Abstract
Objectives
Recent published evidence suggests that adjunctive trazodone treatment may limit serotonin reuptake inhibitor (SRI) response in depressed adolescents in the context of a controlled trial. This study examined the effects of adjunctive trazodone on depression outcome in adolescents in a naturalistic treatment environment.
Methods
We conducted a cohort study through chart review of a clinical sample. Patients in our sample were 15 to 18 years of age treated with either a selective serotonin reuptake inhibitor or serotonin and norepinephrine reuptake inhibitor. The treatment took place in the setting of a partial hospitalization program at a tertiary care centre from 2009–2014. The main outcome measure was the change in Beck Depression Inventory II (BDI-II) score from admission to discharge. We compared this outcome in patients who were exposed to adjunctive trazodone treatment compared to adolescents who did not receive trazodone in the final four weeks of the program.
Results
Exposure to trazodone was significantly associated with non-response to treatment in our sample (n= 35; β1= −7.76; 95% CI −0.52 to – 15.0; p<0.05; R2 = 0.13). In exploring potential confounders, higher baseline BDI-II scores appeared to predict greater change in BDI-II scores from pre- to post-treatment.
Conclusion
In keeping with previous research, we found that trazodone exposure was associated with treatment non-response in adolescents taking SRIs. The findings should be interpreted cautiously since they are limited by small sample size. Future randomized controlled trials of trazodone in samples of adolescents taking SRIs for depression are warranted.
Keywords: adolescent, depression, trazodone, treatment-resistant depression, selective serotonin re-uptake inhibitor
Résumé
Objectifs
De récentes données probantes publiées suggèrent que le traitement d’appoint par trazodone peut limiter la réponse aux inhibiteurs de recaptage de la sérotonine (IRS) chez les adolescents déprimés dans le contexte d’un essai contrôlé. Cette étude a examiné les effets du trazodone d’appoint sur le résultat de la dépression chez des adolescents dans un environnement de traitement naturel.
Méthodes
Nous avons mené une étude de cohorte par un examen des dossiers d’un échantillon clinique. Les patients de notre échantillon, âgés de 15 à 18 ans, étaient traités soit par inhibiteur sélectif du recaptage de la sérotonine, soit par inhibiteur du recaptage de la sérotonine et de la noradrénaline. Le traitement avait lieu dans le contexte d’un programme d’hospitalisation partielle dans un centre de soins tertiaires, de 2009 à 2014. La principale mesure de résultat était le changement de score à l’inventaire de dépression de Beck II (BDI-II) de l’admission au congé. Nous avons comparé ce résultat chez les patients exposés au traitement d’appoint par trazodone par rapport aux adolescents qui n’ont pas reçu de trazodone dans les 4 dernières semaines du programme.
Résultats
L’exposition au trazodone était significativement associée à une non-réponse au traitement dans notre échantillon (n = 35; β1 = −7,76; IC à 95% −0,52 à −15,0; p < 0,05; R2 = 0,13). En explorant les facteurs de confusion potentiels, des scores plus élevés au départ au BDI-II semblaient prédire un changement plus marqué aux scores du BDI-II d’avant le traitement à après.
Conclusion
En accord avec la recherche précédente, nous avons constaté que l’exposition au trazodone était associée à la non-réponse au traitement chez les adolescents prenant des IRS. Les résultats devraient être interprétés avec prudence puisqu’ils sont limités à une petite taille d’échantillon. Les futurs essais randomisés contrôlés de trazodone dans des échantillons d’adolescents prenant des IRS pour la dépression sont justifiés.
Mots clés: adolescent, dépression, trazodone, dépression réfractaire au traitement, inhibiteur sélectif du recaptage de la sérotonine
Introduction
The prevalence of major depressive disorder is estimated to be 4% to 8% among adolescents (Birmaher, Brent, & AACAP Work Group on Quality Issues, 2007). Shamseddeen et al. (2012) found that sleep disturbance is the most common residual symptom in adolescents who failed to respond to acute phase treatment. In one study, depressed adolescents with initial insomnia were twice as likely to have a recurrence of their illness (Emslie et al., 2012). The American Academy of Child and Adolescent Psychiatry practice parameter for treatment of children and adolescents with depressive disorders suggests that trazodone could be used as adjunctive and transient treatment for insomnia (Birmaher et al., 2007). In North America, trazodone is the most commonly prescribed medication for sleep difficulties in adolescents with mood and anxiety disorders (Owens, Rosen, Mindell, & Kirchner, 2010).
