The investigation of clinical and multimodal imaging factors influencing the duration of first, acute, and treatment-naive CSCR episodes by survival analysis showed that higher subfoveal choroidal thickness, higher pigment epithelial detachment or bump at leakage sites, and older age were independent predictors of longer episodes.
Key words: central serous chorioretinopathy, choroid, retinal pigment epithelium, choroidal thickness, optical coherence tomography, fluorescein angiography, indocyanine green angiography, age factors, time factors, steroids
Abstract
Purpose:
To evaluate the influence of clinical and multimodal imaging parameters on the duration of acute central serous chorioretinopathy (CSCR) episodes.
Methods:
Consecutive patients with first, treatment-naïve central serous chorioretinopathy episodes presenting within 20 days of symptoms onset were prospectively included. They were reevaluated 15 days to 20 days later, followed by monthly evaluation for 6 months. Subfoveal choroidal thickness (SFCT), fluorescein leakage intensity on fluorescein angiography, elevation of retinal pigment epithelium (RPE) lesions at leakage sites, focal/multifocal pattern of indocyanine green angiography (ICGA) at baseline, time-dependent pattern of subretinal fluid (SRF) resorption on OCT using volume segmentation, history of corticosteroid intake and mean blood pressure were evaluated using univariate (Log rank test) and multivariate (Cox proportional hazard regression) survival analysis.
Results:
Thirty-one patients were included (26 men, 5 women, mean age: 40.0 ± 8.9 years, range: 24–58), of which 26 (84%) had episode resolution by 6 months. Using univariate analysis, episode duration was longer in cases with subfoveal choroidal thickness ≥500 μm (P = 0.0002), retinal pigment epithelium elevation at leakage sites ≥50 μm (P = 0.033), and a peak in subretinal fluid observed during follow-up (P = 0.013), and there was a near-significant association of intense fluorescein leakage (P = 0.074) with longer episodes. Using multivariate analysis, subfoveal choroidal thickness ≥500 μm (P = 0.017), retinal pigment epithelium elevation at leakage sites ≥50 μm (P = 0.010) and patient age ≥40 years (P = 0.010) were significantly and independently associated to longer episodes. Indocyanine green angiography pattern, corticosteroid intake, and blood pressure did not influence episode duration.
Conclusion:
Older age, higher subfoveal choroidal thickness, and higher degree of retinal pigment epithelium alteration at leakage sites are independent factors of longer acute central serous chorioretinopathy episodes.
Central serous chorioretinopathy (CSCR) is a chorioretinal disorder characterized by serous retinal detachments frequently involving the macula and usually associated with focal pigment epithelial detachments (PED), choroidal hyperpermeability, and increased choroidal thickness. Acute CSCR classically affects middle-aged working male individuals, whose working ability may be compromised by the associated visual burden. Because serous retinal detachments resolve spontaneously within six months in most acute CSCR episodes,1–3 observation without treatment is generally recommended as initial management.4 For cases with persistent serous retinal detachment or severe vision loss, several treatment options are available. Photocoagulation of extramacular leaking points by direct argon2,3 or micropulse laser5,6 can reduce the duration of single episodes. Half-dose or half-fluence verteporfin photodynamic therapy (PDT) may contribute to shorten episode duration.7–12 Oral treatment by mineralocorticoid-receptor (MR) antagonists has also shown beneficial effects.4,13–18 However, the ideal timing for these different interventions still remains to be determined. A better understanding of factors influencing episode duration would help to detect and treat earlier cases at risk for persistence, before the development of photoreceptor and RPE damage because of long-lasting subretinal detachment. Because choroidal vasodilation and leakage through the RPE are key mechanisms leading to CSCR4 and because most of subretinal fluid resorption depends on the pumping capacity of RPE cells, several features involved in choroid/RPE physiology may influence acute episode duration, among which subfoveal choroidal thickness, elevation of PED, intensity of RPE leakage and choroidal hyperpermeability, initial subretinal fluid volume, time-dependent fluid resorption pattern, patient age, history of steroid intake, and arterial blood pressure. Although these factors can be accessed on routine clinical examination and retinal imaging, their influence on episode duration has not been previously investigated.
The aim of this study was to evaluate the influence of these ocular and systemic factors on the duration and resolution of first, treatment-naïve, acute CSCR episodes.
Methods
Subjects
This observational, single-center, prospective study was designed in accordance with the tenets of the Declaration of Helsinki. Data collection and analysis was approved by the Ethics Committee of the Swiss Federal Department of Health (CER-VD no. 19/15). All patients signed an informed consent. Consecutive patients presenting at Jules-Gonin Eye Hospital (Lausanne, Switzerland) with a first episode of acute, unilateral, and treatment-naive CSCR from January 1, 2014 to October 31, 2015 were included. A CSCR episode was defined as the association of visual symptoms (vision impairment, metamorphopsia, micropsia, dyschromatopsia or central scotoma) in the presence of subretinal fluid on spectral-domain optical coherence tomography (SD-OCT) with a leaking site on fluorescein angiography (FA) and choroidal vascular hyperpermeability on indocyanine green angiography (ICGA). Exclusion criteria were: initial presentation later than 20 days after symptoms onset, follow-up shorter than 6 months without resolution, spherical error superior to 2 D, and presence of pigmentary changes on fundoscopy or fundus autofluorescence modifications suggestive of previous CSCR episodes. The follow-up scheme for this observational study included a second visit within 10 days to 20 days of the baseline visit, followed by repeated monthly clinical evaluation for 6 months. Ocular and medical history, including history of corticosteroid intake, ocular examination, arterial blood pressure measured at the initial visit, and the time from symptoms onset (vision loss, metamorphopsia, micropsia, dyschromatopsia, or central scotoma) to the first visit, were recorded. Central serous chorioretinopathy episode resolution was defined as the complete reabsorption of subretinal fluid (SRF) on SD-OCT images acquired as described below. In case of nonresolution of SRF at six months, a rescue therapy was proposed. Laser photocoagulation was performed if the leaking site was located more than 1,000 μm from the foveal center. If laser was not possible, mineralocorticoid-receptor antagonist therapy by oral eplerenone (25 mg daily) or spironolactone (25 mg daily) was administered in the absence of contraindications, and otherwise photodynamic therapy was used.
