Abstract
Background
Understanding the effects of capsule composition and transplantation site on graft outcomes of encapsulated islets will aid in the development of more effective strategies for islet transplantation without immunosuppression.
Methods
Here, we evaluated the effects of transplanting alginate (ALG)-based microcapsules (Micro) in the confined and well-vascularized epididymal fat pad (EFP) site, a model of the human omentum, as opposed to free-floating in the intraperitoneal cavity (IP) in mice. We also examined the effects of reinforcing ALG with polyethylene glycol (PEG). To allow transplantation in the EFP site, we minimized capsule size to 500 ± 17 μm. Unlike ALG, PEG resists osmotic stress, hence we generated hybrid microcapsules by mixing PEG and ALG (MicroMix) or by coating ALG capsules with a 15 ± 2 μm PEG layer (Double).
Results
We found improved engraftment of fully allogeneic BALB/c islets in Micro capsules transplanted in the EFP (median reversal time [MRT], 1 day) versus the IP site (MRT, 5 days; P < 0.01) in diabetic C57BL/6 mice and of Micro encapsulated (MRT, 8 days) versus naked (MRT, 36 days; P < 0.01) baboon islets transplanted in the EFP site. Although in vitro viability and functionality of islets within MicroMix and Double capsules were comparable to Micro, addition of PEG to ALG in MicroMix capsules improved engraftment of allogeneic islets in the IP site, but resulted deleterious in the EFP site, probably due to lower biocompatibility.
Conclusions
Our results suggest that capsule composition and transplant site affect graft outcomes through their effects on nutrient availability, capsule stability, and biocompatibility.
By evaluating the effects of the encapsulated islet grafts with different capsule compositions and transplant sites, the authors suggest that the islet grafts with micro capsules and implanted in vascularized sites may increase clinical efficacy. Supplemental digital content is available in the text.
Transplantation of pancreatic islets has shown great promise in achieving insulin independence and preventing type-1 diabetes complications.1 However, chronic immunosuppression is required to avoid rejection and recurrence of autoimmunity after transplantation. Chronic immunosuppression causes numerous adverse effects. Additionally, despite immunosuppression, 56% of the islet grafts lose function by 3 years after transplantation.1
Immunoisolation of pancreatic islets with biocompatible and permeable capsules may improve islet graft survival and allow for the reduction or total elimination of immunosuppression.2-7 Despite 3 decades of research, effective clinical islet encapsulation has not been achieved for reasons yet to be completely understood. Key capsule parameters, including geometry, composition, and transplant site affect the outcome of encapsulated islet grafts and the optimal combination of such parameters might lead islet encapsulation to success. In this study, we focus on the specific effects of capsule transplant site and composition on graft outcomes while keeping a constant geometry (Figure 1A). We used fixed-diameter spherical microcapsules that can be generated with traditional electrostatic droplet generator technology.
FIGURE 1.

Optimizing fabrication of ALG Micro capsules to minimize the volume of encapsulated islet grafts. A, Schematic of our approach to determine the effects of capsule composition and transplant site on encapsulated graft outcomes. B, Diameter distribution (n = 300) of cell-free 1.2% UP-MVG microcapsules (ALG) fabricated with the optimized parameters (Table 1, bold). C-D, Phase contrast images (C) and confocal images of live (green)/dead (red) stained (D) ALG microcapsules fabricated with optimized fabrication parameters (Table 1, bold) and loading density of pancreatic islets from Lewis Rats equal to 5 k, 15 k, and 30 k IEQ/mL and compared to Naked islets; scale bars 100 μm; nuclei: blue. E-F, GSIR of islets encapsulated using optimized fabrication parameters (Table 1, bold). Micro capsules loaded with 15 k IEQ/mL rat islets (green) are compared to naked islets (black). N = 3 aliquots of 100 IEQ per conditions from a minimum of n = 3 independent experiments. Absolute values of insulin concentration in supernatants after incubation in low glucose (L1), high glucose (HG) and low glucose (L2) (E), and stimulation indexes (F) are indicated. G, Diameter distribution (n = 253 capsules from n = 7 independent experiments) of islet-containing 1.2% UP-MVG microcapsules (ALG) fabricated with the optimized parameters (Table 1, bold).
To evaluate the effects of the transplant site, we used alginate (ALG), a material that has been widely used for islet encapsulation.8-18 Traditional ALG microcapsule diameters range from 600 to 1000 μm with most of the volume being islet-free and biologically nonfunctional material.19 Large amounts of bulk capsule material represent a barrier for transport of critical solutes to the islets, which could lead to core hypoxia and necrosis. Furthermore, large diffusion barriers hamper the transport of glucose and insulin through the capsule leading to a delay in glucose sensing and insulin secretion of the encapsulated islets.20-22 Finally, large capsule size may increase the volume of transplanted material up to 1000 times for capsule diameters of 1000 μm in comparison to naked islets with the assumption that only 50% of capsules contain islets. Therefore, such volumes have so far limited the transplantation site to the intraperitoneal cavity (IP), which, unfortunately, is not an islet-friendly environment.22-25 After transplantation in the IP site, capsules fall by gravity and aggregate in the lower abdomen worsening transport through the capsule. Here, we ask whether transplantation of minimized volumes of encapsulated islets in confined and vascularized sites, like the omental pouch in humans (a site we are currently testing in a phase I/II clinical trial with naked islets and chronic immunosuppression at the University of Miami Diabetes Research Institute) and the epididymal fat pad (EFP) in mice, can ameliorate the outcome of encapsulated allografts and of nonhuman primate (NHP) islet grafts in immunodeficient mice.
