Abstract
Background:
Adequate folate status supports endothelial structure and function. Folic acid (FA), an oxidized synthetic folate, which is present in the plasma of patients consuming fortified food or FA supplements, may impair cellular uptake of physiological, reduced folates. We studied the effect of FA on uptake of the dominant circulatory folate, 5-methyltetrahydrofolate (5MTHF) in endothelial cells.
Methods and Results:
For short-term effects of FA, primary human umbilical vein endothelial cells (HUVECs) were maintained in growth medium containing 200 nM 5MTHF and preincubated with 20 nM FA 10 minutes before the 5MTHF uptake assessment. For long-term effects, HUVECs were cultured for 3 passages in growth medium containing either 200 nM 5MTHF, or a combination of 100 nM 5MTHF and 100 nM FA. 5MTHF uptake was assessed after exposing cells to 200 nM [13C5]-5MTHF, after which intracellular [13C5]-5MTHF was quantified using liquid chromatography/tandem mass spectrometry. Acute FA exposure caused a 57% reduction in 5MTHF uptake compared with control conditions (51 ± 12 vs. 22 ± 7 fmol·min−1·mg−1 protein; P = 0.01). Long-term exposure to FA reduced 5MTHF uptake by 41% (51 ± 12 vs. 30 ± 11 fmol·min−1·mg−1 protein; P = 0.05) and reduced total cellular 5MTHF levels by 47 ± 21% in HUVEC (P = 0.02).
Conclusion:
Unmetabolized FA, which appears in the plasma after consumption of fortified food or FA supplements, may impair uptake of 5MTHF, the dominant bioactive form of folate, in HUVEC.
Key Words: endothelial cells, metabolism, molecular biology, folate
INTRODUCTION
One-carbon metabolism, in which reduced folates and homocysteine play crucial roles, is associated with the pathobiology of cardiovascular disease. Traditionally, under folate deficiency, elevated plasma levels of homocysteine were held responsible for endothelial cell injury and cardiovascular disease.1 However, there is probably an important direct role of reduced folates in maintaining vascular tissue integrity and functionality.2 Large intervention trials in mildly hyperhomocysteinemic subjects almost uniformly used the synthetic oxidized folate derivative folic acid (FA), but have shown no clinical benefit, except possibly on the primary prevention of stroke.3,4 One explanation for this lack of benefit on FA supplementation is the possibility of adverse effects of synthetic FA.5,6 FA is an oxidized folate which normally does not occur in the body and which has no known biological role of its own. However, FA is stable, inexpensive, and is thus widely used in food fortification and tablets for vitamin supplementation. Hepatic dihydrofolate reductase converts FA first to dihydrofolate, then to tetrahydrofolate, after which the latter is enzymatically interconverted to 5-methyltetrahydrofolate (5MTHF), the dominant circulating bioactive folate.6 However, with FA doses as they occur in food fortification and, in particular, vitamin supplements, unmetabolized FA appears in the systemic circulation, sometimes at substantial levels.7–9 In most clinical trials, FA doses were between 0.5 and 5 mg, and plasma concentrations of 10–20 nM FA have been found in these individuals.10,11
Three specific folate transport systems are known to mediate the uptake of folates into cells12: (1) The reduced folate carrier (RFC/SLC19A1) is an organic anion exchanger, (2) the proton-coupled folate transporter (PCFT/SLC46A1) is a unidirectional folate influx transporter which functions as proton-folate co-transporter with optimal transport activity at acidic pH, and (3) folate receptors α and β (FRα and FRβ) bind folates with high affinity and transport them into cells through receptor-mediated endocytosis. FRα and FRβ display high affinity toward both reduced folates and the oxidize folate, FA. Hence, these folate transporters differ in pH optimum (physiological pH for RFC, but pH 5.5 for PCFT) and substrate affinity (RFC has low affinity for FA, whereas PCFT, FRα, and FRβ display a high affinity for FA). Expression patterns of these folate transporters and receptors differ between cell types, but are unknown for endothelial cells.
In this study, we examined whether or not FA inhibits the uptake of the bioactive folate 5MTHF in cultured human endothelial cells.
