Abstract
Buprenorphine maintenance therapy patients frequently have severe postoperative pain due to buprenorphine-induced hyperalgesia and provider use of opioids with limited efficacy in the presence of buprenorphine. The authors report good-to-excellent pain management in 4 obstetric patients using nonopioid analgesics, regional anesthesia, continuation of buprenorphine, and use of opioids with high μ receptor affinity.
The population of patients receiving chronic buprenorphine maintenance therapy (BMT) is large and includes many pregnant women. Most BMT patients are maintained on 1 of 2 sublingual preparations: Suboxone (Indivior Inc, Richmond, VA), a combination of buprenorphine and naloxone, or Subutex, which is solely buprenorphine. Pregnant illicit opioid users with poor access to BMT clinics may also use illicit buprenorphine to manage their own opiate withdrawals. Buprenorphine, an opioid with a long serum half-life and active metabolites, binds tightly to the μ-opioid receptor, and there is a ceiling to the analgesia buprenorphine can provide.1–7 Consequently, parturients who present for cesarean delivery or tubal ligation while receiving BMT may have severe postoperative pain.6,7
Multimodal, rather than opioid-only, analgesia is generally preferable. There are 4 options for preoperative buprenorphine management: (1) continue buprenorphine and add additional postoperative buprenorphine,8 (2) continue baseline buprenorphine and add traditional opioids with high μ receptor affinity,9 (3) reduce the baseline buprenorphine preoperatively,10 or (4) discontinue buprenorphine and start a traditional opioid preoperatively.11–13 This last approach is not practical for many obstetric BMT surgical patients. First, buprenorphine has a half-life of 24–60 hours, so patients presenting for urgent or emergent surgery do not have time preoperatively to achieve complete washout. Second, patients fearing a relapse of their opiate addiction may refuse to abstain from buprenorphine. Third, many obstetricians refuse to ask their pregnant patients to abstain from buprenorphine because they fear unmonitored and untreatable fetal withdrawal symptoms. A multimodal approach to BMT patients was recently described by Anderson et al.14 We utilized a similar multimodal approach in the 4 obstetric cases described.
Each patient gave written permission for the authors to publish this case series.
CASE DESCRIPTIONS
A 22-year-old Gravida 2, Para 1 healthy parturient presented for an elective repeat cesarean delivery. Her only medication was buprenorphine/naloxone (Suboxone) 8 mg/2 mg twice daily. The patient was placed in a seated position, and we inserted a paramedian epidural catheter at T10–11, then inserted a 25-gauge Whitacre spinal needle at L2–3, and injected hyperbaric bupivacaine 15 mg and epinephrine 0.1 mg. The anesthetic level was T4 bilaterally, and the patient was comfortable during the cesarean delivery. Postoperatively, we continued Suboxone 8 mg/2 mg twice daily. We initiated a patient-controlled epidural infusion of bupivacaine 0.0625% at 4 mL/h with a bolus dose of 2 mL and a lockout interval of 30 minutes. The patient also received scheduled ketorolac 30 mg intravenously (IV) every 6 hours for 4 doses, then scheduled ibuprofen 800 mg per os every 8 hours. The patient was able to maintain 5-second leg lifts 5 hours postoperatively and was able to move to a chair with assistance 8 hours postoperatively. One day after surgery, the patient could ambulate without assistance. She received an average of bupivacaine 5 mL/h during the first 24 hours and an average of 4 mL/h during the second 24 hours after the cesarean delivery. Her maximum pain scores were 1/10 during the first 24 hours and 0/10 during the second 24 hours after cesarean delivery. At 48 hours, we stopped the epidural infusion and observed the patient for 2 hours. Her pain score remained 0/10, so we removed the epidural catheter and continued the ibuprofen and the buprenorphine/naloxone (Suboxone).
