Abstract
Objective
To estimate costs attributable to robot-assisted laparoscopic prostatectomy (RALP) as compared with open prostatectomy (OP) and laparoscopic prostatectomies (LP) in a National Health Service perspective.
Patients and methods
Register-based cohort study of 4309 consecutive patients who underwent prostatectomy from 2006 to 2013 (2241 RALP, 1818 OP and 250 LP). Patients were followed from 12 months before to 12 months after prostatectomy with respect to service use in primary care (general practitioners, therapists, specialists etc) and hospitals (inpatient and outpatient activity related to prostatectomy and comorbidity). Tariffs of the activity-based remuneration system for primary care and the Diagnosis-Related Grouping case-mix system for hospital-based care were used to value service use. Costs attributable to RALP were estimated using a difference-in-difference analytical approach and adjusted for patient-level and hospital-level risk selection using multilevel regression.
Results
No significant effect of RALP on resource-use was observed except for a marginally lower use of primary care and fewer bed days as compared with OP (not LP). The overall cost consequence of RALP was estimated at an additional €2459 (95% CI 1377 to 3540, p=0.003) as compared with OP and an additional €3860 (95% CI 559 to 7160, p=0.031) as compared with LP, mainly due to higher cost intensity during the index admissions.
Conclusions
In this study from the Danish context, the use of RALP generates a factor 1.3 additional cost when compared with OP and a factor 1.6 additional cost when compared with LP, on average, based on 12 months follow-up. The policy interpretation is that the use of robots for prostatectomy should be driven by clinical superiority and that formal effectiveness analysis is required to determine whether the current and eventual new purchasing of robot capacity is best used for prostatectomy.
Keywords: cost analysis, economics, prostate cancer, prostatectomy, robot-assisted surgery, robotics and laparoscopy
Strengths and limitations of this study.
A broad healthcare sector perspective with 12 months follow-up of a national cohort.
A strong analytical approach including a quasi-experimental difference-in-difference design in combination with the use of regression-based adjustment for selection.
Adjustment for body mass index could not be undertaken due to this information not being available in national register data.
A proportion of patients had missing values regarding cancer stage but these patients did not seem to be different from patients with complete data.
Introduction
The most common cancer among men older than 50 years is prostate cancer.1 The incidence has increased notably since the diagnostic prostate-specific antigen test was introduced and, in accordance, the incidence of prostatectomy has increased rapidly.1–3 Internationally, the transition from open prostatectomy (OP) to laparoscopic prostatectomy (LP) was much slower than the on-going transition from LP to robot-assisted laparoscopic prostatectomy (RALP), which is today the most frequently used technique in North America and in some parts of Europe.4 As a consequence of the rapid dissemination of RALP, the literature comparing RALP to LP is scarce.
The minimally invasive methods LP and RALP have been found to hold some perioperational advantages over OP such as less bleeding and fewer complications of, for example, urinary incontinence.1 5–8 The literature is, however, not definite in terms of whether these benefits of the minimally invasive approaches can be achieved equally with or without robot support.2 4 9 It has been argued that robot technology has a particular advantage in obese patients but, again, this has been questioned by a recent study demonstrating similar oncological and pathological outcomes when comparing RALP to LP and OP in obese patients.10
In comparison with not using robot support, the use of robot support leads to significantly higher costs due to the capital binding in the robot, maintenance costs and surgical supplies.4 11 12 However, there could be cost savings in the longer-term and in a broader healthcare sector perspective that outweighs the additional cost of the surgical procedure. These could flow from the better process outcomes such as less bleeding and fewer bed days. Despite the obvious relevance of a broader perspective, the literature is characterised by focussing solely on admission costs or just operating costs. The overall consequences of the dissemination of the robot technology to healthcare costs are therefore to a large extent uncertain. The objective of this study is to estimate the costs attributable to RALP as compared with OP and LP in a broad healthcare sector perspective and using a time horizon that allows for clinical manifestation of the postoperative advantages of robot support.
Patients and methods
Design
A national-scale cohort was followed from 1 year before to 1 year after prostatectomy. A quasi-experimental difference-in-difference (DID) design13 was combined with regression to adjust for pretreatment covariates (risk selection into surgical technique).14 Data were collected in connection with a Danish health technology assessment (HTA) of robot-assisted surgery, which this study is a further development of.15
Study population
Consecutive men who underwent prostatectomy in Denmark in the period 1 January 2006 to 1 august 2013 were identified from the National Patient Registry,16 using the procedural codes KKEC00, KKEC00A, KKEC00B, KKEC00C, KKEC01, KKEC01A, KKEC01B, KKEC01C, KZXX00 and ZPW00002. To enhance comparability of the patients an inclusion criterion was that the robot-assisted technique should be available at the given hospital at the time of the prostatectomy.