Retinal Imaging
Imaging was performed after standard pupillary dilation using tropicamide 0.5% drops with the Spectralis (Heidelberg Engineering, Heidelberg, Germany). At all visits, a 20° × 20° 97-sections SD-OCT macular volume, a 30° enhanced-depth imaging (EDI) SD-OCT horizontal scan through the fovea the “automatic real time” averaging set at the maximal value of 100 images, a 30° × 30° fundus infrared reflectance, and a 30° × 30° fundus autofluorescence were acquired. Fluorescein angiography and green indocyanine-green angiography were performed at the baseline visit.
For each case, the site of maximum fluorescein leakage on FA was identified by one observer (FBC). The height of pigment epithelial defects at these sites, consisting in PED or RPE bumps, was measured by a single observer (AD) using the built-in Spectralis software (Heidelberg Eye Explorer, version 1.9.10.0). Elevation was defined as 0 μm when no RPE lesion associated with the leakage site was observed.
The subfoveal choroidal thickness (SFCT) was measured by the same observer on baseline enhanced-depth imaging scans as the axial distance from the RPE to the outer choroid/sclera interface. In cases where the interface was ambiguous, the senior author (FBC) determined the SFCT. The maximal height of subretinal detachment was measured similarly as the axial distance between the RPE and the outer aspect of photoreceptor outer segments.
The macular volume was automatically measured by the software over an Early Treatment Diabetic Retinopathy Study (ETDRS) grid centered on the macula.
Multifocal choroidal hyperpermeability was defined as the presence of several hypercyanescent areas on midphase indocyanine-green angiography (10–12 minutes after dye injection).
Fluorescein Expansion Ratio
The intensity of leakage at the previously identified leakage sites on FA was estimated by quantifying the relative expansion of hyperfluorescence from early phase (40–60 seconds) to midphase (2–2.5 minutes). Angiograms were exported as TIFF files and were processed on Matlab using a semiautomated custom algorithm adapted from the method described by Pryds et al.19 The leakage site was indicated manually, and the borders of the hyperfluorescent area were automatically detected using the grayscale intensity threshold of 0.75 × Imax, where Imax is the maximal fluorescence intensity at the leakage site. The ratio between hyperfluorescent areas at midphase and early phase was calculated to provide the fluorescein leakage ratio.
Subretinal Fluid Volume
The volume of subretinal fluid was calculated at each timepoint using a custom-built algorithm on Matlab (version 2015b; Mathworks, Natick, MA). Briefly, the 97 SD-OCT scans corresponding to the macular volume were exported as PNG files, and the borders of the serous retinal detachment were segmented on each scan using an intensity-based method. After visual verification of the segmentation, the volume was obtained by trapezoidal integration, and a heat map of the subretinal detachment was generated. The kinetics of SRF resorption was then analyzed in each patient by comparing SRF volumes at each timepoint.
Statistical Analyses
Survival analyses were performed using the R software (version 3.1.3; R Foundation for Statistical Computing, R Core Team, 2015, Vienna, Austria. http://www.R-project.org/). The Kaplan–Meier method with log-rank tests was used for univariate analyses, and the Cox proportional hazard method for the multivariate analysis, with the “survival”20 package. Results were expressed in terms of hazard ratio and adjusted hazard ratio, respectively. Parameters resulting in a P value ≤0.2 in the univariate analysis were entered in the multivariate model, followed by stepwise regression with the “MASS”21 package. Survival curves were generated with the “ggplot”22 and “survminer” packages. For each investigated parameter, a dichotomizing value was searched that defined 2 groups with a significant difference in episode resolution rate, under the condition that the smallest group was formed by ≥11 patients (one-third of the study population).
Agreement between segmentation of subretinal fluid volume, maximal subretinal fluid height on SD-OCT, and macular volume was estimated using Cohen's Kappa on R, with the “irr” package.23
Spearman correlation coefficients were used to investigate association between variables on GraphPad Prism (version 5.0f; GraphPad Software, La Jolla, CA). The logarithm of the minimal angle of resolution (LogMAR) was used to calculate visual acuity means. P values ≤0.05 were considered significant.
