Abstract
Background
LncRNA X inactive specific transcript (XIST) was reported to function as an oncogene in nasopharyngeal carcinoma cells (NPC) by sponging miR-34a-5p. However, the role of XIST in modulating the radiosensitivity of NPC cells and its mechanism still remain undefined.
Material/Methods
The expressions of XIST and miR-29c in NPC cells were evaluated by qRT-PCR. CNE1 and CNE2 cells were transfected with si-XIST, pcDNA-XIST, miR-29c mimics, anti-miR-29c, or respective controls by Lipofectamine 2000. The effects of XIST knockdown and miR-29c overexpression on cell proliferation, survival fraction, and γ-H2AX expression were investigated by CCK-8 assay, colony formation assay, immunofluorescence, and Western blot, respectively. Luciferase reporter assay and qRT-PCR analysis were performed to confirm whether XIST interacts with miR-29c and regulates its expression.
Results
XIST was upregulated and miR-29c was downregulated in NPC cells. The expressions of XIST and miR-29c changed reversely in response to irradiation. Knockdown of XIST and miR-29c overexpression both resulted in a dramatic suppression of cell proliferation, a marked enhancement of radiosensitivity, and an obvious increase of γ-H2AX foci formation in NPC cells. Luciferase reporter assay and qRT-PCR analysis demonstrated that XIST interacts with miR-29c and negatively regulates its expression. Moreover, miR-29c inhibition abrogated XIST knockdown-induced cell proliferation inhibition and radiosensitivity increase in NPC cells.
Conclusions
XIST knockdown suppressed cell proliferation and enhanced radiosensitivity of NPC cells by upregulating miR-29c, providing a novel therapeutic target to improve radiotherapy efficiency for patients with NPC.
MeSH Keywords: MicroRNAs; Nasopharyngeal Neoplasms; Radiation Tolerance; RNA, Long Noncoding
Background
Nasopharyngeal carcinoma (NPC), deriving from the epithelial lining of nasopharynx, is a type of malignant head and neck tumor characterized by local aggressiveness and early distant metastasis [1]. NPC is highly prevalent in Southeast Asia and southern China, particularly in Guangdong province, Hong Kong, Philippines, and Thailand, with an estimated incidence rate of approximately 20–50 per 100 000 individuals [2,3]. Owing to the relative sensitivity of NPC to ionizing radiation (IR), radiotherapy is identified as the preferred and curative treatment for primary NPC [4]. Nevertheless, local recurrence and distant metastasis, presenting as persistent or recurrent tumors, still occurs in a proportion of patients due to radioresistance, which remains a main obstacle to long-term survival of NPC patients [5]. Therefore, researches of new effective therapeutic strategies as well as the molecular mechanism of NPC radioresistance are urgently needed.
Recently, it is increasingly recognized that non-coding RNAs (ncRNAs), including microRNA (miRNA) and long non-coding RNA (lncRNA), are a new group of clinical biomarkers and potential therapeutic targets for tumors [6]. lncRNAs, a class of transcripts more than 200 nucleotides in length, lack protein-coding ability. These lncRNAs appear to be involved in the regulation of various biological processes, including cell growth, invasion, and apoptosis, as well as cancer progression [7]. Mounting evidence shows that dysregulated lncRNAs are involved in the malignant transformation and progression of various cancers, including NPC [8,9]. For example, lncRNA maternally-expressed gene 3 (MEG3) was downregulated in NPC tissues and cells and characterized as a tumor suppressor in NPC cells [10]. lncRNA ANRIL was reported to be highly expressed in NPC and promoted NPC development via improving cell proliferation, altering glucose metabolism, and inducing side-population stem-like cancer cells [11]. lncRNA ROR was reported to be upregulated in NPC tissues and promoted cell proliferation, migration, and chemoresistance of NPC [12]. lncRNA X inactive specific transcript (XIST), a product of XIST gene and a master regulator of X-chromosome transcriptional silencing in mammals, was found to be upregulated in several tumors, such as ovarian cancer [13] and breast cancer [14]. A previous document indicates that XIST plays pivotal roles in cell differentiation, proliferation, and genome maintenance of human cells [15]. Moreover, XIST was reported to function as an oncogene in NPC through upregulating E2F3 by sponging miR-34a-5p [16]. However, whether XIST is associated with radioresistance of NPC cells remains unknown.
miRNAs are a type of conserved endogenous ncRNA which can negatively regulate gene expression at the post-transcriptional level by suppressing translation or degrading target mRNAs [17]. It is well documented that miRNA serve as either oncogenes or tumor suppressors to participate in numerous biological processes, including cell proliferation, migration, differentiation, apoptosis, cell cycle, and autophagy [18]. Moreover, these miRNAs play a crucial role in radioresistance by disturbing specific pathways, including sensing DNA damage and apoptosis [19]. It has been demonstrated that aberrant expression of miRNAs is involved in the development of radioresistance in NPC [20]. Previous studies identified that miR-29c was downregulated and functioned as a tumor suppressor in several tumors, including NPC [21,22]. Notably, miR-29c was reported to enhance the sensitivities of human NPC to cisplatin-based chemotherapy and radiotherapy [23].
lncRNAs have been reported to suppress miRNAs expression by acting as molecular sponges or competitive endogenous RNAs (ceRNAs) [24]. In the present study, we investigated the effect of XIST on radiosensitivity of NPC cells and the underlying mechanism involved in the ceRNA regulatory network.
