Supplemental Digital Content is Available in the Text.
Inhibitors of leukocyte elastase inhibit spontaneous and evoked pain behaviors in mouse models of chronic pain of neuropathic, cancer, and diabetic origins.
Keywords: Cancer pain, Neuropathic pain, Diabetic neuropathy, Sivelestat, Elastatinal, SSR-69071, T-cells, Neutrophils
Abstract
Neuropathic pain is an integral component of several chronic pain conditions and poses a major health problem worldwide. Despite emerging understanding of mechanisms behind neuropathic pain, the available treatment options are still limited in efficacy or associated with side effects, therefore making it necessary to find viable alternatives. In a genetic screen, we recently identified SerpinA3N, a serine protease inhibitor secreted in response to nerve damage by the dorsal root ganglion neurons and we showed that SerpinA3N acts against induction of neuropathic pain by inhibiting the T-cell- and neutrophil-derived protease, leucocyte elastase (LE). In the current study, via detailed in vivo pharmacology combined with analyses of evoked- and spontaneous pain-related behaviors in mice, we report that on systemic delivery, a single dose of 3 independent LE inhibitors can block established nociceptive hypersensitivity in early and late phases in the spared nerve injury model of traumatic neuropathic pain in mice. We further report the strong efficacy of systemic LE inhibitors in reversing ongoing pain in 2 other clinically relevant mouse models—painful diabetic neuropathy and cancer pain. Detailed immunohistochemical analyses on the peripheral tissue samples revealed that both T-Lymphocytes and neutrophils are the sources of LE on peripheral nerve injury, whereas neutrophils are the primary source of LE in diabetic neuropathic conditions. In summary, our results provide compelling evidence for a strong therapeutic potential of generic LE inhibitors for the treatment of neuropathic pain and other chronic pain conditions harboring a neuropathic pain component.
1. Introduction
Despite the increasing burden of neuropathic pain on human health and the economy,9,36,49 the understanding of its underlying mechanisms is incomplete and available treatment options are suboptimal.8 Neuropathic pain is an integral component of several other chronic pain conditions, such as cancer pain,12 diabetic neuropathy,39 pain caused by spinal cord injury,18 amongst others, and requires novel therapeutic strategies.50
Using a genome-wide screening approach in rodents, we recently identified SerpinA3N, a serine protease inhibitor of the serpin family secreted by sensory neurons upon nerve injury, to be a key modulator of neuropathic hypersensitivity.46 We further identified a protease, leukocyte elastase (LE), to be the key substrate for SerpinA3N in the dorsal root ganglion (DRG) and we reported that mice lacking LE show deficits in the generation of the neuropathic pain.46 Upon screening a large set of different types of neuronal and nonneuronal cell types, including diverse glia, we identified neutrophils and T-lymphocytes infiltrating into the affected DRG to be the source of LE.46 Thus, our previous analyses suggest that LE is a promising target in neuropathic pain.
However, a number of key questions pertaining to its potential therapeutic relevance remained open. Efficacy was seen with intrathecal delivery only over early phases of mechanical hypersensitivity in the spared nerve injury (SNI) model.46 It is unclear about its systemic efficacy and whether this mechanism pertains to established neuropathic pain, which is clinically more relevant. Moreover, key pharmacological parameters, such as dose-response relationships, efficacy studies, analyses of additional, more specific inhibitors of LE, remain to be clarified. Importantly, from the therapeutic point of view, it is important to clarify whether mechanisms involving LE also contribute to the pathophysiology of diverse types of chronic pain conditions involving a neuropathic component or whether they are restricted to traumatic nerve injury. Finally, given that recruitment of distinct classes of immune cells, including T-cells and neutrophils, in diverse locations of the somatosensory axis in neuropathic conditions has been reported,6,10,17,25,34 it is important to address whether peripheral avenues, which were not explored with respect to LE so far, also contribute to the pathophysiology of neuropathic pain.
Several small molecular weight inhibitors have been recently developed to inhibit LE, which show efficacy in lung injury associated symptoms,1,19,23,29,33,45 amongst which sivelestat is approved for clinical usage in Japan and the Republic of Korea.2 However, none of them have been tested in the context of neuropathic pain so far.
In the current study, we investigated the effect 3 independent LE inhibitors administered systemically on established hypersensitivity in early and late phases of neuropathic pain on peripheral nerve injury in mice. We also investigated the effect of systemically administered LE inhibitors on nociceptive hypersensitivity in cancer pain and diabetic pain, which also involve neuropathic pain components, and addressed clinically important spontaneous pain. Finally, we addressed the sources of LE secretion in neuropathic pain conditions in peripheral tissues in chronic pain models in mice.
2. Materials and methods
2.1. Animal experiments
All animal usage procedures were in accordance with ethical guidelines laid down by the local governing body (Regierungspräsidium Karlsruhe). All behavioral measurements were done in awake, unrestrained, age‐matched, more than 8 weeks old, male and female C57/Bl6 mice. Adult female Balb/c mice were used in a model of cancer-induced bone pain (CIBP). Mice were housed in individually ventilated cages in groups under ambient humidity and light conditions on a 12 hour-light-dark cycle with light commencing at 08:00 am and had ad libitum access to food and water.
2.2. Spared nerve injury
Spared nerve injury was performed as explained previously.11 Briefly, mice were anesthetized with 2% isoflurane and the sciatic nerve and its 3 terminal branches (sural, common peroneal and tibial nerves) were exposed via an incision made directly through the biceps femoris muscle. The common peroneal and tibial nerve were tightly ligated with 5.0 silk thread and sectioned distal to the ligation, removing approximately 3 mm of the distal nerve stump. These 2 nerves were subsequently cut and the sural nerve was left intact. The mice were housed under standard conditions in cages until behavioral experiments. For sham surgery, the same procedure was following except the ligation part.
2.3. Cancer-induced bone pain
Cells of the murine osteolytic breast carcinoma cell line, 4T1-Luc expressing the luciferase reporter gene and female Balb/c strain of mice were used as described previously.7,41 The condyles of the left distal femur of the mouse were exposed following an arthrotomy. A hole was then drilled to create space for the injection needle. Using a 10-μL Hamilton syringe, 1.5 × 105 cells were directly injected into the intermedullary space of the mouse femur in a volume of 5 μL sterile 1X phosphate-buffered saline (PBS). The injection hole was sealed with dental cement to prevent leakage of the tumor cells. Different cohorts of mice were treated with sivelestat (20 mg/kg body weight) or saline in a volume of 100 μL intraperitoneally at day 28 posttumor implantation (PID).
2.4. Streptozotocin model of type 1 diabetic neuropathic pain
Diabetes was induced by administering 5 consequent dosages of streptozotocin (STZ, S0130 Sigma), dissolved in 0.05-M citrate buffer, at the concentration of 60 mg/kg body weight intraperitoneally with 24 hours interval. Mice receiving an equal volume of 0.05 M citrate buffer served as sham controls. Blood glucose was monitored at regular intervals and appropriate units of insulin are supplemented subcutaneously to keep the blood glucose levels under control. Different cohorts of mice were treated with sivelestat (20 mg/kg body weight) or saline in a volume of 100 μL intraperitoneally at 8 W post-diabetes induction.
2.5. Intraperitoneal delivery of drugs
Sivelestat (S7198, Sigma) and Elastatinal (sc-201,272, Santa Cruz biotechnology) were dissolved in saline solution and injected intraperitoneally (i.p.) at a required concentration in 100 μL volume. An equal volume of 1X PBS was injected as vehicle control into a separate group of mice. SSR-69,071 (sc-203702, Santa Cruz biotechnology or 2506, R and D systems) was dissolved in 100% DMSO and injected i.p. at a required concentration in 40 μL volume. An equal volume of 100% DMSO was injected as vehicle control into a separate group of mice.
2.6. Behavioral analyses
In all behavioral tests, the experimenter was blinded to the identity of the drug treatments that mice received.
2.7. Nociceptive tests
Responses to paw pressure were determined using von Frey filaments as described previously.5,46 Briefly, mice were acclimatized to the testing environment for 1 hour per day for at least 2 days prior to the behavioral testing. Then mechanical sensitivity was determined with a graded series of von Frey filaments of 0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1, and 1.4 g strength. In the SNI model, the stimuli were applied within the sural nerve territory (lateral part of the hind paw). Each filament was tested 5 times in increasing order starting with the filament producing the lowest force. For baseline mechanical sensitivity test, all filaments were applied and the number of withdrawals was recorded. Withdrawal frequency was calculated as a percentage of withdrawals out of the total number of von Frey applications per filament. For tactile allodynia, the minimal force filament for which animals presented either a brisk paw withdrawal and/or an escape attempt in response to at least 5 stimuli determined the mechanical response threshold, which was defined as the minimum pressure required for eliciting 60% of withdrawal responses out of 5 stimulations and measured in grams (force application).
For testing cold hypersensitivity, the cold plate test was performed with a Hot-Cold Plate (Bioseb, Vitrolles, France) set at 4°C. The mice were placed on the cold plate for a maximum of 30 seconds and the latency until the first withdrawal response of the injured hind paw was recorded.
In STZ model for type I diabetes, withdrawal latency to infrared heat was measured before and at specified time points following i.p. sivelestat injection according to the Hargreaves method using a Plantar test apparatus (Ugo Basile Inc.).
2.8. Spontaneous pain behavior in cancer pain model
Spontaneous nocifensive behavior in the mice with cancer pain was assessed as explained previously.28,37 Briefly, the mice were placed on an open wire mesh bottom and were allowed to habituate for 20 minutes. Following acclimatization, mice were video recorded and returned back to their cages. Time spent in spontaneous pain-related behavior over the 5 minutes time was recorded manually from the offline video records. Parameters previously reported28 for counting nocifensive behavior were included (1) spontaneous guarding (lifting the affected limb and holding it against the body), (2) flinching, and (3) sporadic hopping or limping (intermittent jumps without using the affected limb while moving). Spontaneous nocifensive behavior was assessed before and at 4 hours following i.p. sivelestat or vehicle injection on day 28 following tumor cell implantation (PID18). The experimenter was blinded to the experimental conditions of the mice.