Data sources
Individual-level register data were extracted from national administrative registries including The Danish National Patient Register,16 The Danish Civil Registration System,17 and The Danish National Health Service Register.18 Costs were drawn from the registries for the diagnosis related grouping system (DRG) and the Danish outpatient grouping system (DAGS).19
Costs
A healthcare sector perspective was applied in this study. Thus, the study included service use within the primary sector (general practitioners, medical specialists, therapists and other privately practicing specialists) and within the hospital sector (inpatient and outpatient hospital-based activity). Primary care service was valuated via the activity-based fees and hospital-based care via the DRG/DAGS-tariffs that were used at the time of service provision. The DRG tariffs for prostatectomy cover the activity from the day of admission to the day of discharge (preparation, surgery, remobilisation and discharge) whereas follow-up visits and other events after discharge,for example, caused by complications, are therefore separately reimbursed. The specific tariffs for prostatectomy are shown in online supplementary table 1. The higher tariff of the robot-assisted surgery (on average €4525) thus refers to the rather expensive instrument kit required for each surgery, robot maintenance costs and longer operating time. The theoretical interpretation of the DRG tariff is an average long-term cost. The influence of the lack of person-individual variation in the DRG tariff as a cost estimate for the admission for prostatectomy was informed by conducting sensitivity analysis where the number of bed days was added as a proxy for cost intensity. Other sensitivity analyses included adjustment for experience with robot and patient volume, as well as restrictions to the two most recent years and exclusion of the tariffs from the costs. Costs are reported in Euros (2014 price year).
bmjopen-2016-015580supp001.pdf (37.4KB, pdf)
Identification of relevant aspects of risk selection
Characteristics that affect the choice of surgical method were identified in a literature review. Patient-level characteristics included age, cancer stage and comorbidity and hospital-level characteristics included organisational structure around the technology such as specialisation of staff. The identified characteristics were defined for the study population based on information from national registries: age (years), tumour size and nodal involvement based on the TNM-classification,20 comorbidity as defined by the Charlson Comorbidity Index,21 geographical region of the treating centre, level of experience by time of surgery (to-date volume of prostatectomies using the particular technology), and organisational structure of the surgical department, referring to whether the robot is used within a single department, used across several departments or used in a robotic centre. Finally, dummies for year of surgery were specified in order to be able to adjust for changes in DRG tariffs over the years.
Statistical analysis
Summary statistics including Pearson's χ2 tests for categorical variables and ANOVA for continuous variables were used to describe patient's characteristics. All analysis followed a DID design where the costs attributable to prostatectomy were estimated as the differences between comparators (OP, LP and RALP) of differences in resource use and costs between 12 month periods before and after prostatectomy.13 To further handle risk selection (as described in the previous section) regression models were used to adjust the DID-estimates for covariates identified to affect selection into surgical technique.14 Regressions were specified as multilevel regressions due to the patient-level being nested in the hospital-level (centres treating more than one patient) in order not to underestimate SE. The validity of regression models was visually inspected based on conventional regression diagnostic plots and found to be robust.
Results are reported as arithmetic means with 95% CI based on bootstrapping with 5000 replicates due to the skewed nature of the data. All tests were two-sided with a 5% significance level. The statistical analyses were performed in Stata SE V.13.1.
Ethics
The study was conducted in accordance with the Person Data Act and hence was approved by relevant authorities (The Danish Data Protection Agency) (Journal number 2007-58-0010). Consent is not required for register-based studies according the Danish Ethical Committee system.
Results
Of the 4309 patients included in this study 52% underwent RALP, 42% underwent OP and 6% underwent LP (cf. online supplementary table 2 for procedure volume overtime). There were 22 conversions from either RALP or LP to OP, which were categorised according to the intended technique. The characteristics of the cohort are shown in table 1. The treatment groups were clinically similar in age, though the RALP group was younger than the OP and LP group (median age 64 vs 65 years) (p<0.001). The choice of surgical technique differed geographically and with regard to the organisation of the robot technology (p<0.001). Cancer severity was routinely registered for a proportion of patients only, which could be due to the fact that nodal involvement and metastases are rarely an issue for prostatectomy candidates. However, in case of no nodal involvement patients were less likely to have received a minimally invasive technique (p≤0.001).
Table 1.
