Abstract
Background
The cohesin loading factor, nipped-B-like protein (NIPBL), is also known as the sister chromatid cohesion 2 (SCC2) human homolog. Recently, we have studied the role of expression levels of NIPBL in cell proliferation and chemotherapy resistance of non-small cell lung cancer (NSCLC) cells in vitro. The aim of this study was to investigate the effects of expression of the cohesin loading factor, NIPBL, on the cell cycle, apoptosis, and autophagy of breast cancer cell lines in vitro.
Material/Methods
Expression levels of the NIPBL in the breast cancer cell lines, MCF7, Bcap37, MDA-MB 453 and MDA-MB 231, were measured using Western blot and flow cytometry. Small interfering RNA (si-RNA) was used to study the biological functions of NIPBL. The cell counting kit-8 (CCK-8) assay and the colony formation assay were used to measure cell proliferation; the wound scratching assay and transwell chamber assay were used to investigate cell invasion and migration.
Results
NIPBL gene and protein expression were upregulated in the MCF7 and Bcap37 cells; si-NIPBL transfection inhibited cell proliferation, invasion, and migration of breast cancer cells. Downregulation of NIPBL arrested cells in the G0/G1 phase of the cell cycle and induced apoptosis and autophagy of breast cancer cells through the caspase3 and mammalian target of rapamycin (mTOR) signaling pathways.
Conclusions
Downregulation of cohesin loading factor NIPBL arrested breast cancer cells in vitro in the G0/G1 phase of the cell cycle and induced apoptosis and autophagy.
MeSH Keywords: Apoptosis, Autophagy, Breast Neoplasms, Cell Cycle
Background
Breast cancer is a highly malignant tumor that results in morbidity in women worldwide [1]. Although improvements in the diagnosis and treatment of breast cancer have been made, breast cancer remains a major cause of cancer-related death among women worldwide [2]. There is still a lack of understanding of the molecular mechanisms leading to breast cancer, which contributes to the high incidence of this disease, but genetic, lifestyle and environmental factors are well-established risk factors for breast cancer [3]. There are now several genes that have been reported to be associated with an increased risk of developing breast cancer, including CHEK2, ATM, BRIP1, TP53, CDH1, PTEN, PALB2 and STK11 [4]. Chemotherapy, endocrine therapy, and targeted therapy are the three major forms of treatment for breast cancer. However, drug resistance has greatly limited the effect of therapy and has influenced the prognosis of patients. Unfortunately, the mechanism of drug resistance in breast cancer remains to be elucidated. The discovery of new genes that may predict the susceptibility to develop malignancy, or that serve as therapeutic targets, or that improve drug sensitivity are important areas of cancer research.
Cohesin is a multiprotein complex that mediates the combination of sister chromatid cohesion and is ubiquitously expressed in humans [5]. The components of the cohesin multi-subunit protein complex consist of the core subunits SMC1A, SMC3, STAG1/2, and RAD21, and the regulatory subunits NIPBL, PDS5A/B, WAPL, CDCA5, and MAU2 [5]. The cohesin loading factor, nipped-B-like protein (NIPBL), is also known as the sister chromatid cohesion 2 (SCC2) human homolog and forms an essential complex with the sister chromatid cohesion 4 (SCC4) that is involved in chromosomal cohesion [6,7].
NIPBL has three main roles: in cohesion in chromosomes; in the regulation of gene expression; and in assisting in the repair of breaks in the DNA double-strand [8–10]. Studies have shown that the related protein or components of the cohesion complex constantly change at the expression and mutation levels in human cancers [11]. Somatic mutations may result in abnormalities in cohesion, and these abnormalities may have a role in the causes of chromosome instability in colorectal tumors [12]. In NIPBL mutants, cohesion complexes form normally but fail to bind with chromosomes [6]. NIPBL frameshift mutations may affect the functions of cohesion complexes in the cell cycle and may lead to tumorigenesis in cancers with high microsatellite instability [13]. Min et al. have suggested that NIPBL is inactivated in gastric and colorectal cancers with high microsatellite instability [13]. Also, recent content in the Cancer Genome Atlas (TCGA) has shown that in 10% of NIPBL mutations are found in endometrial carcinoma [5]. Recently, we have studied the role of expression levels of NIPBL in cell proliferation and chemotherapy resistance of non-small cell lung cancer (NSCLC) cells in vitro and showed that downregulation of NIPBL inhibited the proliferation of NSCLC cells and increased their susceptibility to the effects of cytotoxic agents [14].
