Abstract:
Idiopathic ventricular arrhythmias (IVAs) are relatively common in the general population and usually have a good prognosis. However, frequent premature ventricular contractions (PVCs) can lower the quality of life (in symptomatic cases) and can cause cardiomyopathy and sudden cardiac death. In this report, we demonstrate a novel trigger for IVAs. Melatonin use for treating sleep disorders has increased significantly in recent years. We provide here the first human evidence of its proarrhythmic effect by presenting 2 patients (with normal myocardium) with symptomatic PVCs, while on melatonin. Discontinuation of melatonin stopped PVCs in both patients. Our findings highlight the importance of identifying precipitating factors for IVAs.
Key Words: melatonin, ventricular arrhythmia, outflow tract
INTRODUCTION
Even in patients with a structurally normal heart, symptomatic premature ventricular contractions (PVCs) are relatively common. The majority originate in the ventricular outflow tracts (OTs). Although it is well known that triggered activity is the main underlying mechanism of arrhythmogenesis, precipitating factors for this focal activity remain largely undetected.1
Here, we report on the pineal hormone melatonin (which normally regulates the body's circadian rhythms and sleep–wake cycles) being capable of mediating OT PVCs in the absence of structural heart disease. Melatonin is widely used as a prescription/over-the-counter drug to treat sleep disorders. Based on its pharmacological effect of alleviating sleeping problems, it is rather expected to protect against arrhythmias because of the association between arrhythmias and sleep deprivation. In our patients, however, we observed a clear association between melatonin use and the occurrence of PVCs from the OT. As OT arrhythmias represent more than 10% of overall referrals for electrophysiological studies, our present findings highlight the importance of identifying pharmacons that can mediate OT PVC generation because refraining from these drugs is safer and more cost-effective than trying to treat the disease with antiarrhythmic medication or catheter ablation.1
CASE SERIES
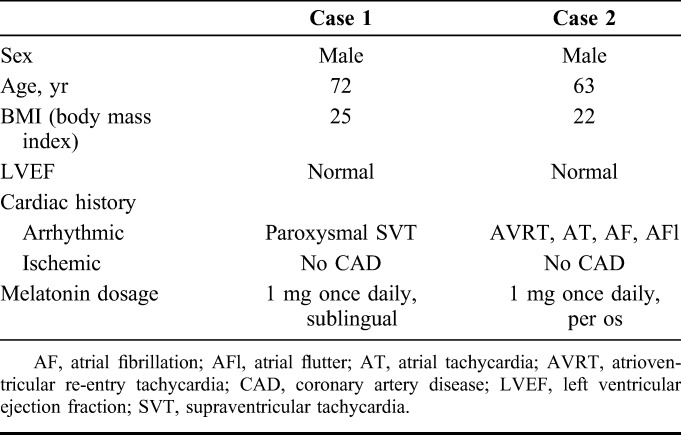
Two patients referred to our department because of palpitations were included in this report. Both patients used melatonin for sleeping problems. Holter and/or implantable loop recorder (ILR) registrations demonstrated PVCs as a cause for their symptoms. The origin of the PVCs was specified either through electrophysiological study or based on QRS morphology on 12-lead electrocardiogram. Patient characteristics are listed in Table 1. A comprehensive literature search in several electronic databases for relevant studies published until January 2017 was conducted. Informed consent was obtained from both patients. Data collection was performed respecting the Health Insurance Portability and Accountability Act 1996.
TABLE 1.
Patient Characteristics
Case 1
The first patient was a 72-year-old man with an uneventful cardiac history (except for a short episode of paroxysmal supraventricular tachycardia in 1980). In August 2014, he was referred to our outpatient clinic because of palpitations despite being on beta-blocker therapy. Other medication used by the patient included sitagliptin (oral antihyperglycaemic), atorvastatin (statin), candesartan (angiotensin II receptor blocker), and metformin (oral antihyperglycaemic). The patient also used melatonin (1 mg once daily, sublingual) because of problems falling asleep.