Treatment of Resistant Depression in Adolescents (TORDIA) was a study investigating treatment options for adolescents whose depression had not improved after one adequate trial of an SSRI (Brent et al., 2008). A surprising result in post-hoc analysis was that sleep medication exposure was associated with a poorer response to treatment (Brent et al., 2008). A follow-up investigation to clarify this finding by Shamseddeen et al. found that youth who received trazodone were six times less likely to respond than those with no sleep medication (p=0.001) (Shamseddeen et al., 2012). In contrast, those treated with other sleep medications had similar rates of response (60.0% vs. 50.4%, p = 0.36) as those who received no sleep medication (Shamseddeen et al., 2012).
Studies of adults with depressive disorders showed findings that may be interpreted as contradictory to Shamseddeen’s findings. Maes et al. (1997) reported that a combination of trazodone and fluoxetine led to greater response rates than using trazodone alone. The combination was also associated with higher meta-chlorophenylpiperazine (mCPP) plasma levels. According to the authors, the antidepressant effect of trazodone may, in part, be attributed to its metabolite meta-chlorophenylpiperazine. mCPP is a partial agonist to 5-HT2C and has moderately high affinity to this receptor. The results of that study suggested that fluoxetine-induced increases in plasma mCPP and trazodone concentrations contributed to the clinical efficacy of the combination of medications.
Maes, Vandoolaeghe, and Desnyder (1996) used a double-blind placebo controlled design and evaluated 33 inpatients who were diagnosed with major depressive disorder. This study used a double-blind placebo controlled design and evaluated 33 inpatients who were diagnosed with major depressive disorder. It showed that 75% of participants who took trazodone 100mg/day in combination with fluoxetine 20mg/day had a clinically significant response compared to 20% of participants who were treated with trazodone in addition to placebo. This suggests that fluoxetine had antidepressant effects despite the presence of trazodone. The Canadian Network of Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder suggests that trazodone has shown superior effects on sleep measures, however, also has highest adverse event rates of somnolence and daytime sedation (Kennedy et al., 2016; Alberti, Chiesa, Andrisano, & Serretti, 2015).
Trazodone is thought to have a mixed agonist and antagonist activity at the serotonin receptors. Trazodone also has weak serotonin reuptake inhibition activity; but for the purpose of clarity in this paper, when we refer to serotonin reuptake inhibitors (SRIs) hereafter, we are excluding trazodone from this class of medications.
Given the large effect size and strong statistical significance observed by Shamseddeen et al. (2012), we hypothesized that the association between adjunctive trazodone treatment and SRI non-response would be detectable in a naturalistic setting. This would support the idea that this relationship is potentially clinically significant. If an association were to be found, we wished to examine whether or not it association could be explained by other baseline characteristics associated with treatment non-response.
Methods
Participants
As a tertiary care clinic, the Youth Program at the Royal Ottawa Mental Health Centre treats adolescents aged 15–18 with severe or refractory psychiatric conditions. Embedded within the Youth Program is a Partial Hospitalization Unit (YPHU). YPHU is an intensive outpatient program for adolescents who are struggling with function in their day-to-day lives secondary to moderate-to-severe mental illness. It often serves as a step-down service from the inpatient unit or a “step-up” service for individuals who need more support than a standard weekly outpatient treatment. The program is voluntary. It runs four days per week from 9:00am to 2:30pm and has two hours of school programming and two hours and 2.5 hours of therapeutic programming including psychotherapy groups and recreational groups. There is also multidisciplinary team involvement.
This was a cohort study through chart review of patients undergoing treatment at the YPHU. Sampling was based on selecting consecutive cases who had received treatment at YPHU. Each patient’s medical record was reviewed by the lead investigator for demographics, past history, medication and course of disease. Information was collected on individual data collection sheets.