Results
Of 35 patients presenting with acute CSCR during the study period, 31 fulfilled the inclusion criteria. There were 26 men and 5 women, with a mean age of 40.0 ± 8.9 years (median: 37.8 years, range: 24.3–58.3 years). After 6 months of follow-up, CRSC episodes were resolved in n = 26 patients (83.9%) and persisted in n = 5 patients (16.1%). Among resolved cases, the mean time from the initial visit to resolution was 83 ± 46 days (median: 83 days, range: 21–180 days). Four cases (12.9%) were resolved after 1 month, 9 cases (29.0%) after 2 months, 16 cases (51.6%) after 3 months, 21 cases (67.7%) after 4 months, and 23 cases (74.2%) after 5 months. A survival curve displaying the time-dependent resolution rate of the 31 cases is displayed in Figure 1.
Fig. 1.
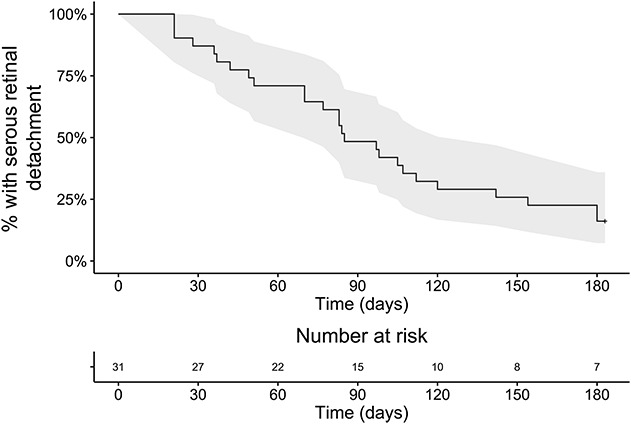
Survival curve showing the time-dependent resolution rate of 31 acute episodes of central serous chorioretinopathy from the time of the initial visit. All patients presented within 20 days after onset of symptoms. By 6 months, resolution was observed in 26 patients. The 95% confidence interval is colored in gray.
Clinical and imaging characteristics are summarized in Table 1. Subfoveal choroidal thickness ranged from 302 μm to 619 μm (mean: 479.9 μm) (Figure 2), the fluorescein expansion ratio ranged from 1.0 to 9.4 (mean: 2.8) (Figure 3), 13 patients had a PED, 14 had a RPE bump, and 4 had no RPE lesion at the leakage site, and the corresponding RPE elevation ranged from 0 μm to 279 μm (mean: 58.1 μm) (Figure 4). When analyzing the kinetics of subretinal fluid resorption using serial SRF volume segmentation on SD-OCT, a peak in SRF volume (higher than the initial value) was observed in n = 13 subjects during follow-up (Figure 5), while n = 18 subjects presented a progressive decrease in SRF volume from the initial visit (Figure 6). The mean time from the first visit to observed SRF volume peak was 42.6 days. A peak in macular volume on SD-OCT was also detected at the same timepoints in these subjects (kappa = 1.0, P < 0.0001), but there was only a moderate agreement in peak detection between subretinal fluid volume and maximal height of subretinal detachment on SD-OCT (kappa = 0.49, P = 0.0013).
Table 1.
Clinical and Imaging Characteristics of 31 Patients With a First Episode of Acute, Treatment-Naive Central Serous Chorioretinopathy
Fig. 2.
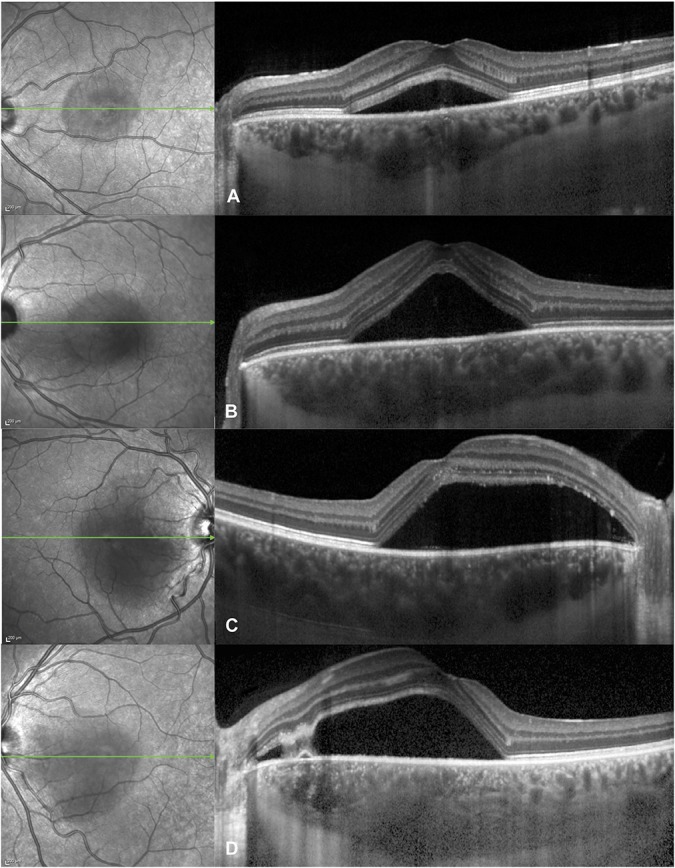
Spectrum of subfoveal choroidal thickness in acute central serous chorioretinopathy, visible on optical coherence tomography scans (right) passing through the fovea as indicated by the green arrows on infrared images (left): (A) 403 mm in a 37-year-old man, (B) 469 mm in a 28-year-old man, (C) 519 mm in a 38-year-old man, and (D) 617 mm in a 34-year-old-man.
Fig. 3.