Unlike polyethylene glycol (PEG), ALG is susceptible to swelling and rupture after transplantation due to osmotic stress leading to loss of immunoisolation and graft rejection.26 Here, we ask whether transplantation of PEG-ALG hybrid 500 μm-diameter micro capsules could ameliorate the outcome of encapsulated islet grafts in mice, especially in the IP site, where the graft is exposed to higher levels of osmotic pressure and mechanical stress than in the EFP site.
MATERIALS AND METHODS
Encapsulation Materials
Micro capsules: ultra-pure medium viscosity sodium alginate (UP-MVG alginate, Novamatrix) at 1.2% w/v gelled with 50 mM calcium chloride (CaCl2). MicroMix capsules: 1.2% UP-MVG-5% w/v PEG, functionalized (75%) with maleimide (MAL) groups (PEG-MAL, 10 kDa, 8-arm, Jenkem Technology custom synthesis) in 1 mL of 1.2% UP-MVG solution. Double capsules: 5% w/v PEG-MAL crosslinked with dithiothreitol (DTT) (OmniPur, Calbiochem) in a 3:1 molar ratio of DTT to PEG.
Islet Isolation
Animal studies were performed under protocol 13-042. Islets from Male BALB/c mice (Jackson Laboratories), Lewis rats (Envigo Laboratories, formerly Harlan) and NHP baboon (The Mannheimer Foundation, Inc., Homestead, FL) were isolated as described elsewhere.27,28
Osmotic Pressure Test
Evaluation of mechanical stability of microcapsules was performed by osmotic pressure testing as previously described.29
Fabrication of Double Capsules
One hundred microliters UP-MVG Micro capsules were suspended in 1 mL of 5% PEG-MAL (water phase). A solution of 50 mL light mineral oil (Sigma Aldrich) and 5% Span80 (Sigma Aldrich) (oil phase) was formed by stirring at 350 rpm for 2'. The water phase was added drop-by-drop to the center of the oil phase while the oil phase was continuously stirred at 350 rpm. Five minutes after addition of the water phase to the oil phase, the DTT solution in DMSO was added to induce PEG-MAL gelation and the stirring speed was increased to 450 rpm. PEG double coating was allowed to crosslink for 15 minutes and secondary beads were removed by filtration through a 250-μm strainer (Thermo Scientific).
In Vitro Assessment of Viability and Functionality of Encapsulated Islets
Static glucose-stimulated insulin release (GSIR) was utilized for assessment of islet function as previously described.30 For viability assessment, naked and encapsulated islets were stained with calcein-AM (live cell marker) and ethidium bromide (dead cell marker) (live/dead viability kit, Molecular Probes), and imaged with a Leica SP5 inverted confocal microscope. Oxygen consumption rate measurements were performed as previously described.31
Engineered Fibrin Gels
Fibrin gels were engineered for promoting rapid revascularization of embedded islets as previously described.32
Diabetes Induction and Islet Transplantation in Mice
Diabetes was induced in islet recipients by a single i.v. injection of streptozotocin (200 mg/kg; Sigma-Aldrich) as previously described.30 For transplantation in the EFP, 750 IEQ islets were distributed uniformly on the surface of the EFP and 20 μL of engineered fibrin gels were then pipetted on the EFP to cover the islets. Graft function was monitored by measuring nonfasting blood glucose (BG) values. Reversal of diabetes was considered when mice maintained at least 3 consecutive BG readings less than 250 mg/dL after islet transplantation. Graft rejection was considered when at least 3 consecutive BG readings greater than 250 mg/dL were detected in those mice that reversed diabetes after islet transplantation. For intraperitoneal (IP) islet transplantation, islets were injected into the peritoneal cavity in a total volume of approximately 0.2 mL. Transplantation in the renal subcapsular space (KD) was performed as previously described.30
Additional information on Materials & Methods can be found in SDC, http://links.lww.com/TP/B339.
RESULTS
Minimizing the Volume of ALG Microcapsules for Transplantation in Confined and Vascularized Sites
We aimed at reducing the total volume of the encapsulated islet graft by (1) minimizing the diameter of the capsules while keeping a homogenous size distribution and (2) maximizing islet-loading density while maintaining coating completeness. Keeping the ALG concentration (1.2% w/v), the potential difference (8.8 kV) and the flow rate (10 μL/min) constant, the average capsule diameter was decreased from 749 ± 35 μm to 279 ± 29 μm by reducing the needle internal diameter from 0.6 mm to 0.17 mm (Table 1). Pancreatic islets have a diameter of 50 to 350 μm. Therefore, we chose 0.4 mm and not 0.17 mm as the internal diameter of the needle to encapsulate the islets. By reducing the flow rate of the alginate solution from 50 to 10 μL/min while keeping the potential difference (8.8 kV) and the internal diameter of the needle (0.4 mm) constant, we were able to decrease the average capsule diameter from 651 ± 12 μm to 526 ± 48 μm (Table 1, Figure 1B).
TABLE 1.
Average diameter and standard deviation (SD) of cell-free ALG micro as a function of the needle internal (ID) and external (OD) diameter (top) and the ALG extrusion flow rate (bottom) in the electrostatic droplet generator process

During the encapsulation process, we compared 3 different islet-loading densities: 500, 1500, and 3000 IEQ suspended in a volume of 100 μL ALG (final islet density: 5 k, 15 k, and 30 k IEQ/mL, respectively). We found that the 5-k IEQ/mL density led to the highest percentage of cell-free capsules, whereas the 30 k IEQ/mL density results in multiple islets per capsule (Figure 1C). Live/dead staining and confocal imaging showed that capsules generated with 15 k IEQ/mL islet density had higher cell viability than capsules with 30 k IEQ/mL islet density (Figure 1D). We also found that 15 k IEQ/mL microencapsulated islets had similar GSIR function as to naked islets (Figures 1E-F) in addition to homogeneous diameters (median, 525 μm; Figure 1G).