The aim of this study was to investigate the effects of expression of the cohesin loading factor, NIPBL, on the cell cycle, apoptosis, and autophagy of breast cancer cell lines in vitro.
Material and Methods
Cell culture
The human breast cancer cell lines MCF7, MDA-MB-231 and MDA-MB-453 cells were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA), and the BACP37 cell was obtained from the Cell Bank at the Chinese Academy of Sciences (Shanghai, China). MCF7, MDA-MB-231, and BACP37 cells were maintained in RPMI 1640 medium (Hangzhou Bio-Pharmaceutical Technology Co., Ltd., China) containing 10% fetal calf serum (FCS) (Sijiqing Biological Engineering Materials Co., Ltd., China) and 100 units/ml penicillin and streptomycin in an atmosphere of 5% CO2 at 37°C. MDA-MB-453 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Hangzhou Bio-Pharmaceutical Technology Co., Ltd, China) containing 10% FCS.
Transfection
NIPBL small interfering RNAs (si-RNAs) were purchased from GenePharma (Shanghai, China). The sequences of the si-NIPBL1 sense strand, si-NIPBL2 and si-NC were: 5′-GCUCGGAACAAAGCAAUUA-3′, 5′-GCGGCAAUGU AUGAUAUAATT-3′ and 5′-GGUUGCCGACUCGUUAAUATT-3′, respectively.
Cells were plated in six-well plates at 2×105/well and cultured overnight to 50–60% confluence. The cells were subsequently transfected with si-NC, si-NIPBL1 and si-NIPBL2 by using Lipofectamine 3000, according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). The silencing effect was examined using Western blot analysis after transfection for 48 h. Cells were harvested after 48 h of transfection for the subsequent experiment.
Cell viability assay
Cell proliferation was assessed using the cell counting kit-8 (CCK-8) assay (Invitrogen, Carlsbad, CA, USA). Approximately 2,000 cells were seeded in each well of 96-well plates, and 10 μL of CCK-8 medium was added to 90 μL of culture medium. After incubation at 37°C for 2 h, the absorbance was measured at 450 nm, and the OD450 value indicated the number of live cells. Cell proliferation in vitro was determined after five days.
Colony formation assay
Cells were trypsinized after transfection for 48 h and then counted and seeded into six-well plates at a concentration of 1,500 cells/well. All samples were incubated for ten days, allowing them to form natural colonies in 2 ml of complete medium. The plates were subsequently washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, and stained with crystal violet (Beyotime, Shanghai, China) for 15 min. Colonies containing more than 50 cells were counted.
Wound healing assay
Uniform wounds were made in the cell culture system by using Ibidi Culture-Insert (Ibidi, GmbH, Munich, Germany, No. 80209). The culture-inserts were placed in the individual wells of a six-well plate. In each reservoir, 5–7×105 cells were cultured in medium with a final volume of 70 μl. The culture-inserts were removed after the cells were sufficiently attached (24 h). The cells were subsequently washed with PBS and then incubated in 2 ml of serum-free medium. The wound areas were monitored under a microscope.
Transwell assay
Diluted matrigel (30 μl) was placed into the bottom of each transwell and then incubated for 1 h at 37°C. The cells (105) were subsequently plated in the upper chamber of the transwell (Corning, USA) and cultured in serum-free medium; 500 μl of medium containing 10% FBS was added to the lower well. After 48 h, the cells were fixed with 4% paraformaldehyde and stained with crystal violet (Beyotime, Shanghai, China). A microscope was used to image and count the attached cells.
Flow cytometric analysis of cell cycle and apoptosis
Cells were harvested after treatment with siRNAs for 48 h and then washed three times with PBS. The cells were detected using a Cell Cycle Staining Kit (MultiSciences, Lianke Biotechnology Co., Ltd., China) for cell cycle analysis, according to the manufacturer’s protocol.
For the assessment of cell apoptosis, both adherent and re-suspended cultured cells were harvested by centrifugation at 2,500 rpm for 5 min. Apoptosis was detected using a fluorescein isothiocyanate (FITC) Annexin-V Apoptosis Detection Kit (Becton Dickinson and Co., USA) according to the manufacturer’s protocol. Apoptosis and cell cycle were both analyzed using a Beckman Coulter Flow Cytometer.
