Abstract
Purpose of review
Understanding the characteristics of transmission during acute HIV infection (AHI) may inform targets for vaccine-induced immune interdiction. Individuals treated in AHI with a small HIV reservoir size may be ideal candidates for therapeutic HIV vaccines aiming for HIV remission (i.e. viremic control after treatment interruption).
Recent findings
The AHI period is brief and peak viremia predicts a viral set point that occurs 4–5 weeks following infection. Robust HIV-specific CD8+ T-cell responses lower viral set points. Phylogenetic analyses of founder viruses demonstrated unique bottleneck selections and specific genetic signatures to optimize for high-fitness variants and successful transmission events. HIV clades, route of transmission and the presence of minor variants may affect vaccine protection. Antiretroviral treatment in AHI results in smaller HIV reservoir size, better CD4+ T-cell recovery and fewer virus escapes.
Summary
The knowledge of untreated and treated AHI informs the development of vaccines, in that preventive vaccines will require broad coverage for multiple clades and antigenic variants associated with unique bottleneck selections. Vaccines that help the host to control viremia could minimize onward transmission. Therapeutic HIV vaccines aimed at HIV remission should be studied in early-treated individuals who have few or no viral escape mutants and a more preserved immune system.
Keywords: acute HIV infection, early antiretroviral therapy, HIV reservoir, HIV vaccine, preventive HIV vaccine, therapeutic HIV vaccine
INTRODUCTION
The understanding of acute HIV infection (AHI) may inform vaccine development by characterizing the pathophysiology of the transmission event and in particular those characteristics of transmission that might be targets for vaccine-induced immune interdiction. Further, understanding the nature of host control of viremia may also help refine vaccine development strategies to minimize onward transmission risk [1▪▪]. Antiretroviral therapy (ART) initiation in AHI leads to a smaller HIV reservoir size and a better preserved immunity compared with later treatment [2]. Therefore, early-treated persons may be ideal candidates for therapeutic HIV vaccines aiming for HIV remission, that is long-term undetectable viremia for an as-yet undefined period (probably of several years) in the absence of ART [3].
In this article, we will describe the characteristics of AHI and how knowledge of untreated and treated AHI could inform vaccine development.
Box 1.
no caption available
ACUTE HIV INFECTION
AHI is associated with a rapid rise in HIV plasma viremia and commonly manifested clinically with reported symptoms of fever, headache and malaise and physical findings of elevated heart rate and lymphadenopathy. The symptoms and signs arise just prior to and at peak viremia and are relatively brief [1▪▪]. Peak viremia is above 1 million copies/ml in the majority of acutely infected persons and is predictive of viral load set point, a key determinant of long-term prognosis [1▪▪,4–7]. Importantly, the viral load set point is established at a median of 31 days (range 18–42 days) following the advent of viremia [1▪▪] (Fig. 1). The AHI period is therefore abrupt and decisive in terms of long-term prognosis for the individual.
