Pneumatic vitreolysis with C3F8 gas is effective in releasing focal vitreomacular traction in a high percentage of eyes with few adverse events, especially with limited vitreomacular traction (within 1 disk area), lack of thick cellophane membranes, no diabetes mellitus, younger age (mean age of 69.1 years vs. 78.1 years), better baseline best spectacle–corrected visual acuity (mean of 20/50 vs. 20/66), small Stage 2 macular hole, and female gender.
Key words: gas injection, ocriplasmin, pneumatic vitreolysis, posterior vitreous detachment, Stage 2 macular hole, syneresis, vitreomacular adhesion, vitreomacular traction
Abstract
Purpose:
To evaluate the outcome of perfluoropropane (C3F8) gas injection for symptomatic vitreomacular traction (VMT) with or without Stage 2 macular hole (MH).
Methods:
A retrospective review of eyes with VMT treated with 0.3 mL of C3F8 gas was performed. Patients avoided the supine position until gas resolution. Patients with small MH maintained partial face-down positioning.
Results:
Forty-nine consecutive patients (50 eyes) with symptomatic VMT underwent pneumatic vitreolysis between 2010 and 2016. A posterior vitreous detachment developed in 43 eyes (86.0%) after a single gas injection, at a median of 3.0 weeks. Twenty-eight of 35 eyes (80.0%) with VMT only and all 15 eyes (100%) with a small Stage 2 MH developed a posterior vitreous detachment, with MH closure in 10 of 15 eyes (66.7%). Median baseline and last best spectacle–corrected visual acuities were 20/50 and 20/40, respectively (P < 0.001). Mean follow-up time was 11.1 ± 9.9 months. Rate of posterior vitreous detachment was reduced with presence of diabetes mellitus (25%) and with thick cellophane membrane (50%). Univariate analysis showed increased VMT release for eyes with VMT extent within 1 disk area (χ2 = 13.1, P = 0.002), eyes with absence of diabetes mellitus (χ2 = 8.8, P = 0.007), and eyes with Stage 2 MH (χ2 = 5.47, P = 0.019); there was a trend between success and lack of thick cellophane membrane (χ2 = 3.32, P = 0.068). Results using logistic regression also showed younger age (P = 0.012), followed by better baseline best spectacle–corrected visual acuity (P = 0.044), lack of diabetes mellitus (P = 0.077), and female gender (P = 0.045) to be predictors of increased VMT release. One VMT-only eye formed a MH and another VMT-only eye developed a retinal detachment. Both eyes responded to vitrectomy.
Conclusion:
Pneumatic vitreolysis with limited face-down position is a viable option for treating VMT with few adverse events. More studies are needed to elucidate its indications, benefits, and risks.
Disorders of the vitreomacular interface consist of a spectrum of anomalous relationships of the posterior hyaloid to the underlying internal limiting membrane.1,2 Vitreomacular adhesion (VMA) occurs when the posterior hyaloid remains attached to the internal limiting membrane centrally. Vitreomacular traction (VMT) is diagnosed when VMA results in traction and distortion of the retinal architecture, inducing ocular symptoms and vision deficit.1–3 Under certain circumstances, tractional forces associated with progression of VMT may lead to the development of a full-thickness macular hole (MH) and further vision loss.1–3 Common management options for symptomatic VMT and early small MHs may include observation, vitrectomy, and intravitreal injection of ocriplasmin. Observation has been shown to be a viable option for managing this condition because recent publications have indicated spontaneous relief of VMT in 30% to 40% of these eyes.4–13 However, no reliable diagnostic methods have yet been proven to predict consistently which eyes may develop spontaneous resolution of VMT without harmful sequelae and which eyes may form a full-thickness macular defect with further vision loss. Thus, observation may not be the best option for managing all cases of VMT, given the inherent risk of MH formation in 60% to 70% of such cases.4–13 However, subjecting all patients with VMA or VMT to a costly trip to the operating room for a vitrectomy is likely excessive, considering the inherent surgical risks even in the most experienced hands and given an eventual 30% to 40% chance of a spontaneous resolution reported in the literature. Thus, an alternative management option deemed to be more proactive than passive observation but less invasive than a vitrectomy would be a welcome addition to the armamentarium for vitreoretinal specialists in managing eyes with VMT. Ocriplasmin (Jetrea; ThromboGenics NV, Leuven, Belgium) seemed to fulfill such a niche when pilot studies and the Trial of Microplasmin Intravitreal Injection for Non-surgical Treatment of Focal Vitreomacular Adhesion (MiVI-TRUST) Trials (TG-MV-006 and TG-MV-007) showed its promotion of a posterior vitreous detachment (PVD).14,15 Ocriplasmin was approved by the Food and Drug Administration for treatment of symptomatic VMT in 2012. The intravitreal administration of this recombinant protein composed of the catalytic domain of human plasmin provides a potential advantage over a vitrectomy for treating VMT because its injection is performed in the office setting. However, its high cost and overall modest success rate of 26.5% in the induction of a PVD limits its clinical utility. In addition, multiple anecdotal adverse events associated with intravitreal injections of ocriplasmin in human eyes have been reported.16–20 They include transient visual loss, dyschromatopsia associated with electroretinographic changes, dislocation of the crystalline lens after zonulolysis, transient blurring of the ellipsoid zone, and dehiscence of the photoreceptor layer documented by spectral-domain ocular coherence tomography (SD-OCT). Such reported complications have dampened the initial enthusiasm for many retinal surgeons in the use of this drug.3 Currently, the manufacturer of ocriplasmin has embarked on a multicenter prospective postmarketing registry for documenting the efficacy and any safety issues associated with ocriplasmin (Ocriplasmin Research to Better Inform Treatment [ORBIT] Study).
Given the concerns regarding ocriplasmin outlined above, pneumatic vitreolysis (PVL) has been suggested as a management alternative for treatment of symptomatic VMT. Chan et al21 first demonstrated and reported the utility of intraocular gas in eliciting a PVD (96%) and closure of small full-thickness MHs (57%) in human eyes in 1995. In this updated study, we investigated a series of 50 eyes treated with intraocular perfluoropropane (C3F8) gas injection and limited face-down positioning for treatment of symptomatic VMT.
Methods
A retrospective study was conducted on consecutive patients who underwent PVL in 1 of the 2 centers (Southern California Desert Retina Consultants and Retinal Consultants of San Antonio) between 2010 and 2016. Inclusion criteria were patients with symptomatic idiopathic VMT with or without a small Stage 2 MH, who received intraocular C3F8 gas injection for the purpose of releasing VMT and resolving the macular defect in case of Stage 2 MH. Exclusion criteria included eyes with previous retinal surgery (with the exception of previous ocriplasmin use), patients with a Stage 2 MH >300 μm, patients with a MH beyond Stage 2, and patients who refused to avoid the supine position during the treatment period. Eyes with conditions that might confound the results were also excluded (e.g., advanced cataracts [more than mild cortical and nuclear sclerotic cataracts], retinal vascular occlusion, optic nerve pathologic conditions, glaucoma, advanced macular pathologic condition [e.g., substantial age-related macular degeneration], macular edema induced by a condition other than VMT, and previous retinal breaks or detachment). An Institutional Review Board exemption was granted by the Western Institutional Review Board (Puyallup, WA) before data collection. This study has complied with the Declaration of Helsinki and all federal and state regulations of the United States, including the Health Insurance Portability and Accountability Act.