Material and Methods
Cell lines and culture
The human NPC cell lines CNE-1 and CNE-2 were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin in a humidified incubator at 37°C with 5% CO2. Primary normal human nasal epithelial line HNEpC was obtained from PromoCell (Heidelberg, Germany) and cultured with commercially available Airway Epithelial Cell Growth Medium (PromoCell) at 37°C under 5% CO2.
Cell transfection
siRNAs against XIST (si-XIST-1, si-XIST-2), siRNA control (si-con), pcDNA-XIST, pcDNA empty vector (vector), miR-29c mimic (miR-29c), miRNA control (miR-con), miR-29c inhibitor (anti-miR-29c), and control inhibitor (anti-miR-con) were purchased from GenePharma Co., Ltd. (Shanghai, China). NPC cells were seeded into 6-well plates and cultured with complete medium without antibiotics at least 24 h prior to transfection. Then, cells were transiently transfected with siRNAs, miRNAs, or pcDNAs, or cotransfected with si-XIST and anti-miR-29c or anti-miR-con using Lipofectamine™ 2000 (Invitrogen). The cells were harvested at different time points for analysis post-transfection.
Irradiation exposure
Cell radiation was performed at room temperature by a Cs-137 irradiator (HWM D-2000; Siemens AG, Munich, Germany) using a 6-MV photon beam at a dose rate of 400 cGy/min.
Quantitative real-time PCR
Total RNA was extracted from NPC or HNEpC cells using TRIzol reagent (Invitrogen). Total RNA (1 μg) was reversely transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) and used for qPCR analysis. qPCR for detection of XIST and miR-29c expressions were performed using SYBR Green PCR master mix (Bio-Rad, Hercules, CA) and TaqMan miRNA assay (Applied Biosystems) on a LightCycler 480 II System (Roche, Indianapolis, IN), respectively. The PCR reaction procedures were carried out as below: 95°C for 10 min, followed by 30 cycles of denaturing at 95°C for 5 s, annealing at 60°C for 30 s, and extension at 60°C for 1 min. The relative gene expression levels were calculated using the 2−ΔΔCt method and normalized to internal control small nuclear RNA U6. For the time-course experiment, NPC cells receiving 4 Gy X-rays were harvested for qRT-PCR analysis every 3 h within 24 h after irradiation.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined with CCK-8 assay using the protocol described previously [25]. Briefly, 1×103 cells/well were plated in triplicate in 96-well culture plates (Costar, Corning, NY), cultured for 24 h and then transfected with si-XIST, miR-29c, si-XIST + anti-miR-29c, or their respective control. At 24, 48, and 72 h after transfection, 10 μl CCK-8 solution (Dojindo Molecular Technologies, Kyushu, Japan) was added to each well. The cells were incubated for an additional 1 h and then cell viability was detected at 450 nm using a VersaMax Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA).
Colony formation assay
Transfected NPC cells were seeded into 6-well plates and exposed to the indicated single doses of irradiation (0, 2, 4, 6, or 8 Gy). Following 2 weeks incubation at 37°C, cells were fixed with 100% methanol and stained with 0.1% crystal violet in absolute ethanol for 15 min. Visible colonies with more than 50 cells were counted under an inverted microscope. The survival fraction (SF) was calculated as the ratio of plating efficiency for irradiated and non-irradiated cells. Plating efficiency was defined as bellow: the number of colonies divided by number of cells seeded ×100%. The data was fitted using classic single-hit multi-target model: y=1-(1-e−D/D0)N to draw cell survival curves. Radiation-associated parameters, including D0 (mean lethal dose), Dq (quasi-threshold dose), and N (extrapolation number), were calculated according to the curve. The sensitivity enhancement ratio (SERD0) is presented as the ratio control group D0 value to treatment group D0 value.
Luciferase reporter assay
Mutant of the XIST sequence was synthesized by PCR using site-directed mutagenesis with the QuickChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The primers used were as follows: XIST, forward: 5′-TAA GGA CTA CTT AAC GGG CT-3′, reverse: 5′-TCA CAT CTG CTC CAC TTG AG-3′. XIST fragments containing the wild-type (WT) miR-29c binding sites and its mutated form were inserted downstream of the Firefly luciferase gene in the pGL3 vector (Promega, Madison, WI), namely pGL3-XIST-wt and pGL3-XIST-mut. For reporter assay, cells were seeded into 24-well plates and cultured for 24 h. Then, cells were cotransfected with 100 ng pGL3 Firefly luciferase constructs, as well as 10 ng pRL-TK Renilla plasmids and miR-29c or miR-con by Lipofectamine™ 2000 (Invitrogen). The luciferase activity was measured using the dual luciferase reporter assay system (Promega) at 48 h after transfection and normalized to Renilla luciferase activity. The relative luciferase activity is expressed as a ratio of Firefly/Renilla luciferase activity.
