Abstract
Cervical cancer is one of the most common malignant tumors in women all over the world. However, the exact etiology of cervical cancer remains unclear. The receptor for activated protein kinase C (RACK1) is reported to be involved in tumorigenesis and tumor progression. Besides, the prognostic value of RACK1 in several kinds of tumors has been identified. However, there are limited studies on the functional role of RACK1 in cervical cancer. In this study, we tested the expression level of RACK1 by immunohistochemistry and western blot technologies and find that it is upregulated in cervical cancer. Colony formation and CCK8 assays indicate that RACK1 promotes cell proliferation in CaSki cervical cancer cells. While the silence of RACK1 decreases the cell proliferation in CCK8 analysis. β-galactosidase staining suggests that RACK1 decreases cell senescence in cervical cancer cells. Invasion and migration assay show that RACK1 promotes the invasion and migration of cervical cancer cells. Also, when RACK1 was silenced, it exerts the opposite result. Furthermore, the mRNA expression levels of MMP-3, MMP-9 and MMP-10 were upregulated in RACK1-overexpressed CaSki cells by qPCR analysis. RACK1 also induces S phase accumulation in cell cycle analysis and suppresses cell apoptosis in cervical cancer cells. Flow cytometry analysis of mitochondria functions suggests that RACK1 increases the mitochondrial membrane potential (Δψm) levels to prevent mitochondrial apoptosis in cervical cancer cells. To explore the possible mechanism of RACK1, we tested and found that RACK1 upregulates the expression of NF-κB, cyclin D1 and CDK4 and downregulates the expression of p53, p38, p21 and STAT1 in cervical cancer cells. These results suggest that RACK1 promotes cell growth and invasion and inhibits the senescence and apoptosis in cervical cancer cells probably by affecting the p53 pathway.
Keywords: cervical cancer, RACK1, senescence, invasion, migration
Introduction
Cervical cancer affects ~500,000 individuals each year in women worldwide. It is the major cause of cancer death in women especially in developing countries (1). As known, persistent infection of high-risk types of human papillomavirus (HPV) is the leading cause of cervical cancer (2–5). Besides, genetic factors and personal lifestyle also involve in the progression from precancerous disease to invasive cervical cancer. However, the exact etiology of cervical cancer remains unclear. Despite the improvement of therapeutic methods and diagnostic tools, the overall prognosis of patients has not been improved (2,6). Many patients do not respond well to any currently available treatments (7). Therefore, exploration of pathogenesis and identification of novel biomarkers is imperative.
The receptor for activated protein kinase C (RACK1) is a homolog of the G protein β-subunit. It was originally identified as an anchoring protein for activated protein kinase C (PKC) in cytoplasm (8,9). RACK1 was reported to be involved in tumorigenesis and tumor progression. Many researchers have demonstrated that RACK1 plays a pivotal role in various biological responses, including signal transduction (10–14), cell growth and migration (15–18), cell apoptosis and chemoresistance (19,20). RACK1 was also used as an excellent predictor for clinical prognosis in hepatocellular carcinoma (HCC) and oral squamous cell carcinoma (OSCC) (21). With more studies on RACK1, it was found that the effect of RACK1 was diverse in different types of tumors. RACK1 was found to be upregulated and to promote tumorigenicity in non-small cell lung cancer (12,22), pulmonary adenocarcinoma (23,24), hepatocellular carcinoma (25), neuroblastoma (18), and esophageal squamous cell carcinoma (17). On the contrary, the expression level of RACK1 was found to be reduced in gastric cancer (13,14,26), Besides, RACK1 decreased tumorigenicity of colon cells (27). It is speculated that RACK1 exert diverse effects in different types of cancers via its interaction with different partners and through various signaling pathways. However, the exact mechanisms of the aberrant expression and opposed roles of RACK1 are still unclear.
In this study, we found that RACK1 is highly expressed in cervical cancer tissues and decreases cell senescence and promotes the invasion and migration of cervical cancer cells. Furthermore, we found that the overexpression of RACK1 upregulates the expression of NF-κB, cyclin D1 and CDK4 and downregulates the expression of p53, p38, p21 and STATA1 in vitro. These results suggest that RACK1 promotes cell growth, invasion and migration in cervical cancer cells probably by affecting the p53 signaling pathway.
Materials and methods
Cell culture and transfection
The human cervical cancer cells, CaSki, were obtained from the ATCC (Manassas, VA, USA) and kept in our laboratory. Cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) (both from Gibco Life Technologies, Grand Island, NY, USA). Cells were grown at 37°C in the presence of 5% CO2. Cells were transfected using Lipofectamine 2000 (Invitrogen by Life Technologies, USA) according to the manufacturer's protocol as we described previously (28). For RACK1-overexpressed stable cells, 1,000 µg/ml G418 (Sigma, St. Louis, MO, USA) was added 48 h after transfection and sustained for >14 days. The maintainance concentration of G418 was 500 µg/ml, and it was used throughout the cell culture. We verified the transfection effectiveness and RACK1-overexpressed stable cells by western blot assay. We also established the transient transfected RACK1-silenced CaSki cells by transfecting with the plasmid of SD.U6/neo/GFP-RACK1-RNAi and SD/U6/neo/GFP-NC using Lipofectamine 2000. Briefly, cells (2×105 cells per well) were seeded 24 h prior to the transfection. For each transfection, 2 µg of SD/U6/neo/GFP-NC or SD.U6/neo/GFP-RACK1-RNAi plasmid was transfected into CaSki cells, respectively. The cells were digested and resuspended for further experiments 24 h to 36 h later.
Patient samples
Twenty-five participants were recruited between 2012 and 2016 at the Third Xiangya Hospital, Central South University (Changsha, Hunan, China). Consent forms were obtained from individual patients, and experimental protocols were approved by the Institutional Review Board of the Third Xiangya Hospital. The clinical stages of cervical cancer were defined according to the International Federation of Gynecology and Obstetrics (FIGO). All subjects enrolled in the study were Chinese. Cervical cancer tissue and corresponding non-tumor normal tissue were collected, and each biopsy sample was divided into two sections, one was submitted to routine histological diagnosis, and the remaining section was used for western blotting and immunohistochemistry experiments.
Immunohistochemistry (IHC) and evaluation of staining
Immunohistochemistry was done using the peroxidase anti-peroxidase technique following a microwave antigen retrieval procedure. Mouse anti-RACK1 was purchased from Santa Cruz Biotechnology (cat. no. sc-17754; Dallas, TX, USA). Antibody against RACK1 (1:200) was overlaid on cervical cancer and corresponding non-tumor normal tissue sections and incubated overnight at 4°C. Secondary antibody incubation (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was performed at room temperature for 30 min. Color reaction was developed by using 3, 3′-diaminobenzidine tetrachloride (DAB) chromogen solution. All slides were counterstained with hematoxylin. Positive control slides were included in every experiment in addition to the internal positive controls. The specificity of the antibody was determined with matched IgG isotype antibody as a negative control.
Sections were blindly evaluated by two investigators in an effort to provide a consensus on staining patterns by light microscopy (Olympus, Japan). RACK1 staining was assessed according to the methods described by Hara and Okayasu with minor modifications (29). Each case was rated according to a score that added a scale of intensity of staining to the area of staining. At least 10 high-power fields were chosen randomly, and >1,000 cells were counted for each section. The intensity of staining was graded on the following scale: 0, no staining; 1+, mild staining; 2+, moderate staining; 3+, intense staining. The area of staining was evaluated as follows: 0, no staining of cells in any microscopic fields; 1+, <30% of tissue stained positive; 2+, between 30 and 60% stained positive; 3+, >60% stained positive. The minimum score when summed (extension + intensity) was, therefore, 0, and the maximum, 6. A combined staining score (extension + intensity) of ≤2 was considered to be a low staining; a score between 3 and 4 was considered to be a moderate staining; whereas a score between 5 and 6 was considered to be a strong staining.
