Abstract
To this day, tuberculosis (TB) continues to pose a significant global health burden. The World Health Organization’s Expanded Programme on Immunization (EPI) recommends the bacille Calmette-Guérin (BCG) vaccine for infants to protect against the haematogenous spread of primary TB and other more severe types of TB infection. We report an eight-month-old boy who presented to the Armed Forces Hospital, Muscat, Oman, in 2015 with a one-month history of intermittent fever associated with a limited range of motion in the right hip area. He was up-to-date with his EPI vaccinations and had no history of exposure to individuals with TB infections. He was initially treated for bacterial septic arthritis; however, a GeneXpert TB assay revealed the presence of Mycobacterium tuberculosis, BCG strain. To the best of the authors’ knowledge, this is the first documented case from Oman of a child with TB hip osteomyelitis due to a BCG vaccination.
Keywords: Tuberculosis, BCG Vaccine, Vaccination, adverse effects, Osteomyelitis, Case Report, Oman
Tuberculosis (TB) is an infection caused by the Mycobacterium tuberculosis bacteria which most commonly affects the lungs, but can involve virtually any part of the human body.1,2 In 2014, the World Health Organization (WHO) reported an incidence of 9.6 million TB cases with over three million deaths, including almost 300,000 children under the age of 15 years.3 The WHO’s Expanded Programme of Immunization (EPI) recommends the bacille Calmette-Guérin (BCG) vaccine to protect against the haematogenous spread of primary TB infections among infants.4,5 This case report describes the rare occurrence of hip tuberculosis in an eight-month-old Omani infant secondary to the BCG vaccination. Although rare, complications following vaccinations should be kept under consideration; however, the risks of the BCG vaccine do not outweigh the potential morbidity and mortality of TB infection.3
Case Report
An eight-month-old Omani male infant presented to the Orthopaedic Emergency Department of the Armed Forces Hospital (AFH), Muscat, Oman, in 2015 with a one-month history of intermittent fever associated with a limited range of motion (ROM) in the right hip area. There was no reported swelling, erythema, history of trauma at the affected site or any other systemic symptoms. He was up-to-date with his EPI vaccinations, including the BCG vaccine which he had received during the first week of life. His parents reported no exposure to individuals with similar symptoms; in addition, none of his family members and neighbours had recently suffered from a chronic cough or unexplained weight loss. He had previously been treated at a secondary local hospital for septic arthritis and prescribed intravenous antibiotics (co-amoxiclav). The parents had taken their child home against medical advice and treated him with oral medications; however, he was admitted shortly afterwards to AFH due to his worsening symptoms.
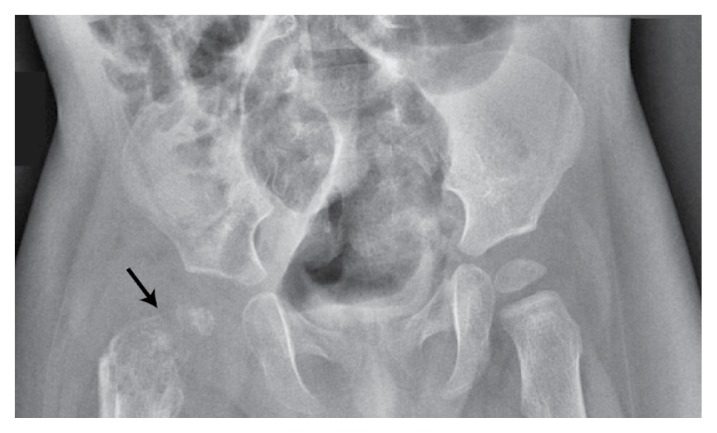
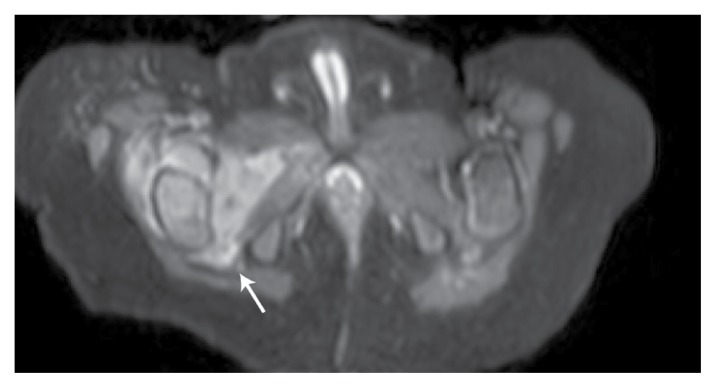
On admission, a physical examination revealed no discrepancies in limb length, with no apparent swelling nor changes in perfusion. However, evidence of limited ROM, especially on flexion and external rotation of the right hip, and tenderness on direct pressure were observed. A blood work-up and complete blood count revealed leukocytosis and increased levels of acute phase reactants. Initial radiographs showed reduced hip joint space with fragmentation and collapse of the right femoral head [Figure 1]. An ultrasound indicated synovial thickening with septations [Figure 2], features consistent with a diagnosis of septic arthritis. Subsequently, the patient underwent an emergency right hip arthrotomy. Intraoperatively, a capsulotomy yielded purulent material with clumps of necrotic tissue and intravenous ceftriaxone was administered. Following the surgery, the patient remained stable with lysis of the febrile episodes. Moreover, there was a gradual full recovery of the ROM of the affected joint. Serial blood counts showed improving acute phase reactant levels and culture studies of samples taken during the capsulotomy did not grow any organisms. A postoperative hip magnetic resonance imaging (MRI) scan revealed residual fluid collection and proximal femur osteomyelitis [Figure 3]. Nevertheless, the patient was discharged after three weeks with a prescription for oral cefdinir to complete the six-week course of antimicrobial therapy.