Immunofluorescence detection of γ-H2AX expression
Cells were seeded into a sterile glass chamber (Becton Dickinson, Franklin Lakes, NJ) and treated with 4 Gy of radiation after adherent growth. Cells were fixed with acetone/methanol (1: 1) and permeabilized using 0.1% Triton-X100 in PBS. Cells were then blocked with 3% bovine serum albumin (BSA) (Sigma, St. Louis, MO) at room temperature for 1 h. Subsequently, cells were incubated with primary antibody against γ-H2AX (Cell Signaling Technology, Beverly, MA) for 2 h. Following 3 washes with PBS, cells were visualized by incubating with Alexa Fluor 488-conjugated goat anti-mouse secondary antibodies (Beyotime Institute of Biotechnology, Haimen, China) for 1 h in the dark. After washing with PBS again, cell nuclei were counterstained with 1 μg/mL of DAPI (Vector Laboratories, Burlingame, CA). Immunofluorescence images were obtained using an Olympus BX53/DP80 microscope (Olympus Corporation, Shinjuku, Japan) at 40× magnification.
Western blot analysis
Total proteins were extracted from cultured cells using RIPA buffer (Beyotime) containing protease inhibitors cocktail (Roche, Switzerland). Protein concentration was quantified using the BCA protein assay kit (Beyotime). Equal amounts of protein were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). After being blocked with 5% nonfat milk in TBST for 1 h at room temperature, the membranes were incubated with primary antibodies, including mouse monoclonal anti-γ-H2AX (No. 2577; 1: 1000 dilution, Cell Signaling Technology, Beverly, MA) and mouse monoclonal anti-GAPDH (No. 97166; 1: 2000 dilution, Cell Signaling Technology) at 4°C overnight, and then by probed with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (sc-2379; 1: 1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h. Reactive protein expression was visualized and detected using a chemiluminescence kit (Millipore).
Statistical analysis
All experiments were repeated at least 3 times independently and results are shown as mean ± standard deviation (SD). SPSS 17.0 statistical software package (SPSS Inc, Chicago, IL) was used for all statistical analysis. Statistical differences were evaluated using the t test or one-way ANOVA. The differences were considered statistically significant at a value of P less than 0.05.
Results
XIST was upregulated and miR-29c was downregulated in response to irradiation in NPC cells
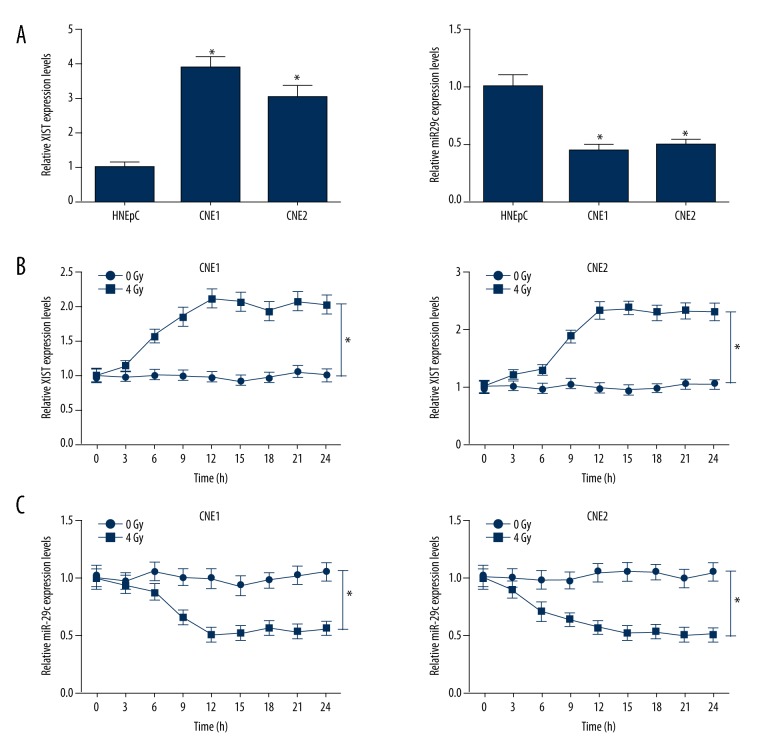
First, the expressions of XIST and miR-29c in NPC and primary normal human nasal epithelial line HNEpC cells were confirmed by qRT-PCR. The results showed that XIST expression was significantly elevated and miR-29c expression was dramatically reduced in NPC cell lines (CNE1 and CNE2) compared with HNEpC cells (Figure 1A). Then, the effect of irradiation on the expressions of XIST and miR-29c was further explored. The expressions of XIST and miR-29c in CNE1 and CNE2 cells were measured every 3 h after 4-Gy irradiation. The qRT-PCR results demonstrated that XIST expression was markedly increased in both CNE1 and CNE2 cells at 6 h after irradiation treatment (Figure 1B). On the contrary, miR-29c was strikingly downregulated 6 h after irradiation (Figure 1C).
Figure 1.