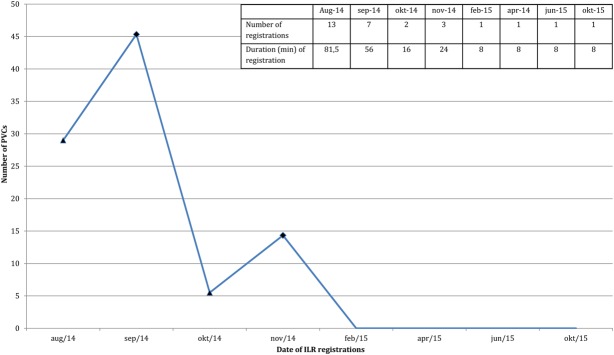
Holter tracings revealed more than 2000 multiform PVCs per 24 hours and ILR registration (Medtronic Reveal LINQ) confirmed PVCs as the cause for the palpitations. Bisoprolol (7.5 mg) was ineffective. The dominant morphology of the PVCs was suggestive of an OT origin on a 12-lead electrocardiogram (Fig. 1). An exercise test showed only occasional PVCs, both during exercise and recovery phase, and without symptoms of angina or ST segment alterations. A normal left ventricular function was seen on echocardiogram. Computed tomography angiography showed no coronary artery disease with a calcium score of zero. Subsequently, 150 mg of flecainide was given in combination with 2.5 mg of bisoprolol, but without any effect. In September and November 2014, he discontinued melatonin resulting in complete cessation of symptoms. In March 2015, after completely abstaining from melatonin, the patient became free from any symptoms. Complete disappearance of PVCs was also confirmed with ILR registration (Fig. 2).
FIGURE 1.
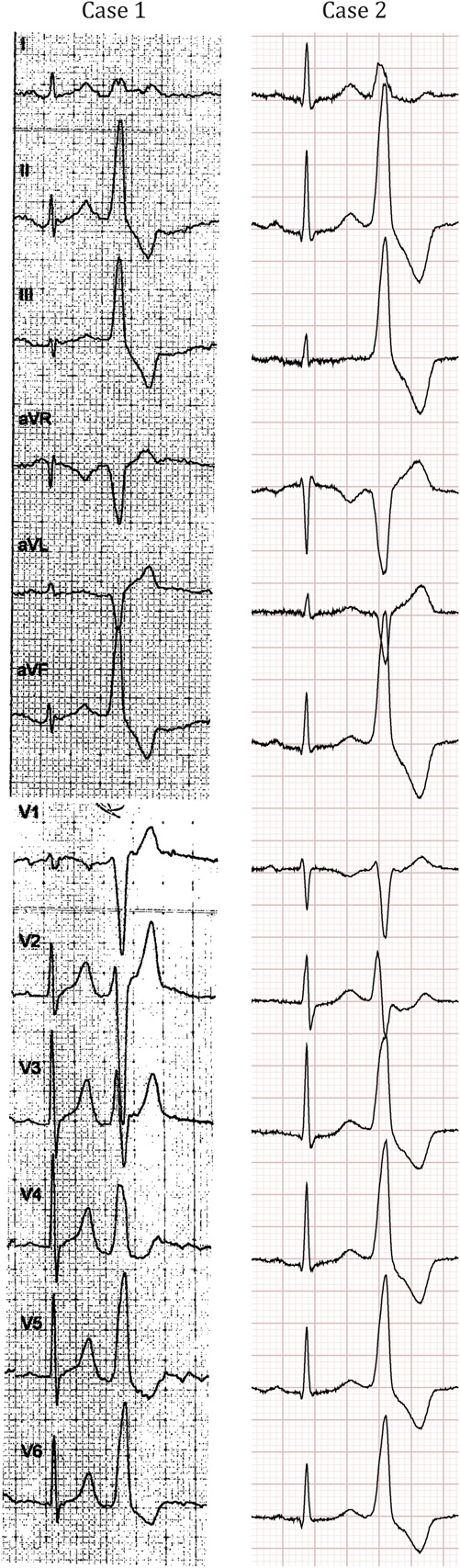
Electrocardiograms of both patients showing PVCs originating from the outflow tracts.