Surgical Technique and Examination
All study eyes underwent PVL. After obtaining an appropriate informed consent, topical 0.5% proparacaine followed by subconjunctival injection of 2.0% lidocaine hydrochloride with or without 0.5% bupivacaine hydrochloride was administered. Sterile prepping with Betadine was performed. Next, a prophylactic paracentesis to remove 0.1 mL to 0.2 mL of aqueous was performed through the limbus with a short 27-gauge or 30-gauge needle connected to a tuberculin syringe. Intravitreal injection of at least 0.2 mL but usually 0.3 mL of filtered C3F8 gas was then performed via the pars plana of the study eye. The intraocular pressure and central retinal arterial perfusion of the surgical eye was monitored before the patient was discharged. All patients were required to avoid the supine position and to lie on one side or the stomach during sleeping hours, until after resolution of the intraocular gas. Patients with a small Stage 2 MH were encouraged to maintain the face-down position as much as possible for at least 3 days to 4 days. Subsequent follow-up visits took place at 1 day, 1 week, and then every 2 weeks to 4 weeks. The examination performed during each clinic visit included best spectacle–corrected visual acuity (BSCVA; defined as visual acuity measurement obtained with the patient's spectacle correction), intraocular pressure measurement, slit-lamp biomicroscopy, indirect ophthalmoscopy, and SD-OCT (Heidelberg Spectralis, Heidelberg, Germany or Carl Zeiss-Meditec Cirrus, Dublin, California).
Data Collection
The key data derived from the retrospective study included baseline and postoperative BSCVA, vitreomacular status, and macular condition, that is, any resolution of VMT because of the development of a PVD, and closure of MH if applicable. The following information was also recorded: patient's demographics (age, gender, right eye vs. left eye), period from intravitreal gas injection to PVD, and follow-up time. In addition, any adverse events that developed after gas injection were recorded, including ocular complications (e.g., retinal breaks or detachment, infection, intraocular pressure spikes, uveitis, hemorrhage, and optic nerve damage) and systemic complications. The outcome measures included development of a PVD, closure of a MH if applicable, and BSCVA of the study eye.
Statistical Methods
Statistical analyses were performed with Statistical Product and Service Solutions (SPSS) version 24 (IBM SPSS, Armonk, NY). Data were summarized using frequency and relative frequency (in percentage) for qualitative variables, whereas mean values, standard deviations, medians, and ranges were calculated for continuous variables. The BSCVA was converted to logarithm of the minimum angle of resolution (logMAR), which is equivalent to logarithm base 10 of the reciprocal of Snellen BSCVA. Both parametric and nonparametric statistics were used in the statistical comparisons. Wilcoxon signed rank test was used when comparing baseline with postoperative BSCVA, whereas two-tailed independent t-test was used to compare the mean age and mean baseline BSCVA between eyes with and without successful release of VMT. Chi-square test of independence (Fisher exact test) and stepwise logistic regression were performed for predictor analysis of baseline factors associated with success of VMT release. Baseline factors analyzed included age, extent of VMT (within 1 disk area [DA], between 1 DA and 2 DA, and >2 DA), initial BSCVA, cellophane maculopathy, diabetes mellitus, Stage 2 MH versus VMT-only, gender, right eye versus left eye, lens status, and previous ocriplasmin use. A P-value of <0.05 was considered to be significant.
Results
Table 1 outlines the demographic distribution of the study subjects at baseline. There were 50 consecutive eyes (49 patients) with symptomatic VMT, who presented to 1 of the 2 study centers and underwent PVL between January 2010 and May 2016. There were 35 women and 14 men. Their mean age was 70.4 ± 6.4 years. All patients included in the study presented with symptomatic VMT in the study eyes. All except seven eyes were phakic. The seven nonphakic eyes had posterior chamber intraocular lenses. Only mild cataracts were found in 15 of the 43 phakic eyes. Of the four patients (eyes) with diabetes mellitus, three had either no diabetic retinopathy or only mild background diabetic retinopathy (mild punctate and blot hemorrhage without maculopathy). Only one eye had proliferative diabetic retinopathy, which failed to respond to PVL. There were four eyes with thick cellophane maculopathy.
Table 1.
Baseline Characteristics
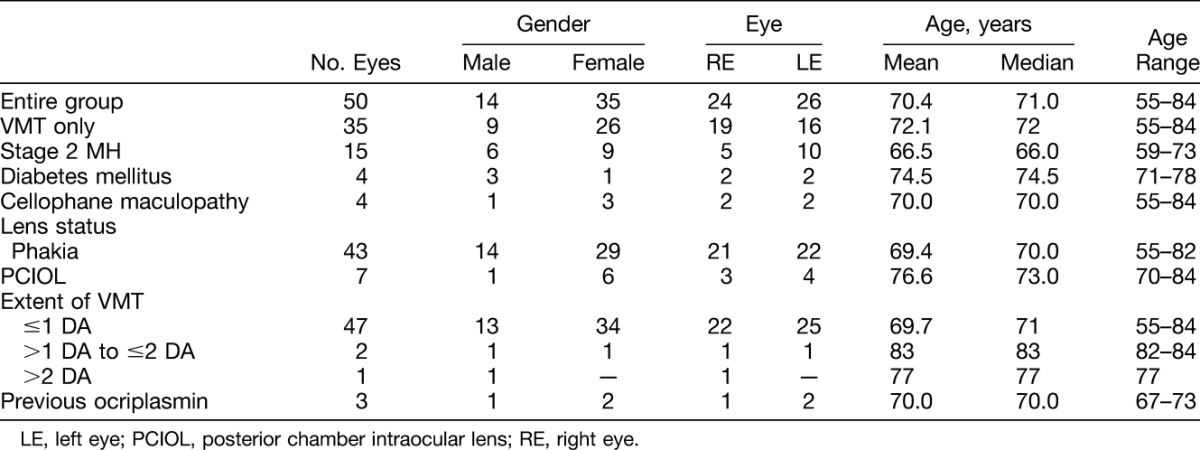
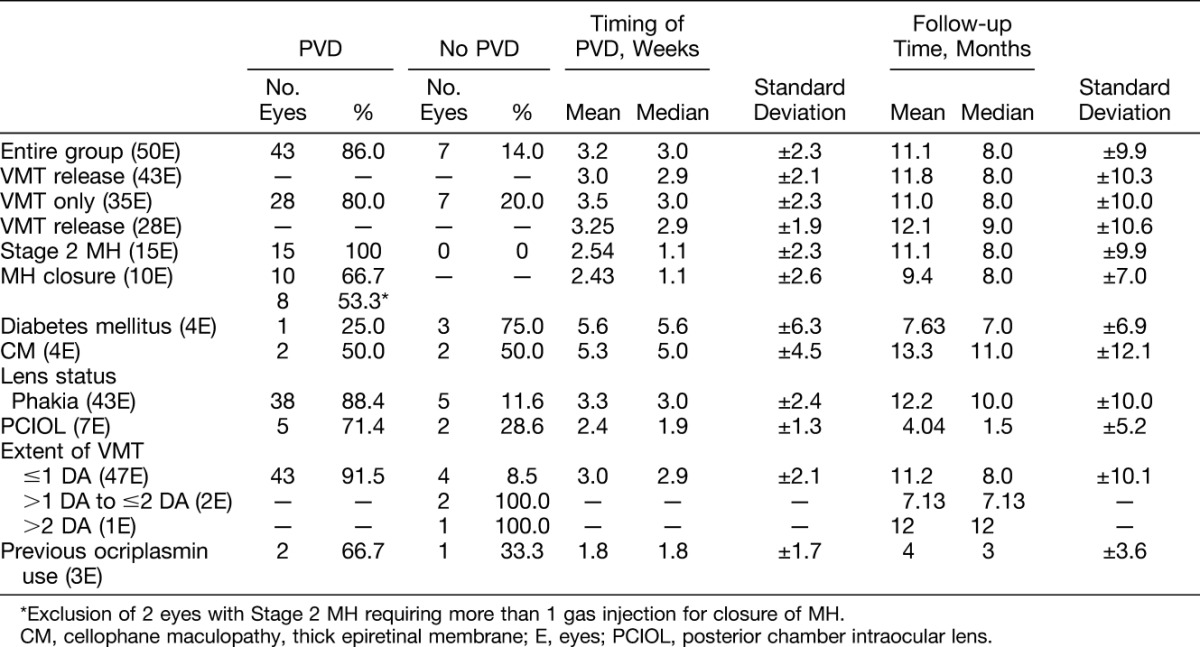
Of the 50 eyes, there were 36 eyes with VMT only and 14 eyes with a Stage 2 MH. Tables 2 and 3 summarize the visual results and anatomical outcomes of the study eyes, respectively. A PVD developed in 43 of 50 eyes (86.0%) after a single gas injection, within a median of 3.0 weeks after intravitreal C3F8 gas injection (range: 4 days to 9 weeks). Regarding the 35 eyes with VMT only, 28 eyes (80.0%) developed a PVD after PVL. All 15 eyes (100%) with a small Stage 2 MH developed a PVD, and there was closure of MH in 10 of these 15 eyes (66.7%) after PVL. Success rate of MH closure was 53.3% when excluding the 2 eyes with repeat gas injections. With the exception of two eyes, study eyes received a single gas injection. One eye with a MH developed a PVD after the initial gas injection but required injection of a second gas bubble for resolving the MH (Case Report 3). Another eye with a MH also developed a PVD after the initial gas injection but required injection of a second gas bubble followed by a third gas bubble before MH closure. When excluding these 2 eyes that required more than 1 gas bubble injection for MH closure, the success rate for MH closure was 8 of 15 or 53.3%. Overall, a complete resolution of all vitreomacular abnormalities was achieved in 38 eyes (76.0%) (28 eyes with baseline VMT only and 10 eyes with baseline small Stage 2 MH) at the end of the follow-up period.