2.9. Voluntary wheel running
Animals were placed individually in cages containing a running wheel and free access to food and water. We started this measurement in the morning around 7 am for a total duration of 4 hours on both testing days. Unrestricted voluntary wheel running activity was digitally recorded using the AWM counter (Lafayette Instrument, LA), which uses an optical sensor to detect the total revolutions of the wheel and is connected to a USB Interface and PC running an AWM Software (Lafayette Instrument, LA). Voluntary wheel running behavior was measured in mice before SNI and day 8 following SNI. Mice were injected with vehicle or i.p. sivelestat at 50 mg/kg body weight on day 8 before placing into the wheel running cage.
2.10. Gait analysis
We performed the Catwalk test as described in detail previously35 using the CatWalk XT version 10.6 gait analysis system (Noldus, Netherlands system). Briefly, mice were habituated to the CatWalk setup and allowed to cross the ramp for 3 sessions. On each testing day, animals were allowed to cross the corridor voluntarily for 3 times and the average of 3 runs was counted as 1 reading. Using the Illuminated footprints technology, paws are captured by a high-speed video camera positioned underneath the glass. The system enables an automatic footprint classification, error correction, interactive footprint measurements, and data segmentation profiling. The parameters analyzed in this study include “Stand” representing the duration of the paw contact to the surface during a run; “Pawprint area” representing the surface of the complete print of a paw; and “Maximal contact area” representing the maximum intensity of the paw during a run. We analyzed the mice before the SNI (basal reading) and at 4 W following SNI. At 4 W, the mice were analyzed before and after 4 hours following i.p. sivelestat at 50 mg/kg or vehicle injection.
2.11. Immunohistochemical and immunofluorescence analysis
Anti-CD3 and anti-Gr1 staining were performed as previously described in detail.46 Briefly, animals were perfused transcardially with PBS followed by 4% paraformaldehyde (PFA). L3 and L4 DRG, sciatic nerve and paws were collected. In the case of SNI, the sciatic nerve was collected the site of ligation including approximately 1 cm of the proximal part. Paw skin was taken out using a biopsy punch. Tissues were postfixed for up to 16 hours in 4% PFA at 4°C. Spinal cords, DRG, and paw skin were stored in 0.5% PFA at 4°C for up to 2 weeks and incubated in 30% sucrose solution overnight at 4°C for cryostat sectioning. Tissue sections were cut at 20, 16, and 25 μm for DRG, sciatic nerve, and paw skin, respectively.
For the immunohistochemical staining using the PE/Cy7 antimouse Ly-6G/Ly-6C (Gr-1) antibody (1:2000; BioLegend, cat. no. 108,416), and for immunofluorescence staining using rat monoclonal anti-CD3 (clone 17A2) antibody (1:100; BD Biosciences, cat. no. 555,273), we followed the protocol described in detail in our previous study.46 Images were captured using an upright microscope (Nikon NiE, Nikon) equipped with Nikon Plan Apo objective set and high-resolution CCD camera (Nikon DS-Ri1, Nikon). Acquisition process was driven by NIS-Elements software 4.1 (Nikon). Fluorescence images were obtained using a laser scanning spectral confocal microscope (Leica TCS SP8 AOBS; Leica Microsystems, Bensheim, Germany).
The numbers of CD3-positive and Gr-1-positive cells within a tissue section were estimated by counting them directly under the microscope. The final value corresponds to the average number of positive cells within every section from the same experimental group. In the case of DRG, SC, and paw skin tissues, serial sections were collected such that each slide contains at least 10 different sections and 1 slide was taken as representative of a mouse for a particular antibody. In the case of sciatic nerve tissue, 6 to 8 tissue sections were collected onto each slide. CD3 or Gr1-positive cells were counted from each section and averaged to represent number of positive cells per section for each individual mouse, which was thus considered 1 biological replicate. In all cases, values from 3 to 5 mice were used to calculate the mean value, SEM, and statistical significance.
2.12. Statistical analyses
For all measurements, data were calculated and presented as mean ± SEM. Two-way analysis of variance for repeated measures followed by Tukey test was used to determine statistical significances in all behavioral experiments. P ≤ 0.05 was considered significant. For all statistical analyses, the appropriate statistical tests were chosen, the data met the assumptions of the test and the variance between the statistically compared groups was similar.
In all of the behavioral analyses described, unless specified otherwise, *denotes P ≤ 0.05 as compared to basal values, †denotes P ≤ 0.05 relative to the corresponding vehicle for each particular time point of analysis; 2‐way analysis of variance of repeated measures was performed followed by Tukey post hoc test and n = 6 to 18 mice per group.
3. Results
3.1. Effects of systemic delivery of sivelestat on mechanical hypersensitivity, cold allodynia, and motor behavior following nerve injury
To understand the effect of systemic sivelestat administration on the early phase of mechanical hypersensitivity in neuropathic conditions, mice were tested behaviorally on postoperative day 8 following SNI (POD8). The mice were then intraperitoneally (i.p.) injected with a single dose of varying concentrations of sivelestat ranging from 0.2 to 50 mg/kg body weight or vehicle. Mechanical hypersensitivity was measured at 1, 3, 6 and 24 hours following i.p. sivelestat or the vehicle injection as the response frequency to the intraplantar application of a range of calibrated von Frey filaments (ie, 0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1, and 1.4 g) (Fig. 1, panel A). In all the mechanical hypersensitivity data presented here, the response frequency from a representative filament of 0.16 g is presented. While both cohorts of mice developed significant mechanical hypersensitivity on day 8 of SNI as compared to basal behavior, the magnitude of mechanical hypersensitivity was significantly lesser at 1 hour and returned to pre-sivelestat levels by 3 hours following sivelestat injection in the mice injected with 0.2 mg/kg sivelestat as compared to the mice injected with vehicle. Mice injected with 2 or 20 mg/Kg of i.p. sivelestat showed significantly lesser mechanical hypersensitivity until 6 hours and displayed comparable mechanical hypersensitivity at 24 hours following sivelestat injection as compared to the mice injected with vehicle. Mice injected with 50 mg/Kg of i.p. sivelestat showed significantly lesser mechanical hypersensitivity until 24 hours following sivelestat injection as compared to the mice injected with vehicle (Fig. 1, panel B). The same observations were also recapitulated by comparing the 60% response threshold (supplemental Fig. 1, panel A; available online at http://links.lww.com/PAIN/A476) or area under the curve of stimulus–response frequency curves for all 8 tested von Frey forces (supplemental Fig. 1, panel B; available online at http://links.lww.com/PAIN/A476). Analyses of withdrawal frequency, mechanical response threshold or mechanical sensitivity data from the paw contralateral to the SNI operated paw demonstrated no change in the mechanical hyperalgesia on day 8 following SNI as compared to basal readings or at 1, 3, 6, or 24 hours following i.p. sivelestat injection as compared to the mice injected with vehicle (supplemental Fig. 2, panels A, B, and C; available online at http://links.lww.com/PAIN/A476).
Figure 1.
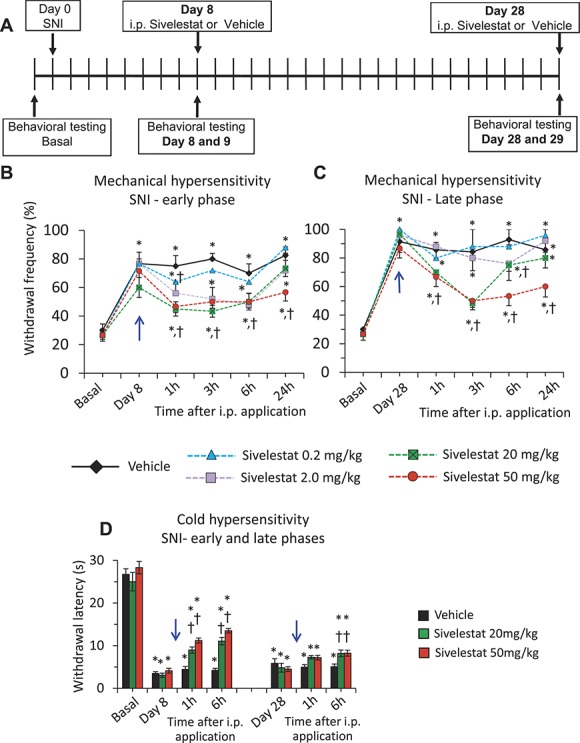
Dose-dependent effects of systemic delivery of sivelestat in the spared nerve injury (SNI) model of neuropathic pain. Analysis of SNI-induced neuropathic mechanical and cold hypersensitivity following intraperitoneal application of leucocyte elastase inhibitor, sivelestat, as compared to the vehicle-injected group. A single dose of 0.2 or 2.0 or 20 or 50 mg/kg body weight sivelestat was injected i.p. on day 8 or day 28 post-SNI (blue arrow). In all panels, *denotes P ≤ 0.05 as compared to basal, †represents P ≤ 0.05 as compared to the vehicle-treated group at respective time point, 2-way analysis of variance of repeated measures followed by Turkey hoc test; n = at least 6 mice per group. (A) Schematic illustration of the experimental protocol followed to analyze the impact of systemic sivelestat on mechanical and cold hypersensitivity at early and late phases following SNI. (B) Paw withdrawal responses to von Frey force of 0.16 g before SNI operation (basal) or at 1, 3, 6, and 24 hours following each dosage of i.p. sivelestat or Vehicle on day 8 following SNI. (C) Paw withdrawal responses to von Frey force of 0.16 g before SNI operation (basal) or at 1, 3, 6, and 24 hours following each dosage of i.p. sivelestat or Vehicle on day 28 following SNI. In panels B and C, n = 20 mice for the vehicle group, 18 mice for the 20 mg/kg group, 6 mice for the 0.2 mg/kg group, and 8 mice for other 2 groups. (D) Spared nerve injury–induced neuropathic cold hypersensitivity (4°C) before and 1 and 6 hours following i.p. sivelestat or vehicle on day 8 and 28 following SNI. n = 12 mice for the vehicle group and 8 each for 20 and 50 mg/kg groups.