β-galactosidase staining
The detection of cellular senescence was performed using a senescence-associated β-galactosidase staining kit (C0602; Beyotime, China) according to the manufacturer's protocol. Images were captured by a light microscope (CKX41; Olympus). The β-galactosidase positive cells (blue) were considered senescent.
Colony formation and CCK8 assay
Eight hundred cells were seeded per well in 6-well plates and cultured for 14 days at 37°C. After incubation, cells were fixed with 4% paraformaldehyde solution and stained with 0.1% crystal violet solution. The number of colonies with >50 cells was counted and photographed. The CCK8 assay was carried according to the protocol (7Sea-Cell Couting kit; 7Sea Biotech, China. Cell suspension (200 µl) was seeded in 96-well cell culture plates at a density of 1,000 cells/well and incubated at 37°C for six days. The density of cells were detected every 24 h. The CCK8 solution (20 µl) was added to each well and incubated at 37°C for 2 h. Then the absorbance at 450 nm was measured using a Paradigm Detection Platform (Beckman Coulter, Brea, CA, USA).
Wound healing assay
For wound healing assay, 5×105 cells were seeded per well in 6-well plates, when the cell density grown to 90%, a straight scratch in the cell monolayer was created by a 10-µl pipette tip. Images of the scratched area (wound) were taken at the time point of 0, 24, 48 and 72 h under a microscope (CKX41; Olympus).
Cell invasion and migration assay
The cell invasion experiment was performed using Corning Matrigel Invasion Chamber in 24-well plate (8-µm pore size; Corning Life Sciences, Lowell, MA, USA). A total of 2.5×104 cells in 500 µl of serum-free medium were added to the top chamber. Growth medium (750 µl) with 20% FBS was added into the lower chamber. After incubation in a humidified incubator with 5% CO2 for 48 h at 37°C, parts of cells were invaded to the lower surface of the upper chamber. Fixed the cells with 4% paraformaldehyde and stained with crystal violet. The cells on the upper surface of the upper chamber were gently removed by a cotton swab. Images of the stained cells were then captured under a microscope at ×100 magnification and cells from at least five randomly selected fields were counted for each experiment. The cell migration assay was performed similarly with the cell invasion experiment by using Falcon Cell Culture Inserts (8-µm pore size; Corning Life Sciences), which without Matrigel coating on the upper surface of the Transwell filters. After 24-h incubation in a humidified incubator, the chamber were fixed with 4% paraformaldehyde and stained with crystal violet. Images were then captured and counted cells from at least five randomly selected fields for each experiment.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and was converted to cDNA using the All-in-One™ First-Strand cDNA Synthesis kit (GeneCopoeia, Rockville, MD, USA). Quantitative real-time PCR was performed using ChamQ™ SYBR® qPCR Master Mix (Vazyme, Nanjing, China), according to the manufacturer's instructions. Data were normalized to the expression level of GAPDH. The primers used in this manuscript were as follows: MMP-2 (left-atgacagctgcaccactgag, right-atttgttgcccaggaaagtg), MMP-3 (left-tgctttgtcctttgatgctg, right-ggaagagatggccaaaatga), MMP-9 (left-ttgacagcgacaagaagtgg, right-gccattcacgtcgtcc ttat) and MMP-10 (left-ggctctttcactcagccaac, right-tcccgaaggaacagattttg).
Flow cytometry analysis of cell cycle and cell apoptosis
Cell cycle and cell apoptosis analysis was carried out by flow cytometry. Cells (5×105/well) were seeded in 6-well cell culture plates and incubated in a humidified atmosphere of 5% CO2 at 37°C. Twenty-four hours later, cells were harvested with 0.5% trypsin and centrifuged. For cell cycle analysis, cells were fixed in ice-cold 70% ethanol overnight at 4°C. After being washed thrice with cold PBS, cells were resuspended in 500 µl of PBS, and 10 µl RNAseA was added for 5 min. Subsequently, 10 µl PI was added into the cell resuspension solution, and incubated for 30 min at 4°C. The cells were finally washed twice with PBS before analysis. Apoptotic analysis was performed using the Hoechst 33342/PI Apoptosis assay kit (BestBio, Shanghai, China). The samples were washed twice with ice-cold PBS and resuspended in 500 µl staining buffer, and then incubated with 5 µl of Hoechst 33342 and 5 µl of PI in the dark for 20 min at 4°C. The cells were finally washed twice with PBS before analysis. Analysis was performed on MoFlo™XDP High-Performance Cell Sorter (Beckman Coulter) and the data were analyzed with the summit v5.2 Software (Beckman Coulter).
Mitochondrial membrane potential detection
The mitochondrial membrane potential (MMP) was detected using a JC-1 fluorescent probe (MultiSciences Biotech Co., Ltd., Hangzhou, China), which is a cationic lipophilic dye that exhibits potential-dependent accumulation in mitochondria. In normal cells, the dye concentrates in the mitochondrial matrix, where it forms red fluorescent aggregates. Any event that dissipates the mitochondrial membrane potential prevents the accumulation of the JC-1 dye in the mitochondria and the dye is dispersed throughout the entire cell leading to a shift from red to green fluorescence. Thus, a change in JC-1 fluorescence emission from red to green indicates depolarization of the mitochondrial membrane. In our experiments, cells were harvested and resuspended in 1 ml warm staining buffer at ~1×106 cells/ml. One microliter of 2 mM JC-1 was added into the cell suspension and incubated at 37°C for 30 min. Washed cells were resuspended in 500 µl PBS. It was then detected by a flow cytometer MoFlo™ XDP (Beckman Coulter). CCCP was used as a positive control and perform standard compensation.
Western blot analysis
Western blot analysis was performed as we described previously (28). In brief, cells and tissues were lysed in RIPA buffer (CWBio, Beijing, China), then 50 µg of lysates were separated in 10% SDS-PAGE gels and transferred onto a PVDF membrane (Hyclone Laboratories, Logan, UT, USA) and then blocked (5% non-fat milk dissolved in TBS-Tween-20) for 1-2 h. The membranes were then incubated overnight at 4°C with primary antibodies. Primary antibodies used in this study include: mouse anti-RACK1 (Santa Cruz Biotechnology, Dallas, TX, USA; 1:1,000 dilution), rabbit anti-c-rel, rabbit anti-stat1, rabbit anti NF-κB-P65 (Immunoway Technology, Newark, DE, USA; 1:1,000 dilution), rabbit anti NF-κB-p65 (phospho-Ser536) polyclonal antibody (Immunoway Technology, Newark, DE, USA; 1:1,000 dilution), mouse anti-CDK4, rabbit anti-cyclin D1, mouse anti-P53, rabbit anti-P21, rabbit anti-MAPK14/P38 (Boster Technology, Wuhan, China; 1:500 dilution), and rabbit anti-cyclin A (Affinity, Ancaster, Canada; 1:1,000 dilution). After washing three times by TBST, the membranes were incubated with anti-rabbit or anti-mouse HRP-conjugated secondary antibody (Santa Cruz Biotechnology; 1:5,000 dilution). The detection was finally performed on the ChemiDoc XRS+ Molecular Imager (Bio-Rad) using the Luminata Forte Western HRP Substrate (Millipore Corp., Billerica, MA, USA). Rabbit anti-GAPDH (Santa Cruz Biotechnology; 1:3,000 dilution) was used as a loading control.