Table 2.
Baseline and Last Visit Best Spectacle–Corrected Visual Acuities
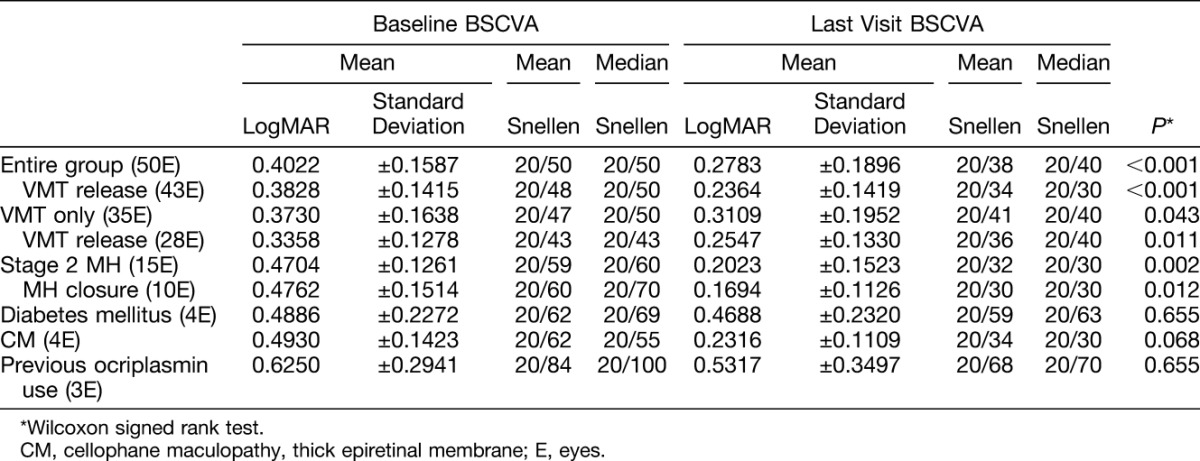
Table 3.
Anatomical Outcomes, Timing of Posterior Vitreous Detachment, and Follow-up Time
The median baseline BSCVA was 20/50 and the median latest BSCVA was 20/40 (P < 0.001, 2-tailed Wilcoxon signed rank test) (Table 2). The mean follow-up time after intravitreal gas injection was 11.1 months (range: 2–40 months).
Failed Cases
Of the 35 eyes with VMT only at baseline, 7 eyes (20.0%) did not achieve release of the VMT. One of these 7 patients with failed PVL had BSCVA of 20/80 at baseline and a history of diabetes mellitus with broad and sticky VMT (>2 DA) in the treated eye at baseline. There was a partial but not complete VMT release. The patient declined further treatment and his BSCVA was 20/50 during the last follow-up visit at 15 months later. Another patient with failure had baseline BSCVA of 20/50 and substantial VMT (between 1 and 2 DA) associated with diabetes mellitus and thick cellophane maculopathy at baseline. A subsequent pars plana vitrectomy with membrane stripping was required to remove the epiretinal membrane and for release of VMT. The BSCVA was 20/100 at 2 months later. A third patient with failure had previous failed response to ocriplasmin first before undergoing PVL. His baseline BSCVA was 20/40 before PVL. He declined further retinal treatment and his BSCVA was deteriorated to 20/100 at 3 months later partially because of a cataract. A fourth patient with failure had a baseline BSCVA of 20/150. He also declined more surgery and his BSCVA remained at 20/150 at the last follow-up visit. A fifth patient with failure had a baseline BSCA of 20/100 and a history of diabetes mellitus, broad VMT (>2 DA), and dense cellophane membrane at baseline. Despite failure of a complete PVD after PVL, there was a partial release of the central portion of the VMT, leading to BSCVA improvement of 20/50 at 12 months after surgery. Of the remaining 2 VMT-only eyes that failed to develop a complete PVD, both underwent a subsequent successful vitrectomy with resolution of VMT and central macular abnormalities, so that the visual acuity was improved from 20/50 to 20/30 for one eye and the visual acuity was 20/40 at baseline and maintained at 20/40 at latest visit for the other eye.
There was failure of closure of 5 small Stage 2 MH (33.3%) despite release of VMT after PVL. All five eyes responded well to a subsequent pars plana vitrectomy with membrane stripping, leading to MH closure in all these eyes. The final BSCVA was 20/40, 20/20, 20/40, 20/30, and 20/70, respectively.
There were three VMT-only eyes that received a previous ocriplasmin injection for resolving symptomatic VMT. All three eyes failed to develop a PVD despite the ocriplasmin injection. Subsequent intravitreal gas injection was successful in resolving VMT in two of these three eyes. Of the two eyes that responded to subsequent gas injection, one eye developed a peripheral retinal break and a retinal detachment despite VMT release after gas injection (see details in next paragraph) and the other eye had developed a Stage 2 MH with residual focal VMT within 24 hours after the initial ocriplasmin injection (Case Report 4). For this eye, subsequent gas injection completed the VMT release and closure of MH after diligent face-down positioning (see Case Report 4 below for more details). The details on the third eye that failed to respond to previous ocriplasmin injection and subsequent gas injection are described above in the summary of the seven failed cases.
Adverse Events
Ocular complications developed in two eyes after PVL. One VMT-only eye developed a Stage 2 MH despite release of the VMT after gas injection. Subsequent pars plana vitrectomy resolved the MH with a final visual acuity of 20/40. Another VMT-only eye had a previous ocriplasmin injection. Despite achieving a PVD after subsequent gas injection, a retinal break associated with a retinal detachment developed in this eye. A pars plana vitrectomy successfully repaired the retinal detachment and the BSCVA was recovered to 20/70 in this eye. There were no other ocular complications and there was also a lack of any systemic adverse events.
Results of Predictor Analysis of Baseline Factors
Success for VMT release was reduced to 25% for eyes with diabetes mellitus and to 50% for eyes with cellophane maculopathy in this study. Univariate chi-square analysis of independence showed a significant relationship between success (VMT release) and the extent of VMT (more successful for VMT ≤1 DA compared with VMT > 1 DA [χ2 = 13.1, P = 0.002, Fisher exact test]), between success and the absence of diabetes mellitus (χ2 = 8.8, P = 0.007, Fisher exact test), and between success and Stage 2 MH (compared with VMT-only) (χ2 = 5.47, P = 0.019). There was a trend between success and lack of thick cellophane membrane (χ2 = 3.32, P = 0.068, Fisher exact test). Furthermore, stepwise logistic regression showed that younger age was the strongest predictor for success (odds ratio [OR] = 0.61, 95% confidence interval [CI] = 0.42–0.90, P = 0.012), followed by better baseline BSCVA (OR = 0.01, 95% CI = 0.001–0.46, P = 0.044), absence of diabetes mellitus (OR = 129.5, 95% CI = 0.6–28,223.8, P = 0.077), and female gender (OR = 6.1, 95% CI = 1.03–36.5, P = 0.045). The other baseline variables that did not show significance on logistic regression included cellophane maculopathy (OR = 6.3, 95% CI = 0.6–62.05, P = 0.115), previous ocriplasmin use (OR = 6.1, 95% CI = 0.4–158.8, P = 0.197), lens status (OR = 0.5, 95% CI = 0.6–4.02, P = 0.50), right eye versus left eye (OR = 0.6, 95% CI = 0.1–3.8, P = 0.60).