In the next set of experiments, we asked the question whether systematically applied sivelestat affords comparable protection against neuropathic mechanical hypersensitivity at late phase's post-SNI. Mice were analyzed for mechanical hypersensitivity at 4 weeks (W) following SNI and same experimental paradigm explained above for the early phase analyses was followed. As observed at POD8 following SNI, both the cohorts of mice exhibited significant mechanical hypersensitivity at 4 W following SNI as compared to basal behavior (Fig. 1, panel C). While the magnitude of mechanical hypersensitivity was unchanged in the mice injected with 0.2 mg/Kg sivelestat, the mice injected with 2 mg/kg sivelestat showed significantly less mechanical hypersensitivity at 6 hours, which returned to pre-sivelestat levels by 24 hours, as compared to the mice injected with vehicle. Mice injected with 20 mg/kg sivelestat showed significantly lesser mechanical hypersensitivity at 1, 3 and 6 hours, and returned to pre-sivelestat levels by 24 hours, following sivelestat injection as compared to mice injected with vehicle. In the case of mice injected with 50 mg/kg sivelestat, the magnitude of mechanical hypersensitivity was significantly less at all the time points tested as compared to the mice injected with vehicle (Fig. 1, panel C). Analyses of the response frequency to higher magnitudes of mechanical stimulation, such as von Frey strength of 1.4 g, which represents nociceptive strength stimulation, also revealed significant reduction in the SNI-induced mechanical sensitivity at 1, 3, and 6 hours following 20 mg/kg i.p. sivelestat injection on day 8 post-SNI (supplemental Fig. 1, panel A; available online at http://links.lww.com/PAIN/A476) and at 3 hours following 20 mg/kg i.p. sivelestat injection at 4 W post-SNI (supplemental Fig. 1, panel B; available online at http://links.lww.com/PAIN/A476) as compared to the vehicle-injected group. The same observations were also confirmed by comparing the 60% response threshold (Suppl. Fig. 2, panels A and B; available online at http://links.lww.com/PAIN/A476) or area under the curve of stimulus–response frequency curves for all 8 tested von Frey forces (supplemental Fig. 2, panels C and D; available online at http://links.lww.com/PAIN/A476). Analyses of the data from the paw contralateral to the SNI operated paw demonstrated no significant change in the mechanical hyperalgesia at 4 W following SNI or at 1, 3, 6 or 24 hours following i.p. sivelestat injection as compared to the basal readings or the mice injected with vehicle, respectively (supplemental Fig. 3, panels A–F; available online at http://links.lww.com/PAIN/A476).
To address potential sex-specific differences in the magnitude of protection afforded by i.p. sivelestat against SNI-induced hypersensitivity, we analyzed male and female mice separately following the i.p. injection of vehicle or sivelestat at 20 mg/kg. Analyses of withdrawal frequency to graded von Frey filaments at 1, 3, 6, and 24 hours following i.p. sivelestat or vehicle injection revealed that both male and female mice demonstrated similar temporal patterns of reduction in mechanical hypersensitivity at early (1 W) and late phases (4 W) post-SNI (supplemental Fig. 4, panels A and B; available online at http://links.lww.com/PAIN/A476).
We then tested the impact of systemically administered sivelestat on cold hypersensitivity that is classically seen in neuropathic pain states. Mice were tested for cold allodynia on day 8 following SNI, which was evident as a significant drop in withdrawal latency towards 4°C temperature (Fig. 1, panel D), then treated with 20 or 50 mg/kg sivelestat i.p. or vehicle and behaviors measured at 1 and 6 hours thereafter. Although vehicle injection did not change behaviors significantly, sivelestat partially, but significantly, alleviated neuropathic cold allodynia at day 8 as well as at 4 W (Fig. 1, panel D). The magnitude of change with sivelestat was considerably stronger in the early phase, as compared to the late phase, of cold allodynia.
3.2. Effects of systemic delivery of sivelestat on changes in gait and voluntary behaviors associated with neuropathic pain
To analyze voluntary activity parameters, mice were placed in a cage with free access to a running wheel and voluntary behavioral parameters were analyzed. To facilitate comparisons over the same window of the circadian rhythm, running wheel data at basal (before SNI) and on day 8 following SNI were always collected for the same 4 hours during the morning. On day 8, either sivelestat or vehicle was injected i.p. before placing the mice into the running wheel environment. Analysis of the number of wheel revolutions made revealed that both the groups of mice have same activity before SNI. Whereas mice injected with vehicle showed significantly lowered running wheel activity at day 8 post-SNI, as published previously,35 mice injected with i.p. sivelestat did not show a decrease over baseline and showed significantly higher activity as compared to the mice injected with vehicle (Fig. 2, panel B).
Figure 2.
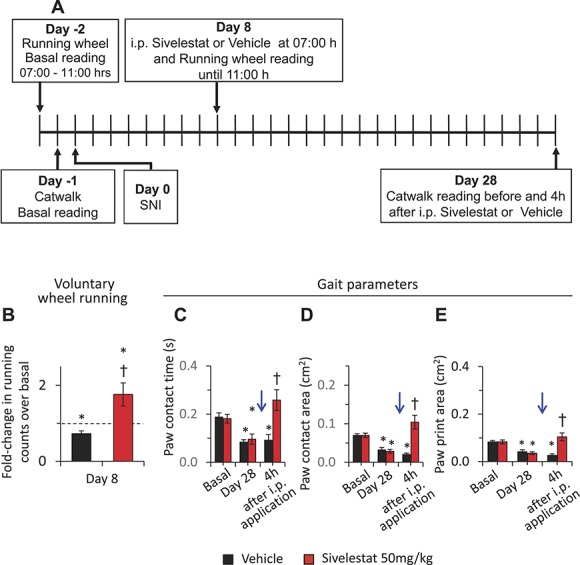
Effects of systemic delivery of sivelestat on voluntary wheel running and gait in neuropathic pain. Analysis of spared nerve injury (SNI)-induced voluntary wheel running behavior and gait following intraperitoneal application of leucocyte elastase inhibitor, sivelestat, as compared to the vehicle-injected group. A single dose of 50 mg/kg body weight sivelestat was injected i.p. on day 8 or day 28 post-SNI (blue arrow). (A) Schematic illustration of the experimental protocol followed to analyze the impact of systemic sivelestat on gait and voluntary wheel running in neuropathic pain conditions. (B) Number of wheel turns performed by the mice in voluntary wheel running experiment in the group of mice injected with vehicle (black bar) or with sivelestat at 50 mg/kg on day 8 following SNI as compared to the basal readings in the respective group of mice. A single dose of 50 mg/kg body weight sivelestat or vehicle was injected i.p. on day 8 post-SNI before placing the mice in voluntary wheel running setup. Cumulative wheel running counts during 3 hours of exposure to the running wheel are presented. *denotes P ≤ 0.05 as compared to basal t test, n = 6 mice per group. (C–E) Three different gait parameters analyzed by catwalk system. Duration of paw contact (C), the intensity of the paw contact measured as the maximum area (D) and area of total paw contact (E) from the paw ipsilateral to the SNI surgery are presented before SNI (basal) and at 4 hours following i.p. sivelestat or vehicle injection on day 28 after SNI. *denotes P ≤ 0.05 as compared to basal, †represents P ≤ 0.05 as compared to the vehicle-treated group at respective time point, 2-way analysis of variance of repeated measures followed by Turkey post hoc test; n = 8 mice per group.
In the next set of experiments, distinct parameters of the animal gait were analyzed before SNI and at 4 W post-SNI before and 4 hours after injection of i.p. vehicle or 50 mg/kg sivelestat. Comparison of the duration of contact with the surface or maximum contact area or print area of the paw ipsilateral to the SNI surgery revealed that the basal values are comparable in both the groups before SNI. At 4 W following SNI, all the 3 parameters were significantly reduced in both the groups of mice as compared to the basal values (Fig. 2, panels C, D, and E). At 4 hours following injection, the mice treated with i.p. sivelestat exhibited significantly higher values as compared to the mice injected with vehicle (Fig. 2, panels C, D, and E). Analysis of same parameters in sham-operated mice before surgery or at 4 hours following i.p. sivelestat at 4 W following sham surgery revealed that i.p. sivelestat had no impact on gait-related parameters in both the ipsi- and contra-lateral paws to the operated paw (supplemental Fig. 5, available online at http://links.lww.com/PAIN/A476). Thus, the observed effects of sivelestat did not arise due to unspecific modulation of gait, but rather by alleviating SNI-induced defects in gait.
3.3. Comparison of the impact of systemic sivelestat and pregabalin on neuropathic pain
In the next set of experiments, we set out to compare the magnitude of protection exerted by the systemic sivelestat to that of pregabalin, one of the prominently used drugs in the clinic for the treatment of neuropathic pain.26,32 For this, the same experimental paradigm explained above for Figure 1 and depicted in Figure 3 panel A was followed on a new cohort of mice. On i.p. injection of sivelestat or pregabalin or vehicle on day 8 following SNI, the withdrawal frequency in the sivelestat- or pregabalin-injected groups was significantly lesser at 1, 3, and 6 hours following i.p. injection as compared to the vehicle-injected group and the analgesic effect was lost when tested at 24 hours after drug administration (Fig. 3, panel B). Analysis of 60% response threshold also revealed the same observations (Fig. 3, panel C). While the protection against the mechanical sensitivity in the pregabalin-injected group lasted for up to 3 hours post i.p. injection, it lasted until 6 hours post i.p. injection in the sivelestat-injected group. In the late phase of neuropathic pain, that is, at 4 W following SNI, the withdrawal frequency in the sivelestat- or pregabalin-injected groups was significantly lesser at 1, 3, and 6 hours, but not at 24 hours, following i.p. injection as compared to the vehicle-injected group (Fig. 3, panel D); similar results were seen on comparing withdrawal thresholds (Fig. 3, panel E), although pregabalin showed a stronger impact than sivelestat on response thresholds at 1 and 3 hours at this late stage post-SNI. Sham-treated mice showed no changes at 8 days or 4 W following sham surgery with either sivelestat or pregabalin treatment (supplemental Fig. 6, panels A–D; available online at http://links.lww.com/PAIN/A476).
Figure 3.
