Supplemental digital content is available in the text.
Key words: somatoform disorders; functional somatic syndromes (not MeSH); sick leave; Health care costs; primary health care; ASL = achieved significance level; BCa = bias-corrected and accelerated; BDS = bodily distress syndrome; CAGE = Cutting down, Annoyance by criticism, Guilty feeling, Eye openers Questionnaire; DREAM = Danish register for evaluation of marginalization; DRG = diagnosis-related group; DSM = Diagnostic and Statistical Manual of Mental Disorders; ES = effect sizes; FIP = functional illness in primary care; FP = family physician; GLM = generalized linear model; ICD-11-PHC = the International Classification of Diseases for Primary Health Care; MCS = mental component summary; PCS = physical component summary; SCL = symptom check list; SCAN = Schedules for Clinical Assessment in Neuropsychiatry; SCID = Structured Clinical Interview for DSM disorders; SF-36 = Short Form Health Survey; SSD = Somatic symptom disorder; WHO = World Health Organization; WHO-CIDI = WHO Composite International Diagnostic Interview
ABSTRACT
Objective
The upcoming International Classification of Diseases, 11th Revision for primary care use suggests inclusion of a new diagnostic construct, bodily (di)stress syndrome (BDS), for individuals with medically unexplained symptoms. We aimed to explore the long-term outcome of BDS in health care costs, work disability, and self-rated health.
Methods
Consecutive patients consulting their family physician for a new health problem were screened for physical and mental symptoms by questionnaires (n = 1785). A stratified subsample was examined with a standardized diagnostic interview (n = 701). Patients with single-organ BDS (n = 124) and multiorgan BDS (n = 35), and a reference group with a family physician–verified medical condition (n = 880) were included. All included patients completed a questionnaire at 3, 12, and 24 months of follow-up. Register data on health care costs and work disability were obtained after 2 and 10 years of follow-up, respectively.
Results
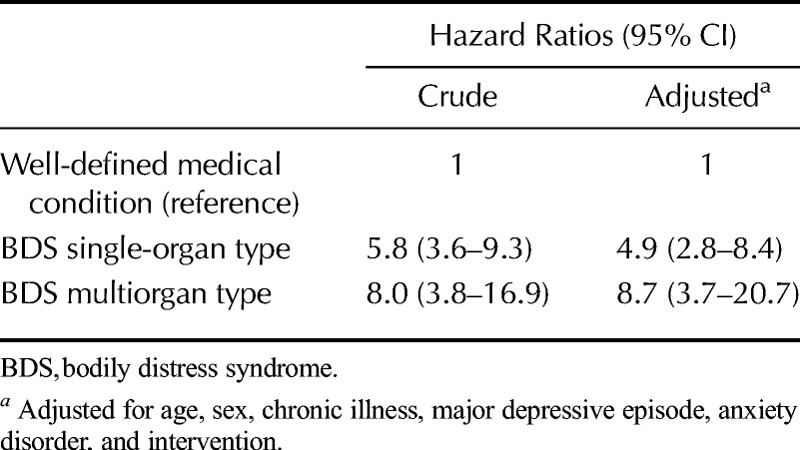
Patients with BDS displayed poorer self-rated health and higher illness worry at index consultation and throughout follow-up than the reference group (p ≤ .001). The annual health care costs were higher in the BDS groups (2270 USD and 4066 USD) than in the reference group (1392 USD) (achieved significance level (ASL) ≤ 0.001). Both BDS groups had higher risk of sick leave during the first 2 years of follow-up (RRsingle-organ BDS = 3.0; 95% confidence interval [CI] = 1.8–5.0; RRmultiorgan BDS = 3.4; 95% CI = 1.5–7.5) and substantially higher risk of newly awarded disability pension than the reference group (HRsingle-organ BDS = 4.9; 95% CI = 2.8–8.4; HRmultiorgan BDS = 8.7; 95% CI = 3.7–20.7).
Conclusions
Patients with BDS have poor long-term outcome of health care costs, work disability, and subjective suffering. These findings stress the need for adequate recognition and management of BDS.
INTRODUCTION
Symptoms that are not attributable to any conventionally defined disease are highly prevalent across all medical settings. Such medically unexplained or functional somatic symptoms represent a spectrum ranging from normal or self-limiting symptoms to severe and persistent conditions (1). Patients with persistent functional somatic symptoms constitute a vast challenge to both the health care system and the social security system; they are characterized by high rates of anxiety and depression (2,3), they are frequent attenders to health care services and contribute to high health care costs (4,5), and they may have an increased risk of sickness absence and work disability (6–9).
Previous studies support the evidence for an unfavorable outcome of conditions involving persistent functional somatic symptoms, but these studies are largely cross-sectional or based on self-reported questionnaires and/or less well-defined diagnostic constructs. Thus, we lack evidence on the long-term consequences of persistent functional somatic symptoms based on well-defined diagnostic criteria.
The field of persistent functional somatic symptoms is characterized by a lack of consistent terminology and overlapping diagnoses. Recently, a new concept of bodily distress syndrome (BDS) was introduced. This concept derives from empirical studies and seems to capture most patients with somatoform disorders and functional somatic syndromes (e.g., fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome) (10,11). The BDS builds on specific physical symptom patterns representing 4 symptom groups: cardiopulmonary, gastrointestinal, musculoskeletal, and general symptoms group. Bodily distress syndrome can be further divided into 2 severity groups: severe multiorgan type and less severe single-organ type (Fig. 1) (11). This new unifying concept has been suggested to replace diagnoses of somatoform disorders and has been incorporated into the current draft of the International Classification of Diseases for Primary Health Care (ICD-11-PHC) (12,13).
FIGURE 1.

Diagnostic criteria for BDS.
As BDS is a newly introduced diagnostic concept, only little knowledge exists on the prognosis of patients with BDS. Therefore, we performed a longitudinal study aiming to determine the long-term outcome of BDS in health care costs, work disability, and self-rated physical and mental health (including illness worry). The original patient sample in which the diagnostic criteria for BDS were developed was used in this study (11).
METHODS
The study was a questionnaire- and register-based follow-up study of a cohort established in 2000 for the Functional Illness in Primary Care (FIP) study comprising data on adult patients who consulted their family physician (FP) for a new health problem. The FIP study was an intervention study focusing on the effect of an educational program for training FPs in recognition and management of patients with functional symptoms (14). The FIP study was carried out in a 2-phase design consisting of a patient screening questionnaire and a standardized psychiatric interview. The FIP study design and the recruitment of patients have been described extensively elsewhere (15). The study was approved by the Science Ethics Committee in the former County of Aarhus, the Scientific Research Evaluation Committee of the Danish College of General Practitioners, and the Danish Data Protection Agency.