Western blot analysis
Cells transfected with siRNAs were harvested via scraping, rinsed three times in PBS, and lysed in mammalian protein extraction buffer containing a cocktail of protease inhibitors. The lysates were transferred into Eppendorf tubes and clarified by centrifugation at 12,000 rpm for 30 min at 4°C. Protein concentration was measured using a bicinchoninic acid (BCA) Protein Assay Kit (ComWin Biotech Co., Ltd., China). Total protein (30 μg) was electrophoresed on an 8% or 12% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) gel and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The membranes were blocked with Tris-buffered saline (TBS)-T (TBS plus 0.1% Tween 20) containing 5% dried skimmed milk powder at room temperature for 2 h, and then immunoblotted with primary antibodies overnight at 4°C. The primary antibodies, included anti-NIPBL antibody (1: 1000, Bethyl Laboratories, Inc., Montgomery, TX, USA), anti-caspase3 antibody, anti-caspase9 antibody, anti-BAX antibody, anti-CDK4 antibody, anti-cyclin D1 antibody (ProteinTech, Europe), anti-LC3 antibody, anti-P62 antibody, anti-poly (ADP-ribose) polymerase (PARP) antibody, anti-mammalian target of rapamycin (mTOR) antibody, anti-p-mTOR antibody, anti-p-4EBP1 antibody (Cell Signaling Technology, USA). Anti-GAPDH antibody (ProteinTech, Europe) was used as a loading control, and signals were detected using a chemiluminescence enzyme immunoassay (CLEIA), according to the manufacturer’s protocol (Biological Industries, Israel).
Statistical analysis
Statistical analysis of data was conducted using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviations (SD), and graphical representations were prepared using GraphPad Prism 5.0. Student’s t-test was used to analyze the difference between groups. P<0.05 indicated statistical significance.
Results
NIPBL was expressed in breast cancer cell lines
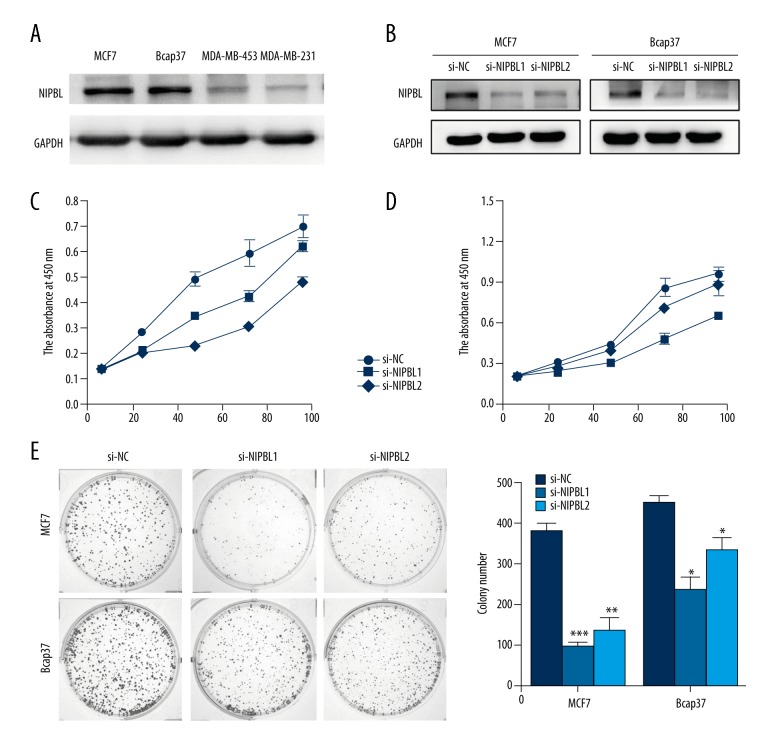
Variations in NIPBL protein expression levels in the breast cancer cell lines MCF7, Bcap37, MDA-MB 453 and MDA-MB 231 were quantitated by Western blot analysis. NIPBL protein was expressed in these four breast cancer lines but was upregulated in MCF7 and Bcap37 cell lines (Figure 1A). Si-NIPBL transfection reduced NIPBL protein expression levels in MCF7 and Bcap37 cells, compared with the expression level in controls, as shown by Western blot analysis (Figure 1B).