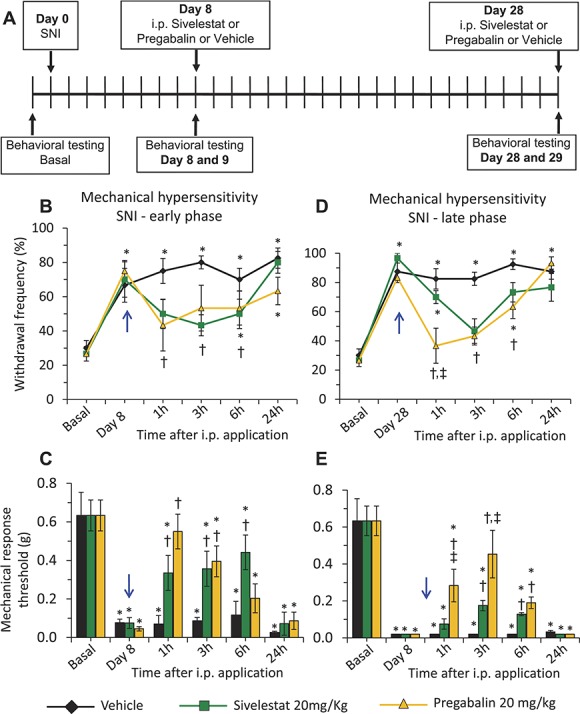
Comparison of effects of systemic delivery of sivelestat or pregabalin on neuropathic pain. Analysis of SNI-induced neuropathic mechanical hypersensitivity following intraperitoneal application of leucocyte elastase inhibitor, sivelestat, or pregabalin as compared to the vehicle-injected group. A single dose of 20 mg/kg body weight sivelestat or pregabalin was injected i.p. on day 8 or day 28 after spared nerve injury (SNI) (blue arrow). In all panels, *denotes P ≤ 0.05 as compared to basal, †represents P ≤ 0.05 as compared to the vehicle-treated group at respective time point, 2-way analysis of variance of repeated measures followed by Turkey post hoc test; n = at least 6 mice per group. (A) Schematic illustration of the experimental protocol followed to analyses the impact of systemic sivelestat or pregabalin on mechanical hypersensitivity in early and late phases following SNI. (B) Paw withdrawal responses to von Frey force of 0.16 g at 1, 3, 6, and 24 hours following each dosage of i.p. sivelestat or pregabalin on day 8 following SNI. n = 8 mice per group. (C) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before SNI (basal) or at 1, 3, 6, and 24 hours following each dosage of i.p. sivelestat or pregabalin or vehicle on day 8 following SNI. n = 8 mice per group. (D) Paw withdrawal responses to von Frey force of 0.16 g at 1, 3, 6 and 24 hours following each dosage of i.p. sivelestat or pregabalin on day 28 following SNI. n = 6 mice per group. (E) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before SNI (basal) or at 1, 3, 6, and 24 hours following each dosage of i.p. sivelestat or pregabalin or vehicle on day 28 following SNI. n = 6 mice per group.
3.4. Effects of other leucocyte elastase inhibitors on neuropathic pain
To judge whether the beneficial effects of sivelestat represent a mechanistic class effect, we then tested other LE inhibitors in the same paradigm. On testing Elastatinal, a potent LE inhibitor,33,43 with the same experimental protocol described for testing sivelestat (Fig. 1, panel A), we observed that mice injected with 20 or 50 mg/kg of Elastatinal showed significantly less mechanical hyperalgesia at 1, 3, 6, and 24 hours following i.p. injection as compared to the mice injected with the vehicle on day 8 post-SNI (Fig. 4, panel A). Analyzing 60% response thresholds showed similar results (Fig. 4, panel B). At 4 W following SNI, mice injected with 20 or 50 mg/kg of Elastatinal showed significantly less mechanical hyperalgesia at 1, 3, and 6 hours, but not at 24 hours, following i.p. injection as compared to the mice injected with vehicle (Fig. 4, panels C and D).
Figure 4.
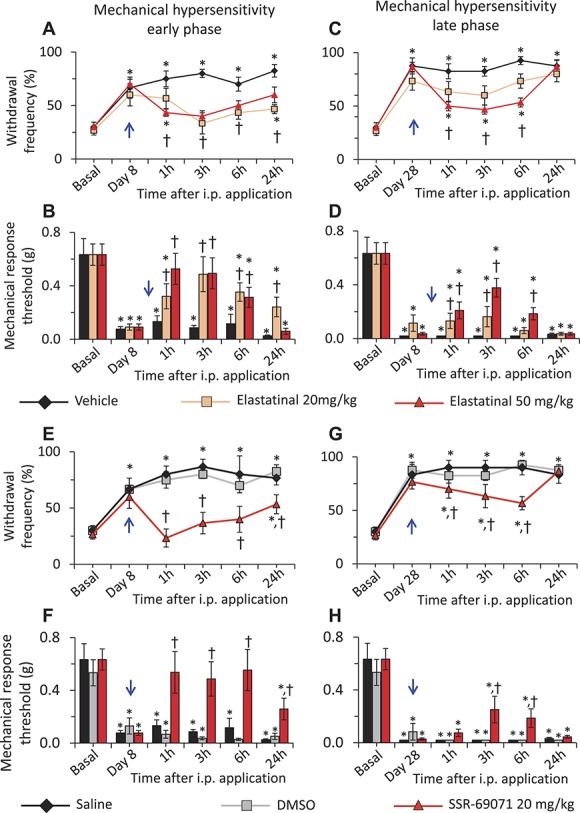
Dose-dependent effects of leucocyte elastase (LE) inhibitors, Elastatinal and SSR-69701, on neuropathic pain. Analysis of SNI-induced neuropathic mechanical hypersensitivity following the intraperitoneal application of LE inhibitors, Elastatinal or SSR-69701 as compared to the vehicle-injected group. A single dose of 20 or 50 mg/kg body weight Elastatinal or 20 mg/kg body weight SSR-69701 was injected i.p. on day 8 or day 28 post-SNI (blue arrow). In all panels, *denotes P ≤ 0.05 as compared to basal, †represents P ≤ 0.05 as compared to the vehicle-treated group at respective time point, 2-way analysis of variance of repeated measures followed by Turkey post hoc test; n = at least 6 mice per group. (A) Paw withdrawal responses to von Frey force of 0.16 g before SNI (basal) and at 1, 3, 6, and 24 hours following each dosage of i.p. Elastatinal or vehicle on day 8 following SNI. (B) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before SNI (basal) or at 1, 3, 6 and 24 hours following each dosage of i.p. Elastatinal or vehicle on day 8 following SNI. (C) Paw withdrawal responses to von Frey force of 0.16 g before SNI (basal) and at 1, 3, 6 and 24 hours following each dosage of i.p. Elastatinal or vehicle on day 28 following SNI. (D) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before SNI (basal) or at 1, 3, 6, and 24 hours following each dosage of i.p. Elastatinal or vehicle on day 28 following SNI. In panels A, B, C and D, n = 14 for the vehicle group and 9 each for the 20 and 50 mg/kg Elastatinal groups. (E) Paw withdrawal responses to von Frey force of 0.16 g before SNI (basal) and at 1, 3, 6 and 24 hours following each dosage of i.p. SSR-69701 or vehicle on day 8 following SNI. (F) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before SNI (basal) or at 1, 3, 6, and 24 hours following each dosage of i.p. SSR-69701 or vehicle on day 8 following SNI. (G) Paw withdrawal responses to von Frey force of 0.16 g before SNI (basal) and at 1, 3, 6, and 24 hours following each dosage of i.p. SSR-69701 or vehicle on day 28 following SNI. (H) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before SNI (basal) or at 1, 3, 6, and 24 hours following each dosage of i.p. SSR-69701 or vehicle on day 28 following SNI. In panels E, F, G, and H, n = 9 for the vehicle group, 6 for the DMSO group, and 8 for the SSR-69071 group.
We then tested the effect of another LE inhibitor, namely SSR-69071,45 on early and late phases of SNI-induced mechanical hypersensitivity. Because this compound is soluble only in 100% DMSO, it was to rule out any potential toxic effects of DMSO on in vivo application in mice. Mice injected with 40 μL of either i.p. Saline or DMSO showed similar mechanical sensitivity (Fig. 4, panels E–H). At 8 days following SNI, mice injected with 20 mg/kg of SSR-69071 showed significantly less mechanical hyperalgesia at 1, 3, 6 and 24 hours following i.p. injection as compared to the mice injected with vehicle (Fig. 4, panel E), which was also true on analyzing response thresholds (Fig. 4, panel F). As with Elastatinal, at 4 W following SNI, the antinociceptive effects of 20 mg/kg of SSR-69071 lasted until 6 hours, but not at 24 hours (Fig. 4, panels G and H).
3.5. Effects of sivelestat on other forms of neuropathic pain caused by cancer or diabetes
We next asked whether systemically applied LE inhibitors are able to exert protection on other forms of chronic pain in which neuropathic pain is an integral component. For this, we chose CIBP28 and the STZ model of type 1 diabetic neuropathic pain.44
Cancer-induced bone pain was induced by injecting osteolytic breast carcinoma cells into the femur and mice exhibited significant mechanical hypersensitivity at 4 W after tumor cell implantation as compared to basal behavior, as reported previously.28,37 On day 28 following tumor cell implantation, mechanical hypersensitivity was measured before and at 1, 3, 6 hours, 1 day, and 3 days after single i.p. injection of sivelestat at 20 mg/kg or vehicle (Fig. 5, panel A). Analyses of withdrawal frequency or 60% response threshold revealed that the magnitude of tumor-induced mechanical hypersensitivity was significantly less until 1 day in mice receiving sivelestat as compared to the mice injected with vehicle (Fig. 5, panels B and C). Importantly, when tested at 4 hours post i.p. sivelestat or vehicle injection, spontaneous nocifensive behavior, such as spontaneous guarding, flinching, sporadic hopping, or limping,28,37 was also significantly reduced in sivelestat-treated mice as compared to vehicle-treated mice (Fig. 5, panel D). Paws contralateral to the tumor cell–injected paw revealed no significant changes in the mechanical sensitivity before or at 1, 3, 6 hours, 1 day, or 3 days following i.p. injection of sivelestat or vehicle (supplemental Fig. 7, panels A and B; available online at http://links.lww.com/PAIN/A476).
Figure 5.