Participants and Setting
In the FIP study, 1785 consecutive patients aged 18 to 65 years attending their family physician (n = 38) for a new health problem during a 3-week period in March and April 2000 gave informed consent to participate and were included. Patients who were of non-Scandinavian origin, who were severely ill or demented, or who were not enlisted with the participating FPs were excluded from participation (15).
Study Design and Procedure
The patients completed a screening questionnaire in the FP's waiting room just before entering the consultation. This questionnaire included the Symptom Check List (SCL-8), which assesses anxiety and depression; the somatization subscale of the SCL-90, which screens for 12 common physical symptoms, the Whitely Index (Whitely-7), which assesses illness worry, and the 4-item Cutting down, Annoyance by criticism, Guilty feeling, Eye openers (CAGE) screening questionnaire for alcohol abuse (16). Included patients also completed the Medical Outcome Study's Short Form Health Survey (SF-36), which assesses physical and mental health (17), and sociodemographic data were obtained. A detailed description of the screening questionnaire is provided elsewhere (16).
A stratified sample consisting of a random selection of one ninth of the patients and all patients with a high score on the screening questionnaires (n = 894) was invited for a psychiatric research interview (Fig. 2). The stratified sampling procedure was used solely for the initial identification of patients with high likelihood of being cases to help reduce the number of noncase interviews. We did not use the information on distress from the questionnaires for categorization of patients or diagnostic purposes; categorization was based on the results of the psychiatric interview. A total of 701 patients (78.4%) accepted to participate in the psychiatric research interview. Patients who had a low score on the screening questionnaire, who were younger, or who were males were more likely to decline participation than other patients (15). For most patients, the interview was performed within a week after the initial contact.
FIGURE 2.

Flow chart. aFPs stated a well-defined medical condition as the main problem in 1009 patients; 296 of these were SCAN interviewed of which 35 fulfilled the criteria for single-organ BDS and 7 fulfilled the criteria for multiorgan BDS and were classified as such.
Psychiatric Research Interview
The interview was based on the Schedules for Clinical Assessment in Neuropsychiatry (SCAN), version 2.1. (18). The SCAN is endorsed by the World Health Organization (WHO) and is a standardized semistructured interview performed by trained medical doctors and covering all types of mental disorders, including a separate section that screens for a wide range of physical symptoms. This section allows the rater to assess whether present symptoms are explained by a medical condition or rather should be seen as functional symptoms, and whether these symptoms are considered to be disturbing for the patient or have been an issue receiving medical attention. Based on the physical symptom screening, diagnoses for a variety of functional somatic syndromes (e.g., chronic fatigue syndrome and fibromyalgia) can be generated. The interviews were performed by 6 physicians who had been certified at the WHO SCAN training center in Aarhus and who had psychiatric, medical, and surgical residency. The interrater reliability between the 6 interviewers were found to be high (kappa = 0.88) for the ICD-10 somatoform disorders and other psychiatric diagnoses (15).
FP Assessment
Immediately after the index consultation, the FP completed a questionnaire inquiring on the presence of chronic physical disease/psychiatric disorders, including categorization of the patient's main health problem as either “Well-defined medical condition” (n = 1009), “Probably well-defined medical condition” (n = 395), “Medically unexplained symptoms” (n = 229), “Psychiatric disorder with physical manifestations” (n = 95), or “No physical health complaints” (n = 39). Family physician's rating of main problem was missing for 18 patients.
Patient Grouping
The SCAN interviews were used to generate a BDS single-organ group and a BDS multiorgan group according to the BDS criteria (11,12). We compared these 2 groups to a reference group of patients with a well-defined medical condition (as rated by their FP). In total, 296 of the 1009 patients who attended due to a well-defined medical condition were SCAN interviewed; 35 patients were found to meet the criteria for single-organ BDS, whereas 7 patients were found to meet the criteria for multiorgan BDS. These 42 patients were included only in one of the BDS groups and excluded from the medical condition group. Ultimately, 880 patients were included in the medical condition group, 124 in the BDS single-organ group, and 35 in the BDS multiorgan group (Fig. 2).
Follow-Up
The patients were asked to complete a mailed questionnaire at 3, 12, and 24 months after the index consultation, including scales measuring self-rated health and illness worry. Register data on health care costs was retrieved for a period of 3 years before index consultation and throughout 2 years after, whereas register data on sick leave and disability were obtained for the 10 years following the index consultation.
Self-Rated Health and Illness Worry
The Physical Component Summary (PCS) and the Mental Component Summary (MCS) from the SF-36 were used as measures of self-rated health (19), and the Whiteley-7 scale as a measure of illness worry (20,21).
Healthcare Costs
The Danish health care system is almost entirely tax financed, and Danish residents are generally offered medical care free of charge. In this study, the costs of primary care, secondary care, and prescribed medication were obtained from the National Health Service Register in the former County of Aarhus. We obtained data from 3 years before index consultation through 2 years after, but the data on prescribed medicine were limited to include only the 6 months immediately before the index consultation due to legal restrictions on registration of medicine use in Denmark. The analyses did not include costs of laboratory tests and x-rays requested in primary care.
All costs for inpatient and daytime admissions and outpatient and emergency ward contacts were extracted from the Danish National Patient Register and the Danish Psychiatric Central Register. Nonpsychiatric costs were calculated as diagnosis-related group case-mix prices by the Diagnosis-Related Group pricing office of the Danish Health and Medicines Authority (2004 fixed prices). Psychiatric hospital care costs were calculated from the average 2004 fixed prices for hospital bed days and outpatient contacts with aid from the finance administration team of the Psychiatric Hospital in Aarhus. All costs were adjusted for time at risk, and the object for analysis was cost per year. Index consultation was included in the 2 years of follow-up.
Work Disability
Danish citizens who have received social benefits or any other welfare payments are registered in the Danish Register for Evaluation of Marginalization (DREAM) (22). Social transfers recorded in DREAM represent 5 categories: benefits to otherwise self-supporting individuals (e.g., statutory maternity pay), benefits related to the labor market (e.g., unemployment benefit or social assistance), temporary health-related benefits (sickness benefit and vocational rehabilitation benefit), permanent health-related benefits (full and partial disability pension), and public (old-age or early) retirement pension. Furthermore, death and emigration of registered individuals are recorded in DREAM.
Danish law stipulates that sickness benefit can be granted for a maximum of 12 months, after which the individual must either return to work, will be eligible for social assistance, or may be awarded disability pension by the municipal authorities. Until a recent restriction, individuals aged 18 to 65 years with permanently reduced work ability were eligible for disability pension. Partial disability pension is granted to individuals with partial loss of working capacity on a permanent basis, and “flexible working” may be arranged, whereas full disability pension is a permanent departure from the labor market.