We concluded that by using an electrostatic droplet generator, we could reduce the volume of standard alginate microcapsules to values that allowed transplantation in the EFP site in mice without impairing viability and function of encapsulated pancreatic islets.
Determining the Effects of the Transplantation Site on the Outcome of ALG Microcapsules in Murine Allografts and Application to NHP Islet Grafts in Immunodeficient Mice
To increase the proangiogenic potential of the EFP site, we used a hydrogel that allows for extended release of proangiogenic factors and their synergistic signaling with extracellular matrix-binding domains in the posttransplant period.32 We found that 750 IEQ naked islets reversed diabetes within 6 days (median reversal time [MRT], 1 day) after transplantation in the engineered EFP site, whereas they did not reverse diabetes after transplantation in the IP site in fully major histocompatibility complex (MHC)-mismatched chemically induced diabetic recipients (MRT, undefined; P < 0.01) (Figures 2A-B). As expected, naked islets were promptly rejected within 27 days in the EFP site (median survival time [MST], 17 days). The same number of islets enclosed in ALG Micro capsules and implanted in the engineered EFP site reversed hyperglycemia as efficiently as naked islets (MRT, 1 day; P = 0.22) (Figures 2A-B) but, unlike naked islets, islets in Micro capsules were able to maintain euglycemia for more than 100 days (MST, undefined; P < 0.01) in absence of immunosuppression (Figures 2A-C). This confirms the effectiveness of ALG Micro capsules in preventing immune rejection. When implanted in the IP site, unlike naked islets, 750 IEQ encapsulated islets were able to reverse diabetes within 7 days (MRT: 5 days, P < 0.001) but they did so with less efficiency than in the EFP site (P < 0.01) (Figures 2A-B). Finally, in the IP site, microencapsulated islets showed a trend toward decreased survival when compared with transplants in the EFP site (MST, undefined; P = 0.08) (Figure 2C).
FIGURE 2.
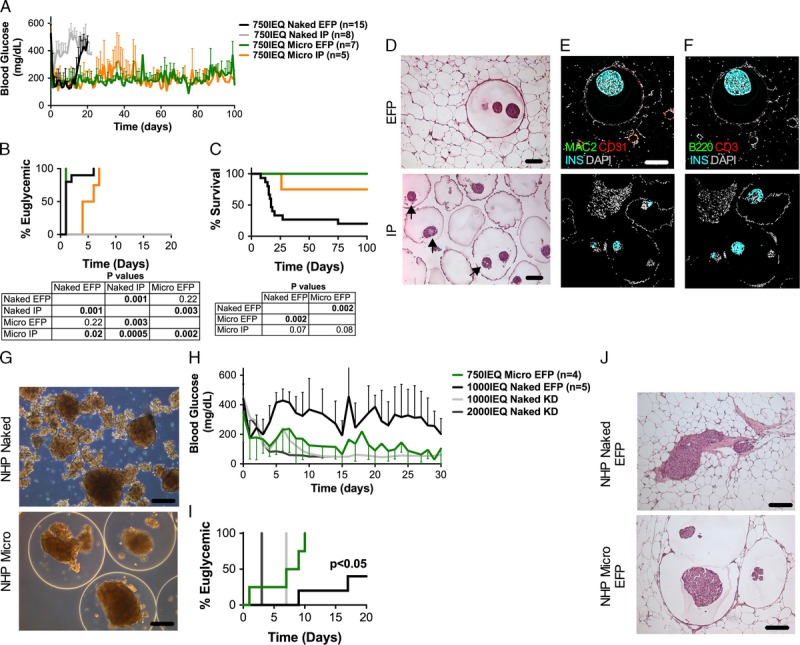
Effects of transplantation site on the outcome of islet allografts encapsulated in optimized ALG microcapsules (Micro) without immunosuppression. The free-floating intraperitoneal (IP) site is compared with the confined and vascularized EFP site. A, Blood glucose of STZ-induced diabetic C57BL/6 mice transplanted with 750 IEQ naked in the EFP (black, n = 15) or IP (grey, n = 8) sites or 750 IEQ microencapsulated (Micro) in the EFP (green, n = 7) or IP (orange, n = 5) sites; all islets from fully MHC-mismatched BALB/c mice donors. B, Percentage of mice that reversed diabetes after transplantation. C, Percentage survival of allografts that reversed diabetes after transplantation. Tables below graphs indicate P values. D-F, Histological evaluation of EFP grafts and of capsules retrieved from the IP site by intraperitoneal lavage, fixed in formalin, embedded in paraffin, and thin sliced (5 μm). Shown are grafts that reversed diabetes and maintained euglycemia for more than 100 days. In H&E-stained sections (D) arrows point at areas of islet central necrosis. Scale bars, 100 μm. Confocal images: host vessels (CD31+, red), macrophages (MAC2+, green) and beta cells (INS+, cyan) are shown in panel (E); T cells (CD3+, red), B cells (B220+, green) and beta cells (INS+, cyan) are shown in panel (F). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (grey). Scale bar 150 μm; (G) Phase contrast images of baboon islets encapsulated in Micro capsules fabricated with optimized fabrication parameters (Table 1, bold) and loading density of 15 k IEQ/mL and compared to Naked islets. Scale bars, 200 μm; (H) Blood glucose of STZ-induced diabetic NOD-scid mice transplanted with 1000 IEQ naked (black, n = 5) or 750 IEQ microencapsulated (Micro, green, n = 4) islets in the EFP and compared to 1000 IEQ (light grey) and to 2000 IEQ (dark grey) naked islets transplanted in the kidney capsule (KD) controls; all islets from baboon nonhuman primate donors. I, Percentage of mice that reversed diabetes after transplantation of baboon islets. J, Histological evaluation of EFP grafts of naked versus Micro encapsulated in the EFP site analyzed 30 days after transplantation in diabetic NOD-scid mice. Scale bars 200 μm.