Figure 1.
Hip X-ray of an eight-month-old boy showing reduced hip joint space with fragmentation and collapse of the right femoral head (arrow).
Figure 2.
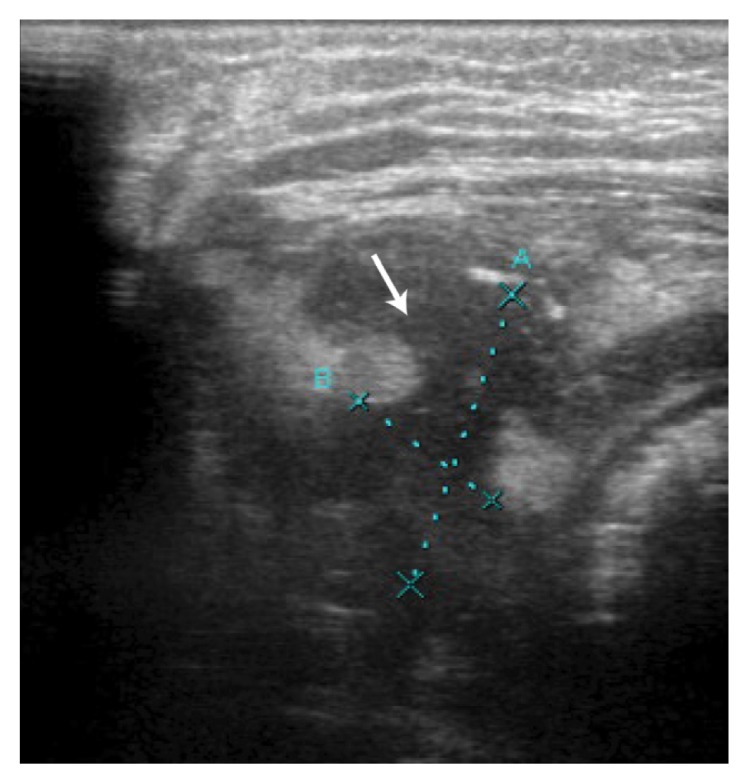
Ultrasonography of the right hip of an eight-month-old boy showing synovial thickening with the presence of septations (arrow).
Figure 3.
Sectional magnetic resonance imaging of the right hip of an eight-month-old boy showing residual fluid collection and proximal femur osteomyelitis (arrow) following an arthrotomy.
Two weeks following discharge, the patient was seen at the AFH outpatient fracture clinic. The mother reported that he cried during movement and when the right hip bore weight. At the follow-up appointment itself, the infant was afebrile but irritable with direct joint tenderness and restricted ROM in the affected limb. A follow-up MRI scan showed significant fluid collection and the patient underwent a repeat extended right hip arthrotomy with washout and placement of a hip spica cast. On histopathology, section tissue samples showed granulomatous inflammation with fragments of multiple epithelioid cells with Langhans-type giant cells surrounded by lymphocytes, a few plasma cells and scattered neutrophils along with congested blood vessels and focal areas of necrosis. Based on the patient’s lack of response to the initial antibiotic therapy, acid-fast bacilli (AFB) staining, GeneXpert testing and fungal cultures were performed. Tuberculin skin testing was negative. While awaiting the test results, intravenous antibiotic therapy was continued.