METHODS
Standards
FA (98% purity) was obtained from Sigma (Deiselhofen, Germany). (6S)-5MTHF (90% purity), [13C5]-5-formyltetrahydrofolate, and [13C5]-5MTHF (isotopic purity >99%) were obtained from Eprova AG (Schaffhausen, Switzerland). [13C5]-5,10-methenyltetrahydrofolate was prepared from [13C5]-5-formyltetrahydrofolate as described previously.13
Isolation of HUVECs and Cell Culture
Primary human umbilical vein endothelial cells (HUVECs) isolated from 6 umbilical cords were grown in folate-free M199 medium (Promocell, Heidelberg, Germany) supplemented with 10% heat-inactivated human serum, 1% penicillin–streptomycin, 10% heat-inactivated calf serum, 5 U/mL heparin, 50 μg/mL endothelial cell growth supplement from cow brain, and 1% glutamine. FA or 5MTHF were added as indicated. HUVECs were plated at a density of 1 × 105 cells in 1.5 mL growth medium in gelatin-coated plates (Merck, Darmstadt, Germany) and maintained at 37°C in a 100% humidified atmosphere of 5% CO2. Culture media were refreshed every other day. After reaching confluence (approximately 6 days), cells were detached by trypsinization and split 1:3 for renewed passages. We used cells which reached the third passage.
Experimental Conditions and Analytical Methods
A—Short-term FA Exposure
Six independent HUVEC isolates were cultured for 3 passages in growth medium containing 200 nM 5MTHF, a concentration that was found to support optimal cell proliferation. Two days before the experiment, 5MTHF concentration in the growth medium was reduced to the more physiological concentration of 20 nM. Before uptake experiments, cells were rinsed twice with Hanks buffered saline solution (HBSS, pH = 7.4). Next, 2 × 105 cells were preincubated with either (1) 20 nM 5MTHF (control) or (2) 20 nM FA in 1.5 mL HBSS containing 1% human serum for 10 minutes at 37°C. Subsequently, cells were incubated for 10 minutes with 200 nM [13C5]-5MTHF. Cells were then placed on ice, rinsed twice with ice-cold HBSS, and harvested by brief trypsinization (1 minute, 37°C). Cells were then washed 3 times with 1 mL ice-cold HBSS and were stored at −80°C until analysis.
B—Long-term FA Exposure
Six HUVEC isolates were cultured for 3 passages in a medium to which (1) 200 nM 5MTHF (control) or (3) 100 nM 5MTHF + 100 nM FA were added. Two days before the experiment, the medium was replaced with a 1.5 mL medium containing 20 nM 5MTHF (control) or FA. Just before the experiment, cells were rinsed twice with HBSS. Cells were then incubated with 200 nM [13C5]-5MTHF in HBSS containing 1% human serum for 10 minutes. Storage and processing of cells were performed as described above.
Sample Clean-up and Analysis
A pellet of 2 × 105 HUVECs was suspended in 500 μL milliQ water and subjected to 3 freeze-thaw cycles, followed by centrifugation for 5 minutes (4°C, 8000 g). To 400 μL of the supernatant (the remainder was used for protein determination using bicinchoninic acid), 22 pmol of [13C5]-5,10-methenyltetrahydrofolate was added as an internal standard, and the mixture was acidified with 50 μL of 6 mM HCl (pH < 1). Three milliliter Hydrophilic-Lipophilic Balance Oasis cartridges (Waters, Milford, MA) were used for sample clean-up after conditioning with 0.5 mL methanol and 1 mL milliQ water. The mixture was applied to the cartridges, washed with 2 mL of milliQ, and subsequently eluted with 0.5 mL methanol containing 1 mg/mL ascorbic acid. The samples were then evaporated to almost complete dryness (nitrogen, 40°C) and dissolved in 100 μL 10 mM formic acid. The [13C5]-5MTHF content was assessed by liquid chromatography/tandem mass spectrometry as described previously.11 Intra-assay coefficient of variation (CV) for [13C5]-5MTHF was <5.6%, and the limit of quantification (signal/noise > 10) was 0.01 pmol.
Determination of Intracellular 5MTHF Concentrations
Endogenous 5MTHF was measured in 2 × 105 cells from all 6 HUVEC isolates after incubation according to experimental condition B using methods described previously.13 Intra-assay CV for intracellular 5MTHF was <1.2%, and limit of quantification (LOQ) (signal/noise) was 3 fmol.