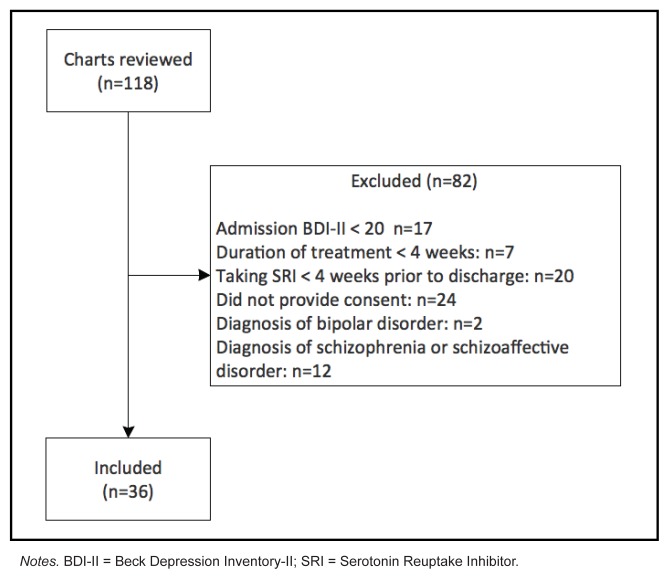
One-hundred and eighteen charts were reviewed in patients consecutively admitted to the YPHU between 2009 and 2014. Thirty-six participants were selected through our ascertainment method (see Figure 1). Participants were included if they were treated with a SRI for at least four weeks prior to discharge, had an admission Beck Depression Inventory II (BDI-II) score of 20 or more (corresponding to moderate-severe depression: Whisman, Perez, & Ramel, 2000), and duration between admission and discharge BDIII of at least four weeks. Participants who were included in the study have signed consent for their data to be used in research. As part of a program evaluation study, each participant had completed self-report measures at admission and at discharge. Potential participants were excluded if they had a diagnosis of schizophrenia, schizoaffective disorder or bipolar disorder. Our study was approved by the Research Ethics Board at our site.
Figure 1.
Ascertainment flow chart
Notes. BDI-II = Beck Depression Inventory-II; SRI = Serotonin Reuptake Inhibitor.
Measures
The following self-report measures were obtained at admission and discharge.
The BDI-II is a 21-item self-report scale designed to assess presence and severity of depressive symptoms. The test-retest reliability is excellent (r=.93). It has displayed construct validity, and it is effective in distinguishing individuals with depression from individuals without depression (Beck, Steer, & Brown, 1996). The BDI-II has been shown in multiple studies to be a reliable measure of depression in adolescents (Uslu, Kapci, Oncu, Ugurlu, & Turkcapar, 2008; VanVoorhis, & Blumentritt 2007; Osman, Kopper, Barrios, Gutierrez, & Bagge, 2004).
The Multidimensional Anxiety Scale for Children 2nd Edition (MASC 2) is a 50-item self-report scale designed to assess anxiety symptoms. Its main factors include physical symptoms, harm avoidance, social anxiety, separation anxiety, generalized anxiety disorder index, and obsessions and compulsions. The MASC 2 has demonstrated excellent reliability with Cronbach’s alpha coefficients, reflecting internal consistency, ranged between 0.89 to 0.92 for the total scores and test-retest reliability ranged between 0.80 to 0.94 (March, 2013).
The Adolescent Alcohol and Drug Involvement Scale (AADIS) is adapted from Mayer and Filstead’s Adolescent Alcohol Involvement Scale and Moberg and Hahn’s Adolescent Drug Involvement Scale. It is designed as a screening tool for alcohol and illicit drug use in adolescents (Moberg, 2003). The AADIS has acceptable internal consistency (Cronbach’s alpha = .85). AADIS scores have high correlations with self-reported levels of drug use (r=.72) and with self-reported severity of drug use (r=.79) (Moberg & Hahn, 1991). A cut-off score of 37 indicates a high likelihood of clinically significant substance use.
Baseline diagnostic categories were derived from clinical impression of the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision (DSM-IV-TR) diagnosis after an assessment by a psychiatrist. Depressive disorders included major depressive disorder with and without psychotic features and dysthymia. Anxiety disorders included generalized anxiety disorder, panic disorder, social anxiety disorder, post-traumatic stress disorder and obsessive-compulsive disorder.
Design
Administration of trazodone was used as the exposure variable. Change in BDI-II score was the outcome variable. The null hypothesis is that that there is no relationship between trazodone exposure and SRI-responsiveness. The alternative hypothesis is that trazodone exposure reduces the likelihood of SRI-responsiveness.
