCl, hydrocodone bitartrate were obtained from the National Institutes of Health National Institute on Drug Abuse (Bethesda, MD) and were each dissolved in pyrogen-free isotonic saline (Hospira, Lake Forest, IL). Ethanol was obtained from AAPER Ethanol and Chemical Co. (Shelbyville, KY) and was diluted with pyrogen-free isotonic saline. Diazepam was obtained from Sigma-Aldrich Corporation (St Louis, MO, USA) and was dissolved in 45% hydroxypropyl beta-cyclodextrin (HPBCD)
5.3. Antinociceptive Testing
Antinociception was assessed using the 56°C warm water tail immersion test performed according to Coderre and Rollman (1983). Before injecting the mice, a baseline (control) latency was determined. Only mice with a control reaction time from 2 to 4 seconds were used. The test latency after opioid treatment was assessed at the peak time point of 20 minutes with a 10-second maximum cut-off time imposed to prevent tissue damage. Antinociception was quantified according to the method of Harris and Pierson (1964) as the percentage of maximum possible effect (%MPE), which was calculated as: %MPE = [(test latency − control latency)/(10 − control latency)]* 100. Percent MPE was calculated for each mouse
5.4. Tolerance Studies
A 7 hour antinociceptive tolerance model for oxycodone and hydrocodone was developed. Mice were injected subcutaneously (s.c.) once every hour for 7 hours (total of 7 injections) with an acute ED80 dose of the opioid (1.25 mg/kg for oxycodone and 6 mg/kg for hydrocodone, as previously determined). An hour after the final dose, mice were administered diazepam or vehicle by intraperitoneal injection and 30 minutes later were challenged with subcutaneous doses of oxycodone or hydrocodone to construct dose-response curves for calculation of ED50 values.. The warm water tail immersion test was performed 20 minutes after the injection of the challenge dose of opioid.
For maximum tolerance to oral administration of oxycodone, the route of administration used by humans, a 4-day antinociceptive tolerance model was developed. Mice were orally gavaged with 256 mg/kg oxycodone twice a day (9 AM & 5 PM) on day 1, 2, and 3. On the fourth day, mice were gavaged only in the morning. A full 24 hours after their last pretreatment dose, baseline latencies were recorded and immediately afterwards diazepam or vehicle was administered by intraperitoneal injection. 30 minutes later, mice were challenged with oral doses of oxycodone to construct dose-response curves for calculation of ED50 values. The warm water tail immersion test began 20 minutes after the administration of the challenge dose.
5.5. Locomotor Activity
Spontaneous motor activity was assessed using activity chambers (Med Associates, St. Albans, VT). Each individual activity chamber has closeable doors and a ventilation system. The interior of the chamber consists of a 27 × 27 cm Plexiglas enclosure that is wired with photo-beam cells connected to a computer console that counts the activity of the animal contained within the enclosure. For locomotor tolerance, a 4-day antinociceptive tolerance model was developed where mice were orally gavaged with 64 mg/kg oxycodone twice a day (9 AM & 5 PM) on day 1, 2, 3, and 4. On the fifth day, mice were habituated to the chamber for 30 minutes before any drug administration. Afterwards, mice were administered an intraperitoneal injection of vehicle or 0.5 mg/kg diazepam and placed in home cage. 30 minutes later, mice were administered 64 mg/kg oxycodone by oral gavage. 10 minutes later, mice were placed in separate activity chambers. Ambulatory counts for spontaneous activity were obtained over a 40-minute time period.
5.6. Statistical Analysis
Opioid dose-response curves were generated for calculation of ED50 values using least-squares linear regression analysis followed by calculation of 95% confidence limits (95% CL) by the method of Bliss (1967). All data are represented as mean ± standard error of the mean. Locomotor data was analyzed using two-way analysis of variance (ANOVA) with factors for time, treatment, and their interactions with locomotor activity followed by Tukey’s post hoc analyses to determine statistical significance (Prism 6). Analyses were considered significant when p < 0.05.
Highlights.
Antinociceptive tolerance developed to oxycodone and hydrocodone
0.5 mg/kg diazepam fully reversed antinociceptive tolerance to oxycodone
2 mg/kg diazepam fully reversed antinociceptive tolerance to hydrocodone
There was no potentiation of acute opioid antinociceptive effects by diazepam
Oxycodone locomotor tolerance was reversed by 0.5 mg/kg diazepam
Acknowledgments
The authors thank David Stevens for technical support performing the experiments needed for this study.
Funding: We gratefully acknowledge support from The National Institute on Drug Abuse grants RO1 DA036975 & DA T32007027
Footnotes
Authorship Contributions:
The authors have no conflicts of interest to report.
Participated in research design: Gonek M., Henderson, G.H., Akbarali, H.I. and Dewey, W.L.
Conducted experiments: Gonek M.
Performed data analysis: Gonek M. and Dewey, W.L.