Independent t-test was performed to compare the mean age and mean baseline BSCVA between those eyes with successful release of VMT versus those eyes with failure to release VMT. The results showed that there was a significant difference in mean age between the 2 eye groups (69.1 ± 5.8 years vs. 78.1 ± 4.5 years, P = 0.03). Also, there was a significant difference in mean baseline BSCVA (0.38 ± 0.14 [20/50] vs. 0.5 ± 0.22 [20/66]; P < 0.001) between the 2 groups.
Selected Case Reports
Case 1 (Successful Release of Symptomatic Vitreomacular Traction Without a Macular Hole)
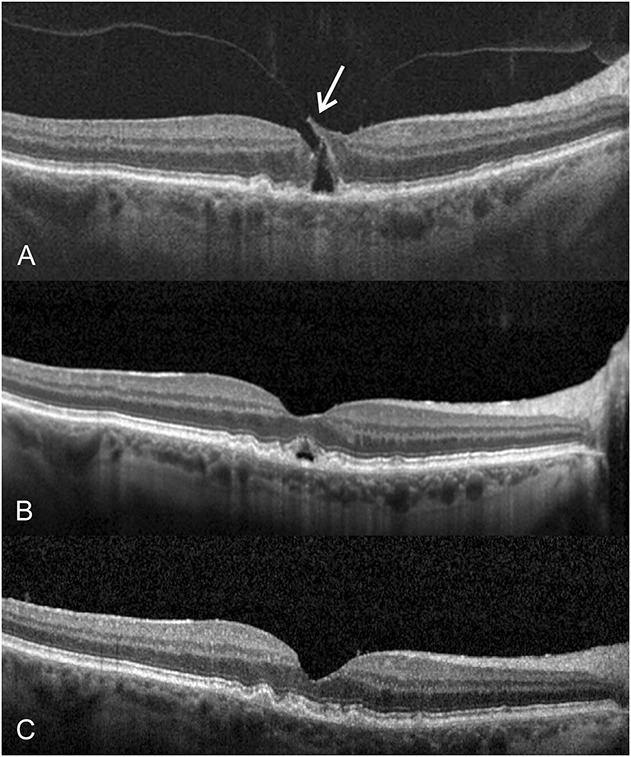
A 72-year-old woman presented in January 2013 with a complaint of metamorphopsia and blurred vision, right eye (Figure 1). Previously in 2004, she responded well to a pars plana vitrectomy for repair of a MH in the left eye. Despite previous successful surgery for her left eye, she reported residual metamorphopsia affecting her left eye. During her examination in January 2013, the BSCVA was 20/40 in the right eye and 20/30 in the left eye. The anterior segment examination showed a clear lens in the right eye and a well-centered and secured posterior chamber intraocular lens in the left eye. Biomicroscopy showed minimal foveal surface irregularities. The SD-OCT showed central VMT measuring slightly more than 1 DA in size in the right eye (Figure 1A). After discussing various management options, she decided to proceed with PVL in the right eye. She received 0.3 mL of 100% filtered C3F8 gas (right eye). At 5 weeks after the intravitreal gas injection, a PVD developed and the VMT was resolved, but there was mild residual perifoveal cystic lesion in the right eye (Figure 1B). There were no complications, including retinal breaks or detachment, in the right eye. At 18 months after treatment, her BSCVA was 20/30 in the right eye and SD-OCT showed clear macular appearance in the right eye (Figure 1C). Amsler grid testing showed normal findings in the right eye.
Fig. 1.
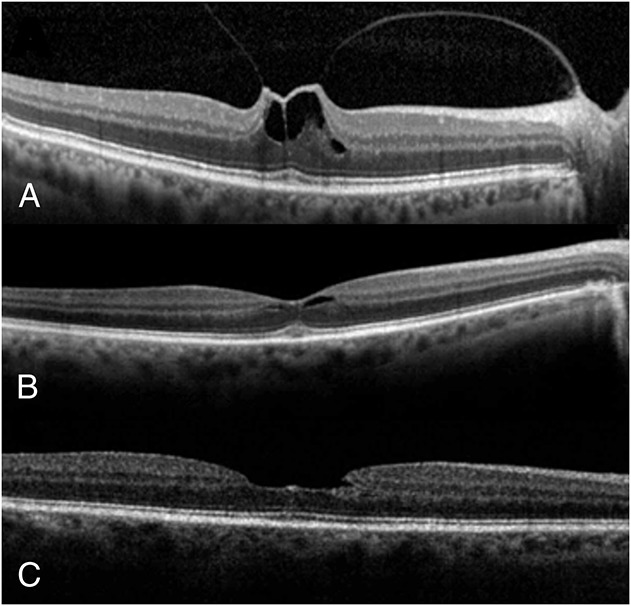
A 72-year-old woman presented with a complaint of blurred vision and metamorphopsia involving her right eye. The BSCVA was 20/40 in the right eye, and there was central VMT of slightly more than 1 DA in size on SD-OCT (A). At 5 weeks after injection of 0.3 mL of C3F8 gas, a PVD developed and the VMT was relieved, but there was mild residual perifoveal cystic lesion in the right eye (B). At 18 months after PVL, there was a normal macular appearance and the BSCVA was 20/30 in the right eye (C).
Case 2 (Successful Release of Vitreomacular Traction and Closure of a Stage 2 Macular Hole)
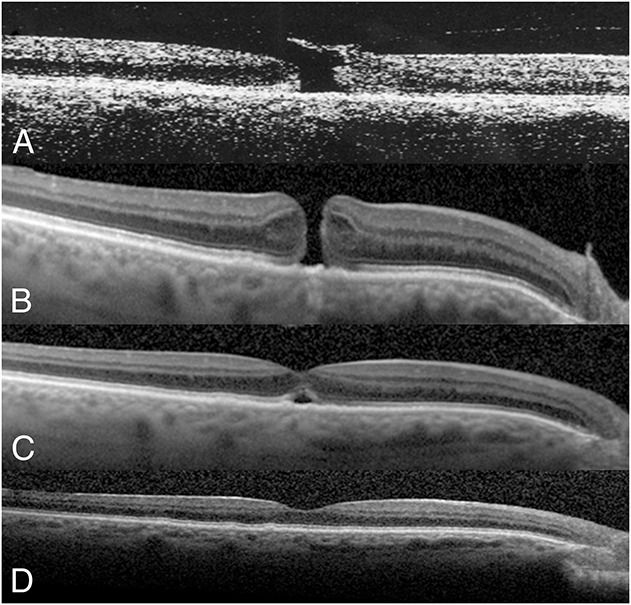
A 71-year-old man without any visual complaints first underwent a retinal examination to rule out age-related macular degeneration on September 23, 2013 (Figure 2). His BSCVA was 20/25 in the right eye and 20/20 in the left eye. The tonometry reading was 20 in each eye. The anterior segment examination showed mild nuclear sclerotic cataracts in both eyes. Biomicroscopy and SD-OCT imaging showed VMA without a macular defect in the right eye (Figure 2A). There were mild macular drusen in both eyes. No retinal treatment was needed at that time. On March 11, 2014, he returned with a new complaint of blurred vision and metamorphopsia in the right eye. His BSCVA was 20/70 in the right eye and 20/20 in the left eye. There were mild nuclear sclerotic cataracts in both eyes. The SD-OCT showed a Stage 2 MH that measured <250 μm in diameter in the right eye (Figure 2A). After discussing various options, he elected PVL for treatment of the Stage 2 MH in the right eye. One week after intravitreal injection of 0.3 mL of C3F8 gas, a PVD developed in the right eye. At the same time, there was closure of the inner portion of the MH but appearance of outer foveal lucency, corresponding to a residual outer foveal cavity in the right eye (Figure 2B). Subsequent follow-up evaluation showed progressive decrease of the outer foveal lucency, consistent with a gradual closure of the outer foveal cyst in the right eye. At 5 months after gas injection, there was complete resolution of the outer foveal lucency in the right eye (Figure 2C). The BSCVA was 20/40 in the right eye.