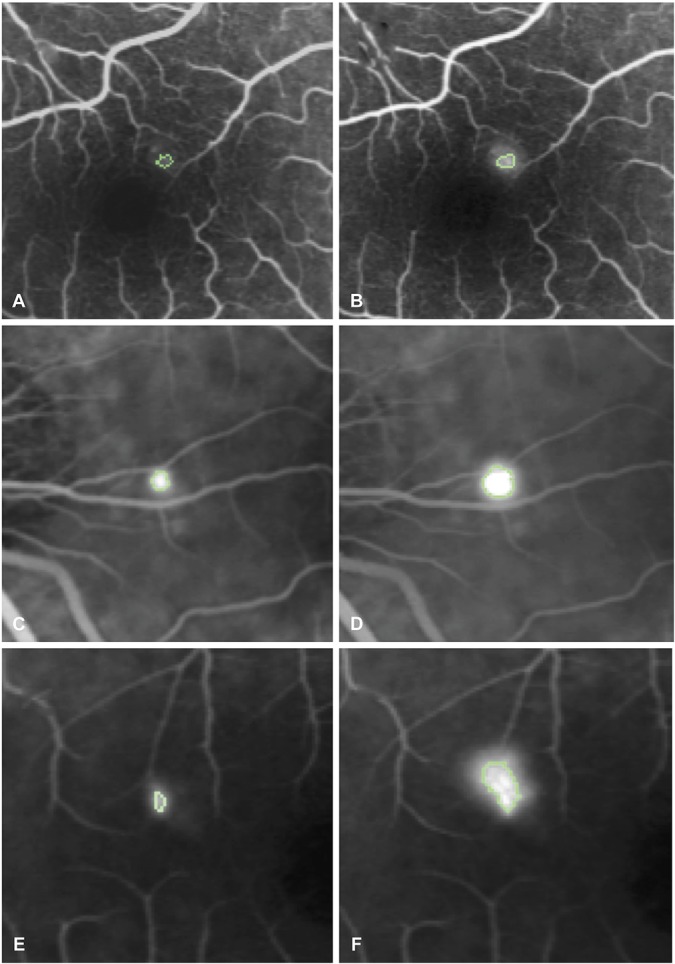
Method to quantify fluorescein leakage expansion on fluorescein angiography from early phase (40–60 seconds) to midphase (2–2.5 minutes). A and B. Early phase (A) and midphase (B) angiograms in a case with a very weak leakage. The hyperfluorescent area corresponding to pixels whose intensity is comprised within 75% of the maximal hyperfluorescence is indicated by a green outline. The fluorescein expansion ratio was calculated as the ratio between the hyperfluorescent areas at midphase and early phase and was 1.32. C and D. Same method applied to early phase (C) and midphase (D) angiograms from a case with intermediate ink-blot leakage pattern, yielding a fluorescein expansion ratio of 3.98. E and F. Same method applied to early (E) and midphase (F) angiograms from a case with intense smokestack leakage pattern, yielding a fluorescein expansion ratio of 6.01.
Fig. 4.
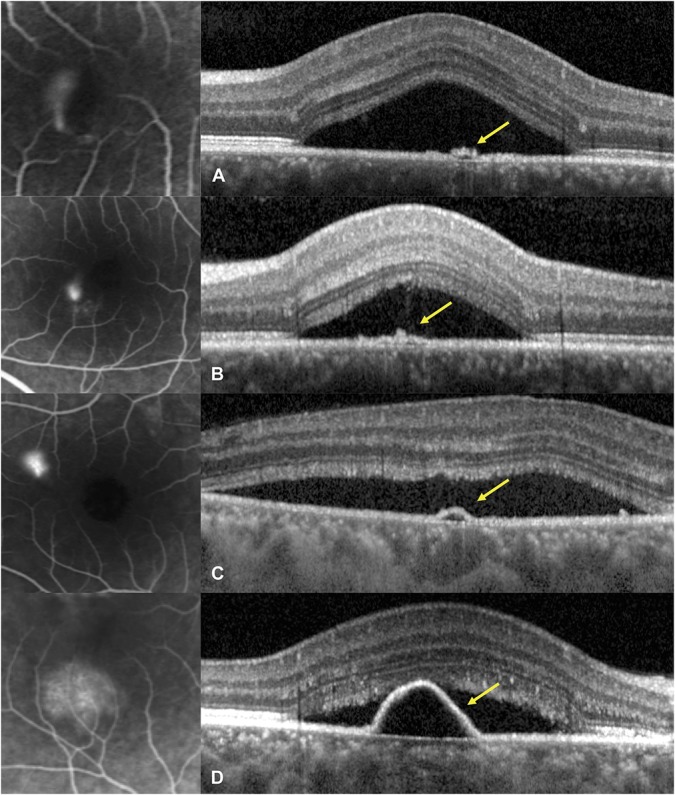
Optical coherence tomography of pigment epithelial lesions (right) at leaking sites on fluorescein angiograms (left) in acute central serous chorioretinopathy. A and B. Pigment epithelial bumps (yellow arrows) with estimated height of 25 mm in a 36-year-old man (A) and 41mm in a 28-year-old man (B). C and D. Pigment epithelial detachments (yellow arrows) with estimated height of 54 mm in a 39-year-old man(C) and 163 mm in a 52-year-old woman (D).
Fig. 5.