Statistical Analysis
STATA (version 13) was used to run statistical analyses. Baseline variables were tabulated. Given small cell sizes, two-sided Fisher exact tests were run to explore potential differences between the exposed groups versus non-exposed groups for dichotomous baseline variables. Continuous variables were examined for normality using visual inspection of quantile normal plots. Medians, inter-quartile ranges and Wilcoxon rank sum tests were used to compare exposure groups for non-parametric data. Means, standard deviations (SD) and t-tests were used for parametric data.
To describe within group changes for the entire sample, paired t-tests were used to test for significant changes on continuous measures (BDI-II and MASC 2) as the data were parametric. Cohen’s d effect sizes were calculated by taking the difference in pre- and post-means and dividing by the pooled standard deviation. Change in sleep disturbance was tested using the Sign-rank test and degree of sleep disturbance as measured by item 16 on the BDI-II was treated as an ordinal variable. Drug use category was treated as a dichotomous variable (using the AADIS cut-off of a score of 37 or higher as “significant drug use”).
Our a priori primary hypothesis was that nightly exposure to trazodone throughout the 4 weeks prior to endpoint would be associated with lack of response as defined by the continuous score on the BDI-II. We tested this hypothesis using univariate linear regression. Namely, we wished to test the equation:
The null hypothesis is that β1 = 0. Ideally, we would have conducted multivariate regression analysis to test for confounding; however, the sample size was too small. We still wished to examine other potential sources of bias. For example, if trazodone is not being prescribed, perhaps it is because the individual is on a sedating adjunctive medication for treatment of depression (e.g. quetiapine, olanzapine, risperidone, mirtazapine). Adjunctive sedating medication exposure was tested to see if it affected outcome. Comorbid anxiety disorder, personality disorder or substance use disorder at baseline may be associated with non-response. Baseline BDI-II score may affect outcome as people with higher scores have further room for improvement, or conversely, may be more treatment-resistant. Sleep disturbance at baseline may predict outcome. Lastly, the amount of time between baseline and endpoint may affect results, as depression may improve simply with the passage of time. Each of these potential sources of bias were tested in univariate linear regression with change in BDI-II score as the outcome. For each regression analysis, the distributions of residuals were checked for approximations of normality and homoscedasticity. Missing data and outliers were removed from analyses (if required, typically this only involved removal of one participant from the entire sample based on visual inspection of quantile normal plots). We also tested whether trazodone exposure was associated with change in anxiety symptoms, as measured by the MASC 2.
Results
We did not find any significant differences in baseline characteristics between the exposed and non-exposed groups (see Table 1). The mean number of days between baseline and endpoint measures was 84 (SD 26.6) with the exception of one “outlier” patient who spanned 295 days between pre- and post-measures. Participants were treated with one of the following medications for at least four weeks before discharge: fluoxetine (n=8), escitalopram (n=8), venlafaxine (n=7), sertraline (n=5), citalopram (n=5), desvenlafaxine (n=2), or duloxetine (n=1). Within-group analyses demonstrated improvement in depression and anxiety scores (see Table 2). In addition to these findings, we observed a significant improvement in sleep disturbance from baseline to endpoint (n=35; z=3.19, p<0.01). We did not observe a significant change in problematic substance use within the overall sample.
Table 1.
Baseline characteristics tabulated across trazodone exposure groups
| Baseline Variable | n | Exposed (% within exposed) | Non-exposed (% within non-exposed) | Total (% of overall total) | Test statistic | p-value |
|---|---|---|---|---|---|---|
| Total sample | 10 (28%) | 26 (72%) | 36 | |||
| Female Gender | 36 | 9 (90%) | 18 (69%) | 27 (75%) | 0.39 | |
| Depressive Disorder | 36 | 7 (70%) | 20 (77%) | 27 (75%) | 0.69 | |
| Anxiety Disorder | 36 | 5 (50%) | 14 (54%) | 19 (53%) | 1.00 | |
| Personality Disorder | 36 | 2 (20%) | 8 (31%) | 10 (28%) | 0.69 | |
| AADIS cut-off (≥37) | 36 | 5 (50%) | 9 (35%) | 14 (25%) | 0.46 | |
| Taking SRI for 4 weeks prior to admission | 34 | 5 (50%) | 19 (73%) | 24 (56%) | 0.40 | |
| BDI-II score | 36 | Median = 28 (IQR 25–35) | Median = 39.5 (IQR 26–49) | Median = 37.5 (IQR 26–46) | z=1.40 | 0.16 |
| MASC 2 score | Mean = 62.3 (SD 10.1) | Mean = 62.3 (SD 16.0) | Mean 62.3 (SD 14.5) | t=0.03 | 0.98 |
Notes. AADIS = Adolescent Alcohol and Drug Involvement Scale; SRI = Serotonin Reuptake Inhibitor; BDI-II = Beck Depression Inventory-II; MASC 2 = Multidimensional Anxiety Scale for Children 2nd Edition; IQR = Inter-quartile Range; SD = Standard Deviation
Table 2.