Grafts retrieved 100 days after transplantation showed that the majority of the microcapsules analyzed (n = 5-7) were intact. The islets within the capsules retrieved from the EFP site had no evidence of degranulation as determined by histological analysis (Figure 2D) and showed well-preserved architecture and strong insulin staining (Figures 2E-F). This is indicative of long-term maintenance of islet viability. Conversely, islets within the capsules that were retrieved from the IP site showed central necrosis (Figure 2D, arrows). Evident vascularization was observed in the perigraft tissue in close proximity but not within the implanted capsules in the EFP site as assessed by CD31 immunofluorescence staining and confocal microscopy (Figure 2E). Lack of CD3+ T cells and B220+ B cells capsule infiltration (Figure 2F) indicates that incomplete survival of capsules in the IP site was not due to loss of immunoisolation.
We conclude that transplantation of islets in ALG microcapsules with minimized volume in a highly vascularized engineered EFP site improves engraftment and long-term function of allogeneic islets when compared with a free floating transplant configuration like the one in the IP site.
Next, we evaluated whether our novel transplantation approach could be translated to transplantation of a marginal mass of baboon NHP islets in immunodeficient and chemically diabetic NOD-scid mice. We found that baboon islets could be encapsulated in Micro capsules with the same protocol we optimized for rodent islets (Figure 2G). More importantly, we found improved engraftment of 750 IEQ baboon islets in Micro capsules (MRT, 8 days) versus 1000 IEQ naked (MRT, 36 days; P < 0.01) islets transplanted in the EFP site. Grafts retrieved 30 days after transplantation showed that islets within Micro capsules had no evidence of degranulation as determined by histological analysis in comparison to naked islet grafts (Figure 2J), which is indicative of maintenance of islet viability.
Design, Fabrication and In Vitro Evaluation of PEG-ALG Hybrid MicroMix and Double Capsules
We found that unlike PEG, ALG capsules were not resistant to osmotic stress—that is, capsule size increased within minutes of incubation either in saline or in H2O and dissolved within 60 minutes (Figures 3A-B). More importantly, addition of PEG to ALG capsules improved the mechanical stability of ALG capsules (Figures 3A-B).
FIGURE 3.
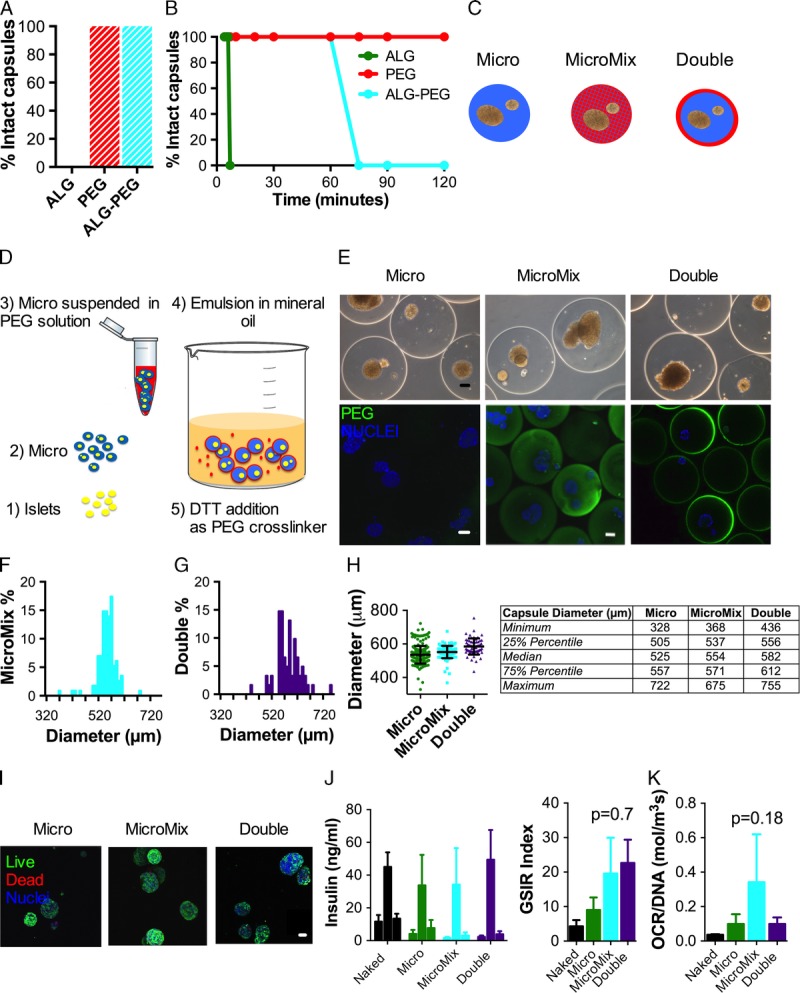
Design, fabrication and in vitro evaluation of PEG-ALG hybrid MicroMix and Double capsules compared to ALG Micro capsules. A-B, Osmotic pressure resistance of cell-free ALG capsules (Micro) compared to PEG capsules and ALG-PEG capsules (MicroMix). Percentage of intact Micro (n = 30) versus PEG (n = 30) versus ALG-PEG (n = 30) capsules after exposure to 2 hrs ddH2O followed by saline buffer for 60 minutes (A) and % intact capsule dependence on time exposure to saline (B). C, Schematic of ALG Micro versus hybrid ALG-PEG Micromix capsules, where PEG and ALG are interlaced, and Double capsules, where PEG is added to the ALG Micro capsule as a thin external layer. D, Schematic of the emulsion procedure for fabrication of Double capsules. E, Representative phase contrast (top) and confocal images (bottom) of Lewis rat islets enclosed in Micro, Micromix and Double capsules stained with anti-PEG antibodies (green). Nuclei are counterstained with Hoechst (blue). Thickness of double capsules was quantified on n = 12 capsules and was found to be 15 ± 2 μm. Scale bars 100 μm. F-H, Diameter distribution of islet-containing MicroMix (blue, F) and Double (purple, G) capsules and direct comparison with Micro capsules (H, P > 0.05). In panel H, statistical analysis of the measured values is presented in the table next to the graph. I, Viability assessment by live (green) and dead (red) staining via confocal imaging of Lewis rat islets enclosed in Micro, Micromix, and Double capsules 48 hours after encapsulation. Nuclei are counterstained with Hoechst (blue). Scale bar 100 μm. J, GSIR of Lewis rat islets encapsulated in Micro (green), MicroMix (blue), Double (purple) capsules and compared to naked islets (black). Absolute insulin secretion and stimulation index are indicated. N = 3 aliquots of 100 IEQ per conditions from a minimum of n = 3 independent experiments. K, Oxygen consumption rate (OCR) normalized to total DNA content of Lewis rat islets encapsulated in Micro (green), MicroMix (blue), Double (purple) capsules and compared to naked islets (black); n = 3 per condition.