Figure 1.
Downregulation of NIPBL expression inhibited the proliferation of breast cancer cells in vitro. (A) Western blot analysis of NIPBL protein level in four human breast cancer cell lines. (B) NIPBL expression in MCF7 and Bcap37 cells infected with si-NIPBL or with control small interfering RNAs (si-RNAs) using Western blot analysis. (C, D) Cell proliferation measured using the cell counting kit-8 (CCK-8) assay. Downregulation of NIPBL expression suppressed breast cancer cell proliferation in vitro. (E) Reduced colony-forming efficiency of si-NIPBL-treated Bcap37 and MCF-7 cells (* P<0.05; ** P<0.01; *** P<0.001). Data are presented as mean ±SD of experiments performed in triplicate.
Downregulation of NIPBL gene expression significantly induced MCF-7 and Bcap37 cell proliferation in vitro
Two efficient siRNAs (si-NIPBL 1 and si-NIPBL 2) were used to downregulate NIPBL expression levels in MCF-7 and Bcap37 cells. The growth of cancer cells was assessed using the cell counting kit-8 (CCK-8) assay and colony formation assay. Compared with the proliferation of control cells, NIPBL was significantly downregulated in MCF-7 and Bcap-37 cells (Figure 1C, 1D). Furthermore, NIPBL-silenced cells showed a significantly reduced ability to form colonies compared with control cells at day 10 (Figure 1E).
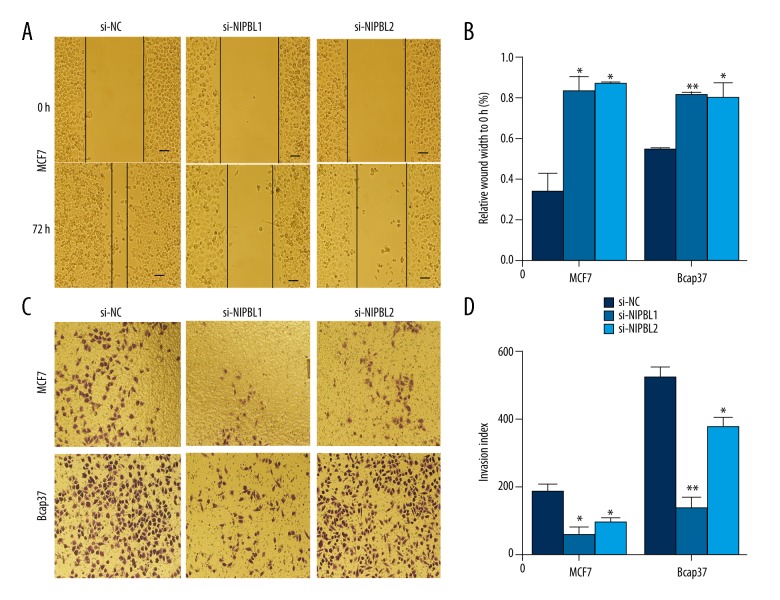
Downregulation of NIPBL gene expression inhibited invasion and migration of breast cancer cells
The wound healing assay showed that downregulation of NIPBL dramatically inhibited cell migration across the wound edge into the scratch area compared with the control group at the same time point (Figure 2A, 2B). The transwell assay showed that reduced NIPBL expression significantly reduced the invasive ability of MCF7 and Bcap37 cells, compared with the control cells (Figure 2C, 2D).
Figure 2.
Cell motility was inhibited by si-NIPBL in scratch assays and invasion assays of breast cancer cells in vitro. The scratch assay shows delayed wound healing. (A, B) Wound distance percentage of MCF-7 for 0 and 72 h after si-NIPBL treatment. (C, D) Invasion assay of MCF7 and Bcap37 cells after 24 h of transfection with si-NIPBL; (* P<0.05: ** P<0.01; *** P<0.001). Data are presented as mean ±SD of experiments performed in triplicate.