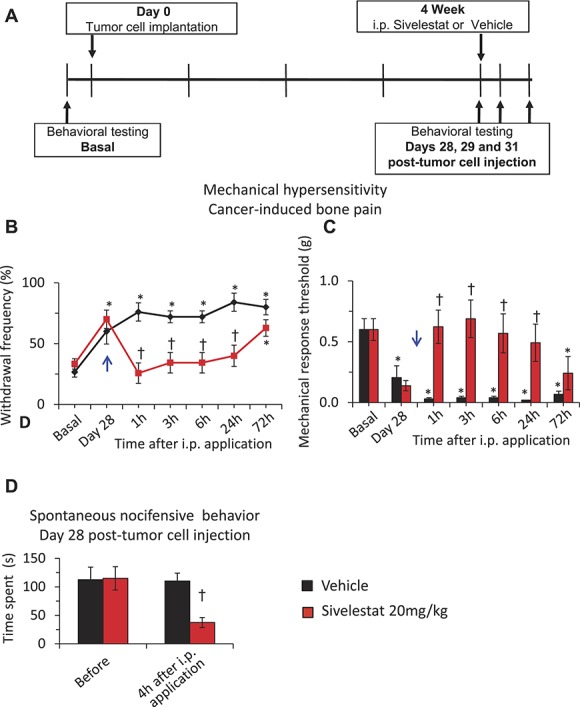
The impact of systemic delivery of sivelestat on neuropathic pain of cancer origin. Analysis of cancer-induced mechanical hypersensitivity following intraperitoneal application of leucocyte elastase inhibitor, sivelestat, as compared to the vehicle-injected group. A single dose of 20 mg/kg body weight sivelestat or an equal volume of vehicle was injected i.p. on day 28 (PID28) following tumor cell implantation in the femur (blue arrow). In all panels, *denotes P ≤ 0.05 as compared to basal, †represents P ≤ 0.05 as compared to the vehicle-treated group at respective time point, 2-way analysis of variance of repeated measures followed by Turkey post hoc test. (A) Schematic illustration of the experimental protocol followed to analyze the impact of systemic sivelestat on mechanical hypersensitivity in CIBP. (B) Paw withdrawal responses to von Frey force of 0.16 g before (basal) and at 1, 3, 6, 1 days and 3 days following 20 mg/kg body weight i.p. sivelestat or vehicle injection on day 28 following tumor cell implantation. (C) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before (basal) or at 1, 3, 6, 1 days and 3 days following 20 mg/kg body weight i.p. sivelestat or vehicle on day 28 following tumor cell implantation. (D) Spontaneous pain behavior calculated as the time spent in nocifensive behavior over 5 minutes duration before and at 4 hours following i.p. sivelestat or vehicle injection on day 28 following tumor cell implantation. In panels B, C, and D, n = 14 mice in the vehicle group and 12 mice in the sivelestat 20 mg/kg group.
In a model of type 1 diabetes induced by multiple treatments with STZ,44 male as well as female mice demonstrated significant mechanical hypersensitivity at 8 weeks after STZ treatment (Fig. 6, panel A). Analyses of withdrawal frequency or 60% response thresholds following i.p. injection of sivelestat at 20 mg/kg or vehicle showed that mice injected with sivelestat showed significantly lesser mechanical hypersensitivity at 1, 3 and 6 hours following i.p. injection as compared to the mice injected with vehicle (Fig. 6, panels A and B). Analyses of the 60% withdrawal threshold, revealed that there was no significant difference in the magnitude of protection afforded by i.p. sivelestat between male and female mice at 1, 3, and 6 hours following i.p. sivelestat as compared to respective vehicle-injected groups (Fig. 6, panel D), suggesting a lack of sex differences with respect to the antinociceptive actions of sivelestat.
Figure 6.
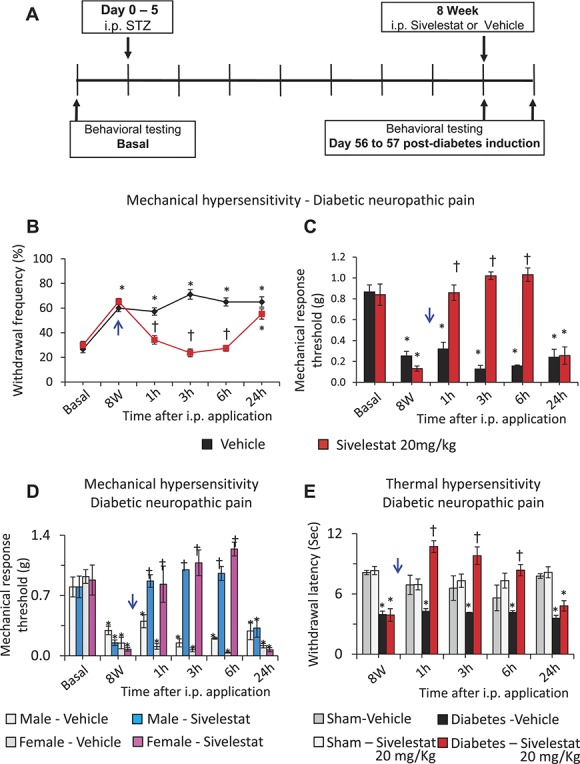
The impact of systemic delivery of sivelestat on neuropathic pain caused by diabetes. Analysis of cancer-induced or diabetic neuropathy-induced mechanical hypersensitivity following intraperitoneal application of leucocyte elastase inhibitor, sivelestat, as compared to the vehicle-injected group. A single dose of 20 mg/kg body weight sivelestat or an equal volume of vehicle was injected i.p. at 8 W following diabetes induction (blue arrow). In all panels, *denotes P ≤ 0.05 as compared to basal, †represents P ≤ 0.05 as compared to the vehicle-treated group at respective time point, 2-way analysis of variance of repeated measures followed by Turkey post hoc test. (A) Schematic illustration of the experimental protocol followed to analyze the impact of systemic sivelestat on mechanical hypersensitivity in neuropathic pain caused by diabetes. (B) Paw withdrawal responses to von Frey force of 0.16 g before diabetes induction and at 1, 3, 6, and 24 hours following 20 mg/kg body weight i.p. sivelestat or vehicle at 8 W following induction of diabetes via streptozotocin (STZ) in male and female mice. n = 18 mice per group. (C) Mechanical response thresholds calculated as von Frey filament strength required to achieve 60% withdrawal frequency before diabetes induction (basal) or at 1, 3, 6, and 24 hours following 20 mg/kg body weight i.p. sivelestat or vehicle at 8 W following induction of diabetes via streptozotocin in male and female mice. n = 18 mice per group. (D) Comparison of the mechanical response thresholds (calculated as von Frey filament strength required to achieve 60% withdrawal frequency) before diabetes induction (basal) or at 1, 3, 6, and 24 hours following 20 mg/kg body weight i.p. sivelestat or vehicle at 8 W following induction of diabetes via streptozotocin in male or female mice. n = 12 for the male group and 6 for the female group. (E) Impact of i.p. sivelestat on thermal hyperalgesia before and at 1, 3, 6 and 24 hours following 20 mg/kg body weight i.p. sivelestat or vehicle at 8 W following induction of diabetes via streptozotocin or in sham control mice. n = 6 mice per group.
In the next experiments, we asked whether i.p. sivelestat can protect the mice from the thermal hyperalgesia caused by diabetes. Analyses of withdrawal latency to calibrated infrared heat applied to the plantar surface (Hargreaves test) revealed that both the cohorts of mice demonstrate significantly lesser withdrawal latency at 8 W postdiabetes induction as compared to sham-treated mice. While the i.p. sivelestat has no impact on the thermal withdrawal latency in sham mice, diabetic mice injected with 20 mg/kg i.p. sivelestat demonstrated significantly lesser thermal hyperalgesia as compared to the mice injected with vehicle at 1, 3 and 6 hours and returned to pre-sivelestat levels by 24 hours following i.p. sivelestat application (Fig. 6, panel E).
3.6. Sources of leucocyte elastase
After confirming the efficacy of systemic sivelestat in attenuating neuropathic pain of distinct origins in male and female mice, we set out to identify possible sources of LE release in the peripheral tissue, that is, lumbar SC, DRG, sciatic nerve, and paw skin. As it is known that LE is exclusively secreted by infiltrating T-lymphocytes or neutrophils,46 we used anti-CD3 or anti-Gr-1 immunoreactivity as markers to identify T-lymphocytes and neutrophils, respectively, using previously characterized antibodies.46
Analyses of immunofluorescence signals revealed large islands of CD3-positive T-cells in the lumbar L3 and L4 DRG isolated from the mice with SNI at day 8 and at 4 W post-SNI, while only a few cells were observed in the DRG isolated from sham-operated mice (Fig. 7, panels C–C1), which was confirmed by quantification and statistical comparison (Fig. 7, panel C2). Along the same lines, significantly higher numbers of CD3-positive T-lymphocytes were observed in the paw skin (Fig. 7, panels A–A2) and the sciatic nerve (Fig. 7, panels B–B2) from mice with SNI as compared to sham-operated mice. In all 3 types of tissues SNI mice, no differences were observed in the number of infiltrating T-lymphocytes between male and female mice (Fig. 7, panels A2, B2, and C2).
Figure 7.