As a measure of work disability, data on temporary (sickness benefit and vocational rehabilitation) and permanent (full and partial disability pension) health-related benefits were retrieved from DREAM for the 3 patient groups. Due to the registration procedure in DREAM, health-related benefits were recorded in weeks.
Data Analysis
Descriptive statistics were used to summarize patients' characteristics at index consultation. The SF-36 PCS and MCS scores were calculated according to the validated Danish version of the SF-36, with higher scores expressing better health (23). The Whiteley-7 was transformed into a scale ranging from zero to 100 by the following expression: (patient raw score − lowest possible score) / (highest possible score − lowest possible score) × 100. Group comparisons were performed by χ2 test for categorical data, Student t test for normally distributed data and Mann-Whitney U test for non-normally distributed data.
To further analyse the development in PCS, MCS, and Whiteley-7 scores over time within and between groups, we estimated mixed models with random intercept for each of these outcomes. The general shape of the models is a group-specific level at index consultation and a group-specific level, that is, one level, at the remaining time points. This was modeled through 2 variables (time and group) with potential interaction effects. The models were graphically depicted, and mean differences with corresponding 95% confidence intervals (CIs) and effect sizes (ESs) were calculated as measures of within-group changes.
We accounted for skewed health care costs with an excess of zeros by estimating sample means of health care costs with bias-corrected and accelerated 95% CIs. Tests of equality of health care cost means for patients with a medical condition and for patients with BDS were done by computing the bootstrap test statistic achieved significance level (ASL) based on 1000 replications (24).
Weekly sick leave status was recorded for each patient throughout the follow-up period, excluding weeks of permanent disability pension, age-related retirement, emigration, or death (missing values). To compare the risk of sick leave between patient groups, we applied a generalized linear model from the binomial family using log-link. Risk ratios (RRs) were used as a measure of association. Corresponding 95% CI were assessed by performing cluster-robust variance estimation to account for the expected dependency of awarded temporary health-related benefits in different weeks for the same patient (25).
The Cox proportional hazard model was used to compare the risk of incident award of full or partial disability pension during 10 years of follow-up among the 3 patient groups. Patient age was used as time scale and was hence appropriately adjusted for. Patients entered the study at the time of the index consultation (delayed entry), that is, they were not observed until the index consultation, and they were censored at the time of death, emigration, or public retirement. The proportional-hazards assumption was graphically assessed using log-log plots. Hazard ratios (HRs) with corresponding 95% CI were used as a measure of association.
In all analyses, patients with a medical condition constituted the reference group. Crude estimates were presented as were estimates adjusted for patient age, sex, chronic illness, comorbid major depressive episode, anxiety disorder, and intervention. Two-sided p values < .05 were considered to be statistically significant, except for analyses of health care costs in which a Bonferroni correction was applied, and the level of significance was set at p < .0025 (2 group comparisons on 5 different measures of costs at 2 different time points). Analyses were conducted by Stata statistical software, version 11.
RESULTS
Patients' Characteristics
Patients in the 2 BDS groups were slightly older, they were more often females, and they were less prone to have finished a higher educational program compared to patients in the medical condition group. Furthermore, patients with BDS had more frequently been granted partial or full disability pension (20.2% and 34.3% vs 3.6%) and thus were less likely to be available for the labor market at index consultation than patients in the medical condition group (Table 1).
TABLE 1.
Patients' Baseline Characteristicsa
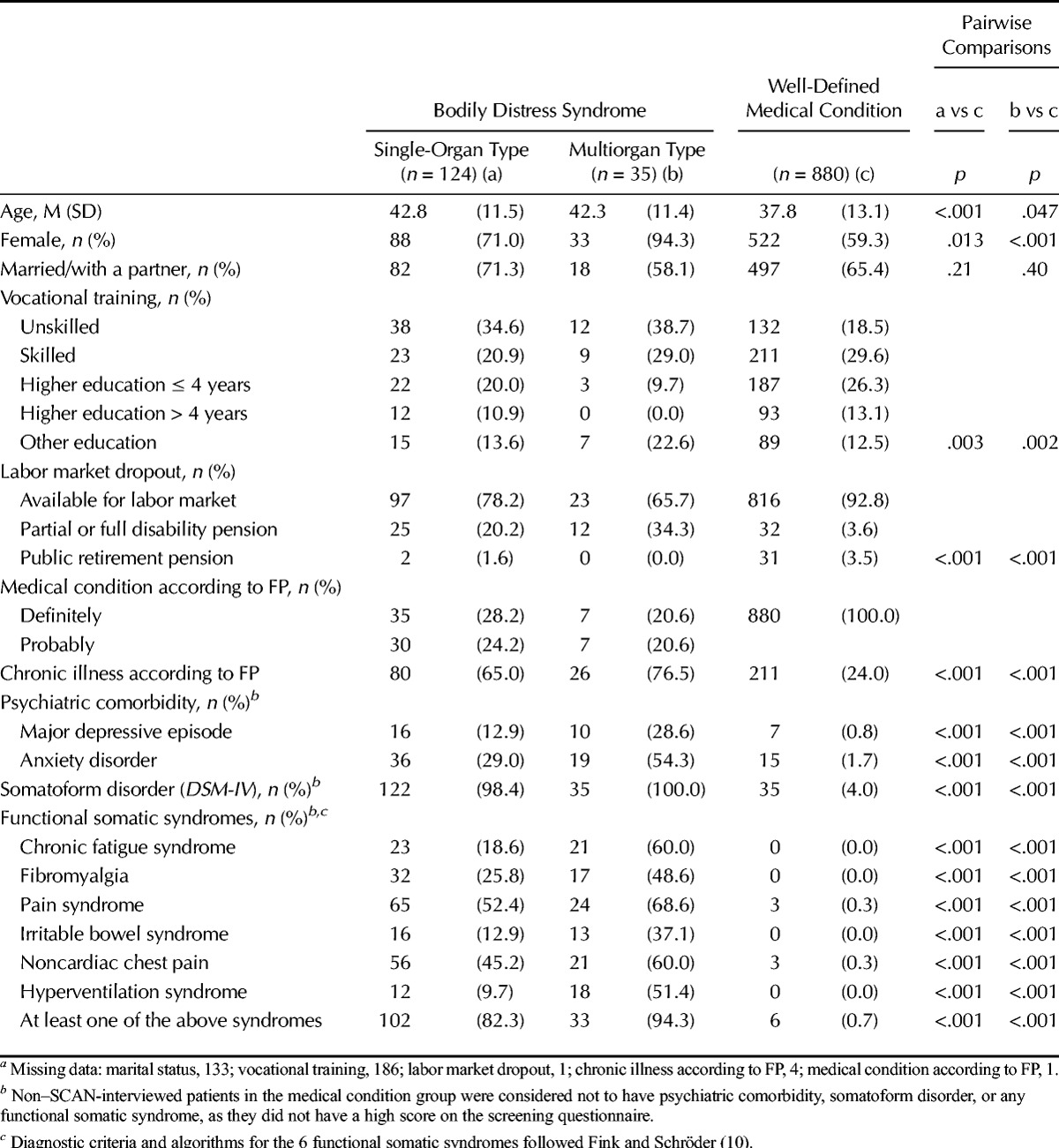
The FPs found more patients in the BDS groups to have a chronic illness compared to the medical condition group. Furthermore, major depressive episode and anxiety disorder (as based on the SCAN interview) were more frequent in the BDS groups. Most of the patients with BDS had at least one functional somatic syndrome: 82.3% of single-organ BDS and 94.3% of multiorgan BDS (Table 1).