Statistical analysis
Differences of non-parametric variables were analyzed by the Mann-Whitney U test. Differences of the quantitative variables between groups were analyzed by Student's t-test using SPSS 11.0 program (SPSS, Chicago, IL, USA). A value of P<0.05 was considered statistically significant.
Results
RACK1 expression is high in cervical cancer
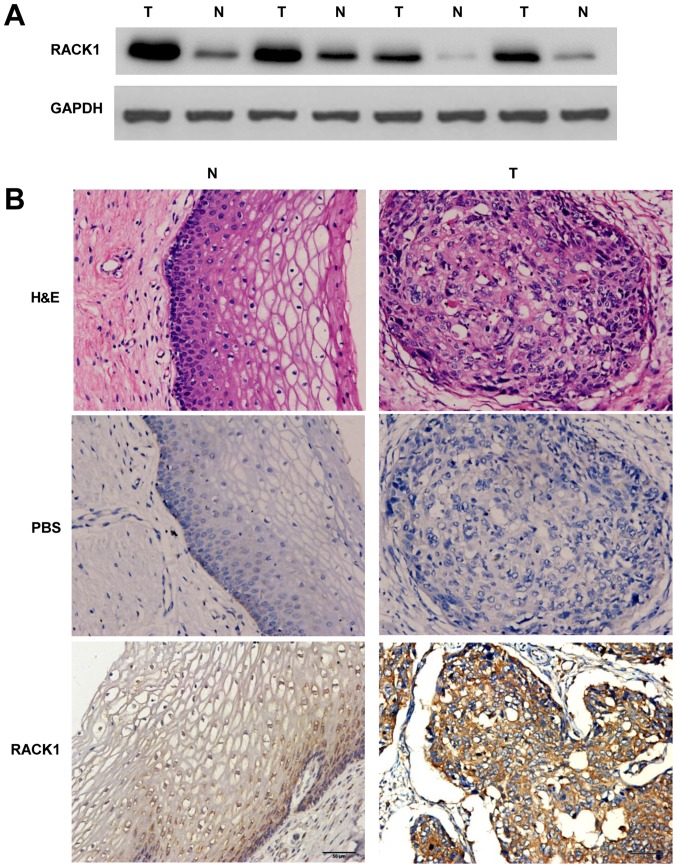
To detect the expression levels of the RACK1 in cervical cancer and the adjacent non-cancerous tissues, western blotting of RACK1 was performed. In comparison with the adjacent non-cancerous tissues, the expression level was identified to be greater in cervical cancer tissues (Fig. 1A). Meanwhile, immunohistochemistry (IHC) was carried out with anti-bodies against RACK1 in cervical cancer and the adjacent non-cancerous tissues. We randomly selected 25 participants who were diagnosed cervical cancer patients, collecting cervical cancer tissues and the adjacent non-cancerous tissues for IHC detecting (Table I). Our study showed a similar pattern in protein expression with western blot results. There was 68% (17/25) high score of RACK1 in cervical cancer tissues and 28.0% (7/25) in the adjacent non-cancerous tissues. The distribution of low score was 8.0% (2/25) and 32.0% (8/25) in cervical cancer and the adjacent non-cancerous tissues, respectively (P=0.012 <0.05) (Fig. 1B and Table II). These results identified that RACK1 is highly expressed in cervical cancer.
Figure 1.
The detection of expression levels of RACK1 in the cervical cancer tissues and the adjacent non-cancerous tissues by western blot analysis and IHC. (A) The expression levels of RACK1 were tested by western blot analysis. T, cervical cancer tissues and N, the adjacent non-cancerous tissues. Data are representative of three independent experiments. (B) Immunohistochemistry analysis of the expression of RACK1 protein was detected in 25 pairs of cervical cancer tissues and the adjacent non-cancerous tissues. Antibody of mouse anti-RACK1 was used; brown grains denote positive signal. Representative images of H&E staining of cervical epithelial tissues and cervical cancer tissues, negative control, RACK1 staining of cervical epithelial tissues and cervical cancer tissues are shown. T, cervical cancer tissues and N, the adjacent non-cancerous tissues. Original magnification (×200).
Table I.
The clinical information of cervical cancer patients contributing to this study.
| Samples | Age (years) | HPV type | Cell type | Differentiation | Stage |
|---|---|---|---|---|---|
| 1 | 57 | 31 | Squamous carcinoma | Moderate | IIa |
| 2 | 45 | 16 | Squamous carcinoma | Moderate | IIb |
| 3 | 62 | 52 | Squamous carcinoma | Moderate | IIa |
| 4 | 51 | 16 | Squamous carcinoma | Moderate | IIb |
| 5 | 65 | 16 | Squamous carcinoma | Moderate | IIb |
| 6 | 48 | (−) | Squamous carcinoma | Moderate | IIa |
| 7 | 52 | 16 | Squamous carcinoma | Poor | IIb |
| 8 | 51 | 16 | Squamous carcinoma | Poor | IIa |
| 9 | 39 | 16 | Squamous carcinoma | Moderate | IIb |
| 10 | 49 | 52 | Squamous carcinoma | Moderate | Ib1 |
| 11 | 50 | 16 | Squamous carcinoma | Moderate | IIa |
| 12 | 47 | 16 | Squamous carcinoma | Moderate | IIb |
| 13 | 50 | 16 | Squamous carcinoma | Moderate | IIa |
| 14 | 52 | 45 | Squamous carcinoma | Moderate | IIa |
| 15 | 45 | 16 | Squamous carcinoma | Moderate | IIa |
| 16 | 52 | 33 | Squamous carcinoma | Moderate | IIa |
| 17 | 46 | 18 | Squamous carcinoma | Moderate | IIb |
| 18 | 40 | 16 | Squamous carcinoma | Moderate | Ib1 |
| 19 | 54 | 16 | Squamous carcinoma | Poor | Ib2 |
| 20 | 50 | 18 | Squamous carcinoma | Poor | IIb |
| 21 | 47 | 16 | Squamous carcinoma | Poor | Ib2 |
| 22 | 62 | 16 | Squamous carcinoma | Moderate | IIb |
| 23 | 40 | 16 | Squamous carcinoma | Moderate | Ib1 |
| 24 | 58 | 16,33 | Squamous carcinoma | Moderate | IIb |
| 25 | 68 | 16 | Squamous carcinoma | Moderate | IIb |
Table II.
The difference of RACK1 expression between cervical cancer and the adjacent non-cancerous tissues.
| Samples | n | Score
|
P-value | ||
|---|---|---|---|---|---|
| Low (0–2) | Moderate (3–4) | High (5–6) | |||
| Cervical cancer | 25 | 2 (8.0%) | 6 (24.0%) | 17 (68.0%) | 0.012a |
| Non-cancerous tissues | 25 | 8 (32.0%) | 10 (40.0%) | 7 (28.0%) | |
Compared with non-cancerous group, P<0.05 by Mann-Whitney U test.