Expression alteration of XIST and miR-29c in NPC cells in response to irradiation. (A) qRT-PCR was performed to examine the expressions of XIST and miR-29c in NPC cell lines (CNE1 and CNE2) and primary normal human nasal epithelial line HNEpC. qRT-PCR was carried out to analyze the expressions of XIST (B) and miR-29c (C) in CNE1 and CNE2 cells at indicated time points after 4-Gy irradiation treatment. * P<0.05.
XIST knockdown suppressed cell proliferation and improved radiosensitivity of NPC cells
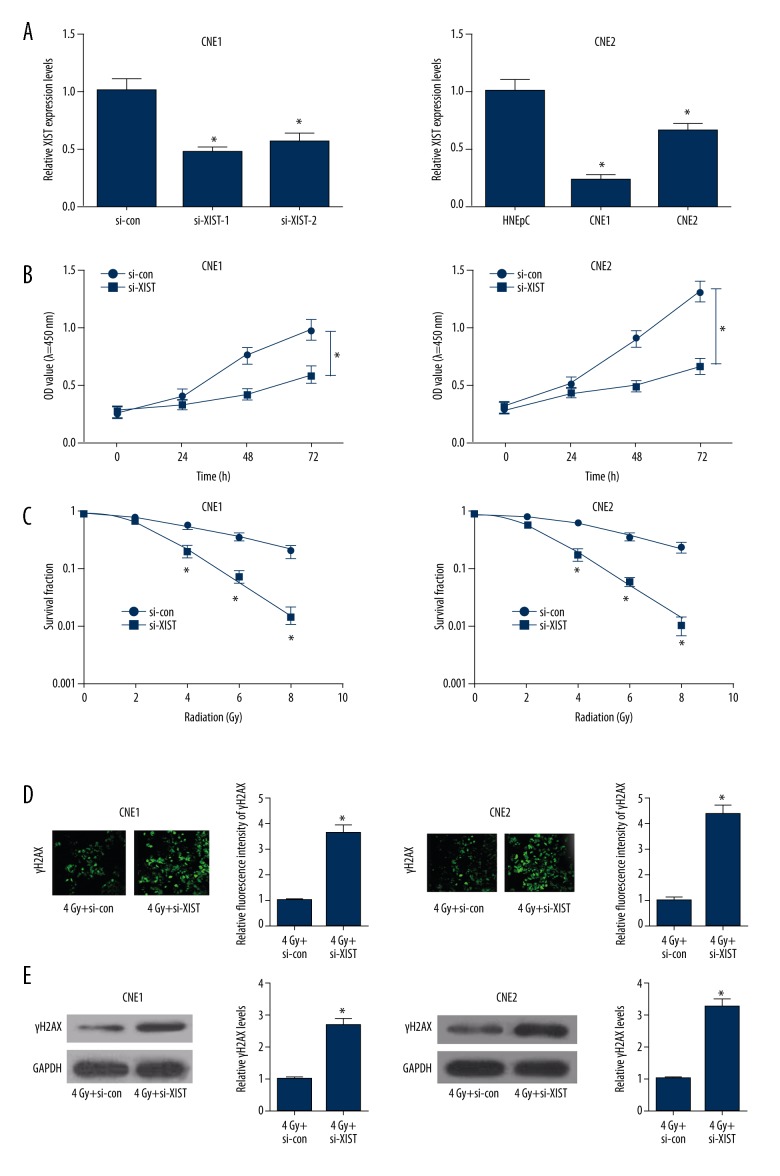
To investigate the role of XIST in radiosensitivity of NPC cells, we used siRNA-mediated knockdown of XIST in NPC cells. The transfection efficiency of si-XIST-1 and si-XIST-2 in NPC cells was verified by qRT-PCR. The results indicated that XIST expression was significantly lower in si-XIST-transfected NPC cells than that in si-control groups (Figure 2A). Moreover, si-XIST-1 transfection resulted in a higher knockdown efficiency (60% in CNE1 and 75% in CNE2) than si-XIST2 (50% in CNE1 and 35% in CNE2). Thus, si-XIST1 was chosen for the next experiments. As demonstrated by CCK-8 assay, si-XIST transfection led to a marked suppression of cell viability at 48 h and 72 h in both CNE1 and CNE2 cells with respect to si-con group (Figure 2B), suggesting that XIST knockdown conspicuously inhibited NPC cell proliferation. Additionally, colony formation assay demonstrated that XIST knockdown resulted in a significant decrease of colony survival fractions in both CNE1 and CEN2 cells (SERD0: 2.573 and 2.086, respectively), indicating that XIST knockdown improved radiosensitivity of NPC cells (Figure 2C). γ-H2AX foci is an indicator of DNA double-strand break (DSB), which is responsible for irradiation-induced cell death, and loss of foci represents the repair of DSB [26]. The effect of XIST on irradiation-induced DNA damage and repair was studied by detecting γ-H2AX expression. Immunofluorescent staining revealed that γ-H2AX foci formation was strikingly increased after 4-Gy irradiation treatment in si-XIST-transfected CNE1 and CNE2 cells (Figure 2D). Consistently, Western blot analysis also suggested that XIST knockdown dramatically increased γ-H2AX level in both CNE1 and CNE2 cells exposed to 4-Gy irradiation (Figure 2E), indicating that XIST knockdown enhanced radiosensitivity of NPC cells by inhibiting DNA damage repair.