FIGURE 2.
Mean PVC number/registration period with ILR mean PVC number during registration period is depicted for each month of ILR use. All registrations were patient activated. Triangles: (re)start of melatonin, rhombuses: discontinuation of melatonin. Embedded table shows the total number and total duration of recordings during 1 month.
Case 2
In May 2012, a 63-year-old man presented with recurrent palpitations after a previous cardiac history of catheter ablation of a left anterolateral accessory pathway, a focal atrial tachycardia and atrial fibrillation (successful catheter ablation in 2011). He also used melatonin (1 mg once daily, per os) because of difficulties falling asleep. Other medications included acetylsalicylic acid (platelet aggregation inhibitor), formoterol (long-acting β2 agonist, 12 µg 2 times daily), fluticasone, and ciclesonide (glucocorticoids).
At follow-up, in July 2012, a 5-day Holter was performed, revealing multiple symptomatic PVCs and nonsustained ventricular tachycardias (nsVTs) with a morphology suggestive of an OT origin (Fig. 1). A coronary angiogram ruled out an ischemic cause of the arrhythmia. Echocardiogram showed a normal left ventricular ejection fraction without any other structural abnormalities. In October 2012, unsuccessful PVC ablation, targeting a right ventricular outflow tract (RVOT) origin, was performed. After a 24-hour Holter revealed a PVC burden of 6% in May 2015, the patient was suggested to stop using melatonin (based on our previous experience with the patient from case 1). A follow-up 24-hour Holter registration showed a complete cessation of PVCs (0% PVC burden), and additionally, the patient became free from any symptoms.
DISCUSSION
This is the first report in the literature that describes evidence for a possible association between melatonin use and the occurrence of idiopathic VAs in humans. Discontinuation of melatonin in 2 patients with OT VAs led to a complete suspension of symptoms and the disappearance of arrhythmias on Holter/ILR registrations.
In the absence of structural heart disease, VAs most commonly arise in the RVOT. Focal triggered activity mediated by delayed after depolarizations (DADs) is believed to account for the generation of these VAs. DADs can be evoked in the presence of various pathological factors (myocardial ischemia, genetic disorders of intracellular Ca2+-handling, etc.), which can cause intracellular Ca2+ overload in myocytes. However, in the absence of such disorders, the mechanism of DAD-mediated arrhythmogenesis is less well understood. Increased sympathetic influence seems to play a role in the generation of DADs in normal myocardium in a cyclic adenosine monophosphate–mediated fashion.2
Several “extrinsic factors” have also been implicated to cause VAs in structurally normal hearts: extensive alcohol caffeine or tobacco use, electrolyte imbalance (hypokalemia), and certain medications represent the main examples. β-receptor activators (catecholamines and synthetic β-agonists) can cause DAD-induced VAs through the elevation of cyclic adenosine monophosphate levels and digitalis causes Ca2+ accumulation and subsequent DADs through the inhibition of the Na+–K+ exchange.2
Based on our observations, melatonin could also belong to the group of mediators that have the potential to precipitate VAs in structurally normal myocardium. In recent years, the clinical use of melatonin has increased significantly. In the United States, its use more than doubled between 2007 and 2012,3 and a similar (or even more significant) increase has been reported in Scandinavian countries.4 In Europe, the availability of melatonin as prescription versus over-the-counter drug varies from country to country. In the United States, melatonin is classified as dietary supplement and therefore available over the counter. Melatonin content of such dietary supplements is not controlled by the Food and Drug Administration (FDA), and therefore concerns may arise regarding the actual melatonin dose of these preparations.