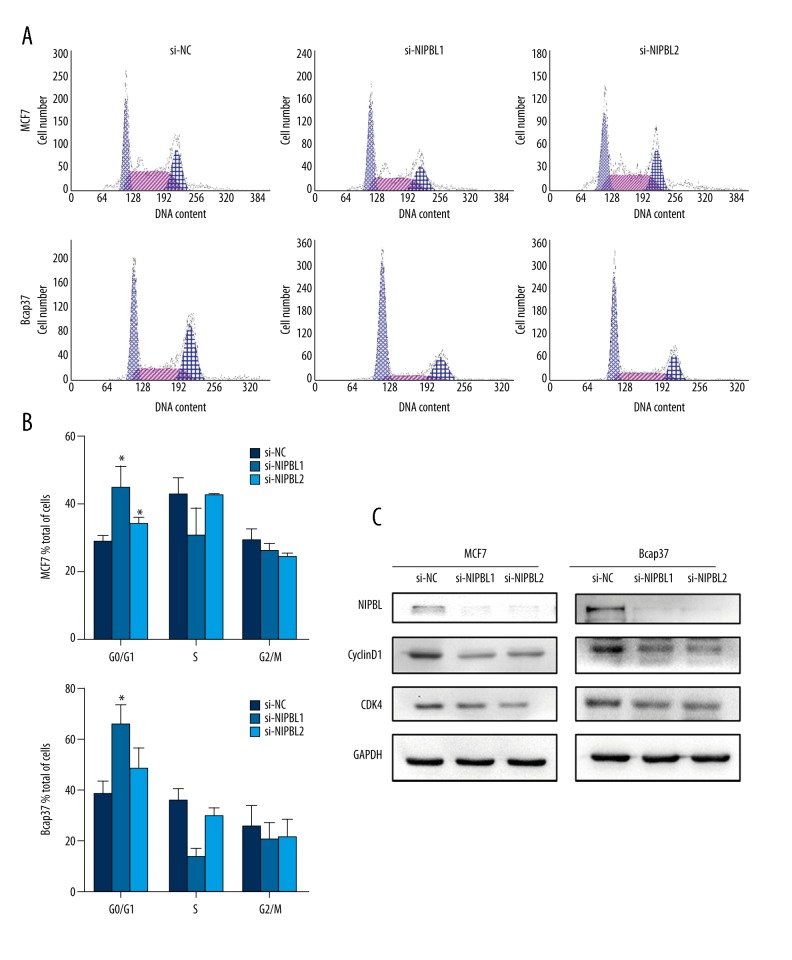
Downregulation of NIPBL expression reduced the arrest of breast cancer cells in the G0/G1 phase
To analyze the effects of NIPBL on the cell cycle of MCF-7 cells and Bcap37 cells, flow cytometry findings showed that, compared with control cells, the percentage of NIPBL-silenced cells in the G0/G1 phase was significantly increased (P<0.05) (Figure 3A, 3B). A comparison of the expression of cell cycle-related proteins was made to confirm this finding. In both cell lines, knockdown of NIPBL induced reduced expression levels of cyclin D1 and CDK4 (Figure 3C). These data indicated that knockdown of NIPBL inhibited breast cancer cell proliferation by arresting the transition from the G0/G1 phase to the S-phase.
Figure 3.
NIPBL silencing induced cell cycle arrest at the G0/G1 phase in breast cancer cells in vitro. (A, B) Flow cytometric analysis of cell cycle of MCF7 and Bcap37 cells. (C) Altered expression of cell cycle-related proteins was identified and confirmed by Western blot analysis. (* P<0.05; ** P<0.01; *** P<0.001). Data are presented as mean ±SD of experiments performed in triplicate.