Within-group clinical changes in the total sample and by exposure group
| Variable | Trazodone Exposure Category | n | Baseline mean (SD) | Endpoint mean (SD) | Test statistic | p-value | Effect size (d) |
|---|---|---|---|---|---|---|---|
| BDI-II | All | 35 | 36 (10.9) | 24.3 (12.0) | t = 6.94 | <0.001 | 1.17 |
| Exposed | 10 | 31.5 (9.9) | 25.3(18.1) | t =2.05 | 0.07 | 0.65 | |
| Non-exposed | 25 | 37.8 (10.9) | 23.9 (9.0) | t = 7.35 | <0.001 | 1.47 | |
| MASC 2 | All | 35 | 62 (SD 14.7) | 55.5 (14.9) | t = 3.97 | <0.001 | 0.68 |
| Exposed | 9 | 62.3 (10.7) | 56.4 (17.4) | t=1.59 | 0.15 | 0.53 | |
| Non-exposed | 26 | 62.3 (16.0) | 55.2 (14.4) | t=3.64 | <0.01 | 0.71 |
Notes. BDI-II = Beck Depression Inventory-II, one outlier removed from non-exposed group; MASC 2 = Multidimensional Anxiety Scale for Children 2nd Edition, one outlier removed from exposed group.
Primary hypothesis
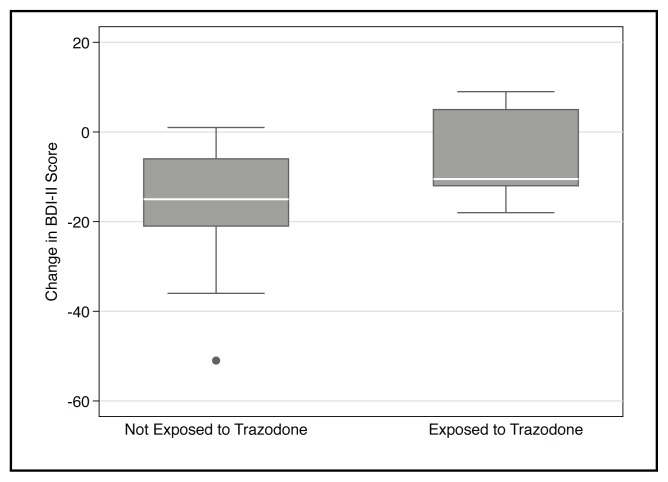
Trazodone exposure was significantly associated with non-response to treatment in our sample (n= 35; β1= −7.76; 95% Confidence Interval (CI) −0.52 to – 15.0; p<0.05; R2 = 0.13). One outlier, which would have favoured our proposed hypothesis, was removed for this analysis. In practice, this translates to a prediction that being exposed to trazodone is associated with reducing the change in BDI-II scores by nearly eight points. A graphical representation of our findings can be seen in Figure 2.
Figure 2.
Box plots of change in depressive symptoms by trazodone exposure
Secondary analyses
Higher baseline BDI-II scores appeared to predict greater change in BDI-II scores from pre- to post-treatment; otherwise, none of the other potential confounders predicted response (see Table 3). We did not find any significant relationship between trazodone exposure and change in anxiety symptoms as measured by the MASC 2.
Table 3.