Self-Rated Health and Illness Worry
Self-rated physical health, mental health, and illness worry are displayed in Table 2. For all 3 scales and at all time points (index and 3-, 12-, and 24-month follow-ups), completers and noncompleters were equally distributed across the 3 patient groups. We did not find any differences in the baseline scores on PCS, MCS, or Whiteley-7 when comparing patients who completed the questionnaires with noncompleters or patients lost to follow-up at any follow-up time points (data not shown).
TABLE 2.
Physical Health, Mental Health, and Illness Worry According to Patient Groups as Measured by Sf-36 and Whiteley-7 (0–100) at Index Consultation and at 3, 12, and 24 Months of Follow-Upa
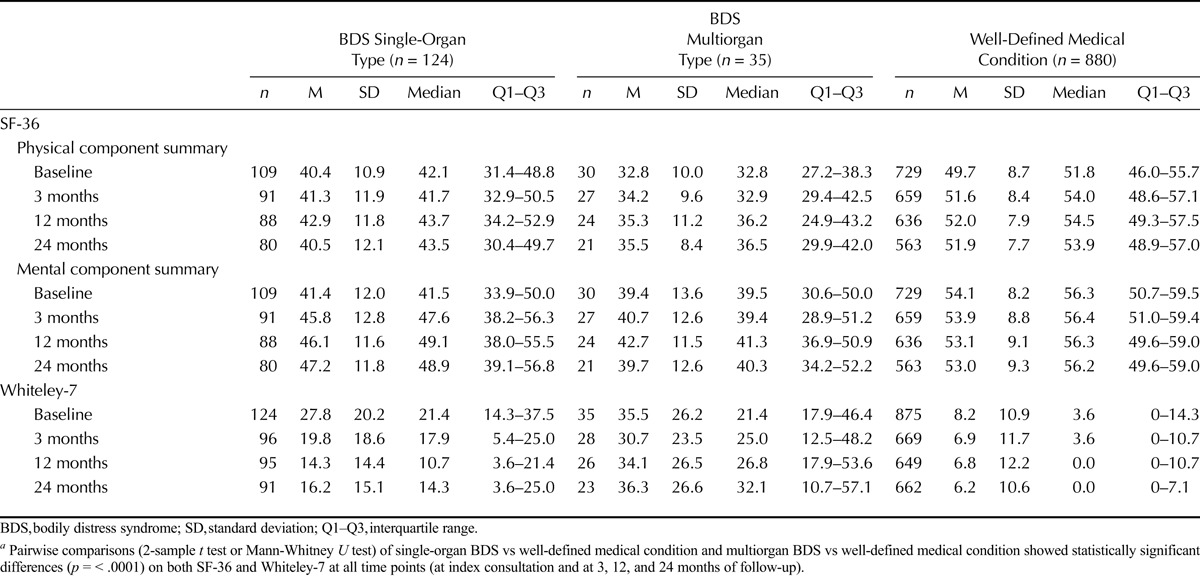
At baseline and all follow-up time points, patients with BDS had lower self-rated physical and mental health and higher illness worry than patients with a well-defined medical condition (Table 2). Within each group, baseline scores were compared with follow-up scores adjusted for age, sex, chronic illness according to the FP, anxiety disorder, major depressive episode, and intervention. From baseline to 24 months follow-up, physical health improved slightly in the multiorgan BDS group (mean difference = 3.9; 95% CI = 0.3–7.5) and in the medical condition group (mean difference = 3.5; 95% CI = 2.2–4.8). Mental health improved only in the single-organ BDS group (mean difference = 4.0; 95% CI = 1.1–7.0), whereas illness worry decreased in both the single-organ BDS group (mean difference = −9.6; 95% CI = −13.6 to −5.7), and the medical condition group (mean difference = −8.0; 95% CI = −9.8 to −6.2). The improvements in self-rated health were rather small (ES = 0.29–0.39), although statistically significant, and only reduced illness worry may be of clinical relevance (ES = 0.70–0.79) (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A329).
Health Care Costs
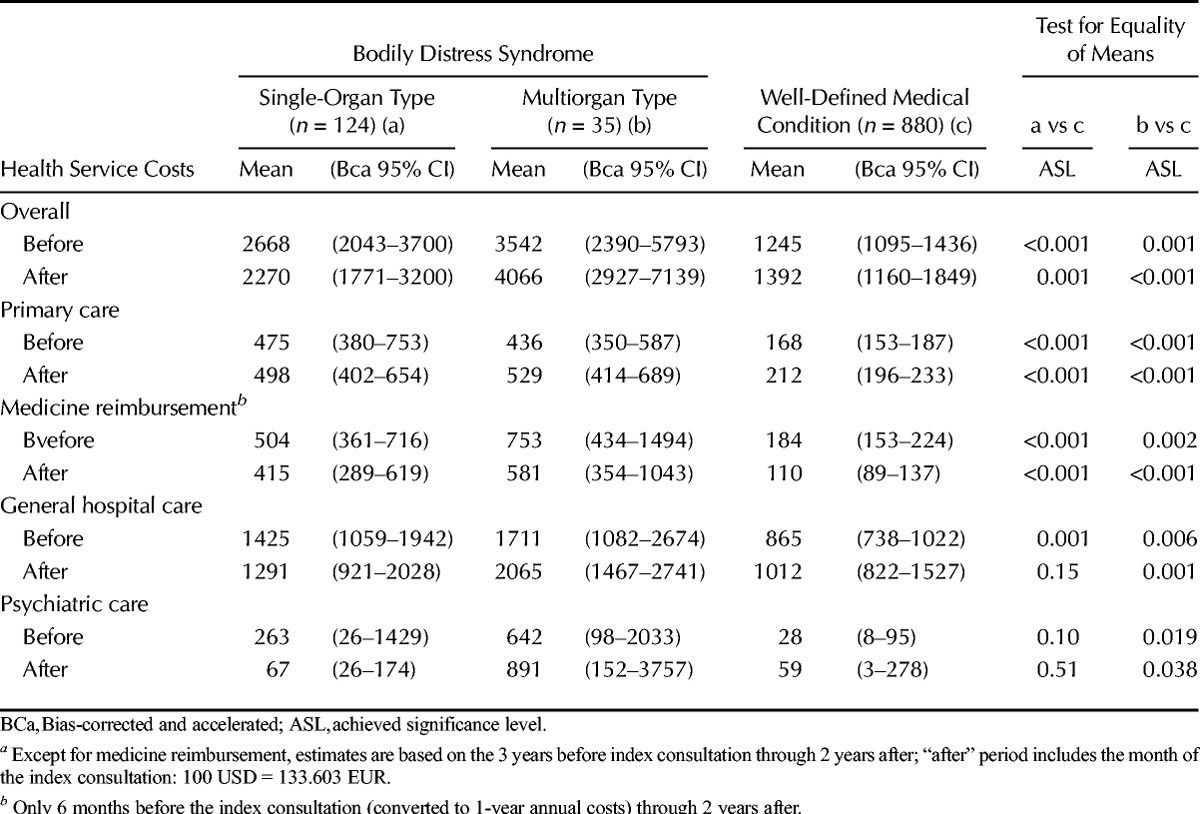
The total annual healthcare costs incurred by the 2 BDS groups were higher than the costs incurred by the medical condition group (follow-up estimates: single-organ BDS: mean = 2270 USD, ASL = 0.001; multiorgan BDS: mean = 4066 USD, ASL < 0.001) (Table 3). Patients with multiorgan BDS generally displayed higher health care costs across all medical settings and types of services (except for psychiatric care) during follow-up, whereas patients with single-organ BDS incurred higher costs only in connection with primary care consultations and prescribed medications. The follow-up costs incurred by general hospital care accounted for 57%, 51%, and 73% of the total costs in the groups with single-organ BDS, multiorgan BDS and medical condition, respectively. Psychiatric care costs accounted for 22% of the total health care costs in the group with multiorgan BDS (service use: n = 8 (23%)), only 3% in patients with single-organ BDS (service use: n = 8 (6%)), and 4% in patients with a well-defined medical condition (service use: n = 7 (<1%)).
TABLE 3.