Real-Time PCR Analysis of RFC, PCFT, FRα, and FRβ
RNA was isolated from 3 HUVEC isolates using an RNeasy kit (Qiagen). cDNA was synthesized from 1.5 μg of total RNA using random primers and SuperScript II Reverse Transcriptase (Invitrogen). Quantitative real-time PCR was performed in a total volume of 25 μL containing 5 μL of cDNA, 300 nM of forward and reverse primers, and 1x SybrGreen PCR Mastermix (Applied Biosystems, Foster City, CA). Primer sets were designed using the Universal ProbeLibrary (Roche Applied Science), according to the GenBank sequences (human RFC messenger RNA NM_194255.1, human PCFT mRNA NM_080669.3, human FRα mRNA NM_016729.2, human FRβ mRNA NM_001113536.1, and human β2-microglobulin (B2M) mRNA NM_004048.2). Samples were run in triplicates on a 7300 Real-time PCR System (Applied Biosystems), and fluorescence was measured during each step. Expression levels were normalized to the reference gene B2M by comparative quantification, using the ΔΔCT method.14 For each sample, ΔCT was calculated (CT, GOI − CT, B2M). The effect of FA on the relative expression of the various folate transporters was calculated as 2−ΔΔCT (ΔΔCT = ΔCFA − ΔC5MTHF).
Statistics
Student's t tests were used to test for statistical differences between the groups. P values <0.05 were considered statistically significant.
RESULTS
FA Inhibits 5MTHF Uptake in HUVECs
5MTHF influx in HUVECs was linear over the first 10 minutes of transport (time points 0, 2, 4, 6, 8, and 10 minutes; Fig. 1) and exhibited Michaelis–Menten kinetics between 40 nM and 25 μM 5MTHF, with a transport Km = 0.25 μM and a Vmax of 0.39 pmol 5MTHF·min−1·mg−1 protein. Under control conditions, influx rates of 5MTHF were 51 ± 12 fmol·min−1·mg−1 protein. Ten minutes of pre-exposure of HUVECs to 20 nM FA significantly inhibited 5MTHF influx by 57% (to 22 ± 7 fmol·min−1·mg−1 protein, P = 0.007; Fig. 2). A similar decrease in 5MTHF influx was observed when 200 nM FA was used (data not shown). Moreover, long-term growth (3 passages) of HUVECs in FA-containing medium repressed 5MTHF influx by 41% (to 30 ± 11 fmol·min−1·mg−1 protein, P = 0.05; Fig. 2).
FIGURE 1.
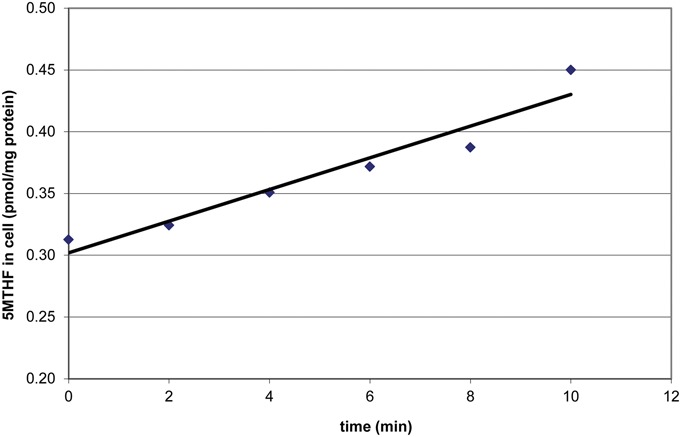
Timecurve 5MTHF Uptake in HUVECs.
FIGURE 2.
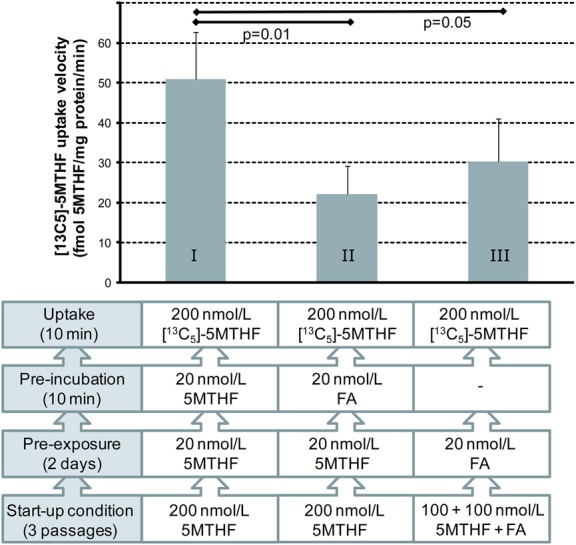
Effect of long- and short-term FA exposure on the uptake of 5MTHF in primary HUVECs. I: control, II: 10 minutes of FA exposure, III: long-term FA exposure. Bars represent mean ± SD for 6 HUVECs isolates.
FA Reduces Intracellular 5MTHF
Consistent with the suppression of 5MTHF uptake after long-term exposure to FA, these HUVECs also displayed 47% ± 21% lower intracellular 5MTHF pools (P = 0.02; Fig. 3). Nonmethyl–reduced folate levels in these cells (∼4% of total folate) remained unchanged.