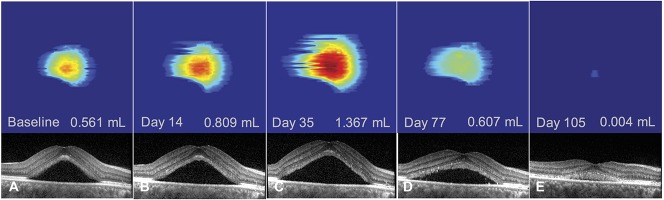
Follow-up of an acute episode of central serous chorioretinopathy in a 52-year-old woman using subretinal fluid volume segmentation on optical coherence tomography. There was an initial increase in subretinal fluid from baseline (A–B) with a peak in subretinal fluid volume at Day 35 (C) and subsequent decrease (D–E) until subretinal fluid resolution at Day 132 (not shown). Note the shape of a pigment epithelial detachment visible on the segmented serous retinal detachment (same patient as Figure 4D).
Fig. 6.
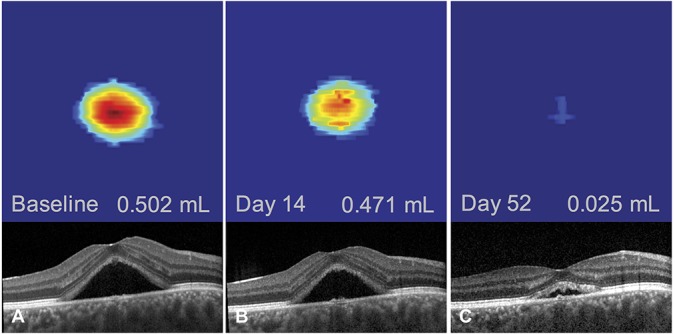
Follow-up of an acute episode of central serous chorioretinopathy in a 35-year-old man using subretinal fluid volume segmentation on optical coherence tomography. No increase in subretinal fluid volume could be observed, with a progressive decrease from baseline (A) to all timepoints (B and C) and resolution at Day 75 (not shown).
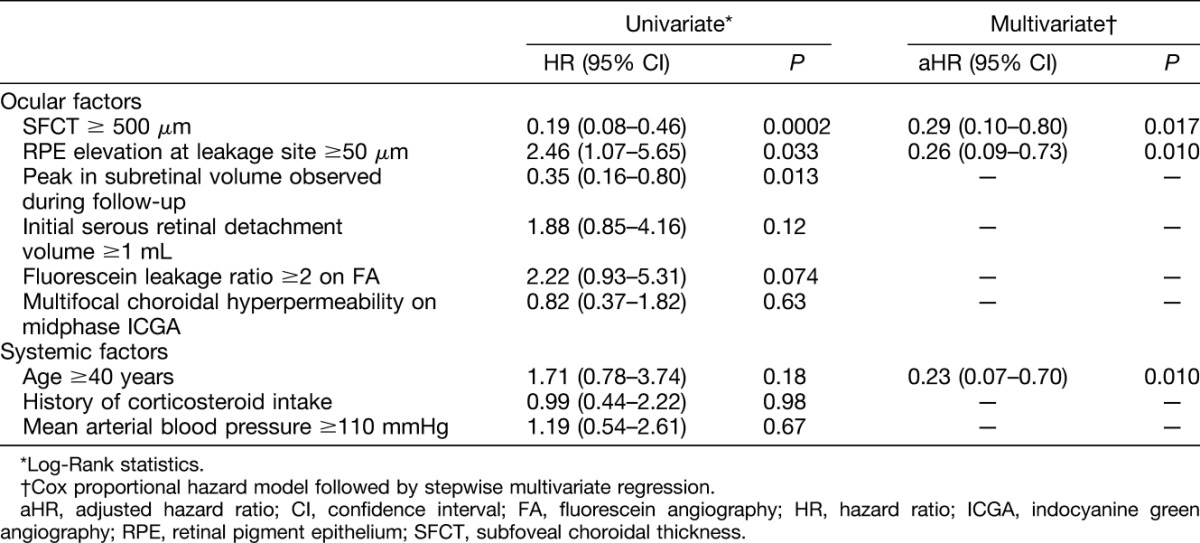
Using univariate survival analysis, the duration of CSCR episodes was longer in patients with SFCT ≥500 μm (P = 0.0002), those with RPE elevation at leakage sites higher than 50 μm (P = 0.033) and those with a peak in subretinal fluid observed during follow-up (P = 0.013). There was a near-significant association of intense fluorescein leakage (fluorescein expansion ratio ≥ 2) with longer episodes (P = 0.074). In contrast, patient age (P = 0.18), initial subretinal fluid volume (P = 0.12), focal/multifocal pattern of choroidal hyperpermeability on indocyanine-green angiography (P = 0.63), history of corticosteroid intake (P = 0.98), or mean arterial blood pressure (P = 0.67) did not have a significant effect on episode durations.
Variables with a significance level ≤0.2 were selected for the Cox multivariate survival model, with the assumption of proportional hazard. After stepwise multivariate regression, SFCT (≥500 μm, P = 0.017), RPE elevation at leakage sites (≥50 μm, P = 0.010), and patient age (≥40 years, P = 0.010) remained independent significant contributors to longer duration of CSCR episodes. Comparative survival curves are displayed in Figures 7 and 8 and detailed survival results are reported in Table 2.
Fig. 7.