Annual Health Care Costs Before and After Index Consultation in USDa
Work Disability
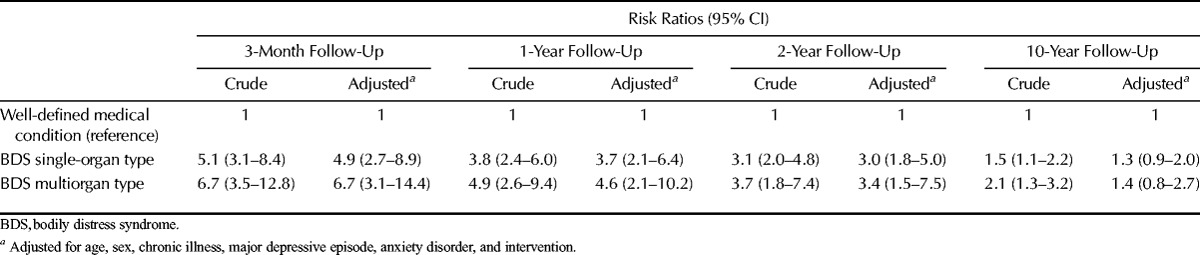
The average risk of sick leave in the first 3 months after the index consultation was higher in patients with single-organ BDS (RR = 4.9; 95% CI = 2.7–8.9) and multiorgan BDS (RR = 6.7; 95% CI = 3.1–14.4) than in patients with a well-defined medical condition (Table 4). The risks decreased over time; after 10 years of follow-up, no statistically significant differences were observed between the groups.
TABLE 4.
Risk of Sick Leave According to Patient Groups During 3 Months, 12 Months, 24 Months, and 10 Years of Follow-Up
Marginalization in terms of permanently reduced or loss of work ability seemed relatively stable over time in the 3 groups (Fig. 3). A higher proportion of patients with BDS received disability pension, most pronounced in the multiorgan BDS group, compared to patients with a well-defined medical condition. It should be noted that migration between categories was evident, for example, patients receiving disability pension (full or partial) were transferred to public retirement no later than the age of 65 years, whereas others who were available for the labor market at the index consultation were awarded disability pension during the follow-up period. During the 10 years of follow-up, patients with BDS were more likely to be excluded from the labor force due to ill health; the multiorgan BDS group was 8 times as likely (HR = 8.7; 95% CI = 3.7–20.7) and the single-organ BDS group 4 times as likely (HR = 4.9; 95% CI = 2.8–8.4) to receive a new award of disability pension than patients with a well-defined medical condition (Table 5).
FIGURE 3.
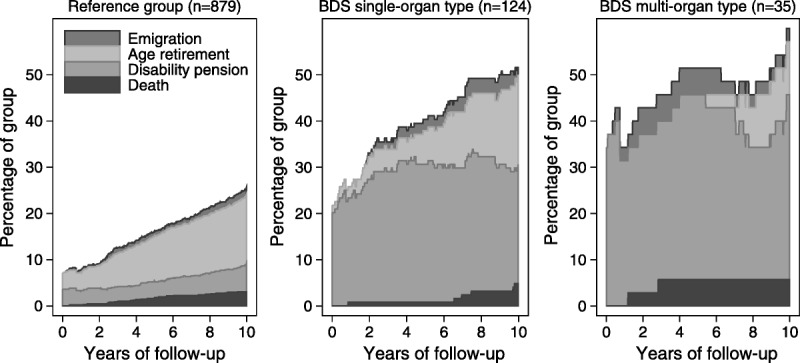
Percentage of patients who left the labor market during 10 years of follow-up according to the 3 patient groups. BDS, bodily distress syndrome.
TABLE 5.
Risk of New Awards of Permanent Health-Related Benefits According to Patient Groups During 10 Years of Follow-Up
DISCUSSION
This study suggests that BDS (both single-organ and multiorgan) is a disabling condition with substantial long-term impact on both the individual and the society. Bodily distress syndrome was associated with lower self-rated health, higher illness worry, higher health care costs, and higher risk of work disability throughout the follow-up period.
Physical component summary scores of approximately 41 and MCS scores of approximately 47 have been demonstrated in samples of primary care patients who meet the criteria for either somatization disorder, abridged somatization disorder, or multisomatoform disorder (26). These scores are comparable to the scores found for patients with single-organ BDS, whereas patients with multiorgan BDS seemed to report considerably more impaired physical and mental health status. The degree of impairment in both BDS groups was remarkably higher than previously found for chronic physical conditions such as arthritis, chronic lung disease, diabetes, and chronic heart disease in the general population (27).
