Abstract
Serum protein fingerprints associated with MGUS and MM and their changes in MM after autologous stem cell transplantation (MM-ASCT, day 100) remain unexplored. Using highly-sensitive Proximity Extension ImmunoAssay on 92 cancer biomarkers (Proseek Multiplex, Olink), enhanced serum levels of Adrenomedullin (ADM, Pcorr= .0004), Growth differentiation factor 15 (GDF15, Pcorr= .003), and soluble Major histocompatibility complex class I-related chain A (sMICA, Pcorr= .023), all prosurvival and chemoprotective factors for myeloma cells, were detected in MM comparing to MGUS. Comparison of MGUS and healthy subjects revealed elevation of angiogenic and antia-poptotic midkine (Pcorr= .0007) and downregulation of Transforming growth factor beta 1 (TGFB1, Pcorr= .005) in MGUS. Importantly, altered serum pattern was associated with MM-ASCT compared to paired MM at the diagnosis as well as to healthy controls, namely by upregulated B-Cell Activating Factor (sBAFF) (Pcorr< .006) and sustained elevation of other pro-tumorigenic factors. In conclusion, the serum fingerprints of MM and MM-ASCT were characteristic by elevated levels of prosurvival and chemoprotective factors for myeloma cells.
Keywords: serum pattern, cytokines, growth factors, proximity extension immunoassay, post-transplant serum pattern
INTRODUCTION
Monoclonal gammopathy of undetermined significance (MGUS) is a precursor lesion to overt multiple myeloma (MM), a clonal B-cell malignancy characterized by excessive multiplication of a plasma cell clone(s) in bone marrow, and accumulation of either a monoclonal immunoglobulin (Ig) (M-protein) or an Ig-free light chain in blood [1]. During the last decade, MM treatment and patient outcomes improved remarkably after the introduction of novel agents and autologous stem cell transplantation (ASCT) [2]. However, even with the best combination of currently available drugs, a cure is not achieved for most MM patients [2, 3]. Better characterization of neoplastic cells and microenvironment in particular myeloma stages is therefore needed as well as clarifying of reason(s) for treatment failure in most MM patients [2, 3, 4]. The neoplastic plasma cells in MGUS and MM share similar genetic abnormalities, probably occurring as early events [5, 6]. The key role in microenvironment play bone marrow stromal cells and other microenvironmental cells that secrete a plethora of cytokines and growth factors after paracrine stimulation and/or direct interaction with neoplastic cells [7]. Moreover, also myeloma cells secrete numerous cytokines and growth factors [8, 9]. The secreted molecules may, in turn, promote homing, migration, proliferation, survival of malignant plasma cells as well as contribute to the bone resorption and drug resistance [10].
Given the key role of cytokines and growth factors in MM pathogenesis, we investigated the complexity of serum microenvironment using novel multiplex highly-sensitive PEA immunoassay on 92 cancer-related proteins followed by pattern-recognition analyses. Besides identification of serum fingerprints distinguishing MGUS and MM, we for the first time compared paired MM samples from the time of diagnosis and after autologous stem cell transplantation (MM-ASCT) as well as MGUS to healthy subjects.
RESULTS
Serum protein fingerprinting in MGUS and MM by PEA immunoassay
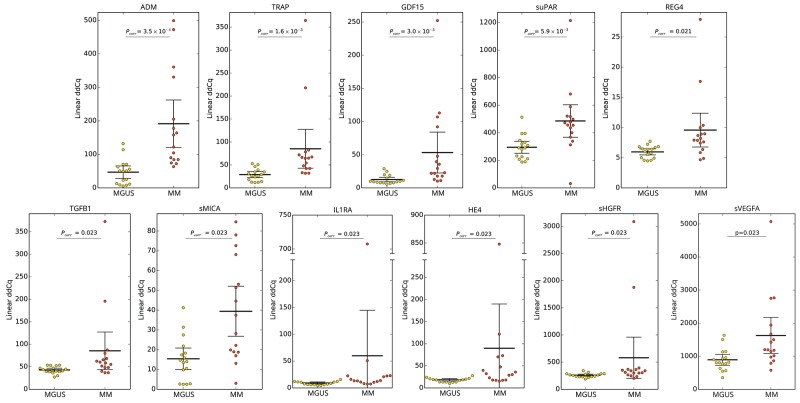
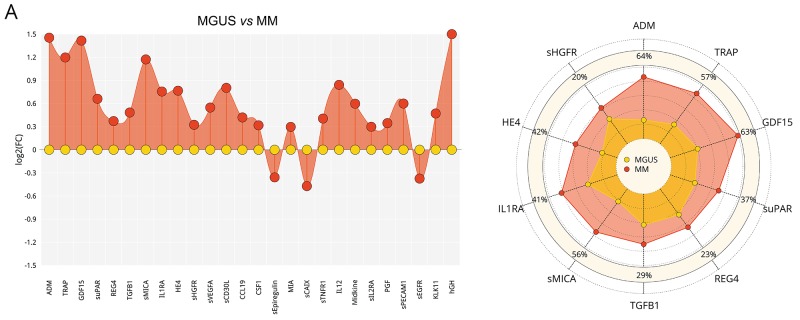
To assess the serum protein fingerprints associated with MGUS and MM, we compared serum protein pattern obtained by PEA immunoassay in MGUS and MM and healthy controls. Of ninety-two analyzed biomarkers (Supplementary Table S1), levels of six biomarkers (sBTC, CA242, sER, GM-CSF, IL2, IL4) were below the Proseek limit of detection (LOD) in all studied groups and were therefore excluded from further analysis. Comparing MGUS and MM, 26 analytes were deregulated between these groups, whereas 13 analytes reached the significance after the adjustment for multiple comparisons (Supplementary Table S2A). The distribution of serum levels of top-ranked proteins (ADM, TRAP, GDF15, suPAR, REG4, TGFB1, sMICA, IL1RA, HE4, sHGFR, sVEGFA; see Table 1A), all found upregulated in MM, is shown in Figure 1. The protein serum fingerprints associated with MGUS and MM and the changes in protein levels between MGUS and MM for top-deregulated analytes are shown in Figure 2A.
Table 1. Serum levels of top-ranked proteins differentiating between A) MGUS vs MM, B) healthy controls vs MGUS, C) healthy controls vs MM, D) MM vs MM-ASCT and E) healthy controls vs MM-ASCT.
| Analyte | Mean Linear ddCq (95% CI) | FC | P | Pcorr | |
|---|---|---|---|---|---|
| A | MGUS | MM | |||
| ADM | 46.9 (28.4-65.4) | 191 (121-262) | 2.80 | 4.0 × 10-6 | 3.5 × 10-4 |
| TRAP | 28.9 (22.3-35.6) | 85.1 (42.8-127) | 2.33 | 3.7 × 10-5 | 1.6 × 10-3 |
| GDF15 | 12.6 (9.19-16.0) | 53.3 (22.5-84.1) | 2.72 | 1.1 × 10-4 | 3.0 × 10-3 |
| suPAR | 295 (251-338) | 486 (368-603) | 1.60 | 2.7 × 10-4 | 5.9 × 10-3 |
| REG4 | 5.96 (5.46-6.46) | 9.58 (6.78-12.4) | 1.30 | 1.2 × 10-3 | .021 |
| TGFB1 | 42.9 (39.0-46.8) | 85.5 (43.7-127) | 1.41 | 1.9 × 10-3 | .023 |
| sMICA | 15.4 (9.98-20.8) | 39.4 (26.8-52.1) | 2.29 | 2.1 × 10-3 | .023 |
| IL1RA | 9.22 (7.23-11.2) | 60.0 (0-145) | 1.71 | 2.6 × 10-3 | .023 |
| HE4 | 18.4 (15.6-21.3) | 89.8 (0-190) | 1.72 | 3.0 × 10-3 | .023 |
| sHGFR | 259 (240-278) | 580 (199-960) | 1.26 | 3.0 × 10-3 | .023 |
| sVEGFA | 892 (728-1056) | 1628 (1082-2173) | 1.47 | 3.0 × 10-3 | .023 |
| B | healthy controls | MGUS | |||
| Midkine | 30.3 (25.8-34.8) | 75.0 (60.8-89.3) | 2.24 | 8.2 × 10-6 | 7.0 × 10-4 |
| THPO | 13.1 (11.7-14.6) | 24.2 (18.8-29.6) | 1.79 | 9.8 × 10-5 | 4.2 × 10-3 |
| sTNFRSF4 | 4.12 (3.29-4.94) | 7.39 (6.05-8.72) | 1.54 | 1.6 × 10-4 | 4.4 × 10-3 |
| sHER4 | 174 (163-184) | 270 (228-311) | 1.45 | 2.4 × 10-4 | 4.5 × 10-3 |
| IFNγ | 1.58 (1.52-1.64) | 2.17 (1.95-2.39) | 1.31 | 3.1 × 10-4 | 4.5 × 10-3 |
| TGFB1 | 61.9 (54.2-69.5) | 42.9 (39.0-46.8) | .71 | 3.7 × 10-4 | 4.5 × 10-3 |
| sPECAM1 | 15.6 (12.6-18.7) | 28.7 (23.3-34.1) | 1.56 | 3.7 × 10-4 | 4.5 × 10-3 |
| sIL17RB | 5.56 (3.84-7.28) | 10.9 (9.09-12.7) | 1.81 | 1.1 × 10-3 | .010 |
| KLK6 | 28.6 (24.5-32.8) | 44.0 (37.9-50.2) | 1.29 | 1.1 × 10-3 | .010 |
| suPAR | 425 (373-476) | 295 (251-338) | .69 | 2.0 × 10-3 | .017 |
| C | healthy controls | MM | |||
| PGF | 59.2 (52.5-66.0) | 137 (97.2-177) | 1.72 | 1.6 × 10-5 | 1.4 × 10-3 |
| GDF15 | 8.34 (6.14-10.5) | 53.3 (22.5-84.1) | 3.69 | 9.8 × 10-5 | 4.2 × 10-3 |
| HE4 | 12.9 (10.4-15.5) | 89.8 (0-190) | 2.56 | 1.6 × 10-4 | 4.4 × 10-3 |
| sTNFR2 | 9.52 (7.93-11.1) | 17.9 (14.3-21.6) | 1.88 | 2.4 × 10-4 | 5.3 × 10-3 |
| CSF1 | 100 (94.4-106) | 195 (115-275) | 1.64 | 3.7 × 10-4 | 5.3 × 10-3 |
| Midkine | 30.3 (25.8-34.8) | 158 (82.9-232) | 3.42 | 3.7 × 10-4 | 5.3 × 10-3 |
| sPECAM1 | 15.6 (12.6-18.7) | 69.6 (17.4-122) | 2.38 | 7.7 × 10-4 | 9.3 × 10-3 |
| CCL19 | 463 (317-609) | 929 (733-1125) | 1.76 | 1.1 × 10-3 | 9.3 × 10-3 |
| sVEGFA | 765 (700-830) | 1628 (1082-2173) | 1.57 | 1.1 × 10-3 | 9.3 × 10-3 |
| IFNγ | 1.58 (1.52-1.64) | 5.17 (0-11.1) | 1.47 | 1.1 × 10-3 | 9.3 × 10-3 |
| D | MM | MM-ASCT | |||
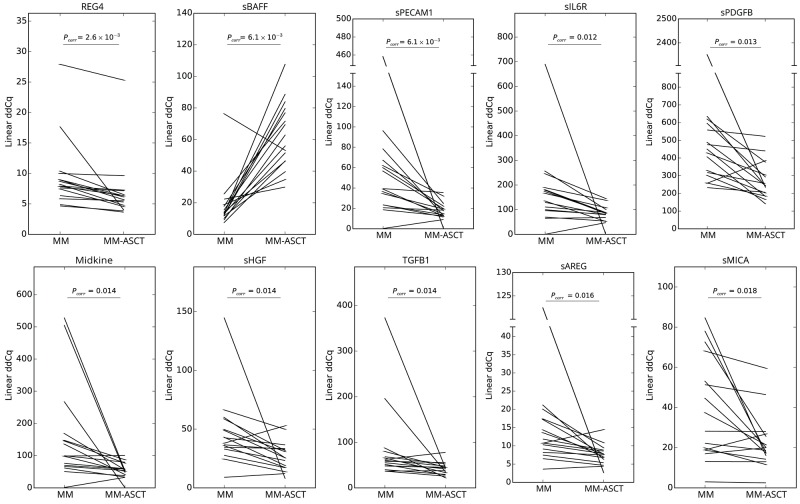
| REG4 | 9.58 (6.78-12.4) | 7.01 (4.50-9.51) | .74 | 3.1 × 10-5 | 2.6 × 10-3 |
| sBAFF | 18.4 (10.5-26.3) | 62.3 (51.8-72.9) | 4.38 | 1.5 × 10-4 | 6.1 × 10-3 |
| sPECAM1 | 69.6 (17.4-122) | 17.3 (13.1-21.6) | .41 | 2.1 × 10-4 | 6.1 × 10-3 |
| sIL6R | 174 (99.3-249) | 81.6 (64.6-98.6) | .55 | 5.8 × 10-4 | .012 |
| sPDGFB | 544 (299-789) | 274 (222-327) | .56 | 7.6 × 10-4 | .013 |
| Midkine | 158 (82.9-232) | 54.5 (42.7-66.3) | .54 | 1.0 × 10-3 | .014 |
| sHGF | 47.1 (32.5-61.6) | 26.9 (20.7-33.1) | .63 | 1.3 × 10-3 | .014 |
| TGFB1 | 85.5 (43.7-127) | 41.6 (34.9-48.3) | .72 | 1.3 × 10-3 | .014 |
| sAREG | 19.0 (5.32-32.8) | 7.52 (6.17-8.87) | .67 | 1.7 × 10-3 | .016 |
| sMICA | 39.4 (26.8-52.1) | 22.5 (15.8-29.2) | .59 | 2.1 × 10-3 | .018 |
| E | healthy controls | MM-ASCT | |||
| sBAFF | 14.1 (12.9-15.3) | 62.3 (51.8-72.9) | 4.32 | 8.2 × 10-6 | 7.0 × 10-4 |
| CSF1 | 100 (94.4-106) | 152 (131-173) | 1.44 | 2.4 × 10-4 | 6.0 × 10-3 |
| sTGFA | 24.4 (19.6-29.2) | 11.9 (9.03-14.8) | .36 | 2.4 × 10-4 | 6.0 × 10-3 |
| TRAP | 32.2 (25.0-39.4) | 71.2 (42.5-100.0) | 1.82 | 3.7 × 10-4 | 6.0 × 10-3 |
| CXCL10 | 130 (61.2-199) | 667 (323-1011) | 4.05 | 5.4 × 10-4 | 6.0 × 10-3 |
| sTNFR2 | 9.52 (7.93-11.1) | 21.2 (17.6-24.9) | 2.45 | 5.4 × 10-4 | 6.0 × 10-3 |
| sTNFRSF4 | 4.12 (3.29-4.94) | 10.9 (7.21-14.6) | 2.22 | 5.4 × 10-4 | 6.0 × 10-3 |
| Flt3L | 254 (225-284) | 522 (411-633) | 2.07 | 7.7 × 10-4 | 6.0 × 10-3 |
| GDF15 | 8.34 (6.14-10.5) | 22.7 (16.5-28.8) | 2.50 | 7.7 × 10-4 | 6.0 × 10-3 |
| HE4 | 12.9 (10.4-15.5) | 43.6 (3.61-83.5) | 1.88 | 7.7 × 10-4 | 6.0 × 10-3 |
| THPO | 13.1 (11.7-14.6) | 28.5 (21.7-35.3) | 2.23 | 7.7 × 10-4 | 6.0 × 10-3 |
FC (Fold Change) between group medians of linear ddCq
*Pcorr value corrected for multiple comparisons (Benjamini-Hochberg correction)
Figure 1. Distribution of serum levels of top-ranked proteins in patients with MGUS vs MM.
Group means are indicated by horizontal bars, error bars indicate 95%CI; Pcorr values for differences between two groups of patients after multiple corrections are stated.
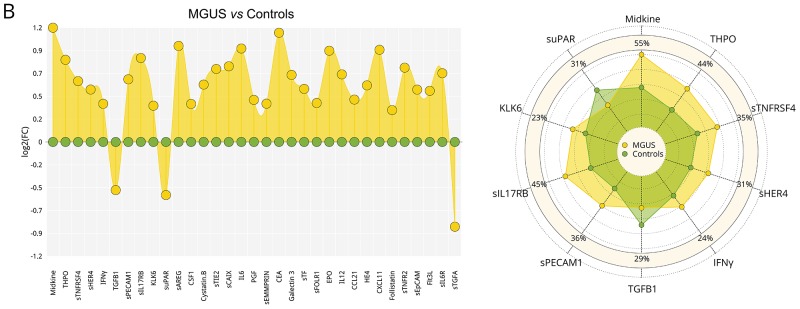
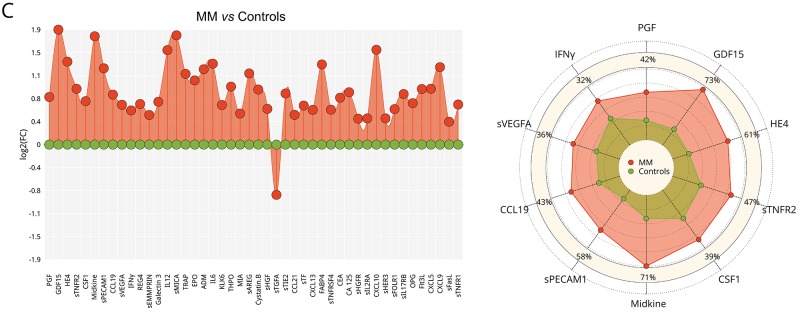
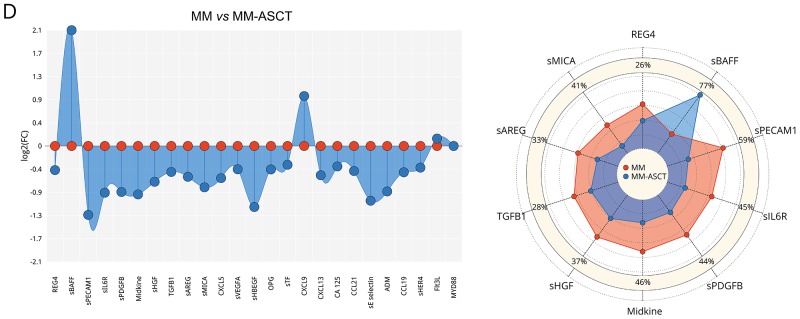
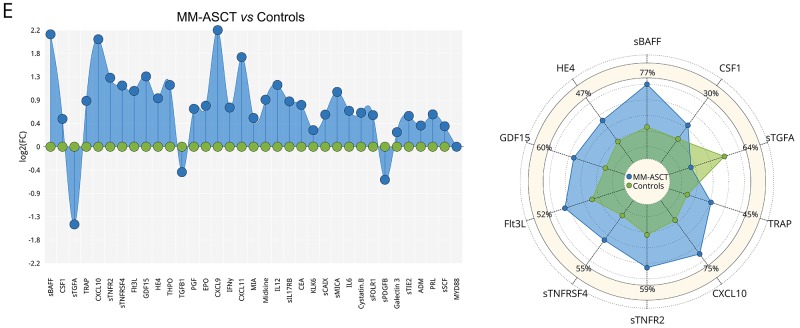
Figure 2. Serum fingerprints and changes in top-deregulated proteins in MGUS, MM, and MM-ASCT.
Fingerprints were presented as FC (fold-change of group medians) of serum levels of deregulated serum proteins between particular groups (P< .05); changes in top-deregulated proteins are presented as percentage of changes between group medians of particular groups: A. MGUS vs MM, B. controls vs MGUS, C. controls vs MM. D. MM vs MM-ASCT and E. controls vs MM-ASCT. MGUS is colored yellow, MM red, MM-ASCT blue, and control subjects green.
Comparison of protein pattern obtained in MGUS and healthy controls revealed deregulation of 33 proteins (Figure 2B), of these 21 reached significance after multiple comparisons (Supplementary Table S2B). The protein levels of top-ranked proteins (midkine, THPO, sTNFRSF4, sHER4, INFγ, TGFB1, sPECAM1, sIL17RB, KLK6, suPAR) are presented in Table 1B and Supplementary Figure S1A.
When comparing MM and controls, we observed deregulation of 46 serum proteins (Figure 2C), of these 41 analytes reached significance after adjustment for multiple comparisons (Supplementary Table S2C). The distribution of serum levels of top-ranked proteins between MM and controls (PGF, GDF15, HE4, sTNFR2, CSF1, midkine, sPECAM1, CCL19, sVEGFA, INFγ; see Table 1C) is shown in Supplementary Figure S1B. The subanalysis based on cytogenetic/FISH analysis was not performed due to the high heterogeneity within the group.
Changes in serum protein pattern in post-transplant MM
To assess the changes in serum protein pattern in MM after ASCT, we compared the post-transplant sera (day 100) with paired samples obtained in MM patients at the time of diagnosis and healthy control subjects. Comparing paired samples from MM-ASCT and MM, the most upregulated protein in post-transplant sera was sBAFF (Pcorr= .006), followed by CXCL9 (Pcorr= .041). Next twenty-one proteins were downregulated (14 proteins after adjustment for multiple comparisons) in MM-ASCT comparing to MM (Figure 2D, Supplementary Table S2D). The top-ranked proteins were: elevated sBAFF and downregulated REG4, sPECAM1, sIL6R, sPDGFB, midkine, sHGF, TGFB1, sAREG, and sMICA in MM-ASCT comparing to MM (Table 1D, Figure 3). Importantly, serum levels of MM-associated pro-tumorigenic factors such as GDF15, CSF1, suPAR, and HE4 did not change after ASCT comparing to sample at the diagnosis (Supplementary Table S2D).
Figure 3. Changes in serum protein levels between paired samples from MM at the diagnosis and after ASCT (day 100).
Pcorr values for differences between two groups of patients after multiple corrections are stated.
To exclude the influence of treatment regime on serum pattern, we assessed the protein profile in subgroups based on ASCT induction regime (IMiD-based/bortezomib-based). We did not detect any differences in the cytokine levels as a function of the induction regime as well as the hematological response (CR, VGPR/PR) on day 100 (Pcorr> .05).
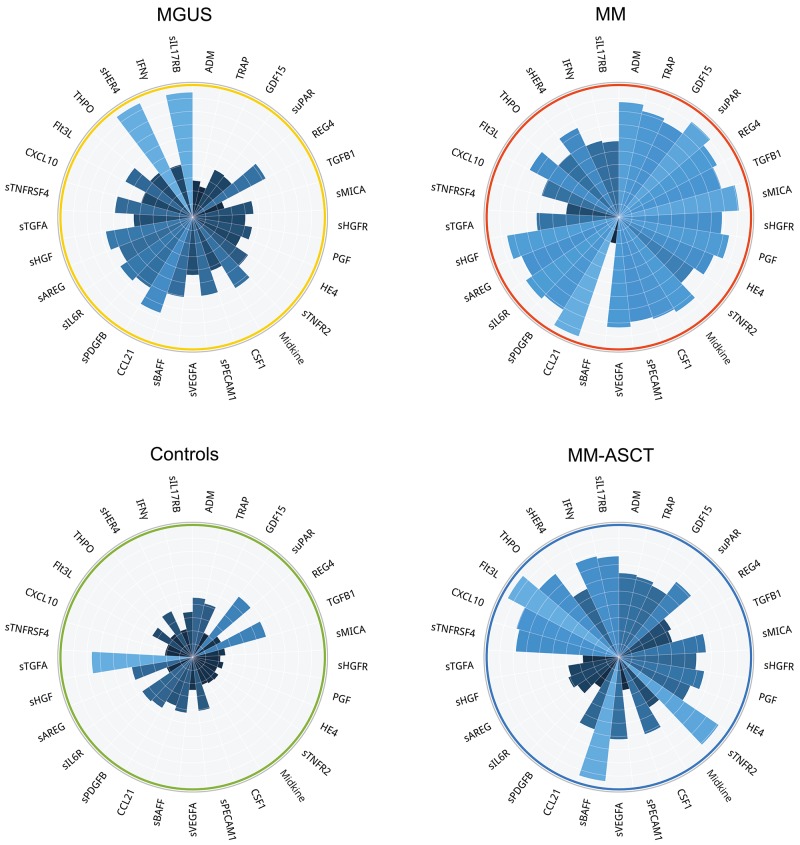
Comparing to healthy subjects, the serum of post-transplant MM patients showed permanently altered pro-tumorigenic signature characteristic by deregulation of 35 proteins (after multiple adjustments: 28 analytes) (Figure 2E, Supplementary Table S2E). Except sTGFA and TGFB1, all deregulated proteins were elevated in MM-ASCT. The top-ranked proteins between MM-ASCT and healthy controls were as follows: sBAFF, CSF1, sTGFA, TRAP, CXCL10, sTNFR2, sTNFRSF4, Flt3L, GDF15, HE4, THPO (Table 1E, Supplementary Figure S1C). The serum protein pattern in MM-ASCT and its comparison to those of healthy controls, MGUS and MM, are presented in Figure 4.
Figure 4. Comparison of protein fingerprints in MGUS, MM, MM-ASCT and healthy subjects for selection of top-deregulated proteins.
Pattern-recognition algorithms
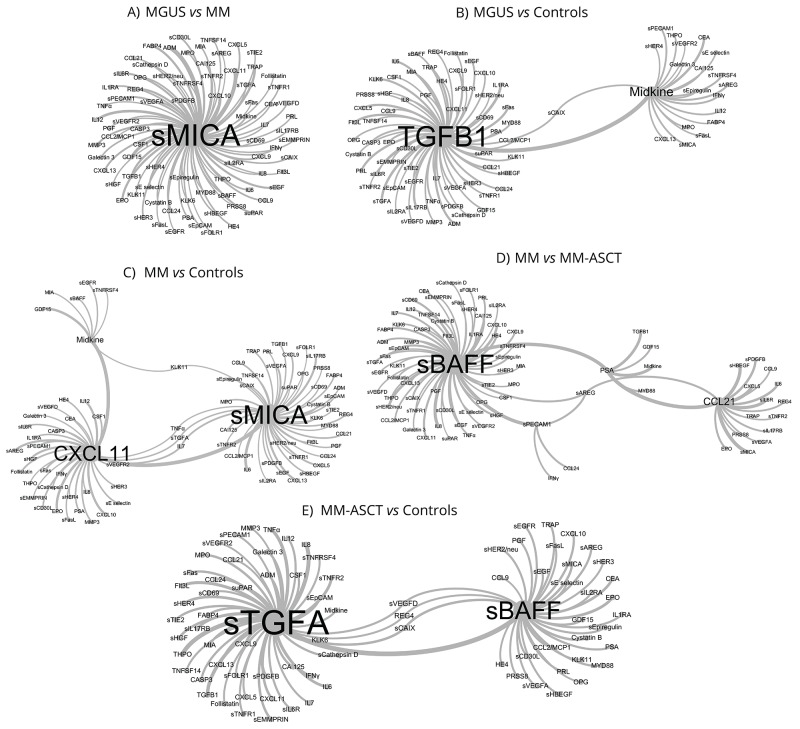
To facilitate the selection of the most promising circulating proteins distinguishing studied groups (MGUS, MM, MM-ASCT, healthy subjects), we applied advanced binary classification algorithm and analyzed co-occurrence of analytes in classification models. The most accurate classification model for separation of MGUS and MM utilized in classification rules most frequently sMICA in combination with other analytes (Figure 5A). In MGUS vs healthy controls, the classification rules used most often TGFB1 and midkine (Figure 5B) and in MM vs healthy controls most often sMICA, CXCL11, and midkine (Figure 5C). The classification model for MM and MM-ASCT used in the classification rules most frequently sBAFF and CCL21 (Figure 5D) and for MM-ASCT and controls used sTGFA and sBAFF (Figure 5E).
Figure 5. Network visualization of classification models obtained by pattern-recognition analysis that identified key serum biomarkers distinguishing between MGUS, MM, and MM-ASCT based on co-occurrence of analytes in classification models.
A. MGUS vs MM, B. controls vs MGUS, C. controls vs MM, D. MM vs MM-ASCT and E. controls vs MM-ASCT. The size of the vertices (font-size) and connections among vertices show those proteins, which were used in classification rules of the particular patient group in the most accurate classification model.
Classification of MGUS, MM, and MM-ASCT
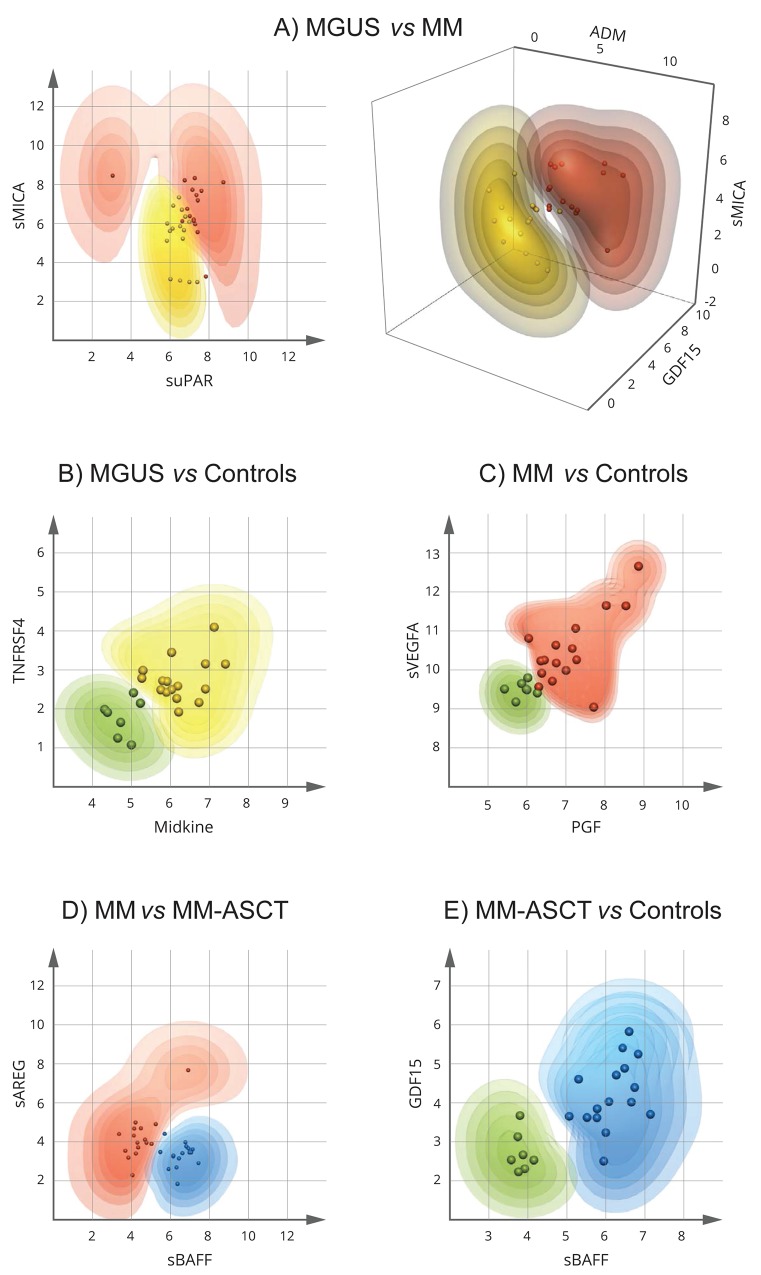
To detect the minimum number and the best combination of serum analytes able to discriminate between MGUS and MM, and MM-ASCT, we applied Multilinear Discriminant Analysis, Naive Bayes classifiers, Random Forests, and extended Support Vector Machine (kSVM). The probability of correct classification to particular patient subgroup (intervals: >90, 90-80, 80-70, 70-60, and 60-50%) was calculated for every combination of two or three analytes from individual patients, and the misclassification error was determined. The best visual separation of studied patient groups was achieved by kSVM and therefore used in further study. The best dual-combination able to discriminate MGUS vs MM was achieved by the combination of sMICA and suPAR, able to separate these groups with a classification error of 0.062 (1 false/16 samples) (Figure 6A). The best triple-combinations for separating MGUS and MM were sMICA-ADM-GDF15 (Figure 6A) as well as the combination of sMICA-ADM-REG4, sMICA-suPAR-REG4, sMICA-suPAR-sHGFR, ADM-suPAR-REG4, TRAP-REG4-sHGFR (data not shown). The triple-combinations increased the probability of correct classification of MGUS and MM; the classification error remained 0.062 (1 false/16). For discrimination of MGUS and MM, MM-ASCT from controls and MM from MM-ASCT, several combinations of only two analytes were sufficient to classify all samples correctly (with no misclassification error). For MGUS vs controls, the combinations were as follows: midkine-sTNFRSF4 (Figure 6B) or midkine-TGFB1, TGFB1-THPO, TGFB1-sHER4, TGFB1-IFNγ, and TGFB1-sIL17RB (data not shown). Best separation of MM and controls was observed for combinations PGF-sVEGFA (Figure 6C) and PGF-midkine. Regarding serum from MM and MM-ASCT, the best analyte pairs able to discriminate these groups were sBAFF-sAREG (Figure 6D) or the combination of sBAFF with sPECAM1 or sHGF (data not shown). For MM-ASCT vs controls, the combination of following analyte pairs resulted in 100% correct separation: sBAFF-GDF15 (Figure 6E) or sBAFF in combination with other analytes such as CSF1, sTGFA, TRAP, sTNFR2, sTNFRSF4 or HE4. Also, the combination of sTGFA with CSF1, sTNFR2 or Flt3L discriminated fully between MM-ASCT and healthy controls (data not shown).
Figure 6. Extended Support Vector Machine (kSVM) analysis for identification of the minimum number and the best combination of proteins distinguishing MGUS, MM, and MM-ASCT.
The dots represent the individual patient data (combinations of two or three analytes). The contour plots show the probabilities (intervals: > 90, 90-80, 80-70, 70-60, and 60-50%) for input data from individual patients to belong to the particular patient group. MGUS is colored yellow, MM red, MM-ASCT blue, and control subjects green. The more saturated color the higher probability of correct classification. A. MGUS vs MM, B. controls vs MGUS, C. controls vs MM D. MM vs MM-ASCT, E. controls vs MM-ASCT.
DISCUSSION
In this study, we investigated the complexity of serum microenvironment in MGUS, MM and MM after ASCT using highly-sensitive PEA immunoassay. We hypothesized that serum of pre-cancer MGUS and MM differ by the presence of pro-tumorigenic factors. Indeed, we detected elevated levels of adrenomedullin (ADM), TRAP, GDF15, TGFB1, suPAR and other pro-tumorigenic proteins in serum of MM patients compared to MGUS. These proteins were already reported in MM but not investigated simultaneously. Pro-angiogenic factor ADM was identified as the most highly upregulated gene in hypoxia-dependent/independent fashion in MM cells, suggesting to be a major driving force for the angiogenic switch during MM evolution [11]. Highly upregulated TRAP is a marker of osteoclasts driving the bone resorption in MM [12]. The crucial role of TGFB1 in MM is supported by the observation that the inhibition of TGF-β signaling by TGF-β type I receptor kinase inhibitor causes a suppression of MM cell growth and an enhancement of bone formation [13]. Regarding GDF15 in MM, high serum levels were associated with poor prognosis [14, 15] and treatment response [16] and osteolysis [17]. GDF15 enhances the tumor-initiating and self-renewal potential of myeloma cells [18], contributes to drug resistance in both stroma-dependent/independent MM cells [14, 15], and promotes osteoclast differentiation while inhibits osteoblast differentiation [17]. Regarding suPAR, high suPAR expression in MM predicts progression, shorter survival and early extramedullary infiltration [19].
In order to detect the most promising circulating protein(s) distinguishing MGUS and MM, we analyzed co-occurrence of analytes in MGUS/MM classification models and identified sMICA as the most useful classifier. High serum levels of sMICA were already detected in MM as an adverse prognostic factor [20, 21], but not elevated in MGUS [20]. sMICA may originate from MICA-expressing MM cells, fibroblasts or other stromal cells upon stimulation [8, 22]. There is evidence that sMICA impairs the function of the NKG2D + T CD8+ and NK cells, contributing to myeloma cell immune escape [20]. Additionally, patients with MGUS, but not MM, generate high-titer anti-MICA antibodies that antagonize the suppressive effects of sMICA [20]. It has been therefore suggested that alterations in the NKG2D pathway by sMICA and anti-MICA antibodies are critically involved in the suppression of innate and adaptive immunity during the progression from MGUS to MM [20]. Importantly, some drugs may reconstitute the capabilities of sMICA-inhibited cytotoxicity of CD8+ and NK cells [23, 24], thus further highlights the potential of NKG2D + T CD8+ and NK cell-mediated immunotherapeutic interventions in MM [24, 25, 26].
Next, we investigated the minimum number and best combination of serum analytes able to discriminate between MGUS and MM. Advanced data mining methods revealed that the combination of sMICA and suPAR separates these groups with a classification error 0.062. The combination of triplets sMICA-ADM-GDF15 or sMICA-suPAR-REG4 increased the probability of correct classification of MGUS and MM with the same classification error (1/16) significantly. Although larger cohort studies are needed to confirm our results, our study nominated sMICA, ADM, GDF15, suPAR, and REG4 as key MM-associated serum proteins able to discriminate MGUS and MM.
Despite new therapies and ASCT increasing remission rates, nearly all MM patients ultimately succumb to disease relapse and progression. Because tumor microenvironment may contribute to these processes, we investigated for the first time serum pattern from paired samples from MM patients from the time of diagnosis and after ASCT (day 100). Interestingly, the post-transplant sera possessed high levels of soluble B-Cell Activating Factor (sBAFF), a survival factor for myeloma cells [9]. Enhanced serum levels of sBAFF, found produced by MM cells, immune and stromal cells [9, 27], correlated inversely with overall survival in MM and resistance to dexamethasone and lenalidomide [27, 28]. Since the elimination of sBAFF in an MM mouse model resulted in a decrease of tumor burden and protected against lytic bone disease [29], the sBAFF signaling represents a promising therapeutic target in MM [27], especially in the setting of post-transplant sBAFF elevation. After ASCT, the MM-associated proteins sMICA and ADM were downregulated but still elevated compared to healthy controls. On the other hand, serum levels of other pro-tumorigenic factors such as GDF15, CSF1, suPAR, and others did not change after ASCT comparing to paired MM sample at the diagnosis. Similar observation was reported in treated MM patients showing that cytokine pattern in those achieving remission is not restored to physiological levels [30], thus suggesting that once an individual has MM, the microenvironment is permanently altered and primed for a relapse. These results highlight the role of microenvironment for treatment success and may explain why MM remains an incurable disease.
We were also interested in MGUS associated serum pattern comparing to healthy controls. Our analysis revealed for the first time that MGUS is characteristic by low levels of TGFB1 and high levels of midkine, a heparin-binding growth factor involved in angiogenic and anti-apoptotic functions and tumor expansion in various cancers [31, 32]. Enhanced gene expression of midkine and other angiogenic factors were already reported in MM [33, 34] and also in this study we detected higher serum levels of midkine in MM vs MGUS. Importantly, lower gene expression of midkine and other angiogenic genes was detected in IMiD-responders compared to non-responders [34]. Elevation of midkine, produced by normal and malignant B-cells, tumor and stromal cells [35, 36], was also reported in other B-cell malignancies such as chronic lymphocytic leukemia and lymphomas [35]. Regarding TGFB1, low levels of TGFB1 were shown to control MM cell growth [13]. Our observations highlight the role of TGFB1 and midkine in the progression of MGUS to MM thus deserving further investigation.
We are aware that this study has several limitations. Because this study was focused on determination of serum protein fingerprinting, we did not analyze plasma bone marrow and did not investigate the functional effect of deregulated proteins. This should be performed in future studies.
In conclusion, we identified serum protein fingerprints associated with MGUS and MM as well changes ongoing in MM after ASCT. The knowledge of serum pattern may contribute to the identification of key myeloma cell survival factors, which may in turn influence treatment response and disease development.
MATERIALS AND METHODS
Study population and materials
The study cohort includes patients with MGUS (n=16) and MM (n=16); all patients were diagnosed according to the criteria of International Myeloma Working Group [37, 38]. Serum samples were taken at the time of diagnosis from previously untreated patients, aliquoted and stored at -80°C until analysis. In all enrolled MM patients, paired serum sample collected at day 100 after ASCT (MM-ASCT) was also analyzed. Patient characteristics are described in Table 2. Serum samples from healthy control subjects (n=7, mean age 51 yrs; range 45-72 yrs, 4 males/3 females) were taken from members of medical staff; all completed a questionnaire regarding their health status. All patients provided written informed consent about the usage of peripheral blood for the purpose of this study. The study was approved by the ethics committee of University Hospital and Palacky University Olomouc.
Table 2. Patient characteristics.
| Parameter | MGUS (n=16) | MM (n=16) |
|---|---|---|
| Age, years, median (min-max) | 59 (46-83) | 57 (39-64) |
| Gender, n, male/female | 8/8 | 5/11 |
| Paraprotein type, n (%) | ||
| IgG | 11 (69) | 7 (44) |
| IgA | 4 (25) | 4 (25) |
| IgD | 1 (6) | 0 (0) |
| Light chain only | 0 (0) | 5 (31) |
| Bone lesions, n, yes/no | 0/16 | 12/4 |
| ASCT Induction regime, n (IMiD-based/bortezomib-based)* | NA | 16 (8/8) |
| Time difference from diagnosis to day 100 after ASCT, days, mean (min-max) | NA | 314 (231-567) |
| Response on day 100 after ASCT, CR/VGPR/PR, n (%) | NA | 11/4/1 (69/25/6) |
*1 patient received tandem ASCT
NA not applicable, CR complete remission, VGPR very good partial remission, PR partial remission
Proximity extension immunoassay
Serum profiles of ninety-two cancer-related proteins were assessed by the Proseek Multiplex Oncology I kit (Olink Bioscience, Uppsala, Sweden) according to the manufacturer’s recommendation. Briefly, serum samples (1 µl) were incubated in the presence of 92 proximity antibody pairs tagged with DNA reporter molecules. Once the pair of antibodies bound to their corresponding antigens, the respective DNA tails formed by proximity extension an amplicon that was quantified by high-throughput real-time PCR (BioMark™ HD System, Fluidigm Corporation). The generated fluorescent signal directly correlates with protein abundance. Olink Wizard (Olink) was used for data normalization: the raw Cq-value (log2 scale) for each data point was normalized by subtracting the Cq-value for the extension control and compared to that of the corresponding background reaction resulting in a ddCq-value [39]. For further analysis, linearized values (2ddCq) were used. For panel description see Supplementary Table S1, for sensitivity and specificity parameters of PEA analysis see Assarsson et al. [39].
Pattern-recognition algorithms
Binary classification by a stochastic nature-inspired symbolic regression method and evolutionary fuzzy-rules [40] was conducted in order to learn symbolic models for particular patient groups (MGUS, MM, MM-ASCT, healthy subjects) based on their serum protein pattern. For each tested patient group pair, the procedure was repeated more than 500 times to accommodate the stochastic nature of the algorithm and to obtain representative results. The most accurate classification models, separating all patients in the correct patient groups, were utilized for the identification of key molecules and those co-occurring in the classification rules characteristic for the particular patient group.
The proteins from the classification rules were further used to form a network model of patient classification with molecules as vertices and co-occurrences in successful classification models as edges. An algorithm based on analysis of the nearest neighbors between the studied molecules was applied to determine vertex and edge weights in the network model [41]. The size of the vertices (font-size) and connections among vertices show those proteins, which were used in classification rules of the particular patient group in the most accurate classification model.
Classification methods for separation of patient groups
To assess the minimum number and best combination of serum analytes distinguishing between the patient groups (MGUS vs MM, MM vs MM-ASCT), we applied several classification methods from the area of information retrieval such as Multilinear Discriminant Analysis, Naive Bayes classifiers, Random Forests, and kSVM to calculate the class probabilities for every input data (combinations of two or three analytes) and to determine the misclassification error [42, 43]. All methods were calculated using R statistical software with package Caret (http://topepo.github.io/caret/index.html).
Statistical analysis
Statistical analyses (Mann-Whitney-Wilcoxon and paired Wilcoxon tests) were performed using R statistical software package (http://www.r-project.org/). All data analyses were performed on linearized expression data (2ddCq) for each protein. A combination of box plots and swarm plots (a one-dimensional hybrid between scatter plot and strip chart) was employed to visualize the distribution of signals across subjects in particular subgroups. Radar charts were created for each pair of compared subgroups to visually assess the quantitative changes in levels of the most significant molecules, determined for each group pair by the Mann-Whitney-Wilcoxon test, and for paired samples (MM and MM-ASCT) by paired Wilcoxon test. P-value for each protein was adjusted for multiple comparisons using the False Discovery Rate (FDR) by the Benjamini-Hochberg procedure. A P-value < .05 was considered as significant.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Abbreviations
- ADM
Adrenomedullin
- ASCT
Autologous stem cell transplantation
- CA125
Cancer antigen 125
- CA242
CA 242 tumor marker
- CCL19
Chemokine (C-C motif) ligand 19
- CCL21
Chemokine (C-C motif) ligand 21
- CEA
Carcinoembryonic antigen
- CR
Complete remission
- CSF1
Colony stimulating factor 1
- CXCL5
C-X-C motif chemokine 5
- CXCL9
Chemokine (C-X-C motif) ligand 9
- CXCL10
C-X-C motif chemokine 10
- CXCL11
C-X-C motif chemokine 11
- CXCL13
Chemokine (C-X-C motif) ligand 13
- EPO
Erythopoietin
- FABP4
Fatty acid binding protein 4
- FDR
False Discovery Rate
- Flt3L
FMS-like tyrosine kinase 3 ligand
- GDF15
Growth differentiation factor 15
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HE4
Human Epididymis Protein 4
- Ig
Immunoglobulin
- IL1RA
Interleukin-1 receptor antagonist
- IL2
Interleukin 2
- IL4
Interleukin 4
- IL12
Interleukin 12
- IMiD
Immunomodulatory drug
- KLK6
Kallikrein-6
- kSVM
Extended Support Vector Machine
- MIA
Melanoma-derived growth regulatory protein
- MGUS
Monoclonal gammopathy of undetermined significance
- MM
Multiple myeloma
- MYD88
Myeloid differentiation primary response 88
- OPG
Osteoprotegerin
- PEA
Proximity extension immunoassay
- PGF
Placental growth factor
- PR
Partial remission
- PRL
Prolactin
- REG4
Regenerating islet-derived protein 4
- SMM
Smoldering multiple myeloma
- TGFB1
Transforming growth factor beta 1
- THPO
Thrombopoietin
- TRAP
Tartrate-resistant acid phosphatase
- sAREG
soluble Amphiregulin
- sBAFF
soluble B-cell activating factor
- sBTC
soluble Betacellulin
- sCAIX
soluble Carbonic anhydrase 9
- sCD30L
soluble CD30 ligand
- sE selectin
soluble E-selectin
- sEGFR
soluble Epidermal growth factor receptor
- sEMMPRIN
soluble Extracellular matrix metalloproteinase inducer
- sEpCAM
soluble Epithelial cell adhesion molecule
- sEpiregulin
soluble Epiregulin
- sER
soluble Estrogen receptor
- sFasL
soluble Fas ligand
- sFOLR1
soluble Folate receptor alpha
- sHBEGF
soluble Heparin-binding EGF-like growth factor
- sHER3
soluble Receptor tyrosine-protein kinase erbB-3
- sHER4
soluble Receptor tyrosine-protein kinase erbB-4
- sHGF
soluble Hepatocyte growth factor/scatter factor
- sHGFR
soluble Hepatocyte growth factor receptor
- sIL2RA
soluble Interleukin-2 receptor alpha chain
- sIL17RB
soluble Interleukin-17 receptor B
- sMICA
soluble MHC class I polypeptide-related sequence A
- sPDGFB
soluble Platelet-derived growth factor subunit B
- sPECAM1
soluble Platelet endothelial cell adhesion molecule
- sSCF
soluble Stem cell factor
- sTF
soluble Tissue Factor
- sTGFA
soluble Transforming growth factor alpha
- sTIE2
soluble Receptor tyrosine kinase Tie2
- sTNFR1
soluble Tumor necrosis factor receptor 1
- sTNFR2
soluble Tumor necrosis factor receptor 2
- sTNFRSF4
soluble Tumor necrosis factor receptor superfamily member 4
- suPAR
soluble Urokinase plasminogen activator receptore
- sVEGFA
soluble Vascular endothelial growth factor A
- VGPR
Very good partial remission
Footnotes
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
GRANT SUPPORT
This work was supported by grant of Ministry of Health of Czech Republic (MZ ČR VES16-32339A), and in part by the Student Grant System, VŠB – Technical University of Ostrava (SP2016/68).
Authorship
P.S., T.P., and R.F. performed experiments; P.G., M.K., and P.K. analyzed the results and made the figures; T.P., V.S., J.M. collected patient material and provided clinical data; E.K., and T.Pa. designed the research; P.S., T.P., and E.K. wrote the manuscript; and T.Pa. suggested improvements to the manuscript.
REFERENCES
- 1.Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S, Munshi N, Palumbo A, Miguel JS, Sonneveld P, Cavo M, Usmani S, Durie BG, et al. International Myeloma Working Group. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28:269–277. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi G, Richardson PG, Anderson KC. Promising therapies in multiple myeloma. Blood. 2015;126:300–310. doi: 10.1182/blood-2015-03-575365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San-Miguel JF, Mateos MV. Can multiple myeloma become a curable disease? Haematologica. 2011;96:1246–1248. doi: 10.3324/haematol.2011.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood. 2011;117:5573–5581. doi: 10.1182/blood-2011-01-270140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Corral L, Sarasquete ME, Beà S, García-Sanz R, Mateos MV, Corchete LA, Sayagués JM, García EM, Bladé J, Oriol A, Hernández-García MT, Giraldo P, Hernández J, et al. SNP-based mapping arrays reveal high genomic complexity in monoclonal gammopathies, from MGUS to myeloma status. Leukemia. 2012;26:2521–2529. doi: 10.1038/leu.2012.128. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125:3049–3058. doi: 10.1182/blood-2014-11-568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakumaran A, Robey PG, Fedarko N, Landgren O. Bone marrow microenvironment in myelomagenesis: Its potential role in early diagnosis. Expert Rev Mol Diagn. 2010;10:465–480. doi: 10.1586/erm.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girlanda S, Fortis C, Belloni D, Ferrero E, Ticozzi P, Sciorati C, Tresoldi M, Vicari A, Spies T, Groh V, Caligaris-Cappio F, Ferrarini M. MICA expressed by multiple myeloma and monoclonal gammopathy of undetermined significance plasma cells Costimulates pamidronate-activated gammadelta lymphocytes. Cancer Res. 2005;65:7502–7508. doi: 10.1158/0008-5472.CAN-05-0731. [DOI] [PubMed] [Google Scholar]
- 9.Moreaux J, Legouffe E, Jourdan E, Quittet P, Rème T, Lugagne C, Moine P, Rossi JF, Klein B, Tarte K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol. 2012;2012:157496. doi: 10.1155/2012/157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocemba KA, van Andel H, de Haan-Kramer A, Mahtouk K, Versteeg R, Kersten MJ, Spaargaren M, Pals ST. The hypoxia target adrenomedullin is aberrantly expressed in multiple myeloma and promotes angiogenesis. Leukemia. 2013;27:1729–1737. doi: 10.1038/leu.2013.76. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca R, Trendle MC, Leong T, Kyle RA, Oken MM, Kay NE, Van Ness B, Greipp PR. Prognostic value of serum markers of bone metabolism in untreated multiple myeloma patients. Br J Haematol. 2000;109:24–29. doi: 10.1046/j.1365-2141.2000.01960.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto T, Abe M. TGF-β-related mechanisms of bone destruction in multiple myeloma. Bone. 2011;48:129–134. doi: 10.1016/j.bone.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 14.Tanno T, Lim Y, Wang Q, Chesi M, Bergsagel PL, Matthews G, Johnstone RW, Ghosh N, Borrello I, Huff CA, Matsui W. Growth differentiating factor 15 enhances the tumor-initiating and self-renewal potential of multiple myeloma cells. Blood. 2014;123:725–733. doi: 10.1182/blood-2013-08-524025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corre J, Labat E, Espagnolle N, Hébraud B, Avet-Loiseau H, Roussel M, Huynh A, Gadelorge M, Cordelier P, Klein B, Moreau P, Facon T, Fournié JJ, et al. Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma. Cancer Res. 2012;72:1395–1406. doi: 10.1158/0008-5472.CAN-11-0188. [DOI] [PubMed] [Google Scholar]
- 16.Tarkun P, Birtas Atesoglu E, Mehtap O, Musul MM, Hacihanefioglu A. Serum growth differentiation factor 15 levels in newly diagnosed multiple myeloma patients. Acta Haematol. 2014;131:173–178. doi: 10.1159/000354835. [DOI] [PubMed] [Google Scholar]
- 17.Westhrin M, Moen SH, Holien T, Mylin AK, Heickendorff L, Olsen OE, Sundan A, Turesson I, Gimsing P, Waage A, Standal T. Growth differentiation factor 15 (GDF15) promotes osteoclast differentiation and inhibits osteoblast differentiation and high serum GDF15 levels are associated with multiple myeloma bone disease. Haematologica. 2015;100:e511–514. doi: 10.3324/haematol.2015.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, Danho C, Laharrague P, Klein B, Rème T, Bourin P. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen J, Wang Q, Wang J, Su GH, Wang J, Guo SH, Liu YA, Wu Z, Liu RF, Li X, Guo XJ, Cao J, Zhang YH, Wang ZY. Analysis of soluble urokinase plasminogen activator receptor in multiple myeloma for predicting prognosis. Oncol Lett. 2015;10:2403–2409. doi: 10.3892/ol.2015.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A. 2008;105:1285–1290. doi: 10.1073/pnas.0711293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebmann V, Schütt P, Brandhorst D, Opalka B, Moritz T, Nowrousian MR, Grosse-Wilde H. Soluble MICA as an independent prognostic factor for the overall survival and progression-free survival of multiple myeloma patients. Clin Immunol. 2007;123:114–120. doi: 10.1016/j.clim.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Choy MK, Phipps ME. MICA polymorphism: biology and importance in immunity and disease. Trends Mol Med. 2010;16:97–106. doi: 10.1016/j.molmed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Klöß S, Chambron N, Gardlowski T, Arseniev L, Koch J, Esser R, Glienke W, Seitz O, Köhl U. Increased sMICA and TGFβ1 levels in HNSCC patients impair NKG2D-dependent functionality of activated NK cells. Oncoimmunology. 2015;4:e1055993. doi: 10.1080/2162402X.2015.1055993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fionda C, Soriani A, Zingoni A, Santoni A, Cippitelli M. NKG2D and DNAM-1 Ligands: Molecular Targets for NK Cell-Mediated Immunotherapeutic Intervention in Multiple Myeloma. Biomed Res Int. 2015;2015:178698. doi: 10.1155/2015/178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther. 2011;18:509–516. doi: 10.1038/gt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dosani T, Carlsten M, Maric I, Landgren O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of myeloma and their uses in immunotherapies. Blood Cancer J. 2015;5:e306. doi: 10.1038/bcj.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hengeveld PJ, Kersten MJ. B-cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J. 2015;5:e282. doi: 10.1038/bcj.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu G, Shen XJ, Pu J, Chu SP, Wang XD, Wu XH, Sun CJ, Zhang X, Zhu BL, Ju SQ. BLyS expression and JNK activation may form a feedback loop to promote survival and proliferation of multiple myeloma cells. Cytokine. 2012;60:505–513. doi: 10.1016/j.cyto.2012.06.317. [DOI] [PubMed] [Google Scholar]
- 29.Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, Tai Y, Chauhan D, Tassone P, Venuta S, Munshi NC, Hideshima T, Anderson KC, Raje N. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007;13:5903–5909. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- 30.Zheng MM, Zhang Z, Bemis K, Belch AR, Pilarski LM, Shively JE, Kirshner J. The systemic cytokine environment is permanently altered in multiple myeloma. PLoS One. 2013;8:e58504. doi: 10.1371/journal.pone.0058504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones DR. Measuring midkine: the utility of midkine as a biomarker in cancer and other diseases. Br J Pharmacol. 2014;171:2925–2939. doi: 10.1111/bph.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang HL, Shen JF, Min LS, Ping JL, Lu YL, Dai LC. Inhibitory effect of midkine-binding peptide on tumor proliferation and migration. Int J Clin Exp Pathol. 2015;8:5387–5394. [PMC free article] [PubMed] [Google Scholar]
- 33.Gao SJ, Li GL. Expression of midkine and vascular endothelial growth factor in bone marrow of patients with multiple myeloma and its significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:1464–1467. [PubMed] [Google Scholar]
- 34.Medinger M, Halter J, Heim D, Buser A, Gerull S, Lengerke C, Passweg J. Gene-expression Profiling in Patients with Plasma Cell Myeloma Treated with Novel Agents. Cancer Genomics Proteomics. 2016;13:275–279. [PubMed] [Google Scholar]
- 35.Cohen S, Shachar I. Midkine as a regulator of B cell survival in health and disease. Br J Pharmacol. 2014;171:888–895. doi: 10.1111/bph.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, Zhu S, Wu M, Han W, Yu Y. Functional receptors and intracellular signal pathways of midkine (MK) and pleiotrophin (PTN) Biol Pharm Bull. 2014;37:511–520. doi: 10.1248/bpb.b13-00845. [DOI] [PubMed] [Google Scholar]
- 37.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 38.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 39.Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krömer P, Owais S, Platoš J, Snášel V. Towards new directions of data mining by evolutionary fuzzy rules and symbolic regression. Comput Math Appl. 2013;66:190–200. [Google Scholar]
- 41.Zehnalova S, Kudelka M, Platos J, Horak Z. Local representatives in weighted networks. IEEE/ACM International Conference on Advances in Social Networks Analysis and Mining (ASONAM) 2014, pp. 870-875. doi: 10.1109/ASONAM.2014.6921688.
- 42.Kuhn M, Johnson K. Applied Predictive Modeling. New York: Springer-Verlag; 2013. [Google Scholar]
- 43.Forte RM. Mastering Predictive Analytics with R. Birmingham. UK: Packt Publishing; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.