Next, we fabricated MicroMix capsules (Figure 3C) with the protocol previously optimized for ALG Micro capsules (Figure 1) and Double coating of ALG Micro capsules with PEG (Double) by a new double emulsion method (described in the method section and Figure 3D). We optimized the emulsion parameters to minimize the thickness of the PEG double coatings and the percentage of PEG-only secondary beads to maintain reduced graft volume and good biocompatibility.33 Immunostaining with an anti-PEG antibody confirmed that PEG was uniformly distributed throughout the capsules in the MicroMix configuration, whereas it was absent in the ALG capsules (negative control) and it formed a thin, uniform layer (15 ± 2 μm thick) on 100% of the Double capsules (Figure 3E). Spherical shape, average diameter, and size distribution of MicroMix and Double capsules were comparable to Micro capsules (Figures 3F-H). Viability (Figure 3I), GSIR (Figure 3J, P > 0.05) and oxygen consumption rate (Figure 3K) of islets encapsulated in Micro, MicroMix, and Double capsules were also comparable.
We concluded that reinforcement of ALG microcapsules with PEG improved capsule stability to osmotic stress without affecting capsule geometry or in vitro viability and function of encapsulated islets.
Determining the Effects of Capsule Composition on the Outcome of PEG-ALG Encapsulated Islet Allografts in Mice
IP Site
When implanted in the IP site, islets within MicroMix capsules engrafted faster (MRT, 2 days) than islets within Micro capsules (MRT, 5 days; P < 0.05) and naked islets (MRT, undefined; P < 0.01) (Figures 4A-B). Survival of islets within MicroMix capsules (MST, undefined) was comparable to islets within Micro capsules (MST, undefined; P = 0.77) (Figure 4C). Islets within Double capsules reversed diabetes only in 4 of 6 mice and showed some delay in reversal (MRT, 12 days), although not significantly different to islets within Micro (P = 0.15) and MicroMix capsules (P = 0.07). Survival of islets within Double capsules was comparable (MST, 94.5 days) to islets within Micro (P = 0.8) and MicroMix (P = 0.62) capsules (Figure 4C).
FIGURE 4.

Effects of composition of PEG-ALG capsules on the outcome of islet allografts in the IP site without immunosuppression. ALG Micro capsules are compared to PEG-ALG hybrid MicroMix and Double capsules containing islets and to naked islets. A, Blood glucose of STZ-induced diabetic C57BL/6 mice transplanted with 750 IEQ naked (grey, n = 7) islets or encapsulated in Micro (orange, n = 4), or MicroMix (blue, n = 4), or Double (purple, n = 6) capsules in the IP; all islets from fully MHC-mismatched BALB/c mice donors. B, Percentage of mice that reversed diabetes after transplantation. C, Percentage survival of allografts that reversed diabetes after transplantation. Tables below graphs indicate P values. D-F, Histological evaluation of grafts retrieved from the IP site by intraperitoneal lavage, fixed in formalin, embedded in paraffin, and thin sliced (5 μm). Shown are grafts that reversed diabetes and maintained euglycemia for more than 100 days. In H&E stained sections (D) arrows point at areas of islet necrosis. Scale bars 100 μm. Confocal images: host vessels (CD31+, red), macrophages (MAC2+, green) and beta cells (INS+, cyan) are shown in panel E; T cells (CD3+, red), B cells (B220+, green) and beta cells (INS+, cyan) are shown in panel F. Nuclei are counterstained with DAPI (grey). Scale bar, 150 μm.