Illness worry and the related interaction with somatic symptom burden have been found to predict health care use (28). Our data may indicate that patients with a medical condition and, to some degree, patients with single-organ BDS, feel reassured after consulting their FP as we observed a decrease in illness worry right after the index consultation in both groups. We found patients with multiorgan BDS to remain highly worried over time. On the one hand, changes in illness worry may reflect changes in symptom status and self-evaluated health. On the other hand, our results may reflect previous findings that indicate that FPs tend to feel more comfortable and more successful with the task of explaining and reassuring patients with medical conditions and less severe functional symptoms, whereas patients with severe and persistent conditions are often found to be burdensome and difficult to manage (29–31).
We found patients with multiorgan BDS to incur higher health care costs across all medical settings (except for psychiatric care) during follow-up, whereas higher costs in patients with single-organ BDS were primarily due to use of primary care and prescribed medications. There may be several explanations for these findings. First, our findings may reflect the gradient in severity between single-organ BDS and multiorgan BDS and different needs of care. Second, our findings may indicate that FPs are better capable of handling patients with single-organ BDS, but need specialist support to manage patients with multiorgan BDS in line with the clinical recommendations (32). The results on psychiatric care costs need cautious interpretation, as very few individuals requested psychiatric services. In general, our findings are consistent with the existing literature, as both former population-based studies and clinical studies demonstrate high health care use and increased costs for patients with functional somatic symptoms or somatoform disorders (4,33,34).
Our study results indicate that BDS is strongly related to work disability. Several studies have found an increased risk of sick leave and/or disability in patients with a high functional symptom burden or functional somatic syndromes (7,35–37). We have previously demonstrated a strong relationship between somatoform disorders and disability pension, a relationship that has also been found in prospective studies of fibromyalgia-associated symptoms and the number of pain sites (38–40). After 10 years of follow-up, we could no longer demonstrate any differences in the risk of sick leave between our patient groups. This may be explained by the higher proportion of patients with BDS who had been granted disability pension. As disability pension is a permanent departure from the labor market, patients who already receive disability pension are not at risk of receiving sick leave benefits. Patients with disabilities and restricted work ability may not necessarily receive health-related benefits, but they may instead be granted unemployment benefit or social assistance. A Dutch study found that sick-listed patients with a high somatic symptom burden and decreased functioning were at higher risk of redundancy (37). As a consequence, our analyses of work disability may only partly describe the seriousness of the problem. Thus, the demonstrated group differences may be of an even greater magnitude.
Somatic symptom disorder (SSD) has been introduced in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (41). The main criterion of the diagnosis is that the patient must present at least one distressing somatic symptom (criteria A). The symptom can be of any origin, that is, even a symptom caused by cancer. In addition, the patient must have excessive thoughts, feelings, or behaviors (e.g., health anxiety) related to the symptom (criteria B) (42). As a consequence, the SSD seems to be a new permutation of the DSM-IV hypochondria diagnosis rather than a replacement of the DSM-IV somatization disorder and related diagnoses. The SSD does not use somatic symptom characteristics (e.g., pattern, number, character, or type) for diagnostic purposes. Consequently, the SSD is a very different construct than the BDS; the BDS is solely defined by somatic symptom patterns, and no emotional or behavioral symptoms are needed for the diagnosis (but they may be important for treatment). The data material used in this study formed part of the study in which the BDS diagnostic construct was developed. Back then, we included only medically unexplained symptoms for diagnostic purposes. However, our 10-year clinical experience with BDS and the results from a new study in primary care indicate that it is obsolete to seek to define each symptom as either medically unexplained or not (43). Rather, the FP should ask for symptoms of BDS to identify the unique symptom patterns or illness picture described in the diagnostic criteria (Fig. 1). As always in medical practice, the FP must also exclude differential diagnoses, that is, other conditions that may present with a similar symptom pattern.
Strengths and Limitations
A major strength of our study is that we had complete follow-up of outcomes related to health care costs, sick leave, and work disability (except for emigrated patients), as our data were obtained from Danish national registers. Danish Register for Evaluation of Marginalization has formerly been found to provide valid and high-quality data on disability pension and sickness benefits (22,44), and the completeness of the registers used for the calculations of health care costs are generally considered to be high because the data are continuously updated by state authorities and are used for reimbursement purposes (45,46). Moreover, our use of register data reduced the risk of recall bias, which may be present in studies relying on self-reported measures of health care use and sick leave. Due to the longitudinal design, a rather high proportion of patients were lost to follow-up on the self-reported outcomes. However, as noncompleters were equally distributed across the 3 patient groups at all follow-up points and that no differences between completers and noncompleters were detected in baseline scores, we expect nonresponse to have had no major influence on results.
Another major strength of the study was that the BDS groups were generated from acknowledged standardized diagnostic interviews. Instead of relying on subjective reports by the FPs or the patients on functional symptoms (47), trained physicians performed a systematic screening for a high number of symptoms and rated these as functional symptoms according to predefined criteria. Compared to other standardized psychiatric research interviews, for example, the Structured Clinical Interview for DSM disorders (SCID) or the WHO Composite International Diagnostic Interview (WHO-CIDI), the SCAN interview is better suited for the research purposes. The SCID and WHO-CIDI are both diagnosis focused and include only symptoms that are relevant for DSM-IV or ICD-10 psychiatric diagnoses (48,49), whereas the SCAN interview is symptom driven with a bottom-up approach focusing on psychopathology rather than on specific diagnoses (18). The SCAN is thus much more comprehensive. In addition, because the SCAN is not bound to any diagnostic system, it is better suited for somatic symptoms and complaints, areas in which the SCID and CIDI are both weak.
As the BDS groups were based on the SCAN interview, these groups are expected to represent highly valid classifications. In the medical condition group, a minority of patients (either randomly selected or with a high score on the screening questionnaire) were SCAN interviewed. Therefore, some of the noninterview patients assigned to the medical condition group may have been undetected cases of BDS. Such misclassification of patients would bias the observed differences toward the null. Therefore, the study results may be conservative estimates, and we have no reason to believe that misclassification poses a particular problem to the validity of our study conclusions. Our choice of control group implied that also patients with a high score on the screening questionnaire were included in the control group. However, these high-scoring control group patients were all SCAN interviewed and distress could not be attributed to BDS; the high scores were probably rather related to their underlying medical condition or mental distress. A general population sample could have constituted an alternative control group, but we would have expected this to have caused even more pronounced findings.
One study limitation was that the applied methods of health care cost analyses did not allow us to adjust for FP-rated chronic illness. The FP rated whether the patient had a chronic illness, but the FP did not make any specification of this illness. Therefore, we could not differentiate between chronic illness due to BDS and chronic illness in the form of a chronic comorbid medical condition. Consequently, we do not know how much of the increased health care costs may be attributed to a comorbid medical condition. However, in the analyses that were adjusted for chronic illness, we saw only minor differences between the crude and the adjusted estimates.