Figure 2.
Effect of XIST knockdown on cell proliferation and radiosensitivity of NPC cells. (A) Transfection efficiency of si-XIST-1 and si-XIST-2 in CNE1 and CNE2 cells was detected by qRT-PCR. (B) CCK-8 assay was performed to determine cell proliferation at 24 h, 48 h, and 72 h in si-XIST- or si-con-transfected CNE1 and CNE2 cells. (C) Colony formation assay was used to measure colony survival rate 2 weeks after CNE1 and CNE2 cells transfected with si-XIST or si-con were exposed to the indicated single doses of irradiation (0, 2, 4, 6, or 8 Gy). (D) CNE1 and CNE2 cells transfected with si-XIST or si-con were irradiated with 4-Gy X-rays and then subjected to immunofluorescent staining for γ-H2AX expression. (E) Western blot analysis of γ-H2AX expression level in CNE1 and CNE2 cells transfected with si-XIST or si-con under 4 Gy irradiation. * P<0.05.
miR-29c overexpression inhibited cell proliferation and increased radiosensitivity of NPC cells
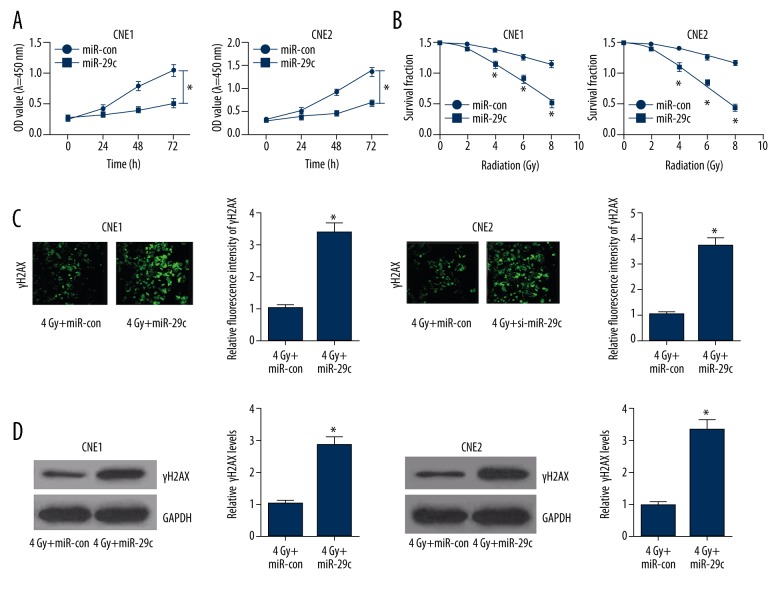
To explore the effect of ectopic expression of miR-29c on radiosensitivity of NPC cells, we established CNE1 and CNE2 cells overexpressing miR-29c by transfecting with miR-29c mimics. CCK-8 assay showed that miR-29c overexpression significantly restrained cell viability at 48 h and 72 h in both CNE1 and CNE2 cells compared to miR-con-transfected cells (Figure 3A). Enforced expression of miR-29c markedly increased radiosensitivity of CNE1 and CNE2 cells (SERD0: 2.164 and 2.395, respectively), as demonstrated by colony formation assay (Figure 3B). Compared with the miR-con group, miR-29c overexpression remarkably promoted γ-H2AX foci formation in CNE1 and CNE2 cells receiving 4-Gy irradiation (Figure 3C). Similarly, γ-H2AX level was dramatically improved in CNE1 and CNE2 cells treated with miR-29c and 4-Gy X-rays, as compared with the miR-con group.
Figure 3.
Effect of miR-29c overexpression on cell proliferation and radiosensitivity of NPC cells. (A) CCK-8 assay was carried out to evaluate cell proliferation at 24 h, 48 h, and 72 h in CNE1 and CNE2 cells transfected with miR-29c or miR-con. (B) The clonogenic survival curves were compared in CNE1 and CNE2 cells transfected with miR-29c or miR-con with the indicated single doses of irradiation (0, 2, 4, 6, or 8 Gy) treatment. (C) Immunofluorescent staining for γ-H2AX expression in CNE1 and CNE2 cells transfected with miR-29c or miR-con under 4-Gy irradiation. (D) Western blot analysis of γ-H2AX level in CNE1 and CNE2 cells transfected with miR-29c or miR-con with 4-Gy irradiation. * P<0.05.