Fig. 2.
A 71-year-old man initially presented with asymptomatic vitreomacular adhesion without a macular defect in his right eye in September 2013. The BSCVA was 20/25 in the right eye. Six months later, he returned with a complaint of blurred and distorted vision in the right eye. His BSCVA had deteriorated to 20/70 in the right eye, and SD-OCT showed central VMT associated with a narrow Stage 2 MH with a retinal flap (arrow) in the right eye (A). At 1 week after injection of 0.3 mL of C3F8 gas and partial face-down positioning, a PVD developed with a partial resolution of the MH. However, there was focal outer foveal lucency, corresponding to a residual outer foveal defect noted on SD-OCT (B). Subsequent visits showed progressive resolution of the outer foveal defect. The BSCVA was improved to 20/40, corresponding to a complete closure of the MH, in the right eye at 5 months after gas injection (C).
Case 3 (“Double Bubbles” for Initially Failed Stage 2 Macular Hole)
A 72-year-old woman presented with VMT associated with a small Stage 2 MH in the right eye on April 30, 2015 (Figure 3). Visual acuity was 20/50 in the right eye (Figure 3A). After a discussion of the therapeutic options, she elected PVL for treating the VMT in the right eye. Within 8 days after receiving 0.3 mL of C3F8 gas, VMT was relieved and there was narrowing of the MH in the right eye. However, the MH did not close (Figure 3B), and she was offered the opportunity to undergo a pars plana vitrectomy. However, she requested injection of a second C3F8 gas bubble instead, which was performed without complications at 10 days later. On Day 4 after the second gas injection with face-down positioning, there was closure of the inner retinal layers of the macular defect in the right eye (Figure 3C). Over the subsequent weeks, there was progressive closure of the outer foveal defect as well. At 6 months after surgery, there was complete closure of MH with visual acuity of 20/30 in the right eye (Figure 3D).
Fig. 3.
A 72-year-old woman presented with VMT associated with a small Stage 2 MH in the right eye (A). The BSCVA was 20/50 in the right eye. After electing PVL for treatment of the VMT, she received 0.3 mL of C3F8 gas in the right eye. Despite relief of VMT and partial narrowing of the foveal defect within 8 days after PVL, the MH did not close (B). She declined a vitrectomy and decided on receiving a second C3F8 gas bubble instead, which was performed for right eye without complications at 10 days later. She maintained face-down positioning and there was closure of inner layers of MH at 4 days after injection of the second gas bubble in the right eye (C). There was further closure of the outer foveal defect in subsequent weeks. At 6 months after surgery, there was complete closure of MH with BSCVA of 20/30 in the right eye (D).
Case 4 (Successful Macular Hole Closure With Pneumatic Vitreolysis After Progression of Vitreomacular Traction–Only to a Stage 2 Macular Hole After Ocriplasmin)
A 67-year-old man presented with a complaint of progressive central visual deficit in the left eye in late August 2013 (Figure 4). The ocular examination showed a BSCVA of 20/25 in the right eye and 20/70 in the left eye. There were mild cortical and nuclear sclerotic cataracts in both eyes. Posterior examination showed VMA in the right eye and a yellowish foveal spot corresponding to central VMT associated with a partial split of the foveal layers, consistent with an impending MH, in the left eye (Figure 4A). He complained of visual defect affecting his left eye, which interfered with his daily visual tasks. After revealing the therapeutic options, he elected treatment with intravitreal ocriplasmin injection, which was performed a week later in September 2013. However, he complained of marked vision loss within 24 hours after ocriplasmin injection. An urgent examination at that time showed that the BSCVA of left eye had decreased to 20/100 because of the formation of a Stage 2 full-thickness MH associated with a narrow apex and a wider base. There was residual VMT in the left eye (Figure 4B). After discussing various options, he elected PVL. Injection of 0.3 mL of C3F8 gas was followed by face-down positioning. Examination performed 4 days later showed a VMT release and closure of the inner and middle layers of the MH with a triangular-shaped outer foveal lucency, consistent with a residual outer foveal cyst (Figure 4C). A reduction in the outer foveal lucency was noted 4 weeks later (Figure 4D) and its resolution corresponding to complete MH closure was noted 6 weeks later (Figure 4E). The BSCVA was recovered to 20/30 in the left eye.
Fig. 4.
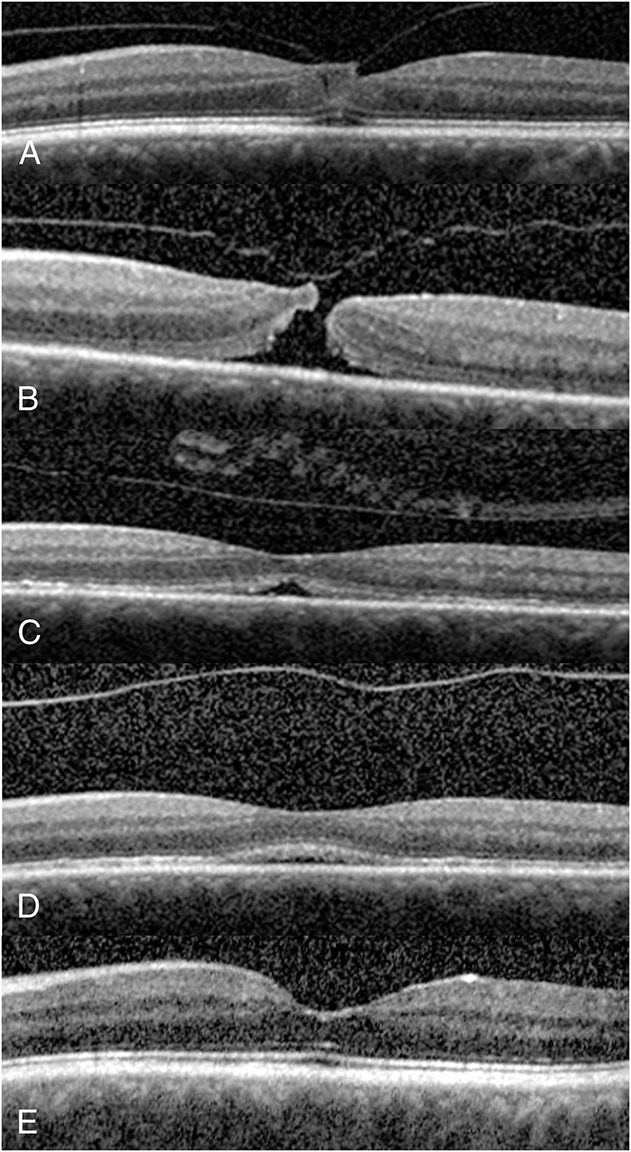
A 67-year-old man complained of progressive central visual deficit of his left eye in August 2013. His BSCVA was 20/70 in the left eye. The SD-OCT showed central vitreomacular adhesion only without symptoms in his right eye, but symptomatic central VMT associated with a partial split of the foveal layers, consistent with a Stage 1 impending MH in the left eye (A). He elected to undergo ocriplasmin injection. Within 24 hours after ocriplasmin injection, he reported further visual loss and an urgent examination showed residual VMT and the progressing of the Stage 1 impending MH to a Stage 2 full-thickness MH with residual VMT in the right eye (B). The BSCVA was deteriorated to 20/100 in the right eye. He then elected to undergo PVL. At 4 days after receiving 0.3 mL of C3F8 gas injection and face-down positioning, a PVD with closure of the inner layers of the MH developed (C). There was a partial lucency of the outer fovea, corresponding to the residual outer foveal defect, in the right eye. At 4 weeks after PVL, there was a decrease in the outer foveal defect (D). At 6 weeks after PVL, there was a complete closure of the MH with BSCVA recovery to 20/30 in the left eye (E).