Histological analysis of grafts that survived more than 100 days after transplantation revealed that the majority of Micro and MicroMix capsules did not present fibrotic outgrowths, capsule damage, and/or fracture (Figure 4D). On the other hand, Double capsules were covered with a 2-layer thick cell overgrowth and presented scattered pockets of inflammatory cells in a portion of the explanted capsules. Islets within Double capsules were fragmented and had a loss of pericapsular membrane, which is indicative of compromised viability and central necrosis (Figure 4D). Immunofluorescence staining confirmed a higher proportion of insulin positive cells in islets enclosed in Micro and MicroMix capsules versus Double capsules (Figures 4E-F). Lack of macrophage (MAC2+, Figure 4E), T and B cell (CD3+ or B220+, respectively, Figure 4F) deposition and penetration in all the capsule compositions suggested that capsules were immunoisolating.
We concluded that addition of PEG to ALG capsules in the MicroMix but not the Double configuration improved islet engraftment in the IP site, whereas long-term islet survival was comparable.
EFP Site
In the EFP site, islets within MicroMix capsules reversed diabetes slightly slower (MRT, 1.5 days) than islets in Micro capsules (MRT, 1 day; P < 0.05) but comparable to naked islets (P = 0.75) (Figures 5A-B). Graft survival within MicroMix capsules (MST, 79 days) was inferior to Micro capsules (MST > 100 days; P < 0.05) and not statistically different than naked islets (MST, 17 days; P = 0.19) (Figure 5C). Islets within Double capsules (MRT, undefined and only in 2/6 recipient mice) reversed diabetes less efficiently than naked islets (P < 0.01), Micro (P < 0.01), and MicroMix capsules (P < 0.05) (Figures 5A-B). Finally, islets within Double capsules (MST, 19 days) displayed poor survival in comparison to Micro capsules (P < 0.05), but was not significantly different from MicroMix (P = 0.53) or naked islets (P = 0.75) (Figure 5C).
FIGURE 5.

Effects of composition of PEG-ALG capsules on the outcome of islet allografts in the EFP site without immunosuppression. ALG Micro capsules are compared to PEG-ALG hybrid MicroMix and Double capsules and to naked islets. A, Blood glucose of STZ-induced diabetic C57BL/6 mice transplanted with 750 IEQ naked (black, n = 15) islets or encapsulated in Micro (green, n = 7), or MicroMix (blue, n = 4), or Double (purple, n = 6) capsules in the EFP; all islets from fully MHC-mismatched BALB/c mice donors. B, Percentage of mice that reversed diabetes after transplantation. C, Percentage survival of allografts that reversed diabetes after transplantation. Tables below graphs indicate P values. D-F, Histological evaluation of EFP grafts fixed in formalin, embedded in paraffin, and thin sliced (5 μm). Shown are grafts that reversed diabetes and maintained euglycemia for more than 100 days. In H&E stained sections (D) arrows point at area of host reactivity. Scale bars 100 μm. Confocal images: host vessels (CD31+, red), macrophages (MAC2+, green) and beta cells (INS+, cyan) are shown in panel E, T cells (CD3+, red), B cells (B220+, green) and beta cells (INS+, cyan) are shown in panel (F). Nuclei are counterstained with DAPI (grey). Scale bar 150 μm. G-I, Biocompatibility of cell-free Micro (G), MicroMix (H) and Double (I) capsules in the EFP site: H&E staining (top) and Masson’s Trichrome (bottom), 7 days after implantation. Scale bar 100 μm.
Histological analysis of grafts surviving for more than 100 days after transplantation demonstrated that Micro and MicroMix capsules were intact and their spherical shape was preserved (Figure 5D). Insulin staining of islets within Micro and MicroMix capsules revealed absence of either degranulation or central necrosis in Micro and MicroMix capsules indicating maintenance of overall viability (Figures 5E-F). Host reaction at the capsule interface was slightly higher in MicroMix versus Micro capsules as indicated by a 1-layer thick cell overgrowth on the surface of MicroMix capsules (Figure 5D). On the other hand, the host inflammatory response to Double capsules was markedly higher as shown by the thicker cellular overgrowth on the surface of those capsules (Figure 5D). Islets within double capsules were fragmented and viability was compromised (Figure 5E). Lack of macrophages (MAC2+, Figure 5E), T and B cells (CD3+ or B220+, respectively, Figure 5F) deposition or penetration in all the capsule compositions indicates that capsules were immunoisolating.
Next, we examined whether lower biocompatibility of MicroMix and Double capsules in comparison to that of Micro capsules was responsible for the reduced islet function. After implantation of empty capsules in the EFP, we found that Micro capsules displayed high biocompatibility with minimal cellular overgrowth and collagen deposition as assessed by trichrome staining (Figure 5G). Inflammatory responses to MicroMix capsules (Figure 5H) were stronger than Micro with slightly higher surface overgrowth. Double capsules, instead, displayed thick cellular overgrowth and fibrotic capsule formation on their surface (Figure 5I).
Overall, we found that the addition of PEG as a reinforcement material to improve ALG stability (MicroMix) showed a benefit in improving islet engraftment when capsules were implanted in the free-floating configuration in the IP site but not in the EFP site. In the EFP confined and highly vascularized site, where the encapsulated islets were placed in direct contact with host tissue, the biocompatibility of the capsule material appeared to be slightly worse and likely affected graft outcome.
DISCUSSION
The success rate of microencapsulated islet allogeneic transplants in preclinical models is encouraging, but lack of translatability of preclinical results in effective clinical protocols is a current hurdle. Gaining a better understanding of the reasons for variability of preclinical results may help identify more effective strategies for better outcomes in future clinical trials.