Comparative survival curves showing the rate of subretinal detachment resolution over time about SFCT (A) and the height of RPE elevations at leakage sites (B) on optical coherence tomography. The duration from initial visit to episode resolution was significantly longer for subfoveal choroidal thickness ≥500 μm (A) and pigment epithelium elevation ≥50 μm (B) both in the univariate and multivariate analyses. P-values from the univariate (log-rank test) and the multivariate analysis (Cox proportional hazard model, between parenthesis) are reported.
Fig. 8.
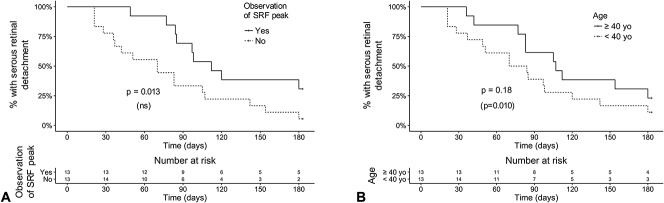
Comparative survival curves showing the rate of subretinal detachment resolution over time about the observation of a peak in SRF on optical coherence tomography (A) and patient age (B). The duration from initial visit to episode resolution was significantly longer for cases with an observed peak in subretinal fluid volume in the univariate analysis only (A). Episodes were also longer for patients aged 40 years or older in the multivariate analysis only (B). P-values from the univariate (log-rank test) and the multivariate analysis (Cox proportional hazard model, between parenthesis) are reported. ns, nonsignificant contributor to the multivariate model.
Table 2.
Factors Influencing the Duration of Acute, Treatment-Naive, First CSCR Episodes by Univariate and Multivariate Survival Analysis
To confirm the robustness of these findings, we repeated the same analyses with the time from symptoms onset (as reported by the patient), instead of the time from the first visit. This modification did not affect the results nor the significance levels in both the univariate and multivariate analyses.
To understand differences between univariate and multivariate results, we investigated possible correlations between variables. There were significant correlations between initial subretinal fluid volume and SFCT (r = 0.36, P = 0.046); between observation of a peak in subretinal fluid and SFCT (r = 0.45, P = 0.010); between the fluorescein expansion ratio and the observation of a peak in subretinal fluid (r = 0.39, P = 0.036), the RPE elevation at leakage sites (r = 0.46, P = 0.026), and the initial subretinal fluid volume (r = 0.58, P = 0.001). There was a near-significant trend between SFCT and fluorescein expansion ratio (r = 0.33, P = 0.082). There was also a near-significant, inverse correlation between patient age and SFCT (r = −0.31, P = 0.09), providing a likely explanation for the nonsignificance of age in the univariate analysis. However, both parameters remained independent, consistently with the results of the multivariate model.
Discussion
In acute CSCR, a serous retinal detachment of the macula is generally not considered a threat for visual function because visual acuity is not or mildly decreased and recovers completely in most cases. But altered quality of vision is a frequent complaint of patients despite normal visual acuity levels and macular microstructure. Electrophysiology studies of acute CSCR demonstrated that abnormal cone function observed during an active episode persists after resoluton.24–27 Poorer recovery is associated with longer symptom duration although the duration threshold before permanent functional damage has not been clearly determined.28 This threshold would help to define the optimal treatment timing for nonresolving cases.
In this study, we have analyzed the natural history of acute, treatment-naive first episodes of CSCR and have correlated the duration of subretinal fluid persistence with clinically available parameters. We have found that longer episode duration was independently associated with higher SFCT, higher elevation of RPE lesions at leakage sites, and older age.
Current hypotheses regarding the pathophysiology of CSCR include choroidal vascular dilation manifesting by choroidal thickening (or pachychoroid),29,30 possibly because of inappropriate activation of the mineralocorticoid pathway,31,32 with concurrent RPE alterations.33 However, the mechanisms involved in SRF resolution or persistence are not fully understood. Genetic studies of nonresolving CSCR cases have reported an association with variants in the Complement Factor H, ARMS 2 and Cadherin 5 genes,34–36 which are expressed by RPE cells.37–39 These findings suggest that RPE changes contribute to the evolution toward nonresolving CSCR. They are consistent with previous fluorescein angiography studies of CSCR40–42 demonstrating that SRF originates from an abnormal passage from the choroid through the RPE, overwhelming the pumping outflow capacity of RPE cells. From this perspective, the association of longer episode duration with higher SFCT and higher elevation of RPE lesions at leakage sites are additional evidence that the degree of choroidal and RPE dysfunction is predictive of the final outcome.
Another interesting finding is the association of episode duration with older age. In aged human maculae, RPE cells increase in size and lose their regular hexagonal shape.43 Studies of aging primate eyes have shown that mitochondrial elongation is observed within RPE cells located of the macular area, an indicator of increased metabolic stress.44 In aged mouse eyes, RPE cells undergo multinucleation because of aborted mitosis, one of the mechanisms of cell death, and the contact with photoreceptor outer segments inhibits RPE cell proliferation.45 Altogether, these observations indicate that the repair capacity of the RPE decreases with aging, particularly in the macula. This supports the notion that older age is associated with longer CSCR episodes, which may ultimately contribute to the chronic epitheliopathy frequently seen in older CSCR patients.46
We are unaware of previous reports relating the time-course of acute CSCR episodes to clinical and multimodal imaging features. In a study of 27 eyes with acute CSCR performed before the OCT era, Klein et al1 related the time-course of the disease to baseline and final fluorescein angiography and repeated fundus examinations. The mean time of resolution was 6 months after symptoms onset, with a maximal observed duration of 12 months. More recently, Pryds et al19 have investigated the fluorescein leakage rate based on early FA frames in cases with typical smokestack leaks, using a method adapted in the present study. They observed variable leakage rates, a finding consistent with our results, but did not correlate with other clinical characteristics. Yang et al47 have described the multimodal correlations between RPE alterations including PED on SD-OCT, FA, and indocyanine-green angiography in CSCR patients but did not relate these findings to the duration of episodes.