RACK1 overexpression promotes the proliferation of cervical cancer cells
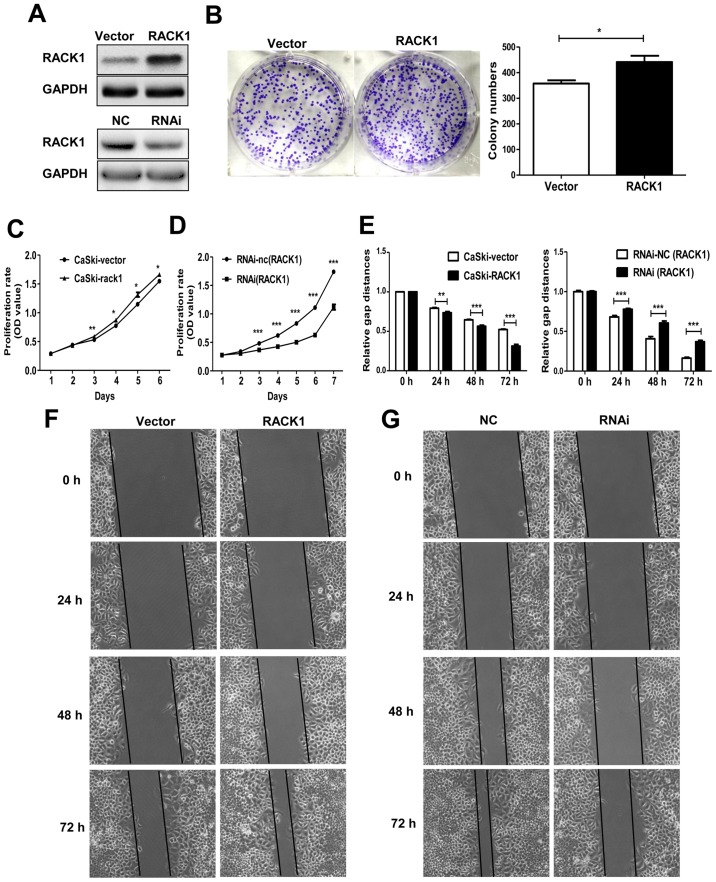
To elucidate the function of RACK1 in the biological behavior of cervical cancer cells, the CaSki cells were transfected with the plasmid pEGFP-N1-RACK1 or control vector pEGFP-N1-vector to generate stable transfected CaSki-RACK1 and CaSki-vector cell lines. After demonstrating the RACK1 expression level by western blot analysis (Fig. 2A), the spontaneous proliferation of CaSki-RACK1 and CaSki-vector cells were determined by the colony formation assay and CCK8 assay, respectively. The colony formation assay showed that RACK1 overexpression promoted the colony formation ability (Fig. 2B, P=0.023). CCK8 analysis showed that RACK1-overexpression promoted cell proliferation in CaSki cells (Fig. 2C). We also performed CCK8 assay on RACK1-silenced CaSki cells. Twenty-four hours after transfection with RACK1-RNAi or the control plasmid RNAi-NC, CaSki cells were digested and resuspended for the CCK8 assay. It showed that the proliferation of CaSki cells significantly decreased after RACK1 was silenced (Fig. 2D). The transfection effect was demonstrated by western blotting (Fig. 1A). Therefore, we considered that RACK1 promoted the proliferation of cervical cancer cells in vitro.
Figure 2.
RACK1 promotes the cell proliferation and migration in cervical cancer cells. (A) Identification the RACK1 expression in RACK1-overexpressed stable CaSki cells and RACK1-RNAi transiently transfected cells by western blot analysis. (B) Colony formation assay. Vector represents cells transfected with the pEGFP-N1 plasmid and RACK1 represents cells transfected with the pEGFP-N1-RACK1 plasmid. (C and D) CCK8 analysis was performed in RACK1-overexpressed CaSki cells and RACK1-silenced CaSki cells. CaSki-vector represents CaSki cells transfected with the pEGFP-N1 plasmid and CaSki-RACK1 represents CaSki cells transfected with the pEGFP-N1-RACK1 plasmid. RNAi-NC represents CaSki cells transfected with RNAi-NC and RNAi represents CaSki cells transfected with RACK1-RNAi. (E) Statistical analysis of wound healing assay. (F) Representative images of wound healing assay performed in RACK1-overexpressed CaSki cells. (G) Wound healing assay was performed in RACK1-silenced CaSki cells. Data are shown as mean ± SD from three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
RACK1 overexpression promotes the cell invasion and migration and inhibits the cell senescence in cervical cancer cells
Cell invasion and migration is an important step during tumor metastasis progress. We detected the migration ability of RACK1-stable expressing CaSki-RACK1 cells and control CaSki-vector cells by wound healing assay in vitro. We found that RACK1 promoted the cell migration in cervical cancer cells (Fig. 2E and F). We also performed the wound healing assay in RACK1-silenced CaSki cells. Twenty-four hours after we transfected with RACK1-RNAi or the control plasmid RNA-NC, CaSki cells were digested and resuspended in 6-well cell culture plate. Images of the scratched area were photographed every 24 h. It showed that the migration rate of CaSki cells decreased after RACK1 was silenced (Fig. 2E and G).
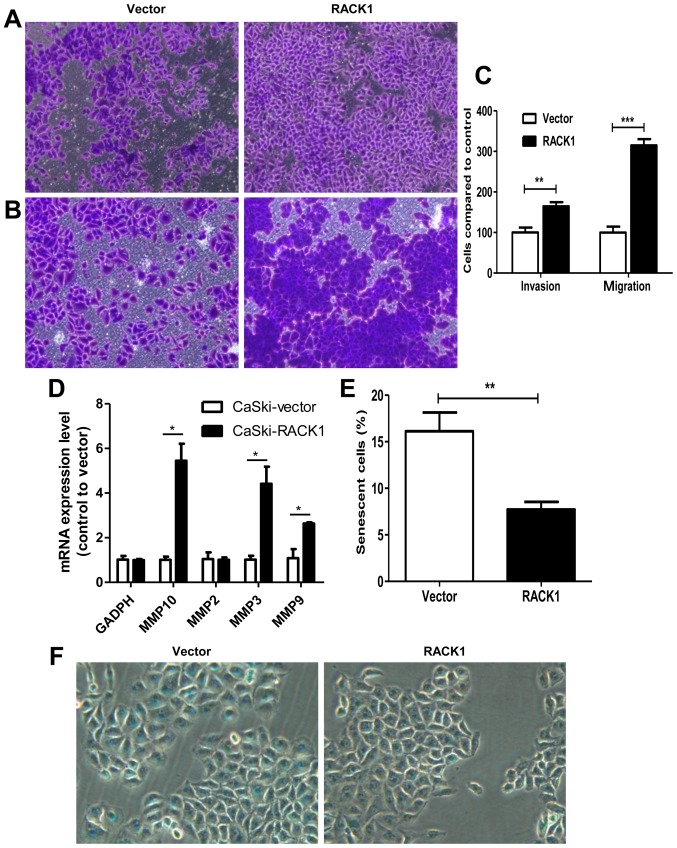
Furthermore, Transwell invasion and migration assays demonstrated that RACK1-overexpressed CaSki cells had a higher invasion and migration capability. For migration assay, 24-h incubation after cell-planting, we found that cells migrated into the downside of the chamber in RACK1-overexpressed CaSki cells were 3 times more than the control group (Fig. 3A and C, P<0.0001). For invasion assay, 48-h incubation after cell-planting, we found that cell invasion speed in RACK1-overexpressed CaSki cells was 1.64 times higher than the control group (Fig. 3B and C, P=0.0015). We further detected the mRNA expression levels of MMP-2, MMP-3, MMP-9 and MMP-10 by qPCR technology. We found that MMP-3, MMP-9 and MMP-10 were upregulated in RACK1-overexpressed CaSki cells (Fig. 3D, P=0.041, 0.016 and 0.02, respectively). The results showed that CaSki-RACK1 cells have higher invasion ability compared to control CaSki-vector cells. This further confirmed that RACK1 may contribute to tumor invasion and migration in cervical cancer.