XIST directly interacted with miR-29c and negatively regulated its expression
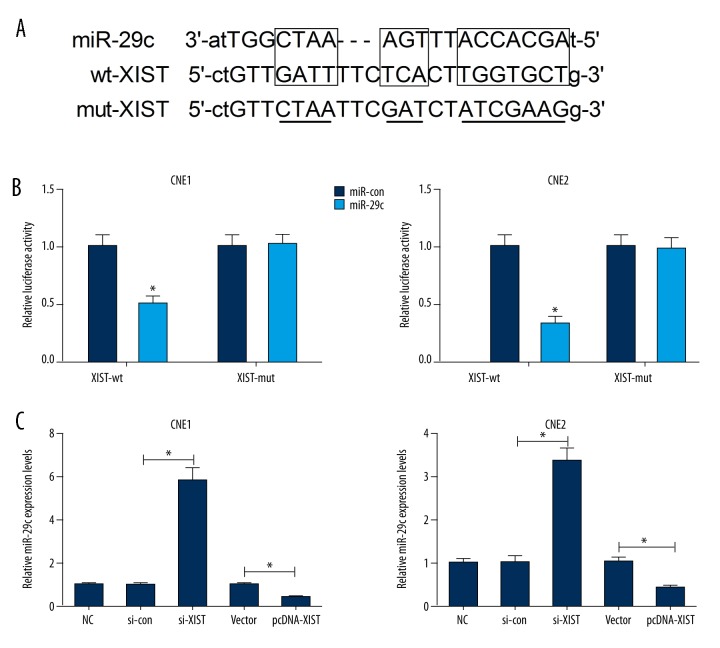
We searched for miRNAs with complementary base pairing with XIST by utilizing the online bioinformatics database (starBase v2.0, http://starbase.sysu.edu.cn/mirLncRNA.php). XIST was presented to harbor binding sequences of miR-29c (Figure 4A). To identify whether miR-29c could directly bind to XIST, we constructed luciferase reporter vectors containing the predicted wild-type or mutant binding sites of miR-29c. Luciferase reporter assay indicated that miR-29c overexpression significantly reduced the luciferase activity of pGL3-XIST-wt in CNE1 and CNE2 cells, but had no obvious inhibitory effect on pGL3-XIST-mut (Figure 4B). In addition, the regulatory effect of XIST on miR-29c expression was further examined by qRT-PCR in CNE1 and CNE2 cells transfected with si-XIST, pcDNA-XIST, or respective controls. As illustrated in Figure 4C, XIST knockdown by si-XIST significantly promoted the expression of miR-29c in CNE1 and CNE2 cells, while XIST overexpression by pcDNA-XIST remarkably suppressed miR-29c expression.
Figure 4.
Mutual effect between XIST and miR-29c. (A) The predicted wild-type or mutant binding sites of miR-29c on XIST. (B) CNE1 and CNE2 cells were cotransfected with miR-29c or miR-con and pGL3-XIST-wt or pGL3-XIST-mut, and luciferase reporter assay was performed. (C) The expression of miR-29c in CNE1 and CNE2 cells transfected with si-XIST, pcDNA-XIST, or respective controls was evaluated by qRT-PCR. * P<0.05.
XIST knockdown suppressed cell proliferation and improved radiosensitivity of NPC cells by upregulating miR-29c
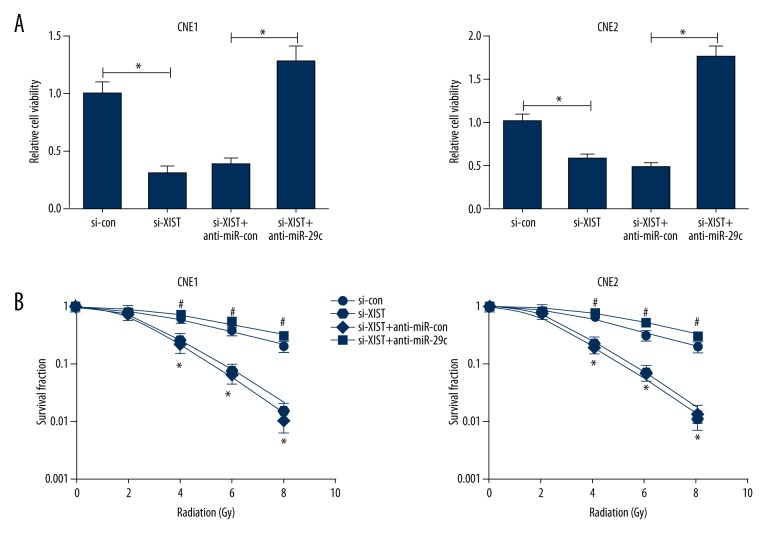
To further explore the possible regulatory mechanism of XIST in radiosensitivity of NPC cells, CNE1 and CNE2 cells were transfected with si-XIST, si-con, si-XIST + anti-miR-29c, or si-XIST + anti-miR-con. As shown in Figure 5A, XIST knockdown led to a marked repression of cell viability in CNE1 and CNE2 cells, while anti-miR-29c significantly abolished this effect. XIST knockdown remarkably reduced survival fractions in CNE1 and CNE2 cells (SERD0: 2.571 and 2.163, respectively), whereas anti-miR-29c dramatically abrogated this effect (SERD0: 0.931 and 0.892, respectively) (Figure 5B), indicating that miR-29c inhibition significantly reversed XIST knockdown-induced increase of radiosensitivity in NPC cells.
Figure 5.
miR-29c downregulation abated the effects of si-XIST on proliferation and radiosensitivity of NPC cells. (A) Cell viability was determined by CCK-8 assay in CNE1 and CNE2 cells transfected with si-XIST or combined si-XIST and anti-miR-29c. (B) CNE1 and CNE2 cells transfected with si-XIST or simultaneous si-XIST and anti-miR-29c were exposed to the indicated single doses of irradiation (0, 2, 4, 6, or 8 Gy), and colony formation assay was used to measure colony survival rate 2 weeks later. * P<0.05, si-XIST vs. si-con; # P<0.05, si-XIST+anti-miR-29c vs. si-XIST+anti-miR-con.