Finally, although we followed the included patients for an extensive period of time, we did not measure patient status of BDS at follow-up. Schedules for Clinical Assessment in Neuropsychiatry interviews were performed at baseline, and no reinterview was made during follow-up. Functional disorders have previously been shown to represent unstable conditions, depending on the diagnostic criteria applied (8,50,51). Based on the present study, we cannot conclude whether patients with BDS continuously fulfilled the criteria for either single-organ or multiorgan BDS or whether their baseline condition worsened or improved over time.
CONCLUSIONS
Patients with single-organ or multiorgan BDS were found to have unfavorable long-term outcomes and to be costly for society. Our study strongly supports the clinical use and the prognostic value of the new concept of BDS. Somatoform disorders and functional somatic syndromes may be treated effectively with combinations of cognitive behavioral therapy, antidepressants, graded exercise, and relaxation techniques (52,53). Correspondingly, promising results have been shown for treatment of severe BDS (54,55). These findings stress the need for improved recognition and implementation of the BDS diagnosis in primary care followed by research on effective treatment of patients with BDS.
Supplementary Material
Acknowledgments
Source of Funding and Conflicts of Interest: The study was funded by the interdisciplinary research program “Health Promotion and Prevention Research” (Sundhedsfremme og forebyggelsesforskning) under the Danish National Research Council, the Health Services of Aarhus County, and the Danish Research Foundation for General Practice (Forskningsfonden for Almen Praksis). The authors report no conflicts of interests.
Footnotes
Supplemental Content
REFERENCES
- 1.Katon W, Lin E, Von Korff KM, Russo J, Lipscomb P, Bush T. Somatization: a spectrum of severity. Am J Psychiatry 1991;148:34–40. [DOI] [PubMed] [Google Scholar]
- 2.Löwe B, Spitzer RL, Williams JB, Mussell M, Schellberg D, Kroenke K. Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry 2008;30:191–9. [DOI] [PubMed] [Google Scholar]
- 3.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med 2003;65:528–33. [DOI] [PubMed] [Google Scholar]
- 4.Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 2005;62:903–10. [DOI] [PubMed] [Google Scholar]
- 5.Burton C, McGorm K, Richardson G, Weller D, Sharpe M. Healthcare costs incurred by patients repeatedly referred to secondary medical care with medically unexplained symptoms: a cost of illness study. J Psychosom Res 2012;72:242–7. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JL, Kroenke K. Prevalence, impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow-up study. Psychosom Med 2008;70:430–4. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke K, Spitzer RL, deGruy FV, 3rd, Hahn SR, Linzer M, Williams JB, Brody D, Davies M. Multisomatoform disorder. An alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Arch Gen Psychiatry 1997;54:352–8. [DOI] [PubMed] [Google Scholar]
- 8.Steinbrecher N, Hiller W. Course and prediction of somatoform disorder and medically unexplained symptoms in primary care. Gen Hosp Psychiatry 2011;33:318–26. [DOI] [PubMed] [Google Scholar]
- 9.Gjesdal S, Bratberg E, Maeland JG. Musculoskeletal impairments in the Norwegian working population: the prognostic role of diagnoses and socioeconomic status: a prospective study of sickness absence and transition to disability pension. Spine (Phila Pa 1976) 2009;34:1519–25. [DOI] [PubMed] [Google Scholar]
- 10.Fink P, Schröder A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. J Psychosom Res 2010;68:415–26. [DOI] [PubMed] [Google Scholar]
- 11.Fink P, Toft T, Hansen MS, Ørnbøl E, Olesen F. Symptoms and syndromes of bodily distress: an exploratory study of 978 internal medical, neurological, and primary care patients. Psychosom Med 2007;69:30–9. [DOI] [PubMed] [Google Scholar]
- 12.Lam TP, Goldberg DP, Dowell AC, Fortes S, Mbatia JK, Minhas FA, Klinkman MS. Proposed new diagnoses of anxious depression and bodily stress syndrome in ICD-11-PHC: an international focus group study. Fam Pract 2013;30:76–87. [DOI] [PubMed] [Google Scholar]
- 13.Ivbijaro G, Goldberg D. Bodily distress syndrome (BDS): the evolution from medically unexplained symptoms (MUS). Ment Health Fam Med 2013;10:63–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Fink P, Rosendal M, Toft T. Assessment and treatment of functional disorders in general practice: the extended reattribution and management model—an advanced educational program for nonpsychiatric doctors. Psychosomatics 2002;43:93–131. [DOI] [PubMed] [Google Scholar]
- 15.Toft T, Fink P, Oernboel E, Christensen K, Frostholm L, Olesen F. Mental disorders in primary care: prevalence and co-morbidity among disorders. Results from the functional illness in primary care (FIP) study. Psychol Med 2005;35:1175–84. [DOI] [PubMed] [Google Scholar]
- 16.Christensen KS, Fink P, Toft T, Frostholm L, Ornbøl E, Olesen F. A brief case-finding questionnaire for common mental disorders: the CMDQ. Fam Pract 2005;22:448–57. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 18.Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N; SCAN. Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry 1990;47:589–93. [DOI] [PubMed] [Google Scholar]
- 19.Ware J, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide, Lincoln, RI: QualityMetric; 2005. [Google Scholar]
- 20.Fink P, Ewald H, Jensen J, Sørensen L, Engberg M, Holm M, Munk-Jørgensen P. Screening for somatization and hypochondriasis in primary care and neurological in-patients: a seven-item scale for hypochondriasis and somatization. J Psychosom Res 1999;46:261–73. [DOI] [PubMed] [Google Scholar]
- 21.Veddegjærde KE, Sivertsen B, Wilhelmsen I, Skogen JC. Confirmatory factor analysis and item response theory analysis of the Whiteley Index. Results from a large population based study in Norway. The Hordaland Health Study (HUSK). J Psychosom Res 2014;77:213–8. [DOI] [PubMed] [Google Scholar]
- 22.Hjollund NH, Larsen FB, Andersen JH. Register-based follow-up of social benefits and other transfer payments: accuracy and degree of completeness in a Danish interdepartmental administrative database compared with a population-based survey. Scand J Public Health 2007;35:497–502. [DOI] [PubMed] [Google Scholar]
- 23.Bjørner JB, Damsgaard MT, Watt T, Bech P, Rasmussen NK, Kristensen TS, Modvig J, Thunedborg K. Dansk Manual til SF-36, København, Denmark: Lif; 1997. [Google Scholar]