Figure 3.
Transwell invasion and migration assay and β-galactosidase staining assay between CaSki-RACK1 cells and CaSki-vector cells. (A) Transwell migration assay. (B) Transwell invasion assay. (C) Statistical analysis of cell number for Transwell invasion and migration assays. Results are presented as mean ± SD from three independent experiments. (D) mRNA expression levels of MMP-2, MMP-3, MMP-9 and MMP-10. (E and F) β-galactosidase staining and statistical analysis of cell numbers for β-galactosidase staining assay. The assay was conducted as three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
Senescence is a stable cell cycle arrest that plays an important role in tumor development or tumor suppression. We further explored whether RACK1 has a function on cell senescence by β-galactosidase staining. As shown in Fig. 3E and F, the number of β-galactosidase-positive cells in control CaSki-vector cells was much higher than RACK1 overexpression CaSki cells (16.12 vs. 7.71%, P=0.0012). It revealed that RACK1 decreased cell senescence in cervical cancer cells.
RACK1 overexpression induces S phase accumulation in cell cycle analysis in cervical cancer cells
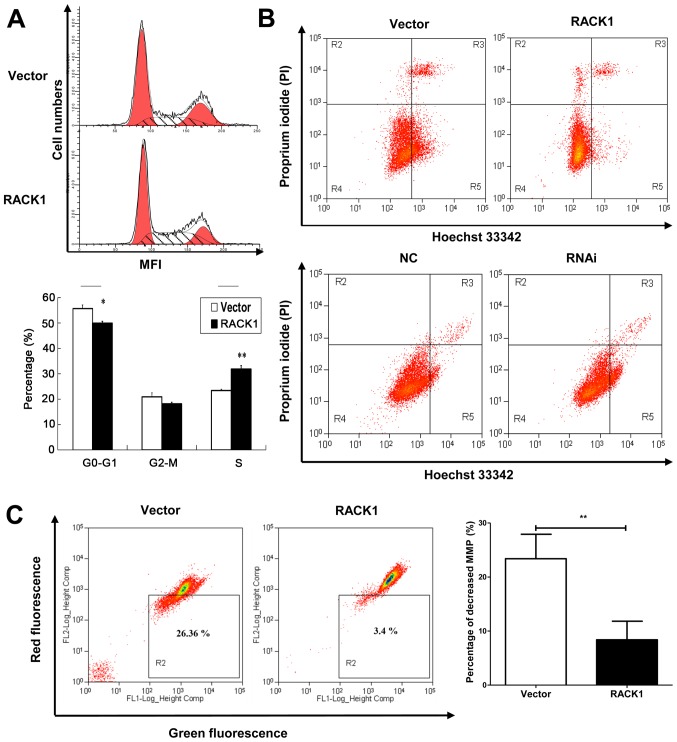
Cell cycle analysis was performed by flow cytometry. Percentages of cells at different stages were statistically analyzed. We observed that RACK1 overexpressed CaSki cells had an S phase accumulation (31.88%), compared to empty vector transfected CaSki cells (23.41%) (Fig. 4A). This indicated that RACK1 may contribute to DNA synthesis in cell cycle and promote cell proliferation.
Figure 4.
Cell cycle analysis, cell apoptosis and the mitochondrial membrane potential were detected by flow cytometry in CaSki cell lines. (A) Flow cytometry analysis of cell cycle between CaSki cells transfected with pEGFP-N1 (vector) and transfected with pEGFP-N1-RACK1. For x-axis, it represents the mean fluorescence intensity (MFI) detected by flow cytometry. For y-axis, it represents cell numbers. Statistical analysis of percentages of cells at different stages (G1, G2 and S) was also performed. (B) Flow cytometry analysis of apoptosis in RACK1-overexpressed CaSki cells (upper image) and RACK1-silenced CaSki cells (lower image). Cells in the gate R3 frame represent late apoptotic cells, and in gate R5 were early apoptotic cells. Data are representative of three independent experiments. (C) The mitochondrial membrane potential (MMP) was detected by JC-1 assay. The loss of mitochondrial membrane potential was represented by the fluorescence change from red to green. Vector indicates CaSki cells transfected with the pEGFP-N1 plasmid, and RACK1 CaSki cells transfected with the pEGFP-N1-RACK1 plasmid. Data are representative of three independent experiments. *P< 0.05; **P<0.01.
RACK1 suppresses cell apoptosis of cervical cancer cells
To further explore the role of RACK1 in cervical cancer, we detected cell apoptosis using Hoechst 33342/PI double stain assay and we analyzed by flow cytometry both the RACK1-overexpressed and RACK1-silenced CaSki cells. Hoechst 33342 stains the condensed chromatin in apoptotic cells more brightly than normal chromatin. Propidium iodide (PI) is only permeant to dead cells and late apoptotic cells. The staining pattern makes it possible to distinguish normal, apoptotic, and dead cell populations. The cells with Hoechst (−)/PI (-) were considered normal, the Hoechst (+)/PI (−) cells were thought to be early apoptotic cells, the Hoechst (+)/PI (+) cells were regarded as late apoptotic cells, and the Hoechst (−)/PI (+) cells were considered to be necrotic cells. As shown in Fig. 4B, in CaSki stable cell lines, the proportion of apoptotic cells in RACK1 overexpressed cells were lower than vector transfected cells (the rate of late apoptotic was 4.53 and 5.99 for RACK1 and vector respectively, and the rate of early apoptotic was 4.60 and 24.23 for RACK1 and vector respectively) (Fig. 4B, upper image). While in RACK1-silenced CaSki cells, the apoptotic rate was higher than the control group (the rate of late apoptotic was 2.27 and 2.00 for RACK1-RNAi and RNAi-NC transfected CaSki cells respectively, and the rate of early apoptotic was 13.60 and 5.47 for RACK1-RNAi and RNAi-NC transfected CaSki cells respectively) (Fig. 4B, lower image). The results implied that RACK1 could suppress cell apoptosis.
RACK1 affects the mitochondrial membrane potential in cervical cancer cells
The dissipation of the mitochondrial membrane potential (Δψm) is known as an early event in apoptosis. So we examined the mitochondrial apoptosis by JC-1 staining. A decline of mitochondrial membrane potential during apoptosis was represented by a decrease in red fluorescence intensity and increase in green fluorescence intensity. As demonstrated in our study, the percentage of cells with loss of Δψm in RACK1-overexpressed CaSki cells was less than control groups (Fig. 4C, P=0.0019). It was demonstrated that RACK1 may inhibit cell mitochondrial apoptosis in cervical cancer cells.
RACK1 overexpression affects the expression of p53, p38, p21, STAT1, NF-κB, cyclin D1 and CDK4 in vitro
To uncover the possible mechanism of RACK1 in cervical cancer, we tested the expression levels of key molecules in p53 signaling pathway by western blot technology. The p53, p38 and p21 were down-regulated in CaSki cells in which Rack1 was overexpressed, whereas, the overexpression of Rack1 downregulated the expression level of STAT1. Furthermore, NF-κB, cyclin D1 and CDK4 was upregulated in CaSki cells in which Rack1 was overexpressed (Fig 5). Our results suggested that RACK1 overexpression may upregulate the expression of NF-κB, cyclin D1 and CDK4 and downregulated the expression of p53, p38, p21 and STAT1 in vitro.