A 23-year-old Gravida 2, Para 1 healthy parturient presented in active labor for urgent repeat cesarean delivery. The patient was on a waiting list for a BMT clinic. While waiting, she illicitly purchased and consumed buprenorphine 4 mg 3 times daily. In the seated position, we performed a combined spinal–epidural anesthetic at L2–3. We injected bupivacaine 15 mg intrathecally and placed a lumbar epidural catheter for postoperative pain relief. The anesthetic level was T3 bilaterally, and the patient was comfortable during the cesarean delivery. We initiated a patient-controlled epidural infusion of bupivacaine 0.0625% at 10 mL/h with a bolus dose of 2 mL and a lockout interval of 15 minutes. We initiated buprenorphine 4 mg every 8 hours. The patient also received scheduled ketorolac 30 mg IV every 6 hours for 4 doses, and then scheduled ibuprofen 800 mg per os every 8 hours. The patient was comfortable with pain scores of 3 to 4/10 for the first 24 hours. Then a mechanical pump problem stopped the infusion and the patient’s pain score increased to 10/10. Comfort and a pain score of 2/10 returned with an epidural bolus of 10 mL bupivacaine 0.25%. We continued the epidural infusion for a second day. We then discontinued the epidural infusion. The patient’s pain remained mild 2 hours later, when we removed the epidural catheter. The main adverse effect with this lumbar epidural analgesia was leg weakness. While the epidural was infusing, the patient required the assistance of 2 nurses to ambulate. The patient was discharged on ibuprofen.
A 34-year-old Gravida 6, Para 5 parturient presented in early labor for repeat cesarean delivery. The patient denied substance abuse. We induced spinal anesthesia with bupivacaine 13.5 mg, fentanyl 25 µg, and morphine 0.1 mg. Intraoperatively, the urine drug screen returned positive for buprenorphine. The patient then admitted to buying illicit buprenorphine and consuming 4 mg every other day. The patient declined our offer to place a thoracic epidural for postoperative pain control. Three hours after surgery, the patient rated her pain as intolerable and reported her pain score as 10/10. We started a hydromorphone IV patient-controlled analgesia (PCA) infusion with a dose of 0.1 mg, lockout of 10 minutes, and maximum hourly dose of 0.6 mg. She received 1.9 mg of hydromorphone over the first 24 hours. The patient also received scheduled ketorolac 30 mg IV every 6 hours for 4 doses, and then scheduled ibuprofen 800 mg per os every 8 hours. After the start of the PCA hydromorphone, the patient’s highest pain score was 5/10. The hydromorphone PCA was discontinued on the second postoperative day, with no increase in pain scores. The patient was discharged on ibuprofen.
A healthy 25-year-old Gravida 2, Para 2 parturient presented 14 hours postpartum for bilateral tubal ligation. Her only medication was buprenorphine 4 mg/day, which she purchased illicitly while waiting for an appointment at a BMT clinic. The patient was seated during intrathecal injection of bupivacaine 15 mg and sufentanil 10 µg. We obtained a T8 sensory level, and the patient was comfortable during the operation. Three hours postoperatively, the patient rated her pain as 4 to 5/10. She received ketorolac 30 mg IV, and her pain decreased to 1 to 2/10. We continued the scheduled ketorolac 30 mg IV every 6 hours and administered buprenorphine 4 mg/day while the patient was in the hospital. The patient’s pain score was 2/10 or lower for the next 24 hours, when she was discharged on acetaminophen and diclofenac.
DISCUSSION
Patients receiving buprenorphine generally have higher opioid use and worse pain control after cesarean delivery than opioid-naive patients.6,7 Published surgical case reports document high postoperative opioid requirements and poor pain control whether or not buprenorphine is held preoperatively (Table 1).7,9,11–13,15–19 Our cesarean delivery patients have had the best analgesia with the least leg weakness when we utilized postoperative thoracic epidural analgesia, as illustrated in case 1.
Table 1.