FIGURE 1.
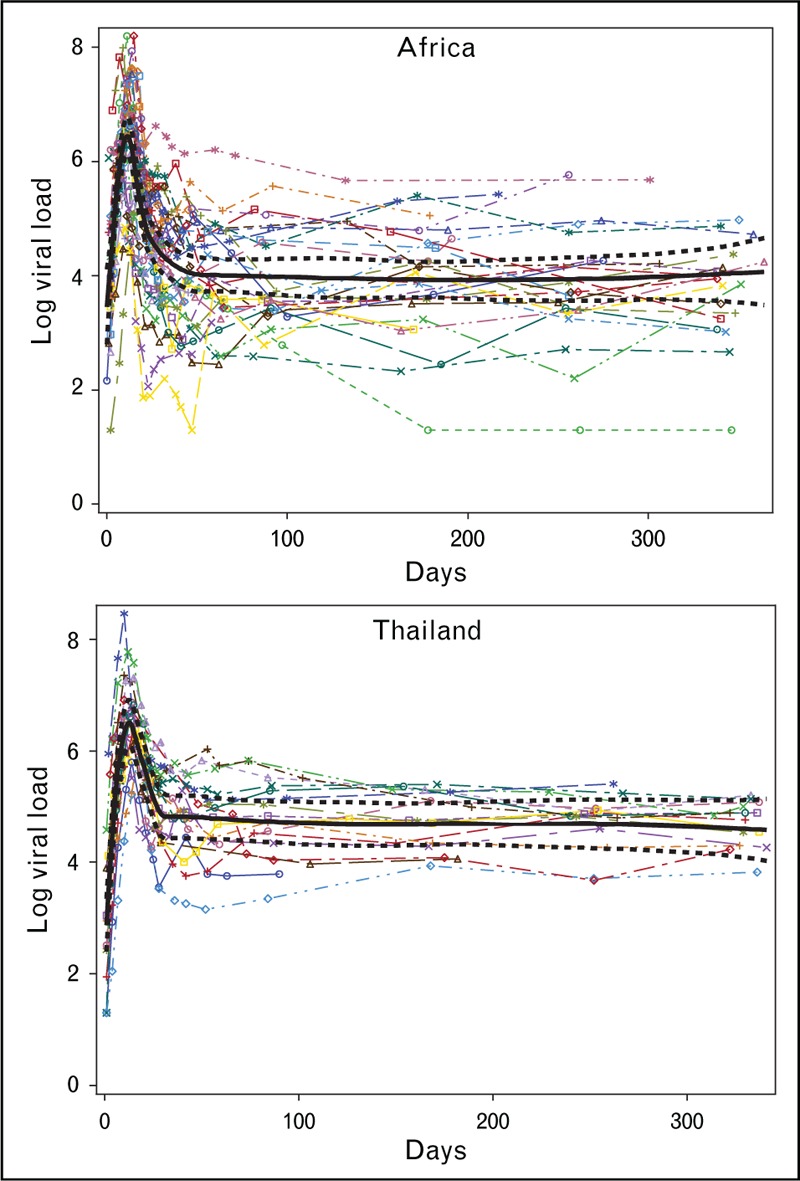
Longitudinal viral load values (log10 copies/ml) are plotted vs. the number of days from the first reactive RNA (day 0) for n = 50 acute cases (33 from Africa and 17 from Thailand) with two or more enzyme immunoassay (EIA) nonreactive/nucleic acid testing (NAT)-reactive samples. Regression splines (in black) with participant-specific slopes and intercepts were fit using the first year of viral load data for each region (Africa and Thailand). Reproduced with permission from [1▪▪].
Definitions of acute, early, chronic and late infection have been proposed using the evolution of HIV plasma viremia, antigenemia, HIV-specific antibody, the advent of a stable set point viral load and the onset of immunodeficiency with loss of host immune control of viremia [8–11]. As a practical matter, the first and most commonly employed staging system for acute and early infection by Fiebig et al.[9] (Table 1) is compromised by the replacement of second-generation immunoassay with newer and more sensitive third-generation (IgM) and fourth-generation (p24 antigen) immunoassays for HIV [8].
Table 1.
Fiebig staging system for acute HIV infection [9]
| Fi | RNA | P24 antigen | IgM-sensitive immunoassay | IgG-sensitive immunoassay | Western blot |
| Fiebig I | + | − | − | − | − |
| Fiebig II | + | + | − | − | − |
| Fiebig III | + | + | + | − | − |
| Fiebig IV | + | +/− | + | − | Indeterminate |
The distinction between acute and early infection has been variably defined but is often related to complete evolution of antibody responses and the advent of stable viral load set point. In closely monitored acute infection cohorts representing multiple HIV subtypes, a stable viral load set point occurs 4–5 weeks after the advent of viremia [1▪▪,12▪].
Diagnosis of AHI is challenging. Most studies seeking HIV infection in the acute stage have relied upon symptoms to prompt clinicians to evaluate a diagnosis with nucleic acid testing. Recent data suggest that although symptoms and signs are common, they may often be brief and mild suggesting that acutely infected individuals may not seek care. Moreover, seroconversion symptoms may be mistaken for other locally prevalent febrile illnesses like malaria [13].
New technologies are affording earlier identification of AHI [1▪▪,12▪]. Twice-weekly HIV nucleic acid testing from small-volume blood collection by finger stick was employed in the RV217 study amongst individuals at risk for HIV in Thailand and East Africa. This resulted in the diagnosis of Fiebig I/II acute infection, prior to peak viremia and seroconversion [1▪▪]. However, this approach is limited by cost and the need to engage high-risk individuals in a clinical setting. The fourth-generation immunoassay is becoming the standard HIV screening test worldwide, and we have shown that it can distinguish two groups of Fiebig I participants with different levels of proviral and viral burden. These very early acutely infected individuals are highly unique and could inform prevention, treatment and cure research [8].