The GeneXpert assay revealed the presence of M. tuberculosis, BCG strain. The patient was immediately prescribed quadruple anti-TB treatment consisting of rifampicin, isoniazid, pyrazinamide and ethambutol. The results of a series of immunodeficiency tests were normal. After two weeks, he was discharged in good health. At a four-week follow-up, there was evidence of mild swelling at the surgical site with improving ROM. The patient remained clinically stable with good tolerance to the oral medications. A hip ultrasound showed nonprogressive fluid collection which was monitored on a regular basis. He completed two months of quadruple anti-TB therapy before continuing treatment with rifampicin, isoniazid and pyridoxine for one year.
Discussion
The BCG vaccine was developed as an attenuated live vaccine derived from virulent strains of the M. bovis species. The vaccine was launched as part of the EPI in 1974 primarily to help reduce the haematogenous spread of TB infections and the development of more severe types of TB infection (e.g. miliary, meningitic and disseminated TB) rather than to prevent primary infection.4,5 In Oman, the EPI was launched in 1981 and included the BCG vaccine to be given intradermally at birth; since its implementation, the number of TB cases per year have markedly decreased from 928 in 1981 to 213 in 2010.6
Before the advent of modern genome analysis techniques, it was difficult to differentiate between virulent mycobacteria and avirulent BCG strains; however, the development of multiplex polymerase chain reaction (PCR) techniques has since provided rapid, sensitive and specific discrimination of BCG vaccine strains.7–11 Specifically, PCR technology has identified distinct genomic regions, designated as RD1, RD2 and RD3, which were found to be absent in the vaccine strains, suggesting that the loss of virulence is due to a particular regulatory mutation in the RD1 region.7–9 This genomic region is present in both human and bovine virulent strains, but absent in all BCG vaccines.8
Rarely, complications from the BCG vaccination arise among 3.3% of cases and manifest around 6–9 months after inoculation.12 A review by Toida et al. reported an incidence of 0.0182 cases per 100,000 BCG vaccinations of dissemination beyond the vaccination site.13 Besides lymphadenitis, which is the most common complication, regional or extraregional localised abscesses and osteomyelitis are uncommon but serious complications following the BCG vaccine.5,12,14–17 Osteomyelitis can affect any bone in the body and usually occurs at a single site. Koyama et al. reported the incidence of BCG-induced osteomyelitis to be 0.2 cases per 100,000 vaccinations.10 A study of Czechoslovakian children with BCG-induced osteomyelitis confirmed by mycobacterial culture noted that the symptoms appeared approximately 17 months after vaccination, with the proximal tibia, distal femur and proximal humerus being most commonly affected.18 In a retrospective study, Kröger et al. found that the age of onset of children with BCG-induced osteomyelitis varied from 0.25–5.7 years and that the most common sites affected were the metaphyses of the long bones, with the lower extremities more commonly affected than the upper.19 A systematic review similarly revealed involvement of the lower limbs to be most common (55.6%), followed by involvement of the axial skeleton (26.0%), the upper limbs (15.4%) and multiple bones (3%).16 An association with immunosuppression has also been documented.12,16 Although the BCG vaccine may be associated with rare complications, the potential morbidity and mortality resulting from a TB infection outweigh them.
In TB osteomyelitis, the bacteria may spread to the synovium, the area around the bone or the bone itself, causing swelling, congestion and the formation of granulation tissue; subsequently, the inflammatory process continues, eventually resulting in bone necrosis and destruction.1 In cases of hip osteomyelitis, this can result in deformity, shortening of the limb, swelling, pathological dislocations and even sinus formation depending on the extent of involvement.1,2 The use of MRI has been useful in the early detection of pathologies, such as oedema and inflammation, and soft tissue abnormalities in the affected joint.2 Table 1 presents a modified classification of hip TB according to disease severity, including synovitis, early arthritis, advanced arthritis and advanced arthritis with subluxation/dislocation.2,20 A number of confirmed BCG-induced osteomyelitis cases have been reported from Europe and Asia.5,14 However, to the best of the authors’ knowledge, the current patient is believed to be the first confirmed case of BCG-induced TB hip osteomyelitis from Oman.