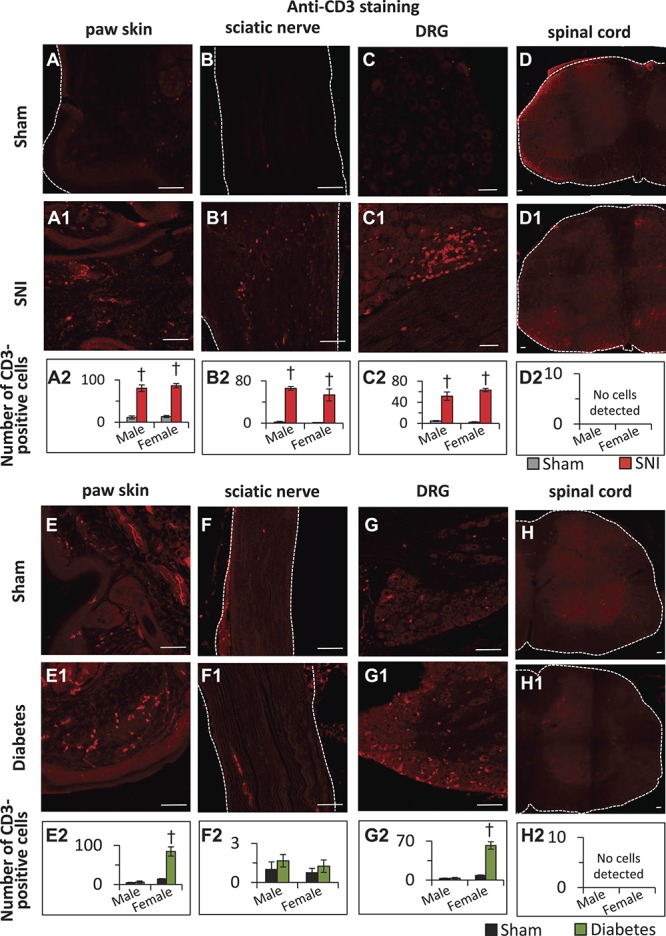
Immunohistochemical characterization of infiltration of T-lymphocytes into peripheral tissue in neuropathic pain and streptozotocin diabetes models on day 28 post-SNI and 8 W post-diabetes induction using an anti-CD3 antibody. (A–D2) Representative images showing the pattern of T-lymphocytes infiltration into mouse paw skin (A–A2), sciatic nerve (B–B2), lumbar dorsal root ganglion (DRG) (C–C2), and lumbar spinal cord (D–D2) isolated on day 28 post-sham or post-spared nerve injury (SNI) surgery. Scale bar in all panels is 75 μm. Panels A2, B2, C2, and D2 represent the quantification of the number of T-lymphocytes infiltrated into paw skin, sciatic nerve, lumbar DRG, or lumbar spinal cord, respectively, isolated from the male and female mice on day 28 post-sham or post-SNI operation. (E–H2) Quantification of the number of T-lymphocytes infiltrated into paw skin (E–E2), sciatic nerve (F–F2), lumbar DRG (G–G2), or lumbar spinal cord (H–H2) isolated from the mice at 8 W following diabetes induction or sham treatment in mice. Panels E2, F2, G2, and H2 represent quantification of the number of CD3-positive T-lymphocytes infiltrated into paw skin, sciatic nerve, lumbar DRG, or lumbar spinal cord, respectively, isolated from the male and female mice at 8 W following diabetes induction. Type 1 diabetes was induced by multiple injections of i.p. streptozotocin. In panels, A2, B2, C2, E2, F2, and G2, †denotes P < 0.05 as compared to sham control; n = 3 to 5 mice per group; 1-way analysis of variance followed by Turkey post hoc test.
In the case of male diabetic mice, while we occasionally detected few infiltrating T-cells into DRG and paw skin, quantitative analyses did not reveal any differences in the number of CD3-positive T-cells infiltrating into the DRG, sciatic nerve or paw skin between diabetic and sham-treated mice at 4, 6, or 8 W post-STZ or vehicle treatment. However, in the case of female diabetic mice, a significant number of CD3-positive cells were found in the paw skin (Fig. 7, panels E–E2) and DRG (Fig. 7, panels G–G2), but not in the sciatic nerve (Fig. 7, panels F–F2). Analyses of lumbar spinal cord tissue isolated from the male and female mice with SNI or diabetes together with corresponding sham controls revealed a lack of detectable numbers of CD3-positive T-lymphocytes in the spinal cord (Fig. 7, panels D–D2 and H–H2).
The number of Gr1-positive neutrophils in the vicinity of the DRG was significantly higher in mice with SNI as compared to sham-operated mice (Fig. 8 panels C1–C2). While there were a negligible number of neutrophils observed in the sciatic nerve isolated from sham-operated mice, sciatic nerves isolated from mice with SNI showed significantly higher number of neutrophils (Fig. 8, panels B1–B2). A large number of neutrophils were observed in the vicinity of ligation. In the case of paw skin, while both the sham and SNI groups of mice exhibited the presence of several neutrophils throughout the paw skin, mice with SNI demonstrated a drastically higher number of neutrophils as compared to sham-operated mice (Fig. 8, panels A–A2). In the case of diabetic mice, the numbers of neutrophils observed to be infiltrating into the paw skin (Fig. 8, panels E1–E2) or the DRG (Fig. 8, panels G1–G2) were significantly higher in diabetic mice than in control mice. However, neutrophils could not be detected in sciatic nerve (Fig. 8, panels F–F2) or lumbar spinal cord (Fig. 8, panels H–H2) sections isolated from either sham or diabetic mice. Analyses of all 3 tissue types isolated from male and female separately revealed lack of sex-specific differences in the pattern or number of neutrophils present in the DRG, sciatic nerve, or paw skin of the mice with SNI or diabetes and the corresponding sham mice.
Figure 8.
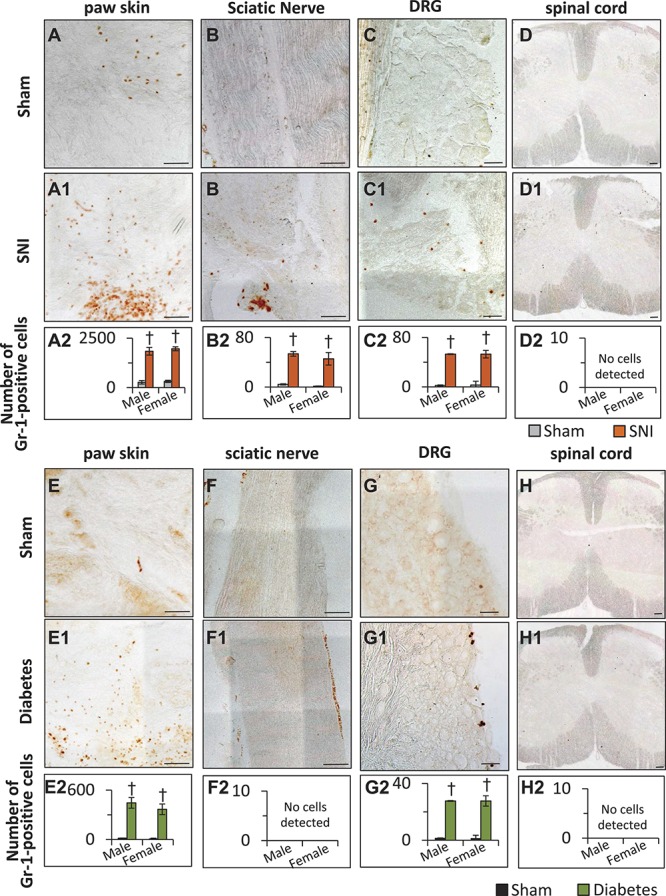
Immunohistochemical characterization of infiltration of neutrophils into peripheral tissue in neuropathic pain and streptozotocin (STZ) diabetes models using an antimouse Ly-6G/Ly-6C (Gr-1) antibody. (A–D2) Representative images to demonstrate the pattern of neutrophil infiltration into mouse paw skin (A–A2), sciatic nerve (B–B2), lumbar dorsal root ganglion (DRG) (C–C2), and lumbar spinal cord (D–D2) isolated at day 28 post-sham or post-spared nerve injury (SNI) surgery. Scale bar in all panels is 75 μm. Panels A2, B2, C2, and D2 represent the quantification of the number of neutrophils infiltrated into paw skin, sciatic nerve, lumbar DRG, or lumbar spinal cord, respectively, isolated from the male and female mice on day 28 post-sham or post-SNI operation. (E–H2) Quantification of the number of neutrophils infiltrated into paw skin (E–E2), sciatic nerve (F–F2), lumbar DRG (G–G2), or lumbar spinal cord (H–H2) isolated from the mice at 8 W following diabetes induction or sham treatment. Panels E2, F2, G2, and H2 represent quantification of the number of Gr-1-positive neutrophils infiltrated into paw skin, sciatic nerve, lumbar DRG, or lumbar spinal cord, respectively, isolated from the male and female mice at 8 W following diabetes induction. Type 1 diabetes was induced by multiple injections of i.p. STZ. In panels A2, B2, C2, E2, and G2, †denotes P < 0.05 as compared to sham control; n = 3 to 5 mice per group; 1-way analysis of variance followed by Turkey post hoc test.
In summary, our immunohistochemical analyses revealed that in the SNI model, T-lymphocytes, and neutrophils infiltrate into DRG, sciatic nerve, and paw skin in both male and female mice as compared to the sham controls. In the mice with STZ-induced diabetic neuropathy, while neutrophils were found to infiltrate into DRG and paw skin of both male and female mice, T-lymphocytes were found to be infiltrating into the DRG, and paw skin of the female but not the male mice as compared to control tissue.
Considering the higher magnitude of protection afforded by systemically applied sivelestat than that observed following intrathecal application46 and infiltration patterns of T-lymphocytes and neutrophils into the peripheral tissue post-SNI, we next set out to test the domains of sivelestat action. To facilitate dosage comparisons between systemic and local administration of sivelestat in either paw skin or in the vicinity of damaged sciatic nerve, we calculated required local injection concentration by scaling the most effective i.p. sivelestat dosage (ie, 20 mg/kg) based on the measured paw weight. Mice were analyzed for mechanical hypersensitivity at 4 W following SNI using the experimental paradigm described above for i.p. injections. At 4 W post-SNI, both the cohorts of mice exhibited significant mechanical hypersensitivity as compared to basal behavior. While the magnitude of mechanical hypersensitivity was unchanged following perisciatic application of sivelestat, (orange bars in Fig. 9), mice injected with intraplantar (i.pl.) sivelestat demonstrated significantly lesser mechanical sensitivity at 1 hour as compared to vehicle-treated mice and returned to pre-sivelestat levels by 3 hours (blue bars in Fig. 9).
Figure 9.
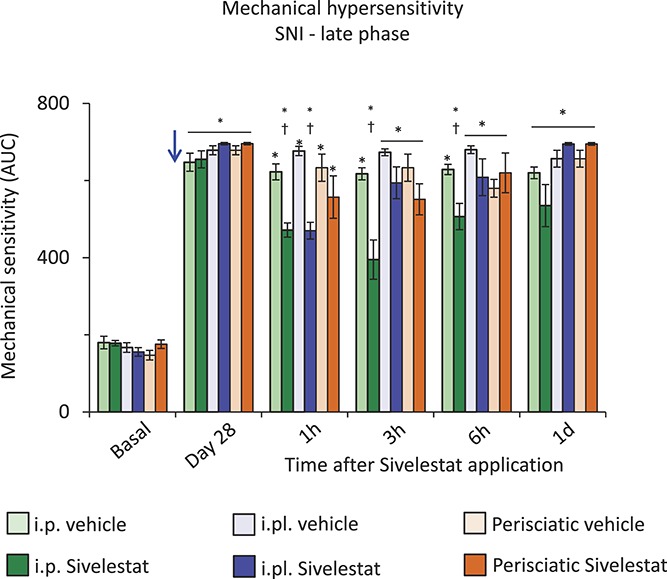
Effect of site-specific delivery of sivelestat in the spared nerve injury (SNI) model of neuropathic pain comparison of mechanical sensitivity expressed as an integral of response frequencies measured with 6 different von Frey forces before SNI (basal) or at 1, 3, 6, and 24 hours following 20 mg/kg sivelestat or vehicle on day 28 following SNI (blue arrow) via intraperitoneal (i.p.) or intraplantar (i.pl.) or perisciatic injections. *denotes P ≤ 0.05 as compared to basal, †represents P ≤ 0.05 as compared to the respective vehicle-treated group, 2-way analysis of variance of repeated measures followed by Turkey post hoc test; n = 7 mice per group.