EARLY EVENTS IN ACUTE HIV INFECTION AND RELEVANCE TO PREVENTIVE HIV VACCINE DEVELOPMENT
Knowledge of the genetic makeup and complexity of HIV-transmitted founder viruses is important in informing vaccine coverage and targets. Acute infection studies so far have offered some optimism that the broad genetic diversity of HIV may not limit vaccine efficacy as there appears to be some limitations on HIV strains that account for transmission events. Phylogenetic analyses of founder viruses in different epidemic settings support the notion of a genetic bottleneck with only a single founder in the large majority of heterosexual transmissions [14–16]. Moreover, founder viruses appear to be phenotypically distinct and specifically more resistant to IFN-α [15,17,18]. HIV transmission in MSM may have a higher proportion of multiple founders, which is thought to relate to a more permissive route of transmission [19]. However, a recent study demonstrated no significant differences in frequencies of single vs. multiple founder viruses in heterosexuals, MSM and people who inject drugs in early stages of AHI [20▪▪]. Such discrepancies may be due to sampling in relation to AHI stages as persons in later stages of acute infection are more likely to have multiple viral strains as escape variants from immune pressure. Interestingly, unique bottleneck selection and specific genetic signatures of transmitted founder viruses were observed between these groups. More selective pressure is observed in lower risk transmission (i.e. heterosexual vs. MSM, insertive anal vs. receptive anal intercourse) possibly to optimize for high-fitness variants that could lead to successful transmission [21]. This supports the rationale for developing vaccines with broad coverage not only for multiple clades, but also for antigenic variants associated with different transmission routes.
More recent evaluation of founder virus diversity using next-generation sequencing approaches targeting regions with changes in sequence over time has identified multiple founders in a high proportion of participants evaluated. This effort has not been applied to a sufficient number of cases to establish with confidence the rate of multiple founders in either heterosexual or homosexual transmission settings. It is not surprising that a more sensitive technique might find additional founders, and many of these additional founding viruses are closely related. Nevertheless, the importance of this finding accrues to the observation that these minor variants contribute to immune escape early in the course of HIV infection [18]. It will be critical to determine if minor variants have different phenotypes from the dominant founder and most importantly, if the diversity might limit HIV vaccine protection.
Preventive vaccines that do not completely block infection could theoretically affect the bottleneck selection process, resulting in transmitted founder viruses with varying fitness. Replicative fitness of transmitted HIV could affect immune activation and proviral DNA in the long-lived memory CD4+ T cells during AHI that may have a bearing on disease progression [22]. Vaccine development could selectively target epitopes that are known to be associated with lower viral set point and better clinical outcomes. Similarly in this era of preexposure prophylaxis (PrEP) use, more bottleneck selective pressure may be needed to optimize viral fitness for a successful transmission event. As PrEP is part of standard preventive package in many settings, individuals who are PrEP failures may harbor viruses with higher fitness, making development of a broadly effective preventive vaccine more challenging. They could also harbor ART-resistant virus with reduced fitness. Understanding the nature of host control of viremia may help refine preventive vaccine development strategies to minimize onward transmission risk such as by lowering viral load levels during acute infection [1▪▪]. The control of HIV viremia is widely considered to be associated with the advent, quality and quantity of HIV-specific CD8+ T cells. Recently, data from a small number of acutely infected women in South Africa indicated that viral load set point correlated with the number of HIV-specific CD8+ T cells. In this experience, a more robust HIV-specific CD8+ T-cell response corresponded to a lower viral load set point, and historical data would suggest an improved long-term course [12▪]. However, there remains substantial plasma viremia, and disease progression is nearly universal even with stronger cellular immune responses.
Although the notion of a genetic or phenotypic bottleneck for HIV transmission has been reassuring to vaccine developers in theory, it has not been readily translated to specific vaccine designs. It may be that the study of acute infection will more decisively inform the role of vaccines for achieving a durable remission in the absence of treatment. In particular, defining the innate and adaptive effector mechanisms associated with the control of acute viremia may inform vaccine therapy designs.