Discussion
After initial description of the technique of PVL in 1995 by Chan et al,21 Costa et al22 and Jorge et al23 reported induction of a PVD (100%) and closure of Stage 2 MHs (83%) with C3F8 gas in a small case series. In addition, Mori et al24 reported induction of a PVD and closure of Stage 2 MHs after injection of pure sulfur hexafluoride (SF6) gas followed by 3 days to 5 days of face-down positioning. In their study, a PVD was achieved in 95% of their study eyes, and successful hole closure was correlated with smaller MHs (<200 μm). In 2013, Rodrigues et al25 reported an initial 40% success rate in release of VMT at 1 month after injection of 100% C3F8 gas in a series of 15 eyes.25,26 In their study, there was release of VMT in another 20% of the study eyes by 6 months. In a case series and meta-analysis of the literature, Arroyo reported in 2015 a success rate ranging from 73% to 87.5% in VMT release using either C3F8 or SF6 gas (J. Arroyo, unpublished data, Retina Society, Paris, France, October 10, 2015). Recently, Steinle et al reported an overall success rate of 83% utilizing C3F8 gas to induce VMT release.27 In a separate assessment, they reported 84% with C3F8 gas, 56% with SF6 gas, and 48% with ocriplasmin in release of VMT in a comparative retrospective case series for treatment of VMT syndrome (N Steinle et al, unpublished data, ARVO 2016, Seattle, May 2, 2016). In 2016, Day et al28 published a success rate of 55.6% using SF6 gas in releasing VMT in a retrospective case series of 9 eyes. Thus, most reports, including our study, show higher success rates associated with C3F8 gas in comparison with SF6 gas for releasing VMT.
Our current study shows that PVL provides a viable alternative to ocriplasmin and vitrectomy for treating selected cases of symptomatic VMT, with an overall success rate of 86% in the release of VMT. The published success rate for ocriplasmin in resolving symptomatic VMT was 26.5% overall in the MIVI-TRUST Trial. The ocriplasmin success rate was increased to 40% for eyes with VMT but without epiretinal cellophane maculopathy, and increased to 60% for eyes with VMT and a MH of <200 μm in diameter.15 The 2-year results of the Ocriplasmin for Treatment for Symptomatic Vitreomacular Adhesion Including Macular Hole (OASIS) Trial reported success of VMT release of 41.7% in the ocriplasmin group and 6.2% in the placebo group.29 In an updated assessment of ocriplasmin performed in recent years, Lim et al reported in the Macula Society Collaborative Retrospective Study a rate of 45% in release of VMT and a rate of 40% for MH closure after ocriplasmin for eyes with VMT (J. Lim et al, unpublished data, ARVO 2016, Seattle, WA, May 2, 2016). In contrast, the published success rate of PVL in resolving VMT has ranged from 56% to >95% in the literature.21–28 Our study shows successful release of VMT in 43 of 50 eyes (86%). For the subset of eyes with a Stage 2 MH, VMT release was achieved in all (100%), and closure of the MH occurred in 10 of 15 eyes (66.7%) after PVL (53.3%, after excluding 2 cases with repeat gas injections). These results are consistent with the favorable outcomes of previous and recent reports associated with C3F8 gas injection for treatment of VMT. Our study also shows few adverse events associated with PVL. There was progression from VMT-only to a Stage 2 MH in one eye and the development of a rhegmatogenous retinal detachment in another VMT-only eye after PVL despite VMT release. However, both eyes responded well to a subsequent pars plana vitrectomy with good visual recovery. For the majority of the study eyes, anatomical and visual successes were achieved with this low-cost procedure in the absence of complications while circumventing the higher expenses and inherent risks of a vitrectomy.
The precise mechanism for the induction of a PVD by intravitreal gas injection is unknown. The potential etiology may include a liquefactant, an interfactant,1,2,30,31 or a combined mechanism of action. We hypothesize that the initial expansion and the subsequent contraction of the intravitreal gas bubble destabilizes the vitreous gel. More precisely, the shifting intravitreal gas bubble enhances liquefaction of the vitreous humor, which has been shown to be a crucial feature in the development of a PVD.21,29 The initial expansion of the intravitreal gas bubble may create a new or enlarge an existing vitreous pocket by stretching the vitreous fibers surrounding the pocket.21,31,32 Syneresis progresses when the liquefied vitreous enters the enlarged vitreous pocket during the resolution phase of the gas bubble, allowing more room for the liquefied vitreous to accumulate in the enlarged vitreous pocket.21 Subsequent eye movements promote the formation of a break on the thinnest portion of the posterior vitreous cortex (anterior to the fovea) surrounded with bulkier mobile vitreous cortex.21,32–35 Finally, the migration of liquefied vitreous into the subhyaloid space through the break in the posterior cortical vitreous leads to a PVD.21 We hypothesize that the long-acting gas bubble acts as a cushion to support the collapsing vitreous, allowing a more gentle process of PVD. This theory provides a plausible explanation for the low frequency of retinal breaks and detachment after PVL in this study and also in previous published reports.21–28 Given that C3F8 gas is associated with a higher success rate for inducing a PVD when compared with SF6 gas, this finding suggests that the duration more than the size of a gas bubble is the key feature in promoting vitreous liquefaction and the subsequent VMT release. Regarding MH, however, it is logical to deduce that a larger size gas bubble provides a better tamponade and greater chance for its closure. After a prophylactic paracentesis to remove aqueous, we typically inject 0.3 mL of C3F8 gas to promote VMT release and enhance MH closure, if applicable.
Regarding the timing of VMT release, the median of 3 weeks and the range from 4 days to 9 weeks in the development of a PVD in our study are consistent with previous reports in the literature.21–25 In addition, although our study shows that the majority of the treated eyes developed a PVD within 4 weeks after intravitreal C3F8 gas injection, a PVD did not develop for 16% of the eyes until 5 weeks to 9 weeks after gas injection. Therefore, one must refrain from prematurely judging failure of PVL and switching to alternative treatment (e.g., a vitrectomy), unless progression of VMT to a Stage 2 MH requires a prompt vitrectomy.
Another important point shown by this study is that all the eyes with a successful release of VMT did not have broad VMA (>2 DA) at baseline. This finding is consistent with the report by Rodrigues et al,25 who found broad VMA and the presence of thick epiretinal fibrosis to be poor prognostic factors for the relief of VMT after intravitreal gas injection. Previous published reports on ocriplasmin also showed a lower success rate of VMT release in the presence of cellophane maculopathy.15,36 Regarding the seven eyes that either failed to develop a PVD or developed only a partial PVD in our series, two had diabetes mellitus, one had thick cellophane maculopathy, and one had both diabetes mellitus and thick cellophane maculopathy. It is possible that eyes with one or both of the above conditions are frequently associated with broader and stronger VMA, which may account for a less robust response to PVL for those eyes.