4. Discussion
The societal and financial burden of the neuropathic pain is enormous4,27,49 and the identification of new therapeutic targets for the management of therapy-resistant neuropathic pain is a major current need.50 Here, we report a high therapeutic potential of systemically administered LE inhibitors for the improvement of neuropathic pain associated phenotypes in preclinical mouse models and report on the cell types that constitute the source of LE in 2 distinct types of neuropathic pain.
The in vivo pharmacology data reported here indicate efficacy with a low, single dose of systemically applied sivelestat in blocking neuropathy-induced mechanical hypersensitivity, a clinically relevant manifestation of several forms of neuropathic pain. Further, since other 2 LE inhibitors that we tested also demonstrated efficacy, we conclude that the observed effects can be clearly allocated to LE blockade and suggest therapeutic promise for the whole class. We observed that the maximal efficacy of sivelestat was lower than that of pregabalin, the current first-line therapy for neuropathic pain, particularly over the chronic phase in the SNI model. However, at early stages post-SNI, the duration of efficacy of sivelestat was longer than that of pregabalin after a single dose (6 hours vs 3 hours with pregabalin). Moreover, Elastatinal, and particularly SSR-69071, were very efficacious, completely reversing neuropathic mechanical allodynia at 8 days after SNI.
It is noteworthy from the point of view of clinical applicability that both the early and late phases of neuropathic hypersensitivity were significantly affected by LE inhibitors, as compared to many drugs which act best in the induction phase of neuropathic pain. This efficacy at chronic stages postnerve injury is mechanistically interesting because both endogenously expressed and exogenously delivered recombinant SerpinA3N was found to alleviate neuropathic mechanical allodynia only up to day 8 after SNI.46 Moreover, the locus of expression and action of SerpinA3N was restricted to the DRG.46 The efficacy shown by LE inhibitors over late stages of SNI upon systemic delivery thereby indicates that LE is also actively involved in mechanisms of neuropathic pain at loci other than DRG neurons. This is indeed consistent with our analyses on T-cell and neutrophil infiltration in the peripheral nerve and the paw in neuropathic pain models.
Systemic sivelestat also demonstrated efficacy in blocking mechanical hypersensitivity in mouse models of bone metastatic pain and painful diabetic neuropathy, which are widespread and clinically challenging to treat. Previous studies report that pregabalin has satisfactory analgesic effects in about 20% of patients suffering from PDN.22 In mouse STZ model, single dose i.p. application of 50 mg/kg pregabalin (equivalent to 314 μmol) was reported to yield a maximum analgesic effect for up to 1 hour, which was neutralized by 4 hours.3 Here, we observed that a single dose of i.p. sivelestat at 20 mg/kg (equivalent to 46 μmol) completely reversed mechanical hypersensitivity in the STZ model for up to 6 hours. sivelestat showed high efficacy in the CIBP model, completely reversing bone metastases-induced mechanical allodynia for at least 24 hours after administration of a single i.p. dose at 20 mg/kg.
Although a majority of literature pertains to the study of mechanical allodynia in the context of neuropathic pain, it is becoming increasingly recognized that other symptoms of neuropathic pain deserve analysis and therapeutic treatment. We observed that a single low dose of systemic sivelestat attenuated cold hypersensitivity, which is a debilitating problem associated with neuropathic pain in regions with a cooler climate. We also addressed recently reported behaviors that are independent of reflexes and represent voluntary actions on part of the animal, some of which also reflect on the overall well-being and motivation of the animal, in keeping with the emotional components of chronic pain.35 SNI is accompanied by striking changes in gait patterns that are associated with the active avoidance of surface contact of the allodynic paw. Here, a single dose of systemic sivelestat improved SNI-induced alterations in gait parameters, such as duration and intensity of paw contact following SNI. Mogil et al. reported that i.p. administration of Morphine (10 mg/kg b.w.), Gabapentin (75 mg/kg b.w.), or topical EMLA (eutectic mixture of 2.5% lidocaine and 2.5% prilocaine) reversed von Frey filament response thresholds but not gait-related parameters on day 14 following SNI in Balb/C mice.31 Another study reported improvement of static and dynamic gait parameters following 7 dosages of 100 mg/kg body weight Gabapentin in the Paclitaxel-induced sensory polyneuropathy model in male C57/Bl6 mice.20 Therefore, it is noteworthy that a single dose of 50 mg/kg b.w. sivelestat reversed both static and dynamic parameters of gait within 4 hours in the current study. Voluntary wheel running in mice is indicative of well-being and motivation in an enriched environment, and it has been recently shown that in the early phase post-SNI, mice show reduced voluntary wheel running behavior, likely due to ongoing pain as well as reduced motivation and well-being.35 (Fig. 2, panel E). Following a single intraperitoneal low dose application of sivelestat, neuropathic mice showed significantly more wheel running, suggesting reduced ongoing pain and thereby improved emotional outcome. The most direct observation supporting the view that LE inhibition blocks on-going pain was made in mice demonstrating nonevoked, spontaneous nocifensive behaviors in the widely used and well-characterized model of breast cancer cell growth in the femur bone.28 Taken together, these observations indicate that the efficacy of LE inhibitors is not restricted to mechanical hypersensitivity but extends to various pathological pain-related behaviors.
We observed a higher efficacy upon i.p. (systemic) delivery of sivelestat than the effect we have previously noted and reported on intrathecal application,46 suggesting additional loci of LE action in neuropathic pain. Complementary to these findings, we observed that local inhibition of LE in the periphery via intraplantar application of sivelestat afforded transient but significant protection against established neuropathic pain, but the magnitude of protection was lower as compared to the same dose of systemic delivery. Taken together, these observations suggest that LE contributes to neuropathic pain manifestation via synergistic effects at peripheral and central domains. These behavioral findings are supported by our observation on increased infiltration of T-cells and neutrophils into sciatic nerve and paw skin of neuropathic mice, in addition to the previously reported infiltration in DRG.46 Previous studies have reported infiltration of T-lymphocytes into peripheral tissues in the early phase of neuropathic pain (ie, 1 W post-SNI).25,30 We observed similar results in DRG, sciatic nerve, and paw skin in the late phase (ie, 4 W post-SNI), suggesting sustained and continuous T-cell infiltration through all stages of neuropathic pain. Despite reports on a higher pool of T-cells in female mice as compared to male counterparts,40 we observed no sex-specific differences in the infiltration patterns of T-cells into the peripheral tissue in mice post-SNI. In diabetic conditions, we observed the infiltration of both T-cells and neutrophils into the DRG and paw skin in the female mice but only of neutrophils in male mice until 8 weeks after injection of STZ. In spinal cord, we observed infiltration of CD3-positive T-cells in male and female mice neither in the SNI model nor in the STZ diabetic model. While our observations are consistent with previous studies reporting a lack of morphological evidence for T-cell infiltration into the spinal cord in neuropathic states in mice,25 one should note that there are studies reporting T-cell infiltration in the ipsilateral spinal cord in some rat models of neuropathic pain.10,17 One study also reported detection of mRNAs coding for T-cell markers in the lumbar spinal cord at 7 days post-SNI in CD-1 mice.40 Thus, whether T-cell infiltration in the spinal cord contributes to plasticity is still unclear. So far, our data clearly point towards a locus of action of LE in the DRG and peripheral tissues.
Several small molecular weight inhibitors were developed to inhibit LE. sivelestat is currently believed to be most potent LE inhibitor with beneficial effects in animal models of lung injury, pneumonia, and spinal cord injury.2,21,24,42 Its pharmacokinetic properties are well studied13–16,38,48 in several animal species including rodents. Clinical studies reported its efficacy in treating acute lung injury–associated symptoms.1,19,29 Elastatinal is one of first identified LE inhibitors33,43 and SSR-69701 is one of recently developed LE inhibitors.23,45,47 They have high potency in inhibiting LE and do not inhibit other proteases such as trypsin, chathepsin, Kallikrein among others.24,43,45 Because the small molecular LE inhibitors tested in this study were originally optimized for airway inflammation,1,19,29 the concentrations required for attenuating neuropathic pain symptoms may have been over or underestimated. Therefore, investigating detailed pharmacokinetic and pharmacodynamic profile and optimizing stoichiometric properties of the LE inhibitors in the context of peripheral and central nervous system will help developing more potent analgesics for the treatment of neuropathic pain.
In summary, our data lay the basis for exploring the therapeutic usage of LE inhibitors in several forms of chronic pain, that is, either resulting from direct traumatic damage to nerves, or harboring a neuropathic component, such as pain caused by cancer growth or metabolic disorders, such as diabetes, and further underline the significance of mechanisms involving T-cells and neutrophils in neuropathic pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
The authors have a patent application pending based in the USA and the EU on the data herein on the use of systemic leukocyte elastase inhibitors against the pain of neuropathic origin (Patent application number: PCT/EP2015/072541).