Figure 5.
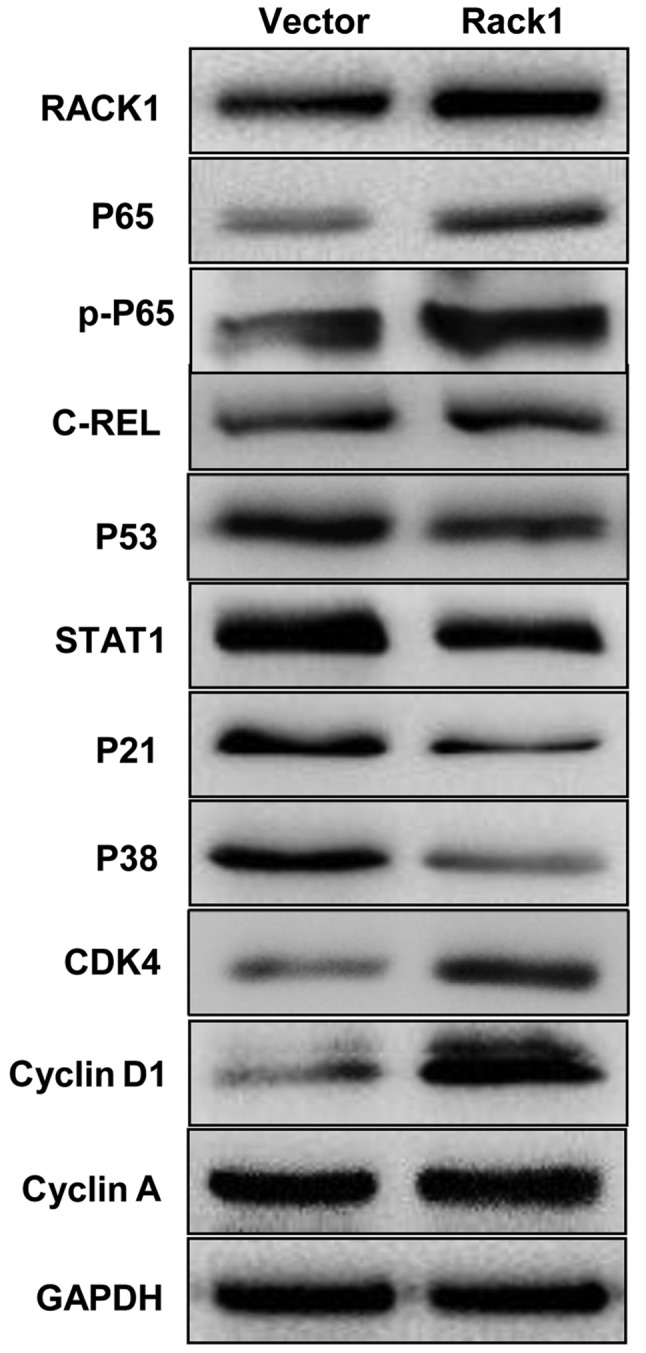
Western blot analysis indicates that RACK1 overexpression affected the expression of p53, p38, p21, STAT1, NF-κB, cyclin D1 and CDK4 of cervical cancer cell in vitro. The protein expression levels of p53, p38, p21, STAT1, NF-κB P65, cyclin D1 and CDK4 in RACK1-overexpressed stable cervical cancer cell line of CaSki were analysed by western blotting. Vector, CaSki cells transfected with the pEGFP-N1 plasmid, Rack1, CaSki cells transfected with the pEGFP-N1-RACK1 plasmid.
Discussion
New cervical cancers (530,000) are diagnosed annually and there are 275,000 deaths from the disease worldwide (http://globocan.iarc.fr/factsheets/cancers/cervix.asp). Persistent infection with high-risk types of human papillomavirus (HPV) is known to be one of the main causes of cervical cancer. However, the exact etiology of cervical carcinoma remains poorly understood. In our study, we found that RACK1 expresses highly in cervical cancer tissues compared with the adjacent non-cancerous tissues. RACK1 may play important roles in tumorigenesis and progression in cervical cancer.
We first detected the expression level of RACK1 in cervical cancer tissues and the adjacent non-cancerous tissues by western blot analysis and immunohistochemistry (IHC). The results showed that RACK1 is higher expressed in the cancer tissues than in the adjacent non-cancerous tissues. Further studies showed that endogenous overexpressed RACK1 promoted the proliferation, invasion and migration, and decreased cell senescence of cervical cancer cells in vitro. Previous studies demonstrated that RACK1 is expressed aberrantly in cancer cells and has diverse effects in different types of tumors (30–34). It can interact with multiple signaling molecules, including Akt, Bcl-2 (35), MCM7 (36), FGFR1 and PKM2 (37). Wang et al found that RACK1 antagonized TNF-α-induced cell death by promoting p38 activation (38). Li et al found that RACK1 was upregulated in proliferating pancreatic ductal adenocarcinoma (PDAC) cells, and involved in regulating cell cycle and apoptosis of PDAC cells by interact with cyclin D1, BCL-2 and caspase-3 (15). In our study, we found that RACK1 induced S phase accumulation in cell cycle analysis and suppressed cell apoptosis in cervical cancer cells. Besides, RACK1 increased the mitochondrial membrane potential (Δψm) levels to prevent mitochondrial apoptosis in cervical cancer cells. As known, dysregulation of the cell cycle underlies the aberrant cell proliferation that characterizes cancer and loss of cell cycle checkpoint control promotes genetic instability (39–43). To further explore the possible mechanism of RACK1 in cervical cancer, we detected the expression levels of some key molecules in p53 signaling pathway by western blot analysis in vitro and found that RACK1 upregulated the expression of NF-κB, cyclin D1 and CDK4 and downregulated the expression of p53, p38, p21 and STAT1. It is well known that the P53-P21-CDK-cyclin D signaling pathway plays a pivotal role in cell cycle regulation. P53 positively regulates the expression of P21, which can bind with cyclin D1 or cyclin A, and inhibit the activity of cyclin-CDK complex regulating the cell cycle (44–47). STAT1 has been reported to have tumor suppressor function and regulate cell apoptosis and cell cycle (48,49). It is a negative regulator of MDM2 and can also act as a coactivator interacting directly with P53 (50). Besides, substantial evidence has demonstrated that NF-κB P65 plays compelling role in cell apoptosis (51–53). Combining with our results, it is confirmed that RACK1 promote cell growth and inhibit cell apoptosis in cervical cancer. We further speculated that possibly RACK1 acts on its effect in cervical cancer by affecting the p53 signaling pathway (Fig. 6). This conjecture requires further experiments to show the regulatory mechanism among these molecules in cell senescence and cell migration and invasion of cervical cancer cells.
Figure 6.