Univariate analyses of baseline factors associated with success of VMT release showed that younger age (mean age of 69 years), better baseline BSCVA (mean of 20/50), VMT extent of within 1 DA, lack of diabetes mellitus, and Stage 2 MH eyes compared with VMT-only eyes to be correlated with success of VMT release. There was also a trend toward a higher rate of VMT release in eyes with a lack of baseline cellophane maculopathy. Further analysis with stepwise logistic regression showed that younger age, followed by better baseline BSCVA, lack of diabetes mellitus, and female gender to be predictors of success for VMT release. Previous studies on the effect of ocriplasmin treatment have also reported that younger age (age of <65 years), lack of cellophane maculopathy, VMT within 1 DA, and Stage 2 MH are strong predictors of success in VMT release.15,36 In our report, the few eyes with VMT extent >1 DA and the few eyes with cellophane maculopathy at baseline could be the reason for not finding a significant association between success on VMT release and these variables when using logistic regression, despite the suggestion of such relationship when using chi-square test. The small sample size may also be the reason for lack of statistical significance in favor of phakic eyes compared with pseudophakic eyes regarding success of VMT release, despite a larger percentage of phakic eyes (88.4%) compared with pseudophakic eyes (71.4%) achieving VMT release in our study, consistent with the report of phakia as a predictor of success by previous studies associated with ocriplasmin treatment.15,36 Regarding female gender as a predictor for VMT release, Palacio et al found in their study quantifying the changes in VMA in healthy human eyes that females have a significantly smaller area of VMA compared to males from the 5th through the 8th decades of life.37 A prior study on ocriplasmin also reported a correlation of female gender with increased success of VMT release.38,39 To the best of our knowledge, the correlation of better baseline BSCVA with greater success of VMT release and the correlation of diabetes mellitus with lower success of VMT release have not been reported previously. Such relationships need validation with further studies.
Regarding the subset of eyes with MHs in our study, it is important to note that we included only eyes with limited Stage 2 MH (within 300 μm in diameter) because a previous study on PVL had shown a poor outcome associated with large MH and MH beyond Stage 2 MH.21,24 In this series, all eyes with successful MH closure had a baseline MH diameter of within 250 μm. Three of the 5 Stage 2 MH that failed to close had a baseline diameter of >250 μm. The successful closure of small MH with PVL is consistent with previous reports.21,24,25 Previous published studies on ocriplasmin14,15,36 also showed an increased success rate for resolving MH of <200 μm in diameter.
In our study, the success rate of MH closure was reduced from 67% to 53% when excluding 2 eyes that required >1 gas injection for closure of the MH. However, the need for repeat gas injections for these eyes in no way detracts from the utility of PVL; in contrast, it actually highlights a valuable asset of PVL, namely, that injection of multiple gas bubbles is a viable option for PVL and repeat gas injection may enhance its success rate. Proper case selection is the key to success for repeat gas injection to induce VMT release. For example, an eye with a narrow focal VMA that fails to respond to initial PVL may respond favorably to a second gas bubble because predictor analysis shows that VMA of within 1 DA in size correlates with a high rate of success for VMT release. Also, although predictor analysis suggested that presence of thick cellophane maculopathy may lower the success rate of PVL, our study shows that VMT release was nevertheless achieved in 50% of eyes with a thick cellophane membrane. Thus, repeat gas injection may be considered for such an eye that fails initial PVL, particularly in the presence of a narrow VMA. For eyes with broad and sticky VMA, it is likely not worthwhile to initiate or repeat PVL.
Regarding an eye with a Stage 2 MH that fails initial PVL, repeat gas injection may be considered in the presence of a narrow Stage 2 MH (within 250 μm), particularly if the patient is highly motivated to maintain face-down positioning after repeat gas injection or if a retinal flap is associated with a Stage 2 MH (similar to Case Report 2). Lim et al have reported enhanced success of MH closure with ocriplasmin for eyes with a retinal flap associated with a narrow MH (J. Lim et al, unpublished data, ARVO 2016, Seattle, WA, May 2, 2016).
It is also interesting to note that besides its potential as a primary procedure for resolving VMT, PVL may also serve as a salvage procedure, as shown by Case Report 4 in our study. Pneumatic vitreolysis was successful in induction of a PVD in two eyes in our series with previous failed response to ocriplasmin. One of these 2 eyes with VMT only at baseline developed a Stage 2 full-thickness MH with persistent VMT within 24 hours after intravitreal ocriplasmin (Case Report 4). Subsequent intravitreal gas injection with face-down positioning led to development of a PVD and closure of the MH.
There were a few adverse events associated with PVL in this study. One eye with VMT only failed to develop a PVD and progressed into a Stage 2 MH instead. Subsequent pars plana vitrectomy led to closure of the MH and BSCVA of 20/30. Another eye with VMT failed initial treatment with ocriplasmin. Subsequent PVL led to VMT release but also development of a retinal detachment. A pars plana vitrectomy was successful in repairing the retinal detachment with BSCVA of 20/70 at 3 months later. There was a lack of persistent uveitis, endophthalmitis, excessive intraocular pressure rise, cataract progression, lenticular dislocation, zonular dehiscence, and systemic complications in this study. With the exception of temporary outer foveal lucency in certain eyes, there was a lack of abnormalities noted on SD-OCT after PVL, that is, disruption of the external limiting membrane and ellipsoid zone, or photoreceptor dehiscence. The lack of substantial SD-OCT defects associated with PVL needs to be validated with a further study using a larger sample size.
There are several limitations associated with this study. The retrospective nature of this study could have introduced confounding factors to the results. The visual acuity measurements were not standardized, although there was a highly significant improvement in the posttreatment visual acuity when compared with baseline. The moderate sample size is another limitation, although there are more eyes included in this report than most of the previous published reports associated with PVL. Also, the lack of concurrent controls may raise the question of the precise success rate of PVL in resolving VMT and closure of small MHs. However, only 10% of control eyes receiving placebo saline injections achieved a PVD in the combined cohorts of the MIVI-TRUST Trial,15 and no more than 30% to 40% of eyes developed a spontaneous PVD in 2 recent reports on the natural history of VMT without therapeutic intervention.12,13 In addition, the sequential development of a PVD in close proximity to an intravitreal gas injection for the majority of the treated eyes in this series is highly suggestive of a causal relationship between PVL and VMT release. Although this study shows that the presence of diabetes mellitus and thick cellophane maculopathy may reduce the success rates of PVL, the limited sample size associated with both conditions precludes a definitive conclusion. Finally, although certain information deduced from our retrospective study may be useful in determining proper case selection for repeat PVL for initially failed cases, further prospective studies are needed to validate these findings.
In conclusion, this study shows that PVL with C3F8 gas is highly capable of releasing focal VMT in select eyes with significant improvement of visual acuity and few adverse events. Posterior vitreous detachment was achieved in 86% of the treated eyes. Closure of small Stage 2 MH was achieved in 64% of treated eyes. For eyes with failed MH closure after PVL, subsequent vitrectomy was successful in closing all MH with good visual outcome. A prospective randomized study with concurrent controls is needed to ascertain the indication, utility, success rate, and safety of PVL.
Footnotes
Presented in part during Annual Meeting of Association of the Macula Society, Miami, FL, February 26, 2016; Annual Meeting of the American Ophthalmological Society, Colorado Springs, CO, May 22, 2016; 30th meeting of the Club Jules Gonin, Bordeaux, France, July 8, 2016; 34th Annual Meeting of the American Society of Retina Specialists, San Francisco, CA, August 13, 2016; 49th Scientific Meeting of the Retina Society, San Diego, CA, September 13, 2016; and Retina Subspecialty Day and Annual Meeting of the American Academy of Ophthalmology, Chicago, IL, October 15 to 16, 2016.
None of the authors have any financial/conflicting interests to disclose.
Design of the study (C.K.C., C.E.M.); Conduct of the study (C.K.C., C.E.M.); Collection, management, analysis (C.K.C., J.N.C., C.E.M., N.D.); Interpretation of the data (C.K.C., J.N.C., C.E.M., N.D.); Preparation, review, and approval of the manuscript (C.K.C., J.N.C., C.E.M., N.D.).