Discussion
It is well known that lncRNAs are emerging as a crucial regulator of diverse cellular processes [27]. Mounting reports have found that lncRNAs are involved in irradiation-induced radioresistance of NPC cells [28–30]. Increasing evidence has indicated XIST is dysregulated in various tumors and is involved in cancer progression. For instance, XIST was demonstrated to be overexpressed and to act as an oncogene by epigenetically repressing KLF2 expression in non-small cell lung cancer [31]. Moreover, XIST was upregulated and functioned as an oncogene in NPC cells through upregulating E2F3, in part through sponging miR-34a-5p [16]. In our present study, we found that XIST was upregulated in NPC cells and irradiation triggered an obvious increase in XIST expression in NPC cells. Furthermore, loss of function implied that XIST knockdown suppressed proliferation and improved radiosensitivity by inhibiting DNA damage repair in NPC cells.
A growing body of evidence has suggested that aberrant expression of miRNAs plays a crucial role in the development of NPC radiosensitivity [20], such as miR-19b-3p [32], miR-24 [33], and miR-378g [34]. Previously, miR-29c was documented to be downregulated in NPC and overexpression of miR-29c inhibited NPC cell migration and invasion in vitro and repressed the formation of lung metastases in vivo [21]. Additionally, it was stated that ectopic restoration of miR-29c enhanced sensitivities of NPC cells to radiation and cisplatin treatment by promoting apoptosis [23]. In our study, we investigated the effects of miR-29c on cell proliferation and radiosensitivity of NPC cells. In accordance with previous studies, our study showed that miR-29c was downregulated in NPC cells and miR-29c expression was decreased after irradiation. Gain of function revealed that miR-29c overexpression led to a dramatic inhibition of cell proliferation and an obvious increase of radiosensitivity by restraining DNA damage repair in NPC cells.
Ample evidence suggests that lncRNAs act as endogenous miRNA sponges that bind to miRNAs and regulate their function. XIST knockdown exerted tumor-suppressive effects by inhibiting cell proliferation, migration, invasion in vitro, and tumor growth in vivo by acting as a molecular sponge of miR-101 to modulate EZH2 expression [35]. XIST can inhibit HCC cell proliferation and metastasis by targeting miR-92b in hepatocellular carcinoma cells [36]. In gastric cancer cells, XIST was reported to promote cell growth and invasion by serving as competing endogenous RNA to repress miR-497 expression [37]. In our study, we found that the expressions of XIST and miR-29c varied inversely in response to irradiation. Mechanistically, we proved the direct binding site of miR-29c on XIST. Rescue experiments further revealed that miR-29c inhibition abolished XIST knockdown-induced cell proliferation suppression and radiosensitivity increase in NPC cells, suggesting that XIST knockdown inhibited proliferation and improved radiosensitivity of NPC cells by upregulating miR-29c.
Conclusions
In conclusion, our study demonstrates that XIST is upregulated and miR-29c is downregulated in NPC cells. XIST expression is increased and miR-29c expression is decreased in response to irradiation. Furthermore, knockdown of XIST suppresses cell proliferation and enhances radiosensitivity of NPC cells by upregulating miR-29c, suggesting that targeting XIST/miR-29c may be a novel strategy to improve radiotherapy for patients with NPC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–54. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 2.Loong HH, Ma BB, Chan AT. Update on the management and therapeutic monitoring of advanced nasopharyngeal cancer. Hematol Oncol Clin North Am. 2008;22(6):1267–78. doi: 10.1016/j.hoc.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Huang L, Liao L, Wan Y, et al. Downregulation of Annexin A1 is correlated with radioresistance in nasopharyngeal carcinoma. Oncol Lett. 2016;12(6):5229–34. doi: 10.3892/ol.2016.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–24. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Zhang R, Claret FX, Yang H. Involvement of microRNA-24 and DNA methylation in resistance of nasopharyngeal carcinoma to ionizing radiation. Mol Cancer Ther. 2014;13(12):3163–74. doi: 10.1158/1535-7163.MCT-14-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury S, Tran N. Circulating microRNAs: Potential biomarkers for common malignancies. Biomark Med. 2015;9(2):131–51. doi: 10.2217/bmm.14.102. [DOI] [PubMed] [Google Scholar]
- 7.Wang KC, Yang YW, Liu B, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472(7341):120–24. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Disco. 2011;1(5):391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang QQ, Deng YF. Genome-wide analysis of long non-coding RNA in primary nasopharyngeal carcinoma by microarray. Histopathology. 2015;66(7):1022–30. doi: 10.1111/his.12616. [DOI] [PubMed] [Google Scholar]
- 10.Chak WP, Lung RW, Tong JH, et al. Downregulation of long non-coding RNA MEG3 in nasopharyngeal carcinoma. Mol Carcinog. 2017;56(3):1041–54. doi: 10.1002/mc.22569. [DOI] [PubMed] [Google Scholar]