Table 1.
| Stage | Clinical findings | Radiological features |
|---|---|---|
| Synovitis | Flexion, abduction and external rotation with apparent lengthening | Haziness of the articular margins and rarefaction |
| Early arthritis | Flexion, adduction and internal rotation with apparent shortening | Rarefaction, osteopaenia and bony erosions in the femoral head, acetabulum or both, without reduction in the joint space |
| Advanced arthritis | Flexion, adduction and internal rotation with shortening | All of the above plus destruction of the articular surface and reduction in the joint space |
| Advanced arthritis with subluxation/dislocation | Flexion, adduction and internal rotation with gross shortening | Gross destruction and reduction of the joint space and a wandering acetabulum |
Most childhood TB infections are contracted as a result of exposure to household contacts with the disease, including parents or other caregivers.21 In the current case, there was allegedly no TB exposure; however, a thorough investigation or field visit to the patient’s locale could not be undertaken to rule out this possibility. A diagnosis of TB should be confimed via the demonstration of tubercle bacilli. The time period between a BCG vaccination and the development of a culture-negative relapsing lesion is an important indication for pathological evaluation.12 Ultrasound-guided aspiration of the synovial fluid may be performed and specimens should be obtained for histopathology, AFB staining, PCR and culture studies. In the present patient, joint aspirates from the right hip were negative for bacterial growth. Hengster et al. found that culture yield percentage decreased with time, with 46% of cases having positive cultures in the first 20 weeks of receiving the BCG vaccination compared to 0% after 20 weeks.22 The use of GeneXpert, a rapid and automated nucleic acid amplification test, confirmed the presence of M. tuberculosis, BCG strain, in the present case.
Due to the rapid dissemination of TB infections among children, prompt initiation of therapy is warranted. Although both surgical and nonsurgical options exist, chemotherapy remains the cornerstone of treatment.1,2 Both the Centers for Disease Control and Prevention in the USA and the WHO emphasise the use of short-course multidrug regimens under directly-observed therapy.3 For skeletal TB, the recommended duration of treatment is usually 12 months, except for those with spinal disease, for whom 18 months of therapy is advised. In paediatric cases, treatment strategies are primarily focused towards preserving anatomy, joint mobility and growth plates.1,2,20,23 First-line anti-TB agents include isoniazid, rifampicin, streptomycin, pyrazinamide and ethambutol. A failure to respond to these medications may warrant the use of second-line agents including capreomycin, kanamycin, ethionamide, cycloserine, and paraaminosalicylic acid.1,2 A lack of response to first-line therapy may be due to several factors, such as inadequate treatment, lack of compliance, an alternate diagnosis or the presence of resistant organisms.
In the current case, the patient underwent an arthrotomy after which his condition seemed to immediately improve. His fever started to lyse and the acute-phase reactants normalised with the continuation of antibiotics. A recent review reported that adequate drainage and thorough debridement can hasten clinical improvement.24 This reduces the bacterial load and removes necrotic tissue, providing a boost to the host’s immune system and a chance for the antibiotics to control the infection.12,24 However, supplemental surgical intervention may be reserved for specific indications, most often to establish the diagnosis or to treat complications of the disease process.2,20
Conclusion
Clinical suspicion of BCG-induced osteomyelitis is warranted in paediatric patients with chronic symptoms of pain, limping, swelling and a limited ROM in the extremities. Imaging and culture studies may guide the clinician, although tissue biopsies and genetic tests confirm the diagnosis. Chemotherapy remains the definitive treatment in such cases. It is important to note that the benefits of the vaccine in terms of reducing TB-related morbidity and mortality overcome the potential risk of BCG-related complications.
ACKNOWLEDGEMENTS
A poster of this case report was presented at the 2nd Annual Congress and Medicare Exposition on Primary Care and General Pediatrics from 19–20 September 2016 in Phoenix, Arizona, USA. An abstract of the poster was published in Health Care: Current Reviews in 2016 (Vol. 4, Suppl. 3, P. 65).
References
- 1.Spiegel DA, Singh GK, Banskota AK. Tuberculosis of the musculoskeletal system. Tech Orthop. 2005;20:167–78. doi: 10.1097/01.bto.0000167745.24646.dc. [DOI] [Google Scholar]
- 2.Saraf SK, Tuli SM. Tuberculosis of hip: A current concept review. Indian J Orthop. 2015;49:1–9. doi: 10.4103/0019-5413.143903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global tuberculosis report 2016. [Accessed: May 2017]. From: www.who.int/tb/publications/global_report/en/
- 4.Hashimoto T. [BCG vaccines for the prevention of tuberculosis in the world]. Kekkaku. 1997;72:629–37. [PubMed] [Google Scholar]
- 5.Lin CJ, Yang WS, Yan JJ, Liu CC. Mycobacterium bovis osteomyelitis as a complication of bacille Calmette-Guérin (BCG) vaccination: Rapid diagnosis with use of DNA sequencing analysis - A case report. J Bone Joint Surg Am. 1999;81:1305–11. doi: 10.2106/00004623-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Oman Ministry of Health. Manual on expanded program on immunization. 3rd edition. [Accessed: May 2017]. From: www.cdscoman.org/uploads/cdscoman/EPI_Manual.pdf.