Potential confounding independent variables tested through univariate regression analyses with change in BDI-II as the outcome
| Independent variable | n | β coefficient | p-value | R2 |
|---|---|---|---|---|
| Adjunctive sedating medication in the 4 weeks prior to endpoint | 34 | 2.33 | 0.50 | 0.01 |
| Comorbid personality disorder at baseline | 34 | 1.06 | 0.78 | 0.00 |
| Degree of sleep disturbance at baseline (item 16 on BDI-II) | 34 | 2.96 | 0.12 | 0.07 |
| Baseline MASC 2 score | 34 | −0.02 | 0.90 | 0.00 |
| Drug use at baseline (AADIS > 37) | 34 | 3.22 | 0.37 | 0.02 |
| Baseline BDI-II total score | 34 | 0.31 | 0.046* | 0.12 |
| Number of days between baseline and endpoint measures. | 33 | −0.04 | 0.58 | 0.01 |
Notes. BDI-II = Beck Depression Inventory-II; MASC 2 = Multidimensional Anxiety Scale for Children 2nd Edition; AADIS = Adolescent Alcohol and Drug Involvement Scale.
Discussion
Our findings are consistent with the results from the study by Shamseddeen et al. (2012), in that trazodone exposure was associated with SRI non-response. This finding is mitigated by the fact that those exposed to trazodone had numerically lower BDI-II scores at baseline. Consequently, it is possible that participants in the trazodone group had less potential for improvement, which could account for the lower levels of response; that is, the results could be due to a “floor effect”. Indeed, our secondary analysis did show that higher baseline BDI-II scores were associated with greater response. Given the observational nature of this study, causal pathways cannot be determined; it could equally be the case that patients who had lower BDI-II scores at baseline had less improvement because they were exposed to trazodone. Interestingly, in the TORDIA study, high baseline BDI-II scores were a potent predictor of treatment resistance at 24-week follow-up; which is the converse of our findings (Emslie et al., 2010). Unfortunately, we did not have the power to conduct a multivariate analysis whereby we could examine interactive effects or robustly test the influence of covariates. We could not find any other confounders to account for the results, though lack of power may account for this.
Serotonin reuptake inhibitors increase the efficacy of serotonin neurons by desensitizing presynaptic serotonin 1A (5HT1A) autoreceptors (Blier, de Montigny, & Chaput, 1990). Serotonin reuptake inhibition occurs immediately following the administration of SRI medication (Gardier, Malagie, Trillat, Jacquot, & Artigas, 1996). The build up of serotonin around 5HTA1 autoreceptors leads to desensitization of the autoreceptors (Blier et al., 1990). Since the autoreceptors function as part of a “negative feedback loop” their desensitization leads to an overall increase in the output of the serotonin neuron (Gardier et al. 1996). This occurs over the span of several weeks. This process explains one of the mechanisms of action of SRI medication (Blier et al., 1990; Gardier et al. 1996). Trazodone is thought to have agonist properties at 5HT1A receptors (Ghanbari, Mansari, & Blier, 2010). We hypothesize that the 5HT1A agonistic action of trazodone interferes with the desensitization of 5HT1A autoreceptors necessary for in SRI response.
Our findings, along with Shamsedden et al. (2012), are in contrast to the results of adult studies (Maes et al., 1997). It is possible that the response-limiting effects of trazodone are unique to younger populations. Indeed, there is substantial evidence that adolescents respond differently to antidepressant medication compared to adults. For example, younger individuals are more likely to have suicide-related symptoms in the context of antidepressants: a meta-analysis (n=99,231) by Stone et al. (2009) examining clinical trials of antidepressants in adults revealed a strong association between increased risk of suicidal symptoms and age of 25 years or younger. Moreover, while tricyclic antidepressants (TCAs) can be quite effective for adults, a meta-analysis of 12 randomized controlled trial comparing the effects of TCAs in children and adolescents concluded that these medications appeared to be no more effective than placebo (Hazell, O’Connell, Heathcote, Robertson, & Henry, 1995). Lastly, in a meta-analysis of 32 randomized controlled trials of adults comparing venlafaxine with other antidepressants, venlafaxine had greater efficacy than SSRI medication (Smith, Dempster, Glanville, Freemantle, & Anderson, 2002); in contrast, the TORDIA study showed that there was no difference in response rates at 12 weeks among adolescents who received venlafaxine compared to an SSRI (Emslie at al., 2010). These studies all point to the importance of considering developmental factors in the effects of psychotropic medications.