Reports in the literature mainly argue for a protective effect of melatonin against arrhythmias. This putative antiarrhythmic effect has been implicated to occur through indirect mechanisms. As a sleep medication, it might be able to alleviate “sleep deprivation–induced arrhythmias.” In addition, by reducing the sympathetic tone, melatonin can also reduce arrhythmia burden caused by sympathetic predominance. A study that analyzed the effect of melatonin in canines on the repetitive extrasystole threshold of the vulnerable period of the ventricular myocardium showed that this threshold was increased by melatonin, thus arguing for a protective effect against arrhythmias.5 The authors proposed that this effect may be achieved by the inhibition of the flow of “arrhythmogenic” sympathetic nerve traffic from the central nervous system to the heart.5 Moreover, through its antioxidant activity melatonin has also been shown to significantly reduce ischemia/reperfusion-induced VAs.6,7
Recent studies describe the expression of melatonin receptors in cardiac tissue.8 The 3 known melatonin receptors are MT1, MT2, and MT3.9 Of these, MT1 and MT2 have been detected in the cardiovascular system.8,10 Through these 2 G-protein–coupled receptors, melatonin might be able to alter the function of key players in the Ca2+-handling machinery (eg, L-type Ca2+ channel, ryanodine receptor, and sarcoplasmic/endoplasmic reticulum calcium ATPase). Although a proarrhythmic mechanism has never been reported before, a presumable direct effect on the myocardium through its receptors could provide the functional basis for a proarrhythmic effect. However, (although less likely) indirect proarrhythmic effects of melatonin should also not be excluded. For instance, a seemingly paradoxical effect of melatonin is the reduction of deeper sleep.11 Through the altered sleep structure, melatonin might exert an indirect proarrhythmic effect. Another side effect of melatonin reported in literature is hypothermia,11 which in turn is thought to be a proarrhythmic condition. This proarrhythmic effect, however, is usually reported in the context of therapeutic hypothermia (between 32 and 36°C). The temperature drop associated with melatonin has been reported to be only 0.28°C after a dose of 5 mg, which therefore represents an unlikely mechanism for arrhythmogenesis in the patients in our current report.
The ultimate effect of melatonin (proarrhythmic vs. antiarrhythmic) might depend on the balance between its indirect versus direct effects, which in turn might be determined by several factors: eg, melatonin dosage in different preparations (especially in dietary supplements), differences in bioavailability, and genetically defined interindividual differences in receptor expression and receptor activity. For instance, it has been shown that there is a substantial person-to-person variability in bioavailability (with up to 25-fold variations in areas under the curve of a single dose in 5 subjects in 1 study).12 Time to maximum melatonin levels and half-life elimination may range between 40 and 90 minutes and 50–120 minutes, respectively.13,14 Hence, our patients might represent a certain population, the members of which might either show greater susceptibility to the proarrhythmic effects of melatonin or possess an altered pharmacokinetics and different bioavailability of this drug. Both of these conditions could lead to the development of symptomatic VAs in response to this medication.
When taking into consideration other possible triggers of PVCs, it is noteworthy to mention that the patient from case 2 used formoterol, which is known to have the potential to cause palpitations. However, the use of this long-acting β2-agonist was constant premelatonin, perimelatonin, and postmelatonin use. In addition, considering the fact that these patients did not have any drug in common on their list of medications (not even 1 from the same group) drug–drug interactions causing the proarrhythmic effect of melatonin seemed unlikely, as well.