EFFECTS OF EARLY ANTIRETROVIRAL THERAPY AND RELEVANCE TO THERAPEUTIC VACCINE DEVELOPMENT
Reservoir establishment occurs early in AHI and reaches a set point within the first 2 months of infection, which in turn determines the size of the reservoir in the chronic stage [23,24]. Persistent infection of long-lived memory CD4+ T cells in the peripheral blood and mononuclear cells in tissues represents a major barrier to HIV remission and cure [25]. Treatment initiated at diagnosis of HIV regardless of stage of infection is now recommended globally for the medical benefit of an HIV-infected person [26]. Initiation of ART during acute infection has been shown to reduce HIV DNA in peripheral blood mononuclear cell or gut mononuclear cell populations [2,27]. This observation is in contrast to the impact of treatment on HIV DNA and other reservoir measurements in persons with chronic HIV infection [28]. A number of studies suggest that low HIV reservoir size is associated with early ART and may be rarely to infrequently associated with spontaneous virological remission [29–31]. In view of these observations, interventions within the early-treated population seem to be the most promising clinical setting for evaluating HIV remission strategies.
It is probable that the induction of robust cellular immune responses and particularly CD8+ T-cell responses with sufficient potency and breadth to reduce the HIV latent reservoir are needed to successfully achieve durable HIV remission. Acutely treated individuals generally have better CD4+ T-cell recovery and a near normal CD4+/CD8+ ratio [32]. The CD8+ T cells during AHI are however highly specific for autologous epitopes, and cross-reactive CD8+ T cells are infrequent [33▪,34]. This suggests that even small changes in viral peptide residues would disrupt variant-specific reactivity leading to inability to control viremia. Immune escape variants present a significant obstacle to immune control but its timing is not well defined. It is thought that viral escape occurs after peak viremia in AHI and increases gradually over the first few years of infection [16], providing an opportunity for preventing escape with the initiation of early ART. Moreover, mutation requires some level of replication, either for point mutation or recombination. As early ART dramatically reduces viral replication, it could prevent mutation development. As multifunctional responses against HIV increase with time, such responses maybe diminished with the rapid decline in viral load after early ART. Antibody responses to HIV are reduced following early treatment as reflected by high rates of seronegativity on diagnostic tests [35]. Broadly neutralizing antibodies emerge after persistent exposure to viremia and are generally not present in the setting of early treatment. Although the quantity of HIV-specific immune responses is lower in early-treated individuals, there may be preservation of certain cellular subsets with long-lived memory phenotypes that express less inhibitory markers [36–38]. Such cells possess effector capabilities to proliferate and perform catalytic function. This may be critical in remission therapies as generating continuous effector memory T-cell immune responses has been associated with clearance of highly pathogenic simian immunodeficiency virus in a nonhuman primate model [39].
To achieve sustained virologic control of HIV-1 (ART-free HIV remission) and to clear virus-expressing cells, it is likely that immune-based therapies will be needed. Therapeutic HIV vaccine trials in chronic HIV infection have been met with little success in regard to clinical benefits [40]. There are strong rationales for studying therapeutic HIV vaccines in early-treated individuals who have few or no viral escape, less CD4+ depletion and more preserved memory T cells. More data are needed on the characteristics of immune responses following treatment initiated at different stages of AHI as responses to therapeutic vaccines may vary depending on the preexisting HIV-specific immune responses.
The optimal timing to consider therapeutic vaccination in the setting of AHI is not well understood [41]. Giving vaccine together with initiating ART during AHI may be beneficial as it might serve to maximize HIV-specific immune responses despite declining native HIV antigen presentation. As even very early treatment with ART in acute infection does not completely reverse the dysfunctional inflammatory response, vaccination during acute infection might skew the development of a robust immune response and/or fuel plasma viremia by activation of CD4+ T cells. The endpoints would be immune responses. Therapeutic vaccines may be administered after a period of sustained viral suppression from ART, and if immune responses are elicited, a closely monitored ART pause could be considered to assess for HIV remission.
It is highly likely that combination treatment with agents to induce HIV expression and thus target latently infected cells for destruction is also required. There are likely sites that harbor HIV latent reservoir, which are not accessible to CD8+ T cells under normal circumstances and so induction of humoral responses that contribute to reduction of the reservoir are also needed. It is likely that this should be evaluated in a clinical trial setting with a view to maximize immune effector mechanism quality and quantity without raising safety concerns.