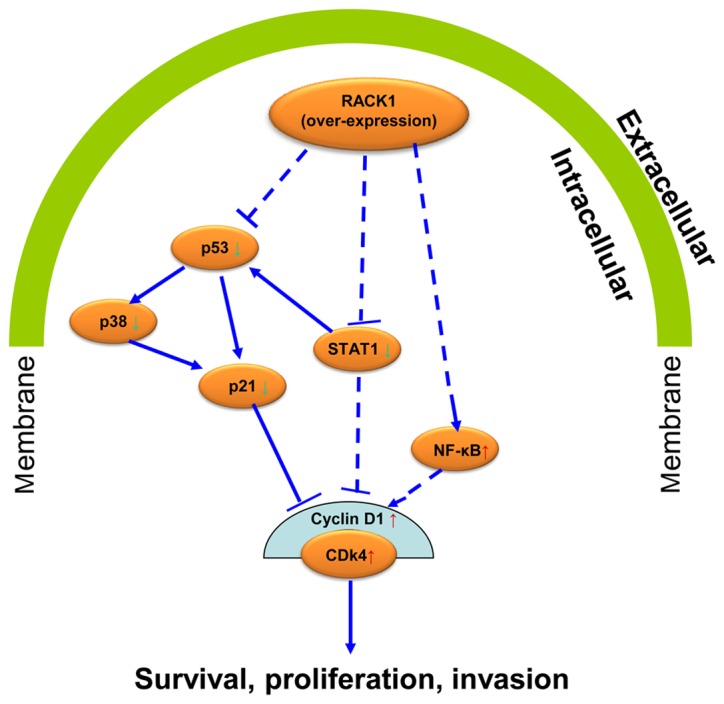
The diagram of a potential signaling pathway that RACK1 regulates the cell proliferation and cell invasion in cervical cancer cells.
Acknowledgments
This study was supported by the National Natural Sciences Foundation of China (81672685, 81402270, 81272975 and 81672993); Key Project of Hunan Provincial Natural Science Foundation (12JJ2044); the Key Planned Science and Technology Project of Hunan Province (2012FJ2014 and 2011FJ3153); the 111 Project (111-2-12); the Natural Science Foundation of Hunan Province (2016JC2035); the Planned Project of Development and Reform Commission of Hunan Province (2012-1493-1); the Planned Project of Department of health of Hunan Province (B2011-030, B2012-029); the Planned Project of Key Subject Construction of the Third Xiangya Hospital, Central South University; the Open-End Fund for the Valuable and Precision Instruments of Central South University. This study was also supported by Hunan Provincial Innovation Foundation for Postgraduate (CX2015B057).
Abbreviations
- RACK1
receptor for activated C kinase 1
- TP53
tumor protein p53
- STAT1
signal transducer and activator of transcription 1
- CDK4
cyclin-dependent kinase 4
- IHC
immunohistochemistry
- GADPH
glyceraldehyde-3-phosphate dehydrogenase
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: Novel functions of E6 and E7 oncoproteins. Rev Med Virol. 2009;19:97–113. doi: 10.1002/rmv.605. [DOI] [PubMed] [Google Scholar]
- 3.Latsuzbaia A, Tapp J, Nguyen T, Fischer M, Arbyn M, Weyers S, Mossong J. Analytical performance evaluation of Anyplex II HPV28 and Euroarray HPV for genotyping of cervical samples. Diagn Microbiol Infect Dis. 2016;85:318–322. doi: 10.1016/j.diagmicrobio.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Tang A, Dadaglio G, Oberkampf M, Di Carlo S, Peduto L, Laubreton D, Desrues B, Sun CM, Montagutelli X, Leclerc C. B cells promote tumor progression in a mouse model of HPV-mediated cervical cancer. Int J Cancer. 2016;139:1358–1371. doi: 10.1002/ijc.30169. [DOI] [PubMed] [Google Scholar]
- 5.Chatzistamatiou K, Moysiadis T, Moschaki V, Panteleris N, Agorastos T. Comparison of cytology, HPV DNA testing and HPV 16/18 genotyping alone or combined targeting to the more balanced methodology for cervical cancer screening. Gynecol Oncol. 2016;142:120–127. doi: 10.1016/j.ygyno.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Scotto L, Narayan G, Nandula SV, Subramaniyam S, Kaufmann AM, Wright JD, Pothuri B, Mansukhani M, Schneider A, Arias-Pulido H, et al. Integrative genomics analysis of chromosome 5p gain in cervical cancer reveals target over-expressed genes, including Drosha. Mol Cancer. 2008;7:58. doi: 10.1186/1476-4598-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowen SE, Neutze DM, Pett MR, Cottage A, Stern P, Coleman N, Stanley MA. Amplification of chromosome 5p correlates with increased expression of Skp2 in HPV-immortalized keratinocytes. Oncogene. 2003;22:2531–2540. doi: 10.1038/sj.onc.1206296. [DOI] [PubMed] [Google Scholar]
- 8.Ron D, Mochly-Rosen D. Agonists and antagonists of protein kinase C function, derived from its binding proteins. J Biol Chem. 1994;269:21395–21398. [PubMed] [Google Scholar]
- 9.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: A dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 10.Peng R, Jiang B, Ma J, Ma Z, Wan X, Liu H, Chen Z, Cheng Q, Chen R. Forced downregulation of RACK1 inhibits glioma development by suppressing Src/Akt signaling activity. Oncol Rep. 2013;30:2195–2202. doi: 10.3892/or.2013.2723. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Meng J, Du Y, Huang Y, Jin Y, Zhang J, Wang B, Zhang Y, Sun M, Tang J. RACK1 promotes the proliferation, migration and invasion capacity of mouse hepatocellular carcinoma cell line in vitro probably by PI3K/Rac1 signaling pathway. Biomed Pharmacother. 2013;67:313–319. doi: 10.1016/j.biopha.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Shi S, Deng YZ, Zhao JS, Ji XD, Shi J, Feng YX, Li G, Li JJ, Zhu D, Koeffler HP, et al. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J Biol Chem. 2012;287:7845–7858. doi: 10.1074/jbc.M111.315416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong-Zheng X, Wan-Li M, Ji-Ming M, Xue-Qun R. Receptor for activated protein kinase C 1 suppresses gastric tumor progression through nuclear factor-κB pathway. Indian J Cancer. 2015;52(Suppl 3):E172–E175. doi: 10.4103/0019-509X.186572. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Min L, Wang X, Zhao J, Chen H, Qin J, Chen W, Shen Z, Tang Z, Gan Q, et al. Loss of RACK1 promotes metastasis of gastric cancer by inducing a miR-302c/IL8 signaling loop. Cancer Res. 2015;75:3832–3841. doi: 10.1158/0008-5472.CAN-14-3690. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Xiao Y, Fan S, Xiao M, Wang X, Chen X, Li C, Zong G, Zhou G, Wan C. RACK1 overexpression associates with pancreatic ductal adenocarcinoma growth and poor prognosis. Exp Mol Pathol. 2016;101:176–186. doi: 10.1016/j.yexmp.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Peng H, Gong PG, Li JB, Cai LM, Yang L, Liu YY, Yao KT, Li X. The important role of the receptor for activated C kinase 1 (RACK1) in nasopharyngeal carcinoma progression. J Transl Med. 2016;14:131. doi: 10.1186/s12967-016-0885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu F, Tao Z, Wang M, Li G, Zhang Y, Zhong H, Xiao H, Xie X, Ju M. RACK1 promoted the growth and migration of the cancer cells in the progression of esophageal squamous cell carcinoma. Tumour Biol. 2013;34:3893–3899. doi: 10.1007/s13277-013-0977-7. [DOI] [PubMed] [Google Scholar]
- 18.Lu F, Zhang C, Wu WJ, Wu YM. RACK1 downregulation suppresses migration and proliferation of neuroblastoma cell lines. Oncol Rep. 2012;27:1646–1652. doi: 10.3892/or.2012.1629. [DOI] [PubMed] [Google Scholar]
- 19.Ruan Y, Sun L, Hao Y, Wang L, Xu J, Zhang W, Xie J, Guo L, Zhou L, Yun X, et al. Ribosomal RACK1 promotes chemoresistance and growth in human hepatocellular carcinoma. J Clin Invest. 2012;122:2554–2566. doi: 10.1172/JCI58488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X, Xue A, Fang Y, Shu P, Ling J, Hou Y, Shen K, Qin J, Sun Y, Qin X. RACK1 overexpression is linked to acquired imatinib resistance in gastrointestinal stromal tumor. Oncotarget. 2016;7:14300–14309. doi: 10.18632/oncotarget.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Zhang B, Jiang L, Zeng X, Chen Y, Feng X, Guo Y, Chen Q. RACK1, an excellent predictor for poor clinical outcome in oral squamous carcinoma, similar to Ki67. Eur J Cancer. 2009;45:490–496. doi: 10.1016/j.ejca.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Li JJ, Xie D. RACK1, a versatile hub in cancer. Oncogene. 2015;34:1890–1898. doi: 10.1038/onc.2014.127. [DOI] [PubMed] [Google Scholar]