FIGURE 3.
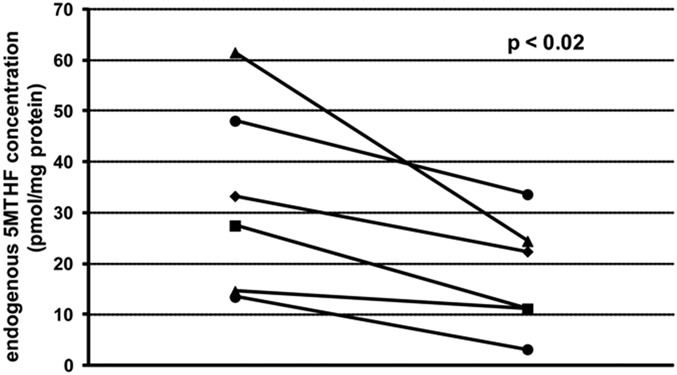
Effect of long-term FA exposure on intracellular 5MTHF concentration in HUVECs. Results depict 6 HUVECs isolates: left is cultured on 200 nM 5MTHF for 3 passages, and right on a combination of 100 nM 5MTHF and 100 nM FA.
Folate Transporter Expression
Real-time PCR was performed for quantification of RFC, PCFT, FRα, and FRβ mRNA levels, relative to the reference gene, β2M (Fig. 4). In HUVECs cultured on the natural folate 5MTHF, RFC and PCFT were significantly expressed; ΔCT: 5 and −0.5, respectively, whereas FRα and FRß were barely detectable (CT: 36 and 40, respectively). After long-term exposure to FA-containing medium, RFC mRNA levels did not change (2−ΔΔCT variation; 1.0–1.1, n = 3). PCFT mRNA levels were decreased in comparison with the same cells that continued on 5MTHF, but this did not reach statistical significance (2−ΔΔCT variation: 1.8–8.0-fold, P = 0.2, n = 3).
FIGURE 4.
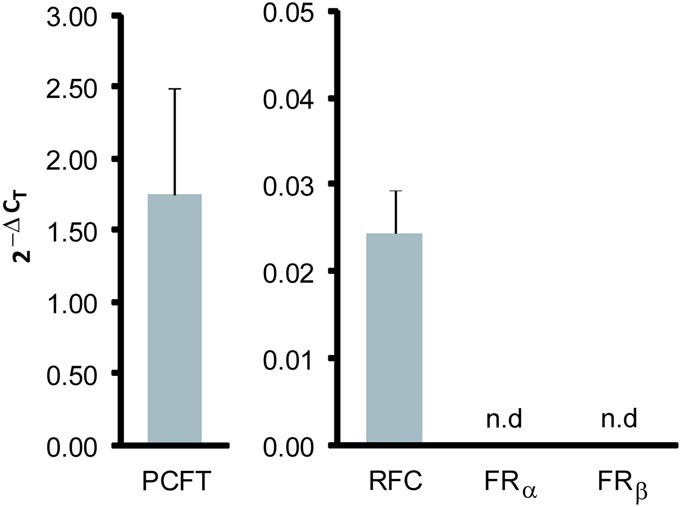
Relative gene expression of folate transporters in primary HUVECs. Results depict the mean relative gene expression levels normalized to βs2M (2-ΔCt ± SD) of 3 HUVEC isolates. n.d., not detectable.
DISCUSSION
Tissue concentrations of 5-MTHF are a key determinant of endothelial cell function in human vasculature.2 Our current study provides the initial necessary proof-of-concept that unmetabolized FA because it occurs in the plasma of subjects taking oral FA supplementation, interferes with endothelial cell uptake of the circulating bioactive folate 5MTHF, and compromises intracellular folate pools. We also found that HUVECs express both the folate transporters RFC and PCFT.