Clinical implications
After two trials of SSRIs and a trial of cognitive-behavioral therapy (CBT), we are not aware of any randomized controlled trials regarding “the next step” for the treatment of depressed adolescents. In their recommendations, Maalouf et al. (Maaslouf, Atwi, & Brent, 2011) suggest looking into augmentation and switching strategies. The findings from our study, along with Shamseddeen’s study, are far from definitive; however, they would suggest that adolescent patients on trazodone who have not responded to standard treatment could be considered for discontinuation of trazodone or switching to another sleep-inducing agent as it may be limiting treatment response. Data is currently limited, however, if more definitive studies support our findings in the future, the option of discontinuing trazodone would be reasonable to explore prior to more invasive treatment options, such as electroconvulsive therapy or options with greater potential for toxicity, such as long-term lithium augmentation treatment.
Research implications
Shamssedden’s findings and our study are observational and so are not conclusive. Given that trazodone is a very commonly prescribed medication for children and adolescents for sleep and there is potential for it leading to treatment non-response, randomized controlled trials of trazodone in samples of adolescents taking SRIs for depression are highly warranted. Moreover, investigators conducting clinical trials of antidepressants in adolescents will also need to consider adjusting for trazodone exposure in participants as it may be affecting outcomes.
Limitations
There are several limitations to the current study. Wide confidence intervals and a low coefficient of determination (R2) are likely functions of the small sample size and limit the implications of our findings. Use of self-reported scale for the primary outcome may be overly influenced by mood state on the day the participant completed the measures. Reporting of symptoms may not be accurate. Use of clinician-rated scales in future studies may assist in mitigating the effects of possible reporting inaccuracy. Lack of randomization of exposure limits the implications of the study results. Medication dosage, total duration of exposure to trazodone and/or SRI, and effects of psychotherapy were not taken into account. Randomized control studies that would address more details about received management would help overcome these limitations. Lastly, the absence of finding other significant confounding factors on univariate analyses (apart from baseline BDI-II scores) may be due to lack of power, as opposed to a true lack of confounding.
Conclusions
Our study concludes that trazodone exposure was associated with lower response to SRI medication in a naturalistic setting; however, this should be interpreted cautiously given the above-mentioned limitations. We could not exclude baseline BDI-II scores as a confounding factor. Randomized controlled trials are lacking in regards to subsequent steps for treatment of adolescent patients who have not remitted after a second trial of an SSRI and a course of CBT. Trazodone is commonly prescribed for insomnia in children and adolescents. Since there is a substantial possibility for this medication to be associated with treatment non-response, future randomized controlled trials assessing its combination with SRI medication is highly warranted.
Acknowledgments / Conflicts of Interest
We thank Dr. Martine Flament, Former Director of the Youth Research Unit at the Royal Ottawa Mental Health Centre, for her general support and insights that greatly assisted the research. We also thank Ms. Selena Walker, Program Evaluation Coordinator at the Royal Ottawa Mental Health Centre, for her administrative assistance and Mr. Nathan Parker, Research Assistant at the Institute of Mental Health, for assistance with formatting. The authors have no financial relationships to disclose.