The rationale for the work presented here was that capsule geometry, composition, and transplant site are the main determinants of the outcome of the encapsulated islet graft. Transport of nutrients to avascular islets relies on passive diffusion. Because diffusion rate depends on the diffusion distance, reducing the capsule diameter from 800 to 500 μm, as we successfully achieved, likely improved transport of nutrients including oxygen, to the islet core, positively impacting islet viability and GSIR function. Nutrient consumption rate inside the capsule is proportional to the number of cells. By comparing the effects of different islet loading densities, we concluded that a 15-k IEQ/mL (3%) loading density was a good compromise between minimizing graft volume and maximizing islet viability. This result represents an improvement over traditional loading densities (0.8%-1.5%).
Reducing the capsule diameter from 800 μm (64-fold increase in graft volume compared with naked islets) to 500 μm (15.6-fold increase in graft volume compared to naked islets) was associated with a 4-fold reduction of the total volume of the encapsulated islet graft and allowed us to transplant encapsulated islets in the confined and highly vascularized EFP site. This allowed us to evaluate the importance of the transplant site in promoting engraftment and long-term survival of encapsulated islets. We have previously shown that presence of pro-angiogenic gels improves the outcome of naked islet grafts in the EFP site.32 Here, we evaluated the potential beneficial effects of transplanting encapsulated islets in a confined site with the highest proangiogenic potential (with inclusion of proangiogenic gels) versus a site where encapsulated islets remain free-floating and cannot get revascularized (IP). Delays in oxygen transport to the islet core in the IP site might have caused the central necrosis phenomenon that we observed in encapsulated islets in the IP but not the EFP site. The fibrotic overgrowth of Double capsules due to poorer biocompatibility observed histologically might have caused a delay in glucose and insulin diffusion through the peritoneal membrane and the capsule and caused loss of a portion of the islet graft. This in turn might be the reason for longer reversal time and the presence of more pronounced BG fluctuations that were observed for Double capsules in the IP site. Lack of T and B cell recruitment and infiltration into the capsules in all conditions further confirmed that reduced long-term graft survival was not due to lack of immunoisolation and immune rejection of transplanted islets, but was likely dependent on islet death because of insufficient transport of nutrients.
Transplantation in the EFP site may not only enhance nutrient transport due to the proximity of host vessels to the encapsulated graft but also may provide protection from mechanical stress at the implant site by confining the graft between 2 mesothelial layers and preventing shear stress due to graft displacement. Both enhanced nutrient transport and protection from mechanical stress are determining factors for long-term survival of encapsulated islets. We found that the more stable MicroMix capsules improved islet engraftment in the IP site where higher mechanical protection is needed but not in the EFP site where higher stability may not be critical for islet function, and where capsule biocompatibility may play a predominant role in determining the outcome of the encapsulated graft. Conversely, Double capsules did not show any improvement in islet engraftment and long-term function over ALG capsules in the IP site. In fact, they had worse outcomes in the EFP site. This is likely due to the fact that any beneficial effects of higher stability conferred by PEG double coatings may not outweigh the observed poor biocompatibility. This is in contrast with our previous studies on conformal coating encapsulation with PEG hydrogels where we did not observe such high fibrotic reaction to thin and conformal PEG coatings. Potentially, worse biocompatibility of Double coatings may come from the presence of secondary particles generated by the double emulsion technology. Secondary particles are smaller and therefore less biocompatible than 500-μm capsules.33 Furthermore, worse biocompatibility of PEG Double coatings may be due to the larger surface area and total volume of PEG double coatings versus conformal coatings, as well as the different transplant site (EFP and IP here vs the kidney capsule in Tomei et al30).
Although the improvement to achieve normoglycemia in mice transplanted in the EFP versus the IP site was a matter of a few days, this could be due to the relatively high dose of islets transplanted in each mouse. Although a few days might seem trivial in mice, the difference in diabetes reversal of a few days with full islet mass may translate into a dramatically bigger effect when suboptimal islet doses are transplanted in larger animals and humans. This is supported by our results from transplantation of a marginal mass of NHP islets in the EFP site of immunodeficient mice showing diabetes reversal and sustained function of baboon islets within Micro capsules optimized to minimize the graft volume. Further work is required to validate our approach and begin to understand the effects of islet encapsulation in autoimmune models of diabetes where additional challenges may require further modifications of our transplantation protocol.
Our results suggest that future clinical trials should be designed to determine whether transplantation of encapsulated islets in clinically relevant confined and vascularized sites, like the omentum, might increase the efficacy of encapsulated islet grafts in humans. We anticipate that application of our findings to improved capsule geometries where coating thickness is minimized will provide additional improvement to the outcome of encapsulated islet grafts and will benefit the field of islet transplantation.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. C. Fraker and Dr. K. G. Asfura for critical discussion on alginate encapsulation and in vitro assessments, to the personnel of the DRI Preclinical Cell Processing and Translational Models Core for their help with islet isolation, transplantation and management of diabetic mice, to the DRI Imaging Core for providing expertise on confocal imaging, and to the DRI Histology core headed by Kevin Johnson for his help with histological processing of all samples.
Footnotes
C.V. and V.M. contributed equally to the work.
Funding was provided by philanthropic funds from the Diabetes Research Institute Foundation, grants from the Juvenile Diabetes Research Foundation (grant 17-2001-268, 17-2010-5 and 17-2012-361), the Fondazione Tronchetti Provera, and the Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico.
A.A.T. is coinventor of intellectual property used in the study and may gain royalties from future commercialization of the technology licensed to Converge Biotech Inc. C.R. is member of the scientific advisory board and stock option holders in Converge Biotech, licensee of some of the intellectual property used in this study. A.T. and R.D.M. are stock option holders in Converge Biotech.