The present study may have practical consequences for the management of acute CSCR patients. Eighty-four percent of consecutive patients demonstrated spontaneous resolution of SRF within 6 months, confirming that observation for up to 6 months is an appropriate initial management. However, patients with SFCT ≥500 μm, PED with elevation ≥50 μm, or age ≥ 40 years may be identified and warned of a higher risk of longer CSCR duration, with a subsequent need for a longer follow-up and/or earlier treatment decision. Although they were not independent risk factors, observation of a peak in SRF during follow-up and to a lesser extent an intense leakage on the baseline FA may also contribute to identify clinically patients at risk of longer episodes. Further studies are required to evaluate whether longer durations of macular serous detachment are associated with worse vision quality and higher risks of CSCR recurrence.
Limitations of this work include the size of the study population because of its prospective nature and the iterative subretinal fluid follow-up that prevented a continuous analysis of subretinal fluid evolution. As a result, peaks in subretinal fluid volume may have been missed, either between follow-up timepoints, or before the initial visit. To minimize this flaw, we excluded patients presenting more than 20 days after symptoms onset and densified the initial follow-up schedule. In addition, we did not consider recently described imaging signs in CSCR such as hyporeflective subretinal lucency,48 loculation of fluid in the posterior choroid,49 or presence of intraretinal hyperreflective foci.50 Finally, SCFT were measured at a single time point at first visit and therefore diurnal variations of SFCT were not considered.
To summarize, we have identified clinical parameters that are significantly associated with longer duration of first acute CSCR episodes. Further functional analyses are required to determine whether these factors could help select patients who should benefit from earlier therapeutic interventions. These parameters could also be useful for the design of future randomized studies of CSCR to limit potential bias.
Footnotes
None of the authors has any financial/conflicting interests to disclose.
References
- 1. Klein ML, Van Buskirk EM, Friedman E, et al. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol 1974;91:247–250. [DOI] [PubMed] [Google Scholar]
- 2. Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1979;63:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol 1983;95:457–466. [DOI] [PubMed] [Google Scholar]
- 4. Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res 2015;48:82–118. [DOI] [PubMed] [Google Scholar]
- 5. Malik KJ, Sampat KM, Mansouri A, et al. Low-intensity/high-density subthreshold micropulse diode laser for chronic central serous chorioretinopathy. Retina 2015;35:532–536. [DOI] [PubMed] [Google Scholar]
- 6. Roisman L, Magalhães FP, Lavinsky D, et al. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surg Lasers Imaging Retina 2013;44:465–470. [DOI] [PubMed] [Google Scholar]
- 7. Salehi M, Wenick AS, Law HA, et al. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev 2015:CD011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim JI, Glassman AR, Aiello LP, et al. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014;121:1073–1078. [DOI] [PubMed] [Google Scholar]
- 9. Ma J, Meng N, Xu X, et al. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol 2014;92:e594–e601. [DOI] [PubMed] [Google Scholar]
- 10. Chan W-M, Lai TYY, Lai RYK, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology 2008;115:1756–1765. [DOI] [PubMed] [Google Scholar]
- 11. Hagen S, Ansari-Shahrezaei S, Smretschnig E, et al. Effect of photodynamic therapy on short-wavelength fundus autofluorescence in eyes with acute central serous chorioretinopathy. Retina 2015;35:223–230. [DOI] [PubMed] [Google Scholar]
- 12. Shiode Y, Morizane Y, Kimura S, et al. Comparison of halving the irradiation time or the verteporfin dose in photodynamic therapy for chronic central serous chorioretinopathy. Retina 2015;35:2498–2504. [DOI] [PubMed] [Google Scholar]
- 13. Bousquet E, Beydoun T, Rothschild P-R, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina 2015;35:2505–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herold TR, Prause K, Wolf A, et al. Spironolactone in the treatment of central serous chorioretinopathy - a case series. Graefes Arch Clin Exp Ophthalmol 2014;252:1985–1991. [DOI] [PubMed] [Google Scholar]
- 15. Salz DA, Pitcher JD, Hsu J, et al. Oral eplerenone for treatment of chronic central serous chorioretinopathy: a case series. Ophthalmic Surg Lasers Imaging Retina 2015;46:439–444. [DOI] [PubMed] [Google Scholar]
- 16. Singh RP, Sears JE, Bedi R, et al. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int J Ophthalmol 2015;8:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghadiali Q, Jung JJ, Yu S, et al. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina 2016;36:611–618. [DOI] [PubMed] [Google Scholar]
- 18. Daruich A, Matet A, Dirani A, et al. Oral mineralocorticoid-receptor antagonists: real-life experience in clinical subtypes of nonresolving central serous chorioretinopathy with chronic epitheliopathy. Transl Vis Sci Technol 2016;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pryds A, Sander B, Larsen M. Characterization of subretinal fluid leakage in central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2010;51:5853–5857. [DOI] [PubMed] [Google Scholar]