Although further mechanistic studies are needed, it seems reasonable to speculate that competitive inhibition of 5MTHF uptake through PCFT by FA may occur because both folates have comparable Km values.12 Downregulation of PCFT after long-term exposure to FA could also contribute to compromised 5MTHF uptake with consequent shrinkage of the intracellular folate pool. Of note, 5MTHF uptake in HUVECs was studied at pH 7.2–7.4, which would favor 5MTHF uptake through RFC, which has a poor affinity for FA. However, although PCFT activity is optimal at pH 5.5, substantial transport activity has been shown to occur at pH 7.2–7.4.12,15,16
Impaired 5MTHF uptake in endothelial cells could occur in vivo in patients taking FA in supplements or food fortification. First, FA concentrations that elicited inhibitory effects (20 nM) are consistent with the folate concentrations found in the plasma after oral FA intake.10 Second, although unmetabolized FA appears in the plasma only briefly (1–2 hours),17 it disappears more slowly from the plasma after prolonged exposure.11 If indeed human endothelial cells have reduced 5MTHF uptake after exposure to FA, diminished cellular folate pools could result. Shrunken intracellular folate pools may have serious deleterious effects including uracil (ie, deoxyuridine triphosphate) misincorporation into DNA as well as inhibition of various S-adenosylmethionine–dependent methylation reactions of DNA, RNA, proteins, and metabolic intermediates.18,19 In endothelial cells, endothelial function and vascular superoxide production may be disturbed by folate deficiency.2
The fact that plasma homocysteine drops after oral FA intake may argue against generalized impairment of cellular 5MTHF uptake. However, it is conceivable that various organs and tissues respond differently to FA, as components of folate homeostasis (ie, folate transporters and intracellular metabolism) will be distinct. Plasma homocysteine is predominantly determined by liver and kidney folate homeostasis, which may not reflect folate metabolism in endothelial cells. A limitation of our study includes the fact that we used only a single endothelial cell model. Ideally, one could have included, for example, human aortic or coronary endothelial cells. Also, our study does not address the potential consequences of FA-mediated–reduced 5MTHF uptake for intracellular folate-dependent metabolism and biosynthetic enzyme activity. Although low 5MTHF appears to be an important determinant of endothelial cell dysfunction,2 future studies are warranted to demonstrate disturbance of endothelial structure or function as an immediate consequence of FA exposure. Such studies may encompass a design similar to our study and look at for example oxidative stress, tetrahydrobiopterin levels, or production of vasoactive molecules, such as nitric oxide. Alternatively, follow-up studies could also involve isolated vessel segments or vascular assessment in vivo, as long as controlled FA exposure is compared with MTHF control.
In conclusion, FA has the ability to inhibit the uptake of the naturally occurring, bioactive folate 5MTHF in endothelial cells through a mechanism that presumably involves interaction with the folate transporter PCFT. As FA consumption leads to unmetabolized FA in the plasma, this might affect endothelial cell folate status, which is of potential concern.
Footnotes
Y. M. Smulders was supported by a grant from the Netherlands Heart Foundation.
The authors report no conflicts of interest.
REFERENCES
- 1.McCully KS. Homocysteine, vitamins, and vascular disease prevention. Am J Clin Nutr. 2007;86:1563S–1568S. [DOI] [PubMed] [Google Scholar]
- 2.Antoniades C, Shirodaria C, Leeson P, et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. [DOI] [PubMed] [Google Scholar]
- 3.Clarke R, Halsey J, Lewington S, et al. ; for the B-Vitamin Treatment Trialists' Collaboration. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170:1622–1631. [DOI] [PubMed] [Google Scholar]
- 4.Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 5.Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. 2011;34:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci U S A. 2009;106:15424–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly P, McPartlin J, Goggins M, et al. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997;65:1790–1795. [DOI] [PubMed] [Google Scholar]
- 8.Plumptre L, Masih SP, Ly A, et al. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr. 2015;102:848–857. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer CM, Sternberg MR, Fazili Z, et al. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. 2015;145:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeiffer CM, Fazili Z, McCoy L, et al. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin Chem. 2004;50:423–432. [DOI] [PubMed] [Google Scholar]
- 11.Obeid R, Kirsch SH, Kasoha M, et al. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism. 2011;60:673–680. [DOI] [PubMed] [Google Scholar]
- 12.Zhao R, Diop-Bove N, Visentin M, et al. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr. 2011;31:177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DE, Kok RM, Teerlink T, et al. Quantitative determination of erythrocyte folate vitamer distribution by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med. 2006;44:450–459. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 15.Kugel Desmoulin S, Wang L, Hales E, et al. Therapeutic targeting of a novel 6-substituted pyrrolo [2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Mol Pharmacol. 2011;80:1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasry I, Berman B, Straussberg R, et al. A novel loss-of-function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood. 2008;112:2055–2061. [DOI] [PubMed] [Google Scholar]
- 17.Kelly P, McPartlin J, Scott J. A combined high-performance liquid chromatographic-microbiological assay for serum folic acid. Anal Biochem. 1996;238:179–183. [DOI] [PubMed] [Google Scholar]
- 18.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ifergan I, Assaraf YG. Molecular mechanisms of adaptation to folate deficiency. Vitam Horm. 2008;79:99–143. [DOI] [PubMed] [Google Scholar]