A.A.T., R.D.M., Y.T., and C.R. designed research; C.V., V.M., M.N., M.M.A., C.V., M.S., and R.D.M. performed research; A.A.T., V.M., and C.V. analyzed data; A.A.T. directed the project, and A.A.T., C.V., V.M., and M.M.A. wrote the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basta G, Calafiore R. Immunoisolation of pancreatic islet grafts with no recipient's immunosuppression: actual and future perspectives. Curr Diab Rep. 2011;11:384–391. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan ES, Vegas A, Anderson DG, et al. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32:827–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuch BE, Keogh GW, Williams LJ, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32:1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faleo G, Lee K, Nguyen V, et al. Assessment of immune isolation of allogeneic mouse pancreatic progenitor cells by a macroencapsulation device. Transplantation. 2016;100:1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang HK, Ham DS, Park HS, et al. Long-term efficacy and biocompatibility of encapsulated islet transplantation with chitosan-coated alginate capsules in mice and canine models of diabetes. Transplantation. 2016;100:334–343. [DOI] [PubMed] [Google Scholar]
- 7.Sasikala M, Rao GV, Vijayalakshmi V, et al. Long-term functions of encapsulated islets grafted in nonhuman primates without immunosuppression. Transplantation. 2013;96:624–632. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann U, Mimietz S, Zimmermann H, et al. Hydrogel-based non-autologous cell and tissue therapy. Biotechniques. 2000;29:564–572 574, 576 passim. [DOI] [PubMed] [Google Scholar]
- 9.Draget KI, Skjåk-Braek G, Smidsrød O. Alginate based new materials. Int J Biol Macromol. 1997;21:47–55. [DOI] [PubMed] [Google Scholar]
- 10.Soon-Shiong P, Desai NP, Sanford PA, et al. Crosslinkable polysaccharides, polycations and lipids useful for encapsulation and drug release. Patent PCT/US92/09364 World International Property Organization. 1993:1–52. [Google Scholar]
- 11.Mazaheri R, Atkison P, Stiller C, et al. Transplantation of encapsulated allogeneic islets into diabetic BB/W rats. Effects of immunosuppression. Transplantation. 1991;51:750–754. [DOI] [PubMed] [Google Scholar]
- 12.Duvivier-Kali VF, Omer A, Parent RJ, et al. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50:1698–1705. [DOI] [PubMed] [Google Scholar]
- 13.Omer A, Duvivier-Kali V, Fernandes J, et al. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79:52–58. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Adcock J, Kühtreiber W, et al. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation. 2008;85:331–337. [DOI] [PubMed] [Google Scholar]
- 15.Soon-Shiong P, Feldman E, Nelson R, et al. Long-term reversal of diabetes in the large animal model by encapsulated islet transplantation. Transplant Proc. 1992;24:2946–2947. [PubMed] [Google Scholar]
- 16.Lanza RP, Jackson R, Sullivan A, et al. Xenotransplantation of cells using biodegradable microcapsules. Transplantation. 1999;67:1105–1111. [DOI] [PubMed] [Google Scholar]
- 17.Lum ZP, Krestow M, Tai IT, et al. Xenografts of rat islets into diabetic mice. An evaluation of new smaller capsules. Transplantation. 1992;53:1180–1183. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Ma X, Zhou D, et al. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98:1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paredes Juárez GA, Spasojevic M, Faas MM, et al. Immunological and technical considerations in application of alginate-based microencapsulation systems. Front Bioeng Biotechnol. 2014;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwald P. A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theor Biol Med Model. 2011;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omer A, Duvivier-Kali VF, Aschenbach W, et al. Exercise induces hypoglycemia in rats with islet transplantation. Diabetes. 2004;53:360–365. [DOI] [PubMed] [Google Scholar]
- 22.De Vos P, Vegter D, De Haan BJ, et al. Kinetics of intraperitoneally infused insulin in rats. Functional implications for the bioartificial pancreas. Diabetes. 1996;45:1102–1107. [DOI] [PubMed] [Google Scholar]
- 23.Colton CK, Avgoustiniatos ES. Bioengineering in development of the hybrid artificial pancreas. J Biomech Eng. 1991;113:152–170. [DOI] [PubMed] [Google Scholar]
- 24.Calafiore R. Perspectives in pancreatic and islet cell transplantation for the therapy of IDDM. Diabetes Care. 1997;20:889–896. [DOI] [PubMed] [Google Scholar]
- 25.Elliott RB, Escobar L, Tan PL, et al. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc. 2005;37:3505–3508. [DOI] [PubMed] [Google Scholar]
- 26.Bhujbal SV, Paredes-Juarez GA, Niclou SP, et al. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J Mech Behav Biomed Mater. 2014;37:196–208. [DOI] [PubMed] [Google Scholar]
- 27.Pileggi A, Molano RD, Berney T, et al. Prolonged allogeneic islet graft survival by protoporphyrins. Cell Transplant. 2005;14:85–96. [PubMed] [Google Scholar]
- 28.Berman DM, O'Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mørch YA, Donati I, Strand BL, et al. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–1480. [DOI] [PubMed] [Google Scholar]
- 30.Tomei AA, Manzoli V, Fraker CA, et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci U S A. 2014;111:10514–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cechin S, Alvarez-Cubela S, Giraldo JA, et al. Influence of in vitro and in vivo oxygen modulation on β cell differentiation from human embryonic stem cells. Stem Cells Transl Med. 2014;3:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Najjar M, Manzoli V, Abreu M, et al. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol Bioeng. 2015. [DOI] [PubMed] [Google Scholar]
- 33.Veiseh O, Doloff JC, Ma M, et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and nonhuman primates. Nat Mater. 2015;14:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