Supplementary Material
Acknowledgements
The authors thank Rose LeFaucheur for secretarial help and Nadine Gehrig, Dunja Baumgartl-Ahlert, Verena Buchert and Barbara Kurpiers for technical assistance. R. Kuner is a principal investigator in the Excellence Cluster CellNetworks, Heidelberg University. The research leading to these results has received funding from a SFB1118 grant from the Deutsche Forschungsgemeinschaft (Project B06, SFB1118) and by funding from the European Seventh Framework Programme (ncRNAPain consortium, FP7/2007-2013) under grant agreement number 602133 to R Kuner. K. K. Bali was partially supported by a post-doctoral fellowship from the Medical Faculty of Heidelberg University. The authors acknowledge support from the Interdisciplinary Neurobehavioral Core (INBC) for the behavioral experiments performed in this study. All the authors read the manuscript in the submitted form.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A476.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Aikawa N, Ishizaka A, Hirasawa H, Shimazaki S, Yamamoto Y, Sugimoto H, Shinozaki M, Taenaka N, Endo S, Ikeda T, Kawasaki Y. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm Pharmacol Ther 2011;24:549–54. [DOI] [PubMed] [Google Scholar]
- [2].Aikawa N, Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag 2014;10:621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Akamine T, Koyanagi S, Kusunose N, Hashimoto H, Taniguchi M, Matsunaga N, Ohdo S. Dosing time-dependent changes in the analgesic effect of pregabalin on diabetic neuropathy in mice. J Pharmacol Exp Ther 2015;354:65–72. [DOI] [PubMed] [Google Scholar]
- [4].Andrew R, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Pract 2014;14:79–94. [DOI] [PubMed] [Google Scholar]
- [5].Bali KK, Venkataramani V, Satagopam VP, Gupta P, Schneider R, Kuner R. Transcriptional mechanisms underlying sensitization of peripheral sensory neurons by granulocyte-/granulocyte-macrophage colony stimulating factors. Mol Pain 2013;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barclay J, Clark AK, Ganju P, Gentry C, Patel S, Wotherspoon G, Buxton F, Song C, Ullah J, Winter J, Fox A, Bevan S, Malcangio M. Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. PAIN 2007;130:225–34. [DOI] [PubMed] [Google Scholar]
- [7].Bloom AP, Jimenez-Andrade JM, Taylor RN, Castaneda-Corral G, Kaczmarska MJ, Freeman KT, Coughlin KA, Ghilardi JR, Kuskowski MA, Mantyh PW. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain 2011;12:698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bouhassira D, Attal N. Translational neuropathic pain research: a clinical perspective. Neuroscience 2016;338:27–35. [DOI] [PubMed] [Google Scholar]
- [9].Breivik H, Eisenberg E, O'Brien T; Openminds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013;13:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci 2009;29:14415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. PAIN 2000;87:149–58. [DOI] [PubMed] [Google Scholar]
- [12].Fallon MT. Neuropathic pain in cancer. Br J Anaesth 2013;111:105–11. [DOI] [PubMed] [Google Scholar]
- [13].Fujimoto H, Imawaka H, Komaba J, Yamamoto R, Hiraku1 S. Studies on the metabolic fate of neutrophil elastase inhibitor ONO-5046·Na(1): plasma concentration profile, distribution and Excretion after single intravenous administration to rats. Drug Metab Pharmacokinet 1997;12:551–65. [Google Scholar]
- [14].Fujimoto H, Imawaka H, Nakade S, Yamamoto R, Hiraku S. Studies on the metabolic fate of neutrophil elastase inhibitor ONO-5046·Na(3): metabolism and binding to serum protein in various animal species. Drug Metab Pharmacokinet 1997;12:576–88. [Google Scholar]
- [15].Fujimoto H, Komaba J, Yamamoto R, Hiraku S. Studies on the metabolic fate of neutrophil elastase inhibitor ONO-5046·Na(4): feto-placental transfer and Excretion into milk. Drug Metab Pharmacokinet 1997;12:589–95. [Google Scholar]
- [16].Fujimoto H, Imawaka H, Junji K, Yamamotos R, Seiji H. Studies on the metabolic fate of neutrophil elastase inhibitor ONO-5046.Na(2): plasma concentration-profile, distribution and Excretion after repeated intravenous bolus administration to rats and effects on metabolizing Enzyme activities. Drug Metab Pharmacokinet 1997;12:566–75. [Google Scholar]
- [17].Gattlen C, Clarke CB, Piller N, Kirschmann G, Pertin M, Decosterd I, Gosselin RD, Suter MR. Spinal cord t-cell infiltration in the rat spared nerve injury model: a time course study. Int J Mol Sci 2016;17:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gruener H, Zeilig G, Laufer Y, Blumen N, Defrin R. Differential pain modulation properties in central neuropathic pain after spinal cord injury. PAIN 2016;157:1415–24. [DOI] [PubMed] [Google Scholar]
- [19].Hayakawa M, Katabami K, Wada T, Sugano M, Hoshino H, Sawamura A, Gando S. Sivelestat (selective neutrophil elastase inhibitor) improves the mortality rate of sepsis associated with both acute respiratory distress syndrome and disseminated intravascular coagulation patients. Shock 2010;33:14–18. [DOI] [PubMed] [Google Scholar]
- [20].Huehnchen P, Boehmerle W, Endres M. Assessment of paclitaxel induced sensory polyneuropathy with “Catwalk” automated gait analysis in mice. PLoS One 2013;8:e76772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iwamoto S, Higashi A, Ueno T, Goto M, Iguro Y, Sakata R. Protective effect of sivelestat sodium hydrate (ONO-5046) on ischemic spinal cord injury. Interact Cardiovasc Thorac Surg 2009;8:606–9. [DOI] [PubMed] [Google Scholar]
- [22].Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis 2015;6:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kapui Z, Varga M, Urban-Szabo K, Mikus E, Szabo T, Szeredi J, Batori S, Finance O, Aranyi P. Biochemical and pharmacological characterization of 2-(9-(2-piperidinoethoxy)-4-oxo-4H-pyrido[1,2-a]pyrimidin-2-yloxymethyl)-4-(1-met hylethyl)-6-methoxy-1,2-benzisothiazol-3(2H)-one-1,1-dioxide (SSR69071), a novel, orally active elastase inhibitor. J Pharmacol Exp Ther 2003;305:451–9. [DOI] [PubMed] [Google Scholar]
- [24].Kawabata K, Suzuki M, Sugitani M, Imaki K, Toda M, Miyamoto T. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem Biophys Res Commun 1991;177:814–20. [DOI] [PubMed] [Google Scholar]
- [25].Kim CF, Moalem-Taylor G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res 2011;1405:95–108. [DOI] [PubMed] [Google Scholar]
- [26].Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience 2016;338:183–206. [DOI] [PubMed] [Google Scholar]
- [27].Langley PC, Van Litsenburg C, Cappelleri JC, Carroll D. The burden associated with neuropathic pain in Western Europe. J Med Econ 2013;16:85–95. [DOI] [PubMed] [Google Scholar]
- [28].Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011;115:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miyoshi S, Hamada H, Ito R, Katayama H, Irifune K, Suwaki T, Nakanishi N, Kanematsu T, Dote K, Aibiki M, Okura T, Higaki J. Usefulness of a selective neutrophil elastase inhibitor, sivelestat, in acute lung injury patients with sepsis. Drug Des Devel Ther 2013;7:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004;129:767–77. [DOI] [PubMed] [Google Scholar]
- [31].Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Langford DJ, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain 2010;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Okura A, Morishima H, Takita T, Aoyagi T, Takeuchi T, Umezawa H. The structure of elastatinal, an elastase inhibitor of microbial origin. J Antibiot (Tokyo) 1975;28:337–9. [DOI] [PubMed] [Google Scholar]
- [34].Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience 2000;101:745–57. [DOI] [PubMed] [Google Scholar]
- [35].Pitzer C, Kuner R, Tappe-Theodor A. Voluntary and evoked behavioral correlates in neuropathic pain states under different social housing conditions. Mol Pain 2016;12:1744806916656635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schaefer C, Mann R, Sadosky A, Daniel S, Parsons B, Nieshoff E, Tuchman M, Nalamachu S, Anschel A, Stacey BR. Burden of illness associated with peripheral and central neuropathic pain among adults seeking treatment in the United States: a patient-centered evaluation. Pain Med 2014;15:2105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, Augustin HG, Kuner R. A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell 2015;27:780–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shintani T, Takamoto M, Sawada M, Aishita H, Nakagawa T. Simultaneous determination of human neutrophil elastase inhibitor (ONO-5046) and its metabolite in plasma and urine by direct injection column-switching HPLC. J Pharm Biomed Anal 1994;12:397–405. [DOI] [PubMed] [Google Scholar]
- [39].Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract 2014;14:167–84. [DOI] [PubMed] [Google Scholar]
- [40].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18:1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Srivastava K, Hu J, Korn C, Savant S, Teichert M, Kapel SS, Jugold M, Besemfelder E, Thomas M, Pasparakis M, Augustin HG. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer Cell 2014;26:880–95. [DOI] [PubMed] [Google Scholar]
- [42].Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E, Nishisho T. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem 2001;78:1064–72. [DOI] [PubMed] [Google Scholar]
- [43].Umezawa H, Aoyagi T, Okura A, Morishima H, Takeuchi T. Letter: elastatinal, a new elastase inhibitor produced by actinomycetes. J Antibiot (Tokyo) 1973;26:787–9. [DOI] [PubMed] [Google Scholar]
- [44].Vareniuk I, Pavlov IA, Obrosova IG. Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia 2008;51:2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Varga M, Kapui Z, Batori S, Nagy LT, Vasvari-Debreczy L, Mikus E, Urban-Szabo K, Aranyi P. A novel orally active inhibitor of HLE. Eur J Med Chem 2003;38:421–5. [DOI] [PubMed] [Google Scholar]
- [46].Vicuna L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, Prokosch S, Riva P, Griffin RS, Njoo C, Gehrig S, Mall MA, Arnold B, Devor M, Woolf CJ, Liberles SD, Costigan M, Kuner R. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med 2015;21:518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Warner P, Green RC, Gomes B, Strimpler AM. Non-peptidic inhibitors of human leukocyte elastase. 1. The design and synthesis of pyridone-containing inhibitors. J Med Chem 1994;37:3090–9. [DOI] [PubMed] [Google Scholar]
- [48].Watanabe F, Sato M, Kato A, Murakami T, Higashi Y, Yata N. First-pass metabolism of ONO-5046 (N-[2-[4-(2, 2-Dimethylpropionyloxy)phenylsulfonylamino]benzoyl]-aminoacetic acid), a novel elastase inhibitor, in rats. Biol Pharm Bull 1997;20:392–6. [DOI] [PubMed] [Google Scholar]
- [49].Witt EA, Kenworthy J, Isherwood G, Dunlop WC. Examining the association between pain severity and quality-of-life, work-productivity loss, and healthcare resource use among European adults diagnosed with pain. J Med Econ 2016;19:858–65. [DOI] [PubMed] [Google Scholar]
- [50].Wolkerstorfer A, Handler N, Buschmann H. New approaches to treating pain. Bioorg Med Chem Lett 2016;26:1103–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