Wrote or contributed to the writing of the manuscript: Gonek M., Henderson, G.H., Akbarali, H.I. and Dewey, W.L.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergman SA, Wyn RL, Williams G. Diazepam enhances fentanyl and diminishes meperidine antinociception. Anesth Prog. 1988;35:190–194. [PMC free article] [PubMed] [Google Scholar]
- Bliss CI. Statistics in Biology. McGraw-Hill Book Company; New York: 1967. [Google Scholar]
- Chen LH, Hedegaard H, Warner M. Drug-poisoning Deaths Involving Opioid Analgesics. 2014:1–8. [PubMed] [Google Scholar]
- Coderre TJ, Rollman GB. Naloxone hyperalgesia and stress-induced analgesia in rats. Life Sci. 1983;32:2139–2146. doi: 10.1016/0024-3205(83)90103-0. [DOI] [PubMed] [Google Scholar]
- Harris LS, Pierson AK. Some Narcotic Antagonists In The Benzomorphan Series. J Pharmacol Exp Ther. 1964;143:141–148. [PubMed] [Google Scholar]
- Hill R, Lyndon A, Withey S, Roberts J, Kershaw Y, MacLachlan J, Lingford-Hughes A, Kelly E, Bailey C, Hickman M, Henderson G. Ethanol Reversal of Tolerance to the Respiratory Depressant Effects of Morphine. Neuropsychopharmacology. 2016;41:1–37. doi: 10.1038/npp.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull LC, Gabra BH, Bailey CP, Henderson G, Dewey WL. Reversal of morphine analgesic tolerance by ethanol in the mouse. J Pharmacol Exp Ther. 2013;345:512–9. doi: 10.1124/jpet.112.202184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. The Effect of Protein Kinase C and G Protein-Coupled Receptor Kinase Inhibition on Tolerance Induced by -Opioid Agonists of Different Efficacy. Pharmacology. 2010;332:1127–1135. doi: 10.1124/jpet.109.161455.ance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Velázquez G, López-Muñoz FJ, Fernández-Guasti A. Parallel anxiolytic-like and antinociceptive actions of diazepam in the anterior basolateral amygdala and dorsal periaqueductal gray. Brain Res. 2010;1349:11–20. doi: 10.1016/j.brainres.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Jiménez-Velázquez G, López-Muñoz FJ, Fernández-Guasti A. Participation of the GABA/benzodiazepine receptor and the NO-cyclicGMP pathway in the “antinociceptive-like effects” of diazepam. Pharmacol Biochem Behav. 2008;91:128–133. doi: 10.1016/j.pbb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Glick SD. Dopamine Release and Metabolism in Nucleus Accumbens and Striatum of Morphine-Tolerant and Nontolerant Rats. Biochem Pharmacol Regimens, Pretreat. 1993;46:341–347. doi: 10.1016/0091-3057(93)90362-w. [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD. Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125:8–18. doi: 10.1016/j.drugalcdep.2012.07.004.Polydrug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Sensitization to Repeated Morphine Injection in the Rat: Possible Involvement of A10 Dopamine Neurons. J Pharmacol Exp Ther. 1987:204–212. [PubMed] [Google Scholar]
- Lau BK, Vaughan CW, Lau BK, Vaughan CW. Descending modulation of pain: The GABA disinhibition hypothesis of analgesia ScienceDirect Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol. 2014;29:159–164. doi: 10.1016/j.conb.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Llorente J, Withey S, Rivero G, Cunningham M, Cooke A, Saxena K, McPherson J, Oldfield S, Dewey WL, Bailey CP, Kelly E, Henderson G. Ethanol reversal of cellular tolerance to morphine in rat locus coeruleus neurons. Mol Pharmacol. 2013;84:252–60. doi: 10.1124/mol.113.085936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Treatment Agency for Substance Misuse [NTA] Does the combined use of heroin or methadone and other substances increase the risk of overdose? Res Brief. 2007:27. [Google Scholar]
- Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT. Oxycodone and morphine have distinctly different pharmacological profiles: Radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain. 2007;132:289–300. doi: 10.1016/j.pain.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Piepponen TP, Honkanen A, Kivastik T, Zharkovsky A, Turtia A, Mikkola JAV, Ahtee L. Involvement of Opioid mu 1 -Receptors in Opioid-Induced Acceleration of Striatal and Limbic Dopaminergic Transmission. 1999;63:245–252. doi: 10.1016/s0091-3057(98)00241-x. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E, Guidotti A. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. 2006;103:4275–4280. doi: 10.1073/pnas.0600329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan AK, McDonald JS, Tejwani GA. Differential effects of intrathecal midazolam on morphine-induced antinociception in the rat: role of spinal opioid receptors. Anesth Analg. 1991;73:124–131. doi: 10.1213/00000539-199108000-00004. [DOI] [PubMed] [Google Scholar]
- Sierralta F, Miranda HF. Analgesic effect of benzodiazepines and flumazenil. Gen Pharmacol. 1992;23:739–742. doi: 10.1016/0306-3623(92)90158-G. [DOI] [PubMed] [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. 2007;127:129–139. doi: 10.1016/j.pain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Papadopoulou Z. Effects of Diazepam-Infrasounds Combination on Locomotor Activity and Avoidance Behaviour of Rats. 1980;12:767–771. doi: 10.1016/0091-3057(80)90164-1. [DOI] [PubMed] [Google Scholar]
- Tan KR, Uwe R, Lüscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34:188–197. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooked White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–72. doi: 10.1046/j.1360-0443.1999.9479612.x. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. 2009;101:107–114. doi: 10.1016/j.drugalcdep.2008.11.013. [DOI] [PubMed] [Google Scholar]