Case Reports Describing Postoperative Analgesia in Patients Receiving Buprenorphine
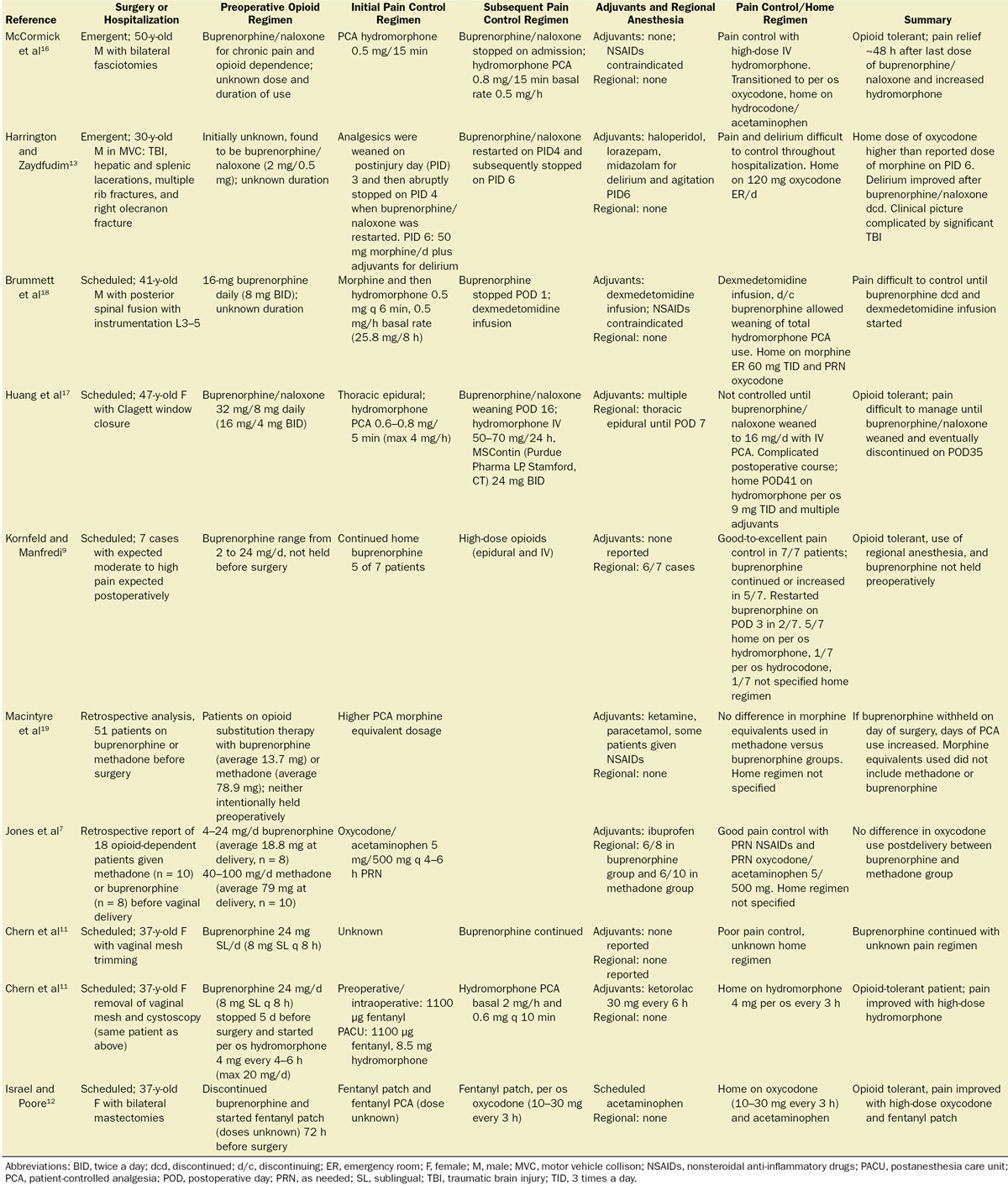
Buprenorphine is an agonist at both the μ-opioid (Ki= 0.2157 nM) and the nociceptin (opioid receptor-like 1) (Ki= 285 nM) receptors, and an antagonist at the κ-opioid and δ-opioid receptors.2,20 Buprenorphine binds more tightly to the μ-opioid receptor (ie, has a lower Ki or equilibrium inhibition constant) than most commonly used μ-opioid agonists (Table 2).3 Opioids with low Ki values have greater binding affinity at the μ receptor but not necessarily greater potency than other opioids. We believe that better analgesia in BMT patients can be achieved by using opioids with Ki values close to the Ki of buprenorphine (such as sufentanil or hydromorphone) rather than opioids that bind more weakly to the μ-opioid receptor (Table 2). High-dose hydromorphone was used successfully in several published case reports.11,16 However, other authors have reported pain control ranging from poor-to-excellent with the use of hydromorphone, fentanyl, or morphine (Table 1). Some authors report poor pain control until discontinuation of the buprenorphine.17,18 Factors other than opioid-binding properties, such as the preoperative buprenorphine dose, the nature of the surgery, and the use of regional anesthesia and nonopioid pain control adjuvants, are also important to consider during postoperative pain management.