References
- 1. Sebag J. Is pharmacologic vitreolysis brewing? Retina 2002;22:1–3. [DOI] [PubMed] [Google Scholar]
- 2. Sebag J. Pharmacologic vitreolysis, premise and promise of the first decade. Retina 2009;29:871–874. [DOI] [PubMed] [Google Scholar]
- 3. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013;120:2611–2619. [DOI] [PubMed] [Google Scholar]
- 4. Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol 1995;119:55–61. [DOI] [PubMed] [Google Scholar]
- 5. Sulkes DJ, Ip MS, Baumal CR, et al. Spontaneous resolution of vitreomacular traction documented by optical coherence tomography. Arch Ophthalmol 2000;118:286–287. [PubMed] [Google Scholar]
- 6. Lecleire-Collet A, Muraine M, Siahmed K, Brasseur G. Spontaneous resolution of vitreomacular traction associated with diabetic macular edema. Eur J Ophthalmol 2004;14:430–433. [DOI] [PubMed] [Google Scholar]
- 7. Odrobina D, Michalewska Z, Michalewski J, et al. Long-term evaluation of vitreomacular traction disorder in spectral-domain optical coherence tomography. Retina 2011;31:324–331. [DOI] [PubMed] [Google Scholar]
- 8. Hwang DJ, Park KH, Woo SJ. Spontaneous resolution of vitreomacular traction syndrome with persistent vitreofoveal adhesion observed on spectral-domain optical coherence tomography. Can J Ophthalmol 2012;47:e17–e19. [DOI] [PubMed] [Google Scholar]
- 9. Almeida DRP, Chin EK. Spontaneous resolution of vitreomacular traction in two patients with diabetic macular edema. Case Rep Ophthalmol 2014;5:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theodossiadis GP, Grigoropoulos VG, Theodoropoulou S, et al. Spontaneous resolution of vitreomacular traction demonstrated by spectral-domain optical coherence tomography. Am J Ophthalmol 2014;157:842–851. [DOI] [PubMed] [Google Scholar]
- 11. Dimopoulos S, Bartz-Schmidt KU, Gelisken F, et al. Rate and timing of spontaneous resolution in a vitreomacular traction group: should the role of watchful waiting be re-evaluated as an alternative to Ocriplasmin therapy. Br J Ophthalmol 2015;99:350–353. [DOI] [PubMed] [Google Scholar]
- 12. John VJ, Flynn HW, Smiddy WE, et al. Clinical course of vitreomacular adhesion managed by initial observation. Retina 2014;34:442–446. [DOI] [PubMed] [Google Scholar]
- 13. Tzu JH, John VH, Flynn HW, et al. Clinical course of vitreomacular traction managed initially by observation. Ophthalmic Surg Lasers Imaging Retina 2015;46:571–576. [DOI] [PubMed] [Google Scholar]
- 14. Stefanini FR, Mauricio M, Falabella P, et al. Profile of ocriplasmin and its potential in the treatment of vitreomacular adhesion. Clin Ophthalmol 2014;8:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med 2012;367:606–615. [DOI] [PubMed] [Google Scholar]
- 16. Freund KB, Shah SA, Shah VP. Correlation of transient vision loss with outer retinal disruption following intravitreal ocriplasmin. Eye (Lond) 2013;27:773–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fahim AT, Khan NW, Johnson MW. Acute panretinal structure and functional abnormalities after intravitreous ocriplasmin injection. JAMA Ophthalmol 2014;132:484–486. [DOI] [PubMed] [Google Scholar]
- 18. Tibbetts MD, Reichel E, Witkin AJ. Vision loss after intravitreal ocriplasmin: correlation of spectral-domain optical coherence tomography and electroretinography. JAMA Ophthalmol 2014;132:487–490. [DOI] [PubMed] [Google Scholar]
- 19. Kim JE. Safety and complications of ocriplasmin. Ocriplasmin, ocriplasmin, oh, how safe art thou? JAMA Ophthalmol 2014;132:379–380. [DOI] [PubMed] [Google Scholar]
- 20. Hager A, Seibel I, Riechardt A, et al. Does ocriplasmin affect the RPE-photoreceptor adhesion in macular holes? Br J Ophthalmol 2015;99:635–638. [DOI] [PubMed] [Google Scholar]
- 21. Chan CK, Wessels IF, Friedrichsen EJ. Treatment of idiopathic macular holes by induced posterior vitreous detachment. Ophthalmology 1995;102:757–767. [DOI] [PubMed] [Google Scholar]
- 22. Costa RA, Cardillo JA, Morales PH, et al. Optical coherence tomography evaluation of idiopathic macula hole treatment by gas-assisted posterior vitreous detachment. Am J Ophthalmol 2001;132:264–266. [DOI] [PubMed] [Google Scholar]
- 23. Jorge R, Costa RA, Cardillo JA, et al. Optical coherence tomography evaluation of idiopathic macula hole treatment by gas-assisted posterior vitreous detachment. Am J Ophthalmol 2006;142:869–871. [DOI] [PubMed] [Google Scholar]
- 24. Mori K, Saito S, Gehlbach PL, Yoneya S. Treatment of stage 2 macula hole by intravitreal injection of expansile gas and induction of posterior vitreous detachment. Ophthalmology 2007;114:127–133. [DOI] [PubMed] [Google Scholar]
- 25. Rodrigues IA, Stangos AN, McHugh DA, Jackson TL. Intravitreal injection of expansile perfluoropropane (C3F8) for the treatment of vitreomacular traction. Am J Ophthalmol 2013;155:270–276. [DOI] [PubMed] [Google Scholar]
- 26. Johnson MW. How should we release vitreomacular traction: surgically, pharmacologically, or pneumatically? Am J Ophthalmol 2013;155:203–205. [DOI] [PubMed] [Google Scholar]
- 27. Steinle NC, Dhoot DS, Quezada RC, et al. Treatment of vitreomacular traction with intravitreal perfluoropropane (C3F8) injection. Retina 2016. Sep 27. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28. Day S, Martinez JA, Nixon PA, et al. Intravitreal sulfur hexafluoride injection for the treatment of vitreomacular traction syndrome. Retina 2016;36:733–737. [DOI] [PubMed] [Google Scholar]
- 29. Dugel PU, Tolentino M, Feiner L, et al. Results of the 2-year Ocriplasmin for Treatment for Symptomatic Vitreomacular Adhesion Including Macular Hole (OASIS) randomized trial. Ophthalmology 2015;123:2232–2247. [DOI] [PubMed] [Google Scholar]
- 30. Sebag J. Pharmacologic vitreolysis (Guest Editorial). Retina 1998;18:1–3. [DOI] [PubMed] [Google Scholar]
- 31. Sebag J. Molecular biology of pharmacologic vitreolysis. Trans Am Ophthalmol Soc 2005;103:473–494. [PMC free article] [PubMed] [Google Scholar]
- 32. Foos RY, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology 1982;89:1502–1512. [DOI] [PubMed] [Google Scholar]
- 33. Thresher RJ, Ehrenberg M, Machemer R. Gas-mediated vitreous compression: an experimental alternative to mechanized vitrectomy. Graefes Arch Clin Exp Ophthalmol 1984;221:192–198. [DOI] [PubMed] [Google Scholar]
- 34. Miller J, Lean JS, Miller H, Ryan SJ. Intravitreal expanding gas bubble. A morphologic study in the rabbit eye. Arch Ophthalmol 1984;102:1708–1711. [DOI] [PubMed] [Google Scholar]
- 35. Eisner G. Biomicroscopy of the Peripheral Fundus; An Atlas and Textbook. Berlin, Germany: Springer-Verlag; 1973:106–107. [Google Scholar]
- 36. Haller JA, Stalmans P, Benz MS, et al. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology 2015;122:117–122. [DOI] [PubMed] [Google Scholar]
- 37. Palacio AC, Gupta A, Nesmith BL, et al. Vitreomacular adhesion evolution with age in healthy human eyes. Retina 2016. Jun 15. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38. Chatziralli I, Theodossiadis G, Parikakis E, et al. Real-life experience after intravitreal ocriplasmin for vitreomacular traction and macular hole: a spectral-domain optical coherence tomography prospective study. Graefes Arch Clin Exp Ophthalmol 2016;254:223–233. [DOI] [PubMed] [Google Scholar]
- 39. Prospero Ponce CM, Stevenson W, Gelman R, et al. Ocriplasmin: Who is the best candidate? Clin Ophthalmol 2016;10:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]