- 7.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–82. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talbot EA, Williams DL, Frothingham R. PCR identification of Mycobacterium bovis BCG. J Clin Microbiol. 1997;35:566–9. doi: 10.1128/jcm.35.3.566-569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kearns AM, Magee JG, Gennery A, Steward M, Graham C, Seiders PR, et al. Rapid identification of Mycobacterium bovis BCG by the detection of the RD1 deletion using a multiplex PCR technique. Int J Tuberc Lung Dis. 1999;3:635–8. [PubMed] [Google Scholar]
- 10.Koyama A, Toida I, Nakata S. [Osteitis as a complication of BCG vaccination]. Kekkaku. 2009;84:125–32. [PubMed] [Google Scholar]
- 11.Okada M, Kobayashi K. [Recent progress in mycobacteriology]. Kekkaku. 2007;82:783–99. [PubMed] [Google Scholar]
- 12.Gharehdaghi M, Hassani M, Ghodsi E, Khooei A, Moayedpour A. Bacille Calmette-Guérin osteomyelitis. Arch Bone Jt Surg. 2015;3:291–5. doi: 10.22038/ABJS.2015.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toida I, Nakata S. [Severe adverse reactions after vaccination with Japanese BCG vaccine: A review]. Kekkaku. 2007;82:809–24. [PubMed] [Google Scholar]
- 14.Chan PK, Ng BK, Wong CY. Bacille Calmette-Guérin osteomyelitis of the proximal femur. Hong Kong Med J. 2010;16:223–6. [PubMed] [Google Scholar]
- 15.Khotaei GT, Sedighipour L, Fattahi F, Pourpak Z. Osteomyelitis as a late complication of bacille Calmette-Guérin vaccination. J Microbiol Immunol Infect. 2006;39:169–72. [PubMed] [Google Scholar]
- 16.Corrales IF, Cortés JA, Mesa ML, Zamora G. [Sternal osteomyelitis and scrofuloderma due to BCG vaccination]. Biomedica. 2003;23:202–7. doi: 10.7705/biomedica.v23i2.1212. [DOI] [PubMed] [Google Scholar]
- 17.Lin WL, Chiu NC, Lee PH, Huang AS, Huang FY, Chi H, et al. Management of bacillus Calmette-Guérin osteomyelitis/osteitis in immunocompetent children: A systematic review. Vaccine. 2015;33:4391–7. doi: 10.1016/j.vaccine.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Marík I, Kubát R, Filipský J, Galliová J. Osteitis caused by BCG vaccination. J Pediatr Orthop. 1988;8:333–7. doi: 10.1097/01241398-198805000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Kröger L, Korppi M, Brander E, Kröger H, Wasz-Höckert O, Backman A, et al. Osteitis caused by bacille Calmette-Guérin vaccination: A retrospective analysis of 222 cases. J Infect Dis. 1995;172:574–6. doi: 10.1097/01241398-198805000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Moon MS, Kim SS, Lee SR, Moon YW, Moon JL, Moon SI. Tuberculosis of hip in children: A retrospective analysis. Indian J Orthop. 2012;46:191–9. doi: 10.4103/0019-5413.93686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams LV, Starke JR. Tuberculosis disease in children. [Accessed: May 2017]. From: www.uptodate.com/contents/tuberculosis-disease-in-children.
- 22.Hengster P, Sölder B, Fille M, Menardi G. Surgical treatment of bacillus Calmette Guérin lymphadenitis. World J Surg. 1997;21:520–3. doi: 10.1007/PL00012279. [DOI] [PubMed] [Google Scholar]
- 23.Mohideen MA, Rasool MN. Tuberculosis of the hip joint region in children. S Afr Orthop J. 2013;12:38–43. [Google Scholar]
- 24.Calhoun JH, Manring MM, Shirliff M. Osteomyelitis of the long bones. Semin Plast Surg. 2009;23:59–72. doi: 10.1055/s-0029-1214158. [DOI] [PMC free article] [PubMed] [Google Scholar]