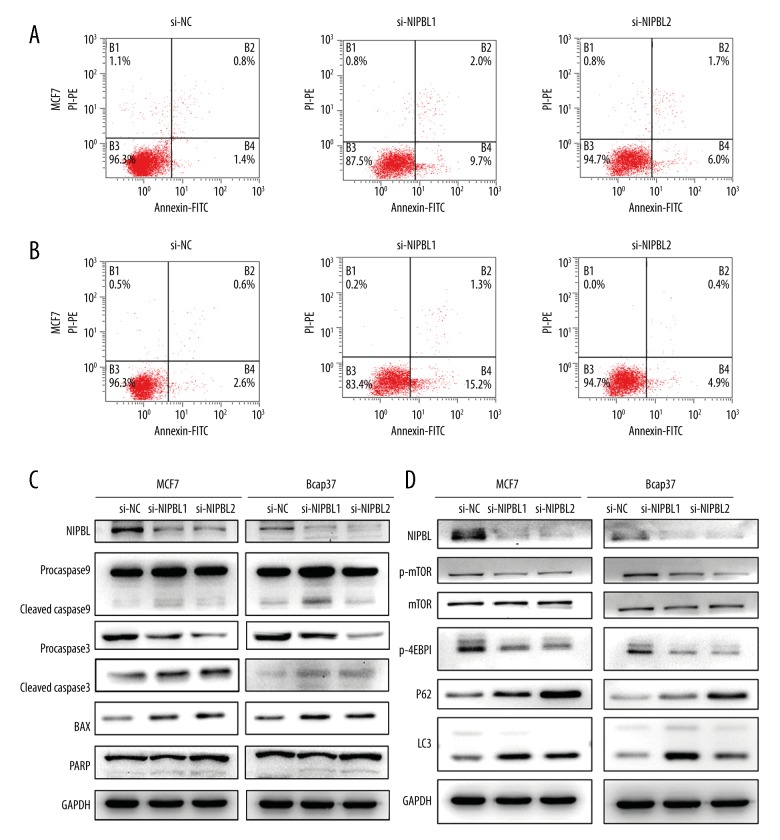
Downregulation of NIPBL induced apoptosis of MCF-7 and Bcap37 cells
To further investigate whether the inhibitory effect of si-NIPBL on MCF-7 and Bcap37 cell proliferation was caused by cell apoptosis, flow cytometry showed that the apoptosis rates of the treated cells were greater than those of the control groups (Figure 4A, 4B). Western blot analysis showed that the expression levels of cleaved poly (ADP-ribose) polymerase (PARP), cleaved caspase 3, cleaved caspase 9 and the apoptosis regulator, BAX (a member of the Bcl-2 family), were increased in the NIPBL-silenced group (Figure 4C). These data indicated that knockdown of NIPBL inhibited breast cancer cell proliferation by promoting cell apoptosis.
Figure 4.
NIPBL silencing promoted cell apoptosis and influenced autophagy in breast cancer cell lines in vitro. (A, B) Flow cytometric analysis of cell cycle of MCF7 and Bcap37 cells. (C) Immunoblot analysis of apoptosis proteins in MCF7 and Bcap37 cells. (D) Cleavage of caspase 3, caspase 9, poly (ADP-ribose) polymerase (PARP), and expression of the apoptosis regulator, BAX (a member of the BCl-2 family), were identified and confirmed by Western blot analysis. (E) Immunoblot analysis of autophagy proteins in MCF7 and Bcap37 cells.
Downregulation of the NIPBL gene induced autophagy of breast cancer cells
To investigate whether NIPBL regulated autophagy, the expression levels of LC3B and P62 proteins, common markers for cell autophagy, were increased following si-NIPBL (Figure 4D). To further elucidate the potential mechanisms of NIPBL on autophagy, we investigated whether the mammalian target of rapamycin (mTOR) signaling pathway was involved in si-NIPBL-induced autophagy of MCF-7 and Bcap37 cells. As shown in Figure 4D, the levels of phosphorylated mTOR level decreased, and the expression level of the mTOR downstream target p-4EBP1 (Thr70) was significantly reduced after treatment with si-NIPBL.
Discussion
Previous studies have demonstrated the roles of NIPBL in loading cohesin onto chromosomes, in gene expression and DNA repair [8–10]. The role of NIPBLs in lung cancer and colorectal cancer cell lines have been reported previously, but studies in breast cancer remain to be undertaken. In this study, we performed a series of in vitro experiments involving breast cancer cells to explore the biological functions of NIPBL and its underlying mechanisms. The findings of this study have shown that in human breast cancer cell lines NIPBL downregulation demonstrated the following mechanisms, all of which may be relevant to the cause, progression, or prognosis of breast cancer, including apoptosis, cell cycle arrest, and autophagy. The findings of this showed that downregulation of NIPBL reduced cell proliferation, invasion, and migration (Figures 1, 2). Also, flow cytometry showed that NIPBL knockdown in vitro induced apoptosis and G0/G1 cell cycle arrest (Figure 3A–3C). The findings of this study also showed that si-NIPBL-induced apoptosis was activated by the intrinsic mitochondrial-mediated caspase pathway. Finally, the findings of this study showed that knockdown of NIPBL promoted apoptosis of breast cancer cells in vitro, possibly via inhibition of autophagosome-lysosome fusion (Figure 4D).