CONCLUSION
There is controversy in respect to the relative proportion of new infection attributable to acute and early infection. There are cohort and phylogenetic data along with mathematical models supporting an important role for acute and early infection but the ranges vary widely and some models assert a negligible contribution of acute infection to incidence [11,42,43]. To some extent, the variant models reflect the importance of viral load as a dominant factor predictive of transmission risk. This is particularly true in view of recently published data identifying the short duration of acute viremia. However, other features of the virus–host interaction may extend the period of increased transmissibility beyond the first 4–5 weeks of viremia. These include the absence of host neutralizing antibody for the first several months and the gradual adaptation of HIV to sustained host immune surveillance.
If acute and early infection represents an important contribution to annual community incidence, there will be a need to either mobilize convenient, inexpensive tools to identify these cases and initiate treatment. Alternatively, a vaccine that reduces peak viremia and reduces overall viral load set point would contribute to a reduction of infectiousness even if the vaccine is only partially effective in affording protection from HIV infection. In diagnosed acutely infected individuals, defining how immune responses evolve over time and how they relate to reservoir size and viral diversity will increase our understanding of critical host–viral interactions and inform the development of therapeutic vaccine development for HIV remission and cure.
Finally, identifying a reasonably large proportion of acutely infected persons efficiently would require frequent testing in the field in high-risk settings. The hope is that insights from this unique and eventful era of HIV infection will inform more broadly applicable interventions including both preventive and therapeutic vaccines.
Acknowledgements
The given work was supported by cooperative agreements (W81XWH-07-2-0067 and W81XWH-11-2-0174) between The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the United States Department of the Army. J.A. was partially funded by NIAID R01AI114236. We thank Ms Oratai Butterworth for her assistance in preparing this manuscript.
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Robb ML, Eller LA, Kibuuka H, et al. Prospective study of acute HIV-1 infection in adults in east Africa and Thailand. N Engl J Med 2016; 374:2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was a prospective study that demonstrated viral dynamics during acute infection. Peak viremia occurred 13 days following first detectable HIV RNA and it correlated with viral set point that was established by day 31.
- 2.Schuetz A, Deleage C, Sereti I, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014; 10:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks SG, Lewin SR, Ross AL, et al. International AIDS Society global scientific strategy: towards an HIV cure. Nat Med 2016; 22:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley CF, Barbour JD, Hecht FM. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J Acquir Immune Defic Syndr 2007; 45:445–448. [DOI] [PubMed] [Google Scholar]
- 5.Lavreys L, Baeten JM, Chohan V, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis 2006; 42:1333–1339. [DOI] [PubMed] [Google Scholar]
- 6.Lefrere JJ, Roudot-Thoraval F, Mariotti M, et al. The risk of disease progression is determined during the first year of human immunodeficiency virus type 1 infection. J Infect Dis 1998; 177:1541–1548. [DOI] [PubMed] [Google Scholar]
- 7.Lindback S, Karlsson AC, Mittler J, et al. Viral dynamics in primary HIV-1 infection. Karolinska Institutet Primary HIV Infection Study Group. AIDS 2000; 14:2283–2291. [DOI] [PubMed] [Google Scholar]
- 8.Ananworanich J, Fletcher JL, Pinyakorn S, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology 2013; 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–1879. [DOI] [PubMed] [Google Scholar]
- 10.McMichael AJ, Borrow P, Tomaras GD, et al. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 2010; 10:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med 2011; 364:1943–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Ndhlovu ZM, Kamya P, Mewalal N, et al. Magnitude and kinetics of CD8(+) T cell activation during hyperacute HIV infection impact viral set point. Immunity 2015; 43:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was a prospective study of acutely infected women showing rapid and high magnitude of HIV-induced CD8+ T-cell responses during acute infection that was associated with subsequent immune control.
- 13.Sullivan PS, Fideli U, Wall KM, et al. Prevalence of seroconversion symptoms and relationship to set-point viral load: findings from a subtype C epidemic. AIDS 2012; 26:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baalwa J, Wang S, Parrish NF, et al. Molecular identification, cloning and characterization of transmitted/founder HIV-1 subtype A, D and A/D infectious molecular clones. Virology 2013; 436:33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrish NF, Gao F, Li H, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A 2013; 110:6626–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008; 105:7552–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton-May AE, Dibben O, Emmerich T, et al. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 2013; 10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kijak GH, Sanders-Buell E, Chenine AL, et al. Cryptic multiple HIV-1 infection revealed by early, frequent, and deep sampling during acute infection. AIDS Res Hum Retroviruses 2014; 30 (S1):A58–A60. [Google Scholar]
- 19.Li H, Bar KJ, Wang S, et al. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog 2010; 6:e1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Tully DC, Ogilvie CB, Batorsky RE, et al. Differences in the selection bottleneck between modes of sexual transmission influence the genetic composition of the HIV-1 founder virus. PLoS Pathog 2016; 12:e1005619. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the unique bottleneck selection and specific genetic signatures of transmitted founder viruses by risk groups with more selective pressure observed in lower risk transmission to optimize transmission.