- 23.Zhong X, Li M, Nie B, Wu F, Zhang L, Wang E, Han Y. Overexpressions of RACK1 and CD147 associated with poor prognosis in stage T1 pulmonary adenocarcinoma. Ann Surg Oncol. 2013;20:1044–1052. doi: 10.1245/s10434-012-2377-4. [DOI] [PubMed] [Google Scholar]
- 24.Nagashio R, Sato Y, Matsumoto T, Kageyama T, Satoh Y, Shinichiro R, Masuda N, Goshima N, Jiang SX, Okayasu I. Expression of RACK1 is a novel biomarker in pulmonary adenocarcinomas. Lung Cancer. 2010;69:54–59. doi: 10.1016/j.lungcan.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Wang W, Wang J, Feng J, Wang Q, Jin J, Lv M, Li X, Li Y, Ma Y, et al. Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology. 2013;57:140–151. doi: 10.1002/hep.25978. [DOI] [PubMed] [Google Scholar]
- 26.Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long LY, Li G, Ji XD, Shi S, Guan DX, et al. RACK1 suppresses gastric tumorigenesis by stabilizing the β-catenin destruction complex. Gastroenterology. 2012;142:812–823.e15. doi: 10.1053/j.gastro.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 27.Mamidipudi V, Dhillon NK, Parman T, Miller LD, Lee KC, Cartwright CA. RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. Oncogene. 2007;26:2914–2924. doi: 10.1038/sj.onc.1210091. [DOI] [PubMed] [Google Scholar]
- 28.Liao S, Xiao S, Zhu G, Zheng D, He J, Pei Z, Li G, Zhou Y. CD38 is highly expressed and affects the PI3K/Akt signaling pathway in cervical cancer. Oncol Rep. 2014;32:2703–2709. doi: 10.3892/or.2014.3537. [DOI] [PubMed] [Google Scholar]
- 29.Hara A, Okayasu I. Cyclooxygenase-2 and inducible nitric oxide synthase expression in human astrocytic gliomas: Correlation with angiogenesis and prognostic significance. Acta Neuropathol. 2004;108:43–48. doi: 10.1007/s00401-004-0860-0. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Cui M, Teng H, Wang F, Yu W, Xu T. Silencing the receptor of activated C-kinase 1 (RACK1) suppresses tumorigenicity in epithelial ovarian cancer in vitro and in vivo. Int J Oncol. 2014;44:1252–1258. doi: 10.3892/ijo.2014.2274. [DOI] [PubMed] [Google Scholar]
- 31.Shen F, Yan C, Liu M, Feng Y, Chen Y. RACK1 promotes prostate cancer cell proliferation, invasion and metastasis. Mol Med Rep. 2013;8:999–1004. doi: 10.3892/mmr.2013.1612. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Guo Y, Feng X, Wang Z, Wang Y, Deng P, Zhang D, Wang R, Xie L, Xu X, et al. Receptor for activated C kinase 1 (RACK1): A regulator for migration and invasion in oral squamous cell carcinoma cells. J Cancer Res Clin Oncol. 2012;138:563–571. doi: 10.1007/s00432-011-1097-7. [DOI] [PubMed] [Google Scholar]
- 33.Dave JM, Kang H, Abbey CA, Maxwell SA, Bayless KJ. Proteomic profiling of endothelial invasion revealed receptor for activated C kinase 1 (RACK1) complexed with vimentin to regulate focal adhesion kinase (FAK) J Biol Chem. 2013;288:30720–30733. doi: 10.1074/jbc.M113.512467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandin V, Senft D, Topisirovic I, Ronai ZA. RACK1 function in cell motility and protein synthesis. Genes Cancer. 2013;4:369–377. doi: 10.1177/1947601913486348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu B, Wang C, Chen P, Wang L, Cheng Y. RACK1 promotes radiation resistance in esophageal cancer via regulating AKT pathway and Bcl-2 expression. Biochem Biophys Res Commun. 2017;491:622–628. doi: 10.1016/j.bbrc.2017.07.153. [DOI] [PubMed] [Google Scholar]
- 36.Fei L, Ma Y, Zhang M, Liu X, Luo Y, Wang C, Zhang H, Zhang W, Han Y. RACK1 promotes lung cancer cell growth via an MCM7/RACK1/Akt signaling complex. Oncotarget. 2017;8:40501–40513. doi: 10.18632/oncotarget.17120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou C, Chen T, Xie Z, Qin Y, Ou Y, Zhang J, Li S, Chen R, Zhong N. RACK1 forms a complex with FGFR1 and PKM2 and stimulates the growth and migration of squamous lung cancer cells. Mol Carcinog. 2017 Apr 18; doi: 10.1002/mc.22663. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Zhou S, Wang JY, Cao J, Zhang X, Wang J, Han K, Cheng Q, Qiu G, Zhao Y, et al. RACK1 antagonizes TNF-α-induced cell death by promoting p38 activation. Sci Rep. 2015;5:14298. doi: 10.1038/srep14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M. Targeting cell cycle regulation in cancer therapy. Pharmacol Ther. 2013;138:255–271. doi: 10.1016/j.pharmthera.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226:352–364. doi: 10.1002/path.3022. [DOI] [PubMed] [Google Scholar]
- 41.Aarts M, Linardopoulos S, Turner NC. Tumour selective targeting of cell cycle kinases for cancer treatment. Curr Opin Pharmacol. 2013;13:529–535. doi: 10.1016/j.coph.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schafer KA. The cell cycle: A review. Vet Pathol. 1998;35:461–478. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 44.Waga S, Li R, Stillman B. p53-induced p21 controls DNA replication. Leukemia. 1997;11(Suppl 3):321–323. [PubMed] [Google Scholar]
- 45.Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: Cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Li D, Dai C, Yang X, Wang F, Yu X, Xiao X Tang S. Critical role of p21 on olaquindox-induced mitochondrial apoptosis and S-phase arrest involves activation of PI3K/AKT and inhibition of Nrf2/HO-1 pathway. Food Chem Toxicol. 2017;108:148–160. doi: 10.1016/j.fct.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 48.Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cell Signal. 2007;19:454–465. doi: 10.1016/j.cellsig.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Zhang Y, Yun H, Lai R, Su M. Correlation of STAT1 with apoptosis and cell-cycle markers in esophageal squamous cell carcinoma. PLoS One. 2014;9:e113928. doi: 10.1371/journal.pone.0113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J Biol Chem. 2004;279:5811–5820. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis. 2000;59(Suppl 1):i6–i16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkins ND. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer. 2012;12:121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]