Cohesin loading or unloading onto sister chromatids is a dynamic process that begins in the early stages of the G1 phase of the cell cycle [5]. Cohesin cannot be loaded onto chromosomes at any point in the cell cycle in NIPBL with sister chromatid cohesion 2 (SCC2) or 4 (SCC4) human homolog mutations, as this phenomenon results in cohesin accumulation in late G1 phase [6]. Although NIPBL is recognized to be a cohesin loading factor that loads cohesin onto chromosomes, the role of NIPBL on the cell cycle in human cancer has rarely been studied. However, the findings from the present in vitro study have shown that NIPBL silencing induced G0/G1 phase arrest in breast cancer cells. Cyclin D1 is an important regulator of G1 progression, and cyclin D/CDK complexes directly phosphorylate Rb; phosphorylated Rb (p-Rb) promotes the transition from the G0/G1 phase to the S-phase of the cell cycle [15]. The results of this study indicate that G0/G1 phase arrest occurs as a result of inhibition of the cyclin D1/p-Rb pathway.
Programmed cell death (PCD) is classified into apoptosis (type-I PCD) and autophagic cell death (type-II PCD) [16]. The role of NIPBL in autophagy has not yet been reported. Autophagy is a self-degradation process involving degradation of cytosolic components and organelles by lysosomes [17]. The results of this study have shown that NIPBL knockdown induced autophagic cell death, and that downregulation of NIPBL induced increased expression levels of LC3B-II and p62 (Figure 4E). Autophagy involves several stages that include: induction and formation of the autophagosome; the fusion of the autophagosome with lysosomes; and breakdown of the autophagic body [18]. LC3-II expression is correlated with the number of autophagosomes, and the SQSTM1 gene encodes the sequestosome-1 protein, also known as ubiquitin-binding protein p62, a selective autophagic substrate that is degraded in autolysosomes [17,19]. The results of this study showed that the expression levels of LC3-II and P62 increased after si-NIPBL treatment, indicating that NIPBL silencing promoted the induction and formation of autophagosome, and inhibition of autophagosome-lysosome fusion, a situation known as ‘autophagic flux.’ However, this preliminary in vitro finding should be confirmed in further studies using the RFP-GFP-LC3 assay. Also, the mammalian target of rapamycin (mTOR) signaling pathway is involved in si-NIPBL-mediated induction of autophagy, by acting as a negative regulator of autophagy, with inhibition of mTOR activity being a crucial step in the induction of autophagy in eukaryote cells [20]. The results of this study showed that phosphorylated mTOR levels were decreased following si-NIPBL treatment.
Cohesin-defective cells present many features that are potentially important drivers of malignancy; these features include impaired DNA damage repair, genomic instability, and gene expression anomalies [11]. Cohesin-defective cells are sensitive to DNA-damaging drugs and radiation [21,22]. Drosophila studies have shown that NIPBL regulates gene expression, and this regulatory function of NIPBL is important in early development [9]. Whether NIPBL plays a role in drug resistance or treatment in breast cancer is unclear and requires evaluation in controlled clinical studies. Further studies should be undertaken to detail the molecular mechanisms of apoptosis and autophagy induced by NIPBL silencing in breast cancer cells.
Conclusions
The findings of this in vitro study have shown that downregulation of cohesin loading factor NIPBL arrested breast cancer cells in vitro in the G0/G1 phase of the cell cycle and induced apoptosis and autophagy. Further controlled clinical studies are recommended to determine whether NIPBL is a potential diagnostic or predictive biomarker or therapeutic target for human breast cancer.