Table 2.
μ-Opioid Receptor Binding Affinities (Ki) for Commonly Used Opioids and Antagonists

Activation of the nociceptin receptor antagonizes the analgesia provided by activation of the μ-opioid receptor. Because of this, the pain relief provided by increasing doses of buprenorphine creates a bell-shaped curve in mice, with the greatest analgesia at moderate buprenorphine doses and less pain relief at higher doses.20 Morphine does not have this ceiling effect.20 The buprenorphine dose associated with maximum postoperative analgesia has not been determined in BMT patients. However, a nonoperative study in opioid-addicted volunteers reported maximum buprenorphine analgesia at a daily dose of 4 to 8 mg.21
Heroin addicts and patients maintained on either methadone or buprenorphine exhibit hyperalgesia, as measured by withdrawal latency in the cold pressor test, when compared to opioid-naive controls.22,23 This hyperalgesia dissipates very slowly with opioid abstinence; chronic pain patients still demonstrated hyperalgesia 121 ± 23 weeks after discontinuation of methadone or buprenorphine.23
In the described cases, patients reached a point 48–72 hours after surgery beyond which they needed only buprenorphine and nonsteroidal anti-inflammatory agents for pain control. Thus, outpatient full agonist opioids may not be needed for many BMT patients after cesarean delivery or postpartum tubal ligation. Outpatient Suboxone can only be prescribed by physicians specifically licensed to do so. Therefore, we did not provide outpatient prescriptions for Suboxone. Ketamine and dexmedetomidine have been successfully added to multimodal analgesia in BMT patients.18,19 We did not use either drug but encourage other clinicians to consider doing so.
The American Academy of Pediatrics supports breastfeeding in BMT patients.24 The concentrations of buprenorphine and its metabolites are low in human milk and are low or undetectable in the plasma of 14-day-old breastfeeding infants of BMT patients.25 The incidence of neonatal abstinence syndrome is lower in infants of mothers receiving methadone or buprenorphine who are breastfed than in those who are fed formula.26
In summary, because of the high affinity of buprenorphine for the µ receptor, activation of the nociceptin receptor, and buprenorphine-induced hyperalgesia, BMT patients have severe postoperative pain more frequently than opioid-naive patients. However, good-to-excellent postoperative pain control can be achieved in many obstetric buprenorphine patients if supplemental opioids are carefully chosen and regional anesthesia is used appropriately.
DISCLOSURES
Name: Barbara L. Leighton, MD.
Contribution: This author helped care for all 4 patients, obtained consent for publication from the patients, wrote the first draft of the article, and did the final editing of the manuscript.
Name: Lara W. Crock, MD, PhD.
Contribution: This author helped review the literature summarized in Table 1, created Table 1, and prepared an intermediate draft of the article.
This manuscript was handled by: Honorio T. Benzon, MD.
Footnotes
Funding: None.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
REFERENCES
- 1.Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2:395–402.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown SM, Holtzman M, Kim T, Kharasch ED. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115:1251–1260.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe DA, McMahon Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59:385–390.. [DOI] [PubMed] [Google Scholar]
- 4.Poisnel G, Quentin T, Barré L, Coquerel A, Debruyne D. Competitive displacement binding assay on rat brain sections and using a beta-imager: application to mu-opioid ligands. J Neurosci Methods. 2006;154:60–67.. [DOI] [PubMed] [Google Scholar]
- 5.Chen KY, Chen L, Mao J. Buprenorphine-naloxone therapy in pain management. Anesthesiology. 2014;120:1262–1274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer M, Paranya G, Keefer Norris A, Howard D. Intrapartum and postpartum analgesia for women maintained on buprenorphine during pregnancy. Eur J Pain. 2010;14:939–943.. [DOI] [PubMed] [Google Scholar]
- 7.Jones HE, O’Grady K, Dahne J, et al. Management of acute postpartum pain in patients maintained on methadone or buprenorphine during pregnancy. Am J Drug Alcohol Abuse. 2009;35:151–156.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Book SW, Myrick H, Malcolm R, Strain EC. Buprenorphine for postoperative pain following general surgery in a buprenorphine-maintained patient. Am J Psychiatry. 2007;164:979. [DOI] [PubMed] [Google Scholar]
- 9.Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: a case series. Am J Ther. 2010;17:523–528.. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1–11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chern SY, Isserman R, Chen L, Ashburn M, Liu R. Perioperative pain management for patients on chronic buprenorphine: a case report. J Anesth Clin Res. 2013;3:1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israel JS, Poore SO. The clinical conundrum of perioperative pain management in patients with opioid dependence: lessons from two cases. Plast Reconstr Surg. 2013;131:657e–658e.. [DOI] [PubMed] [Google Scholar]
- 13.Harrington CJ, Zaydfudim V. Buprenorphine maintenance therapy hinders acute pain management in trauma. Am Surg. 2010;76:397–399.. [PubMed] [Google Scholar]
- 14.Anderson TA, Quaye ANA, Ward EN, Wilens TE, Hilliard PE, Brummett CM. To stop or not, that is the question: acute pain management for the patient on chronic buprenorphine. Anesthesiology. 2017;126:1180–1186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen LE, Stone GL, Matson CA, Tybor DJ, Pevear ME, Smith EL. Total joint arthroplasty in patients taking methadone or buprenorphine/naloxone preoperatively for prior heroin addiction: a prospective matched cohort study. J Arthroplasty. 2016;31:1698–1701.. [DOI] [PubMed] [Google Scholar]
- 16.McCormick Z, Chu SK, Chang-Chien GC, Joseph P. Acute pain control challenges with buprenorphine/naloxone therapy in a patient with compartment syndrome secondary to McArdle’s disease: a case report and review. Pain Med. 2013;14:1187–1191.. [DOI] [PubMed] [Google Scholar]
- 17.Huang A, Katznelson R, de Perrot M, Clarke H. Perioperative management of a patient undergoing Clagett window closure stabilized on Suboxone® for chronic pain: a case report. Can J Anaesth. 2014;61:826–831.. [DOI] [PubMed] [Google Scholar]
- 18.Brummett CM, Trivedi KA, Dubovoy AV, Berland DW. Dexmedetomidine as a novel therapeutic for postoperative pain in a patient treated with buprenorphine. J Opioid Manag. 2009;5:175–179.. [DOI] [PubMed] [Google Scholar]
- 19.Macintyre PE, Russell RA, Usher KA, Gaughwin M, Huxtable CA. Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesth Intensive Care. 2013;41:222–230.. [DOI] [PubMed] [Google Scholar]
- 20.Lutfy K, Eitan S, Bryant CD, et al. Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23:10331–10337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372.. [PubMed] [Google Scholar]
- 22.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146.. [DOI] [PubMed] [Google Scholar]
- 23.Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143–149.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132:e796–e809.. [DOI] [PubMed] [Google Scholar]
- 25.Jansson LM, Spencer N, McConnell K, et al. Maternal buprenorphine maintenance and lactation. J Hum Lact. 2016;32:675–681.. [DOI] [PubMed] [Google Scholar]
- 26.Welle-Strand GK, Skurtveit S, Jansson LM, Bakstad B, Bjarkø L, Ravndal E. Breastfeeding reduces the need for withdrawal treatment in opioid-exposed infants. Acta Paediatr. 2013;102:1060–1066.. [DOI] [PubMed] [Google Scholar]