- 20. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 21. Venables WN, Ripley BD. Modern Applied Statistics With S. 4th ed New York, NY: Springer; 2002. [Google Scholar]
- 22. Wickham H. ggplot2. New York, NY: Springer New York; 2009. Available at: http://link.springer.com/10.1007/978-0-387-98141-3. Accessed June 16, 2016. [Google Scholar]
- 23. Cohen JA. Coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 24. Goyal JL, Ghosh B, Sangit V, et al. Pattern ERG in central serous retinopathy. Doc Ophthalmol 2015;130:141–147. [DOI] [PubMed] [Google Scholar]
- 25. Shimada Y, Imai D, Ota Y, et al. Retinal adaptability loss in serous retinal detachment with central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2010;51:3210–3215. [DOI] [PubMed] [Google Scholar]
- 26. Yip YWY, Ngai JWS, Fok ACT, et al. Correlation between functional and anatomical assessments by multifocal electroretinography and optical coherence tomography in central serous chorioretinopathy. Doc Ophthalmol 2010;120:193–200. [DOI] [PubMed] [Google Scholar]
- 27. Chappelow AV, Marmor MF. Multifocal electroretinogram abnormalities persist following resolution of central serous chorioretinopathy. Arch Ophthalmol 2000;118:1211–1215. [DOI] [PubMed] [Google Scholar]
- 28. Bae S, Jin K, Kim H, Bae SH. Clinical parameters related to metamorphopsia outcome in patients with resolved central serous chorioretinopathy using M-CHARTS: retrospective cohort study. BMC Ophthalmol 2015;15:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina 2016;36:499–516. [DOI] [PubMed] [Google Scholar]
- 30. Gallego-Pinazo R, Dolz-Marco R, Gómez-Ulla F, et al. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol 2014;3:111–115. [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest 2012;122:2672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. [DOI] [PubMed] [Google Scholar]
- 33. Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol 2010;149:361–363. [DOI] [PubMed] [Google Scholar]
- 34. Schubert C, Pryds A, Zeng S, et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat 2014;35:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miki A, Kondo N, Yanagisawa S, et al. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology 2014;121:1067–1072. [DOI] [PubMed] [Google Scholar]
- 36. de Jong EK, Breukink MB, Schellevis RL, et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology 2015;122:562–570. [DOI] [PubMed] [Google Scholar]
- 37. Cheng Y, Huang L, Li X, et al. Genetic and functional dissection of ARMS2 in age-related macular degeneration and polypoidal choroidal vasculopathy. PLoS One 2013;8:e53665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chu PG, Grunwald GB. Identification of an adhesion-associated protein of the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1990;31:847–855. [PubMed] [Google Scholar]
- 39. Kanan Y, Siefert JC, Kinter M, Al-Ubaidi MR. Complement factor H, vitronectin, and opticin are tyrosine-sulfated proteins of the retinal pigment epithelium. PLoS One 2014;9:e105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshioka H, Sugita T. Fluorescein fundus photographic studies on the central serous retinopathy. II. Fluorescein fundus photographic findings in the full course of this disease. Kurume Med J 1969;16:1–12. [DOI] [PubMed] [Google Scholar]
- 41. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967;63:1–139. [PubMed] [Google Scholar]
- 42. Coscas G, Aubry JP. Evolutive angiographic aspects of central serous chorioretinopathies [in French]. Bull Mem Soc Fr Ophtalmol 1972;85:169–187. [PubMed] [Google Scholar]
- 43. Rashid A, Bhatia SK, Mazzitello KI, et al. RPE cell and sheet properties in normal and diseased eyes. Adv Exp Med Biol 2016;854:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gouras P, Ivert L, Neuringer M, Nagasaki T. Mitochondrial elongation in the macular RPE of aging monkeys, evidence of metabolic stress. Graefes Arch Clin Exp Ophthalmol 2016;254:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen M, Rajapakse D, Fraczek M, et al. Retinal pigment epithelial cell multinucleation in the aging eye - a mechanism to repair damage and maintain homoeostasis. Aging Cell. 2016;15:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996;103:2070–2079; discussion 2079–2080. [DOI] [PubMed] [Google Scholar]
- 47. Yang L, Jonas JB, Wei W. Optical coherence tomography-assisted enhanced depth imaging of central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2013;54:4659–4665. [DOI] [PubMed] [Google Scholar]
- 48. Yannuzzi NA, Mrejen S, Capuano V, et al. A central hyporeflective subretinal lucency correlates with a region of focal leakage on fluorescein angiography in eyes with central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina 2015;46:832–836. [DOI] [PubMed] [Google Scholar]
- 49. Spaide RF, Ryan EH. Loculation of fluid in the posterior choroid in eyes with central serous chorioretinopathy. Am J Ophthalmol 2015;160:1211–1216. [DOI] [PubMed] [Google Scholar]
- 50. Lee H, Lee J, Chung H, Kim HC. Baseline spectral domain optical coherence tomographic hyperreflective foci as a predictor of visual outcome and recurrence for central serous chorioretinopathy. Retina 2015;36:1372–1380. [DOI] [PubMed] [Google Scholar]