Comparison of descriptive characteristics (n (% of treatment group))
| Feature | RALP (n=2241) | OP (n=1818) | LP (n=250) | p Value |
| Age (median (25%–75% quartile)) | <0.001 | |||
| 64 (60–67) | 65 (61–68) | 65 (61–68) | ||
| Region | <0.001 | |||
| Capital Region of Denmark | 1097 (49) | 1272 (70) | 120 (48) | |
| Region of Southern Denmark | 121 (5) | 123 (7) | 12 (5) | |
| Central Denmark Region | 554 (25) | 264 (15) | 77 (31) | |
| North Denmark Region | 470 (21) | 160 (9) | 39 (16) | |
| Organisation type* | <0.001 | |||
| Within department | 878 (39) | 1009 (55) | 101 (41) | |
| Across departments | 470 (21) | 160 (9) | 39 (16) | |
| Robotic centre | 894 (40) | 650 (36) | 108 (44) | |
| Tumour size | 0.521 | |||
| T0-T2 | 847 (38) | 649 (36) | 81 (33) | |
| T3-T4 | 324 (14) | 265 (15) | 37 (15) | |
| Ta & Tis | 0 (0) | 1 (0) | 0 (0) | |
| Missing data | 1071 (48) | 904 (50) | 130 (52) | |
| Nodal involvement | <0.001 | |||
| N0 | 304 (14) | 489 (27) | 46 (19) | |
| N1-N3 | 40 (2) | 41 (2) | 3 (1) | |
| Missing data | 1898 (85) | 1289 (71) | 199 (80) | |
| Metastases | 0.001 | |||
| No | 652 (29) | 565 (31) | 46 (19) | |
| Yes | 0 (0) | 1 (0) | 0 (0) | |
| Missing data | 1590 (71) | 1253 (69) | 202 (81) | |
| CCI | 0.401 | |||
| 0 | 3 (0) | 1 (0) | 0 (0) | |
| 1 | 0 (0) | 1 (0) | 0 (0) | |
| 2 | 2230 (99) | 1810 (100) | 245 (99) | |
| 3 | 4 (0) | 2 (0) | 2 (1) | |
| 6 | 5 (0) | 5 (0) | 1 (0) |
*Organisation type refers to whether the robot is used within a single department, used across several departments or used in a robotic centre.
CCI, Charlson Comorbidity Index; LP, laparoscopic prostatectomy; OP, open prostatectomy; RALP, robot-assisted laparoscopic prostatectomy; Ta, tumour without invasion; Tis, carcinoma in situ.
Service use per patient, including length of stay, and the unadjusted mean costs of the patients’ healthcare are depicted in table 2,3 respectively. All treatment groups had statistically significant higher service use in the year following the surgery. No differences were found when comparing RALP to LP but OP was associated with 2.6 extra bed days and slightly higher primary care service use (0.5 more contacts) compared with RALP. This was, however, not reflected in the costs, as RALP was associated with the highest costs primarily caused by differences in inpatient care (table 3).
Table 2.
Healthcare service use in relation to prostatectomy
| Hospital-based care | ||||
| Primary care | Outpatient | Inpatient | ||
| No of contacts | No of admissions | No of admissions | Length of stay | |
| OP | ||||
| Before | 11.0 | 08.1 | 00.0 | 01.0 |
| After | 12.0 | 09.1 | 01.1 | 06.1 |
| Difference | 0.8 (0.5 to 1.0) | 1.0 (0.6 to 1.4) | 1.3 (1.2 to 1.4) | 5.5 (5.2 to 5.9) |
| LP | ||||
| Before | 10.0 | 07.1 | 00.0 | 01.0 |
| After | 11.0 | 08.1 | 01.0 | 04.1 |
| Difference | 0.8 (0.1 to 1.5) | 0.7 (−0.3 to 1.6) | 1.1 (1.0 to 1.2) | 3.6 (2.7 to 4.4) |
| RALP | ||||
| Before | 10.1 | 07.1 | 00.0 | 00.1 |
| After | 11.0 | 09.0 | 01.1 | 03.1 |
| Difference | 0.3 (0.1 to 0.5) | 1.2 (0.8 to 1.5) | 1.2 (1.1 to 1.2) | 3.0 (2.7 to 3.3) |
| Robot attributable service use | ||||
| Compared with OP | −0.5 (−0.8 to 0.1) | 0.2 (−0.4 to 0.7) | −0.1 (−0.2 to 0.0) | −2.6 (−3.0 to 2.1) |
| Compared with LP | −0.5 (−1.3 to 0.2) | 0.5 (−0.5 to 1.5) | 0.1 (−0.1 to 0.2) | −0.6 (−1.5 to 0.3) |
Values are mean per patient with 95% CI.
Before refers to the 12 months prior to the index surgery and after refers to the 12 months after the index surgery including the day of surgery.
LP, laparoscopic prostatectomy; OP, open prostatectomy; RALP, robot-assisted laparoscopic prostatectomy.
Table 3.
Healthcare costs in relation to prostatectomy
| Costs | Hospital-based care | |||
| Primary care | Outpatient | Inpatient | Total | |
| OP | ||||
| Before | 442 | 2720 | 1551 | 4714 |
| After | 429 | 3432 | 11 429 | 15 