- 21.Carlson JM, Schaefer M, Monaco DC, et al. HIV transmission. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science 2014; 345:1254031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claiborne DT, Prince JL, Scully E, et al. Replicative fitness of transmitted HIV-1 drives acute immune activation, proviral load in memory CD4+ T cells, and disease progression. Proc Natl Acad Sci U S A 2015; 112:E1480–E1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun TW, Engel D, Berrey MM, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 1998; 95:8869–8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ananworanich J, editor HIV DNA Set Point Remains Elevated in Untreated vs. Treated Acutely Infected Thais 23rd Conference on Retroviruses and Opportunistic Infections; 2016 February 22–25, 2016; Boston, Massachusetts. [Google Scholar]
- 25.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multitargeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JP, Hurst J, Stohr W, et al. HIV-1 DNA predicts disease progression and posttreatment virological control. eLife 2014; 3:e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Posttreatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persaud D, Luzuriaga K. Absence of HIV-1 after treatment cessation in an infant. N Engl J Med 2014; 370:678. [DOI] [PubMed] [Google Scholar]
- 32.Thornhill J, Inshaw J, Kaleebu P, et al. Brief Report. Enhanced normalisation of CD4/CD8 ratio with earlier antiretroviral therapy at primary HIV infection. J Acquir Immune Defic Syndr 2016; 73:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪.Du VY, Bansal A, Carlson J, et al. HIV-1-specific CD8 T cells exhibit limited cross-reactivity during acute infection. J Immunol 2016; 196:3276–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that the CD8+ T-cell response during acute infection is highly specific for autologous epitopes; therefore, polyvalent vaccines will be required for optimal coverage of immune escape variants that will inevitably occur.
- 34.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 2009; 206:1253–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Souza MS, Phanuphak N, Pinyakorn S, et al. Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection. AIDS 2015; 29:793–800. [DOI] [PubMed] [Google Scholar]
- 36.Lecuroux C, Girault I, Urrutia A, et al. Identification of a particular HIV-specific CD8+ T-cell subset with a CD27+ CD45RO-/RA+ phenotype and memory characteristics after initiation of HAART during acute primary HIV infection. Blood 2009; 113:3209–3217. [DOI] [PubMed] [Google Scholar]
- 37.Takata H, Kessing C, Muir R, Colby D, Phanuphak N, Haddad EK, et al. editors. Differentiation of Effector CD8 T Cells Toward Short Lived Cells Lacking Memory Potential During Acute HIV Infection. Keystone Symposia; 2016 March 20—24, 2016; Olympic Valley, CA. [Google Scholar]
- 38.Jensen SS, Fomsgaard A, Larsen TK, et al. Initiation of antiretroviral therapy (ART) at different stages of HIV-1 disease is not associated with the proportion of exhausted CD8+ T cells. PLoS One 2015; 10:e0139573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen SG, Piatak M, Jr, Ventura AB, et al. Immune clearance of highly pathogenic SIV infection. Nature 2013; 502:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graziani GM, Angel JB. HIV-1 immunogen: an overview of almost 30 years of clinical testing of a candidate therapeutic vaccine. Expert Opin Biol Ther 2016; 16:953–966. [DOI] [PubMed] [Google Scholar]
- 41.Carcelain G, Autran B. Immune interventions in HIV infection. Immunol Rev 2013; 254:355–371. [DOI] [PubMed] [Google Scholar]
- 42.Suthar AB, Granich RM, Kato M, et al. Programmatic implications of acute and early HIV infection. J Infect Dis 2015; 212:1351–1360. [DOI] [PubMed] [Google Scholar]
- 43.Smith MK, Rutstein SE, Powers KA, et al. The detection and management of early HIV infection: a clinical and public health emergency. J Acquir Immune Defic Syndr 2013; 63 Suppl 2:S187–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]