- 11.Zou ZW, Ma C, Medoro L, et al. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7(38):61741–54. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Gu M, You B, et al. Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci. 2016;107(9):1215–22. doi: 10.1111/cas.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren C, Li X, Wang T, et al. Functions and Mechanisms of Long Noncoding RNAs in Ovarian Cancer. Int J Gynecol Cancer. 2015;25(4):566–69. doi: 10.1097/IGC.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 14.Huang YS, Chang CC, Lee SS, et al. Xist reduction in breast cancer upregulates AKT phosphorylation via HDAC3-mediated repression of PHLPP1 expression. Oncotarget. 2016;7(28):43256–66. doi: 10.18632/oncotarget.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engreitz JM, Pandya-Jones A, McDonel P, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341(6147):1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song P, Ye LF, Zhang C, et al. Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene. 2016;592(1):8–14. doi: 10.1016/j.gene.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Bode AM, Cao Y, Dong Z. Regulatory mechanisms and clinical perspectives of miRNA in tumor radiosensitivity. Carcinogenesis. 2012;33(11):2220–27. doi: 10.1093/carcin/bgs235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Ai Q, Cao H, Liu Q. MiR-185-3p and miR-324-3p predict radiosensitivity of nasopharyngeal carcinoma and modulate cancer cell growth and apoptosis by targeting SMAD7. Med Sci Monit. 2015;21:2828–36. doi: 10.12659/MSM.895660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, Tang LL, Sun Y, et al. MiR-29c suppresses invasion and metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer Lett. 2013;329(2):181–88. doi: 10.1016/j.canlet.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Wang CM, Wang Y, Fan CG, et al. miR-29c targets TNFAIP3, inhibits cell proliferation and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma. Biochem Biophys Res Commun. 2011;411(3):586–92. doi: 10.1016/j.bbrc.2011.06.191. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JX, Qian D, Wang FW, et al. MicroRNA-29c enhances the sensitivities of human nasopharyngeal carcinoma to cisplatin-based chemotherapy and radiotherapy. Cancer Lett. 2013;329(1):91–98. doi: 10.1016/j.canlet.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–69. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun C, Liu Z, Li S, et al. Down-regulation of c-Met and Bcl2 by microRNA-206, activates apoptosis, and inhibits tumor cell proliferation, migration and colony formation. Oncotarget. 2015;6(28):25533–74. doi: 10.18632/oncotarget.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn J, Hayman TJ, Jamal M, et al. The mTORC1/mTORC2 inhibitor AZD2014 enhances the radiosensitivity of glioblastoma stem-like cells. Neuro Oncol. 2014;16(1):29–37. doi: 10.1093/neuonc/not139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–27. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Fan H, Liu Y, et al. Curcumin enhances the radiosensitivity in nasopharyngeal carcinoma cells involving the reversal of differentially expressed long non-coding RNAs. Int J Oncol. 2014;44(3):858–64. doi: 10.3892/ijo.2013.2237. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Li T, Wei G, et al. The long non-coding RNA NEAT1 regulates epithelial to mesenchymal transition and radioresistance in through miR-204/ZEB1 axis in nasopharyngeal carcinoma. Tumour Biol. 2016;37(9):11733–41. doi: 10.1007/s13277-015-4773-4. [DOI] [PubMed] [Google Scholar]
- 30.Jin C, Yan B, Lu Q, et al. The role of MALAT1/miR-1/slug axis on radioresistance in nasopharyngeal carcinoma. Tumour Biol. 2016;37(3):4025–33. doi: 10.1007/s13277-015-4227-z. [DOI] [PubMed] [Google Scholar]
- 31.Fang J, Sun CC, Gong C. Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem Biophys Res Commun. 2016;478(2):811–17. doi: 10.1016/j.bbrc.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Huang T, Yin L, Wu J, et al. MicroRNA-19b-3p regulates nasopharyngeal carcinoma radiosensitivity by targeting TNFAIP3/NF-kappaB axis. J Exp Clin Cancer Res. 2016;35(1):188. doi: 10.1186/s13046-016-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang M, Xiao J, Wang J, et al. MiR-24 enhances radiosensitivity in nasopharyngeal carcinoma by targeting SP1. Cancer Med. 2016;5(6):1163–73. doi: 10.1002/cam4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin T, Zhou F, Zhou H, et al. MicroRNA-378g enhanced radiosensitivity of NPC cells partially by targeting protein tyrosine phosphatase SHP-1. Int J Radiat Biol. 2015;91(11):859–66. doi: 10.3109/09553002.2015.1096028. [DOI] [PubMed] [Google Scholar]
- 35.Chen DL, Ju HQ, Lu YX, et al. Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res. 2016;35(1):142. doi: 10.1186/s13046-016-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang LK, Yang YT, Ma X, et al. MicroRNA-92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non-coding RNA XIST. Cell Death Dis. 2016;7:e2203. doi: 10.1038/cddis.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma L, Zhou Y, Luo X, et al. Long non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 axis in gastric cancer. Oncotarget. 2017;8(3):4125–35. doi: 10.18632/oncotarget.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]