References
- Alberti S, Chiesa A, Andrisano C, Serretti A. Insomnia and somnolence associated with second-generation antidepressants during the treatment of major depression: A meta-analysis. Journal of Clinical Psychopharmacology. 2015;35(3):296–303. doi: 10.1097/JCP.0000000000000329. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio, TX, 78204-2498: 1996. [Google Scholar]
- Birmaher B, Brent D Work Group on Quality Issues AACAP. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(11):1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Chaput Y. A role for the serotonin system in the mechanism of action of antidepressant treatments: Preclinical evidence. Journal of Clinical Psychiatry. 1990;51(4):14–20. [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, … Birmaher B. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA. 2008;299(8):901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Kennard BD, Mayes TL, Nakonezny PA, Zhu L, Tao R, … Croarkin P. Insomnia moderates outcome of serotonin-selective reuptake inhibitor treatment in depressed youth. Journal of Child and Adolescent Psychopharmacology. 2012;22(1):21–28. doi: 10.1089/cap.2011.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD, … Kennard B. Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. American Journal of Psychiatry. 2010;167(7):782–789. doi: 10.1176/appi.ajp.2010.09040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: Recent findings from in vivo microdialysis studies. Fundamental & Clinical Pharmacology. 1996;10(1):16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Ghanbari R, El Mansari M, Blier P. Sustained administration of trazodone enhances serotonergic neurotransmission: In vivo electrophysiological study in the rat brain. Journal of Pharmacology and Experimental Therapeutics. 2010;335(1):197–206. doi: 10.1124/jpet.110.169417. [DOI] [PubMed] [Google Scholar]
- Hazell P, O’Connell D, Heathcote D, Robertson J, Henry D. Efficacy of tricyclic drugs in treating child and adolescent depression: A meta-analysis. BMJ. 1995;310(6984):897–901. doi: 10.1136/bmj.310.6984.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, … McInerney SJ. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder Section 3. Pharmacological Treatments. The Canadian Journal of Psychiatry. 2016;61(9):540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf FT, Atwi M, Brent DA. Treatment-resistant depression in adolescents: Review and updates on clinical management. Depression and Anxiety. 2011;28(11):946–954. doi: 10.1002/da.20884. [DOI] [PubMed] [Google Scholar]
- Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. Journal of Affective Disorders. 1996;41(3):201–210. doi: 10.1016/s0165-0327(96)00089-4. [DOI] [PubMed] [Google Scholar]
- Maes M, Westenberg H, Vandoolaeghe E, Demedts P, Wauters A, Nells H, Meltzer HY. Effects of trazodone and fluoxetine in the treatment of major depression: Therapeutic pharmacokinetic and pharmacodynamic interactions through formation of meta-chlorophenylpiperazine. Journal of Clinical Psychopharmacology. 1997;17(5):358–364. doi: 10.1097/00004714-199710000-00004. [DOI] [PubMed] [Google Scholar]
- March JS. Multidimensional anxiety scale for children (MASC2) North Tonawanda, NY: Multi-Health Systems; 2013. [Google Scholar]
- Moberg DP. Screening for alcohol and other drug problems using the Adolescent Alcohol and Drug Involvement Scale (AADIS) Madison: Center for Health Policy and Program Evaluation, University of Wisconsin-Madison; 2003. [Google Scholar]
- Moberg DP, Hahn L. The adolescent drug involvement scale. Journal of Child & Adolescent Substance Abuse. 1991;2(1):75–88. [Google Scholar]
- Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory--II with adolescent psychiatric inpatients. Psychological Assessment. 2004;16(2):120–132. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- Owens JA, Rosen CL, Mindell JA, Kirchner HL. Use of pharmacotherapy for insomnia in child psychiatry practice: A national survey. Sleep Medicine. 2010;11(7):692–700. doi: 10.1016/j.sleep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Shamseddeen W, Clarke G, Keller MB, Wagner KD, Birmaher B, Emslie GJ, … Brent DA. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. Journal of Child and Adolescent Psychopharmacology. 2012;22(1):29–36. doi: 10.1089/cap.2011.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: A meta-analysis. The British Journal of Psychiatry. 2002;180(5):396–404. doi: 10.1192/bjp.180.5.396. [DOI] [PubMed] [Google Scholar]
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, … Rochester G. Risk of suicidality in clinical trials of antidepressants in adults: Analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009;339:b2880. doi: 10.1136/bmj.b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslu RI, Kapci EG, Oncu B, Ugurlu M, Turkcapar H. Psychometric properties and cut-off scores of the Beck Depression Inventory-II in Turkish adolescents. Journal of Clinical Psychology in Medical Settings. 2008;15(3):225–233. doi: 10.1007/s10880-008-9122-y. [DOI] [PubMed] [Google Scholar]
- VanVoorhis CRW, Blumentritt TL. Psychometric properties of the Beck Depression Inventory-II in a clinically-identified sample of Mexican American adolescents. Journal of Child and Family Studies. 2007;16(6):789–798. [Google Scholar]
- Whisman MA, Perez JE, Ramel W. Factor structure of the Beck Depression Inventory-Second Edition (BDI-ii) in a student sample. Journal of Clinical Psychology. 2000;56(4):545–551. doi: 10.1002/(sici)1097-4679(200004)56:4<545::aid-jclp7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]