- 24.Efron B, Tibshirani R. An Introduction to the Bootstrap, New York: Chapman & Hall/CRC; 2003. [Google Scholar]
- 25.Rogers W. Regression standard errors in clustered samples. Stata Technical Bulletin 1994;3:19–23. [Google Scholar]
- 26.Dickinson WP, Dickinson LM, deGruy FV, Candib LM, Main DS, Libby AM, Rost K. The somatization in primary care study: a tale of three diagnoses. Gen Hosp Psychiatry 2003;25:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Alonso J, Ferrer M, Gandek B, Ware JE, Jr., Aaronson NK, Mosconi P, Rasmussen NK, Bullinger M, Fukuhara S, Kaasa S, Leplège A; IQOLA Project Group. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004;13:283–98. [DOI] [PubMed] [Google Scholar]
- 28.Tomenson B, McBeth J, Chew-Graham CA, Macfarlane G, Davies I, Jackson J, Littlewood A, Creed FH. Somatization and health anxiety as predictors of health care use. Psychosom Med 2012;74:656–64. [DOI] [PubMed] [Google Scholar]
- 29.Epstein RM, Hadee T, Carroll J, Meldrum SC, Lardner J, Shields CG. "Could this be something serious?" Reassurance, uncertainty, and empathy in response to patients' expressions of worry. J Gen Intern Med 2007;22:1731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.olde Hartman TC, Hassink-Franke L, Lucassen P, van Spaendonck K, van Weel C. Explanation and relations. How do general practitioners deal with patients with persistent medically unexplained symptoms: a focus group study. BMC Fam Pract 2009;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid S, Whooley D, Crayford T, Hotopf M. Medically unexplained symptoms—GPs' attitudes towards their cause and management. Fam Pract 2001;18:519–23. [DOI] [PubMed] [Google Scholar]
- 32.Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet 2007;369:946–55. [DOI] [PubMed] [Google Scholar]
- 33.Andersen NL, Eplov LF, Andersen JT, Hjorthøj CR, Birket-Smith M. Health care use by patients with somatoform disorders: a register-based follow-up study. Psychosomatics 2013;54:132–41. [DOI] [PubMed] [Google Scholar]
- 34.Barsky AJ, Ettner SL, Horsky J, Bates DW. Resource utilization of patients with hypochondriacal health anxiety and somatization. Med Care 2001;39:705–15. [DOI] [PubMed] [Google Scholar]
- 35.Escobar JI, Golding JM, Hough RL, Karno M, Burnam MA, Wells KB. Somatization in the community: relationship to disability and use of services. Am J Public Health 1987;77:837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris AM, Orav EJ, Bates DW, Barsky AJ. Somatization increases disability independent of comorbidity. J Gen Intern Med 2009;24:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoedeman R, Blankenstein AH, Krol B, Koopmans PC, Groothoff JW. The contribution of high levels of somatic symptom severity to sickness absence duration, disability and discharge. J Occup Rehabil 2010;20:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markkula R, Kalso E, Huunan-Seppälä A, Koskenvuo M, Koskenvuo K, Leino-Arjas P, Kaprio J. The burden of symptoms predicts early retirement: a twin cohort study on fibromyalgia-associated symptoms. Eur J Pain 2011;15:741–7. [DOI] [PubMed] [Google Scholar]
- 39.Kamaleri Y, Natvig B, Ihlebaek CM, Bruusgaard D. Does the number of musculoskeletal pain sites predict work disability? A 14-year prospective study. Eur J Pain 2009;13:426–30. [DOI] [PubMed] [Google Scholar]
- 40.Rask MT, Rosendal M, Fenger-Grøn M, Bro F, Ørnbøl E, Fink P. Sick leave and work disability in primary care patients with recent-onset multiple medically unexplained symptoms and persistent somatoform disorders: a 10-year follow-up of the FIP study. Gen Hosp Psychiatry 2015;37:53–9. [DOI] [PubMed] [Google Scholar]
- 41.Dimsdale JE, Creed F, Escobar J, Sharpe M, Wulsin L, Barsky A, Lee S, Irwin MR, Levenson J. Somatic symptom disorder: an important change in DSM. J Psychosom Res 2013;75:223–8. [DOI] [PubMed] [Google Scholar]
- 42.Toussaint A, Murray AM, Voigt K, Herzog A, Gierk B, Kroenke K, Rief W, Henningsen P, Löwe B. Development and validation of the Somatic Symptom Disorder-B Criteria Scale (SSD-12). Psychosom Med 2016;78:5–12. [DOI] [PubMed] [Google Scholar]
- 43.Budtz-Lilly A, Fink P, Ørnbøl E, Vestergaard M, Moth G, Christensen KS, Rosendal M. A new questionnaire to identify bodily distress in primary care: the 'BDS checklist'. J Psychosom Res 2015;78:536–45. [DOI] [PubMed] [Google Scholar]
- 44.Stapelfeldt CM, Jensen C, Andersen NT, Fleten N, Nielsen CV. Validation of sick leave measures: self-reported sick leave and sickness benefit data from a Danish national register compared to multiple workplace-registered sick leave spells in a Danish municipality. BMC Public Health 2012;12:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen JS, Olivarius Nde NF, Krasnik A. The Danish National Health Service Register. Scand J Public Health 2011;39:34–7. [DOI] [PubMed] [Google Scholar]
- 46.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39:30–3. [DOI] [PubMed] [Google Scholar]
- 47.Rief W, Rojas G. Stability of somatoform symptoms—implications for classification. Psychosom Med 2007;69:864–9. [DOI] [PubMed] [Google Scholar]
- 48.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry 1988;45:1069–77. [DOI] [PubMed] [Google Scholar]
- 49.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry 1992;49:624–9. [DOI] [PubMed] [Google Scholar]
- 50.Leiknes KA, Finset A, Moum T, Sandanger I. Overlap, comorbidity, and stability of somatoform disorders and the use of current versus lifetime criteria. Psychosomatics 2008;49:152–62. [DOI] [PubMed] [Google Scholar]
- 51.Simon GE, Gureje O. Stability of somatization disorder and somatization symptoms among primary care patients. Arch Gen Psychiatry 1999;56:90–5. [DOI] [PubMed] [Google Scholar]
- 52.Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med 2007;69:881–8. [DOI] [PubMed] [Google Scholar]
- 53.Schaefert R, Hausteiner-Wiehle C, Häuser W, Ronel J, Herrmann M, Henningsen P. Non-specific, functional, and somatoform bodily complaints. Dtsch Arztebl Int 2012;109:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fjorback LO, Arendt M, Ornbøl E, Walach H, Rehfeld E, Schröder A, Fink P. Mindfulness therapy for somatization disorder and functional somatic syndromes: randomized trial with one-year follow-up. J Psychosom Res 2013;74:31–40. [DOI] [PubMed] [Google Scholar]
- 55.Schröder A, Rehfeld E, Ornbøl E, Sharpe M, Licht RW, Fink P. Cognitive-behavioural group treatment for a range of functional somatic syndromes: randomised trial. Br J Psychiatry 2012;200:499–507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