Although idiopathic VAs are believed to follow a benign clinical course, some patients can be highly symptomatic (especially the ones with high PVC/nsVT burden) and in rare cases life-threatening VAs can occur. In addition, frequent PVCs/nsVT can precipitate a potentially reversible form of cardiomyopathy (even in asymptomatic patients). Therapy is only warranted for symptomatic patients or asymptomatic individuals with signs of decline of left ventricular systolic function, attributable to frequent PVCs/nsVTs. Medical therapy has proven to have limited efficacy. Catheter ablation, however, is a more effective treatment; although procedural success and complication rates may be highly dependent on the site of origin; with lower efficacy and higher complication rates reported for more uncommon sites of origin (left ventricular outflow tract, aortic cusps).1 Still, lower success rates may also be attributed to incomplete understanding of the arrhythmia mechanism and lack of knowledge on possible precipitating factors. Therefore our findings highlight the importance of the awareness of the potential for melatonin to cause VAs, and should prompt physicians to specifically search for melatonin use in patients with symptomatic PVCs or with high PVC burden on Holter, as melatonin use might often be unreported by many patients (especially in countries where it is available over the counter). In addition, these observations should initiate further studies aiming to identify other possible precipitating factors for VAs in a normal myocardium, and to clarify the underlying mechanisms of their effects. In our opinion, this approach would reduce the costs and increase the safety of the clinical treatment of patients with idiopathic ventricular arrhythmias because although the complication rates of ablation procedures are reported to be low, they are nevertheless not negligible (especially for foci located outside the RVOT) and include major complications such as tamponade, stroke, and coronary artery damage. Therefore identifying reversible causes (similar to melatonin medication) for idiopathic VAs would enable clinicians to offer better treatment strategies and avoid unnecessary and potentially harmful therapy.
ACKNOWLEDGMENTS
As native English speaker, R. Alloway revised the manuscript for language.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (ESC). Endorsed by: association for european paediatric and congenital cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 2.Issa Z, Miller JM, Zipes DP. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald's Heart Disease. 2nd ed Philadelphia, PA: Elsevier Health Sciences; 2012. [Google Scholar]
- 3.Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015;10:1–16. [PMC free article] [PubMed] [Google Scholar]
- 4.Hartz I, Handal M, Tverdal A, et al. Paediatric off-label use of melatonin–a register linkage study between the Norwegian prescription database and patient register. Basic Clin Pharmacol Toxicol. 2015;117:267–273. [DOI] [PubMed] [Google Scholar]
- 5.Blatt CM, Rabinowitz SH, Lown B. Central serotonergic agents raise the repetitive extrasystole threshold of the vulnerable period of the canine ventricular myocardium. Circ Res. 1979;44:723–730. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi H, Fujiki A, Tani M, et al. Role of sympathovagal balance in the initiation of idiopathic ventricular tachycardia originating from right ventricular outflow tract. Pacing Clin Electrophysiol. 1997;20:2371–2377. [DOI] [PubMed] [Google Scholar]
- 7.Diez ER, Prados LV, Carrion A, et al. A novel electrophysiologic effect of melatonin on ischemia/reperfusion-induced arrhythmias in isolated rat hearts. J Pineal Res. 2009;46:155–160. [DOI] [PubMed] [Google Scholar]
- 8.Ekmekcioglu C, Thalhammer T, Humpeler S, et al. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res. 2003;35:40–44. [DOI] [PubMed] [Google Scholar]
- 9.Witt-Enderby PA, Bennett J, Jarzynka MJ, et al. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 2003;72:2183–2198. [DOI] [PubMed] [Google Scholar]
- 10.Ekmekcioglu C, Haslmayer P, Philipp C, et al. Expression of the MT1 melatonin receptor subtype in human coronary arteries. J Recept Signal Transduct Res. 2001;21:85–91. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–131. [PubMed] [Google Scholar]
- 12.Waldhauser F, Waldhauser M, Lieberman HR, et al. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–313. [DOI] [PubMed] [Google Scholar]
- 13.Gooneratne NS, Edwards AY, Zhou C, et al. Melatonin pharmacokinetics following two different oral surge-sustained release doses in older adults. J Pineal Res. 2012;52:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen LP, Werner MU, Rosenkilde MM, et al. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol Toxicol. 2016;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]