Footnotes
Source of support: This work was supported by National Natural Science Foundation of China (81672597), Ministry of Health and Department of Health in Zhejiang (2015PYA001), the Natural Science Foundation of Zhejiang Province (LY16H160039) and Zhejiang TCM Research Grant (2014ZB069)
Conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Donepudi MS, Kondapalli K, Amos SJ, Venkanteshan P. Breast cancer statistics and markers. J Cancer Res Ther. 2014;10:506–11. doi: 10.4103/0973-1482.137927. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Yang F, Li W, et al. miR-29a suppresses MCF-7 cell growth by downregulating tumor necrosis factor receptor 1. Tumour Biol. 2017;39:1010428317692264. doi: 10.1177/1010428317692264. [DOI] [PubMed] [Google Scholar]
- 4.Tedaldi G, Tebaldi M, Zampiga V, et al. Multiple-gene panel analysis in a case series of 255 women with hereditary breast and ovarian cancer. Oncotarget. 2017;8(29):47064–75. doi: 10.18632/oncotarget.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill VK, Kim JS, Waldman T. Cohesin mutations in human cancer. Biochim Biophys Acta. 2016;1866:1–11. doi: 10.1016/j.bbcan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciosk R, Shirayama MA, Tanaka T, et al. Cohesin’s binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Molecular Cell. 2000;5:243. doi: 10.1016/s1097-2765(00)80420-7. [DOI] [PubMed] [Google Scholar]
- 7.Mannini L, Lamaze CF, Cucco F, et al. Mutant cohesin affects RNA polymerase II regulation in Cornelia de Lange syndrome. Sci Rep. 2015;5:16803. doi: 10.1038/srep16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsett D. Roles of the sister chromatid cohesion apparatus in gene expression, development, and human syndromes. Chromosoma. 2007;116:1–13. doi: 10.1007/s00412-006-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorsett D. Cohesin, gene expression and development: Lessons from Drosophila. Chromosome Res. 2009;17:185–200. doi: 10.1007/s10577-009-9022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oka Y, Suzuki K, Yamauchi M, et al. Recruitment of the cohesin loading factor NIPBL to DNA double-strand breaks depends on MDC1, RNF168 and HP1gamma in human cells. Biochem Biophys Res Commun. 2011;411:762–67. doi: 10.1016/j.bbrc.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Tomaszewski JM, McKay MJ. Can corruption of chromosome cohesion create a conduit to cancer? Nat Rev Cancer. 2011;11:199–210. doi: 10.1038/nrc3018. [DOI] [PubMed] [Google Scholar]
- 12.Barber TD, McManus K, Yuen KW, et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci USA. 2008;105:3443–48. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MS, An CH, Chung YJ, et al. NIPBL, a cohesion loading factor, is somatically mutated in gastric and colorectal cancers with high microsatellite instability. Dig Dis Sci. 2013;58:3376–78. doi: 10.1007/s10620-013-2808-5. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Ying Y, Shan L, et al. Enhanced expression of cohesin loading factor NIPBL confers poor prognosis and chemotherapy resistance in non-small cell lung cancer. J Transl Med. 2015;13:153. doi: 10.1186/s12967-015-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S, Li X, Xia D, et al. Cepharanthine induces autophagy, apoptosis and cell cycle arrest in breast cancer cells. Cell Physiol Biochem. 2017;41(4):1633–48. doi: 10.1159/000471234. [DOI] [PubMed] [Google Scholar]
- 16.Bursch W, Ellinger A, Gerner C, et al. Programmed cell death (PCD): Apoptosis, autophagic PCD, or others? Ann NY Acad Sci. 2000;926:1–12. doi: 10.1111/j.1749-6632.2000.tb05594.x. [DOI] [PubMed] [Google Scholar]
- 17.Cui J, Gong Z, Shen HM. The role of autophagy in liver cancer: Molecular mechanisms and potential therapeutic targets. Biochim Biophys Acta. 2013;1836:15–26. doi: 10.1016/j.bbcan.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang K, Lu Y, Xie F, et al. Cationic liposomes induce cell necrosis through lysosomal dysfunction and late-stage autophagic flux inhibition. Nanomedicine. 2016;11:3117. doi: 10.2217/nnm-2016-0289. [DOI] [PubMed] [Google Scholar]
- 20.Chang HJ, Ro SH, Jing C, et al. mTOR regulation of autophagy. FEBS Letters. 2010;584:1287–95. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atienza JM, Roth RB, Rosette C, et al. Suppression of RAD21 gene expression decreases cell growth and enhances cytotoxicity of etoposide and bleomycin in human breast cancer cells. Mol Cancer Ther. 2005;4:361–68. doi: 10.1158/1535-7163.MCT-04-0241. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Balakrishnan K, Malaterre J, et al. Rad21-cohesin haploinsufficiency impedes DNA repair and enhances gastrointestinal radiosensitivity in mice. PLoS One. 2010;5:e12112. doi: 10.1371/journal.pone.0012112. [DOI] [PMC free article] [PubMed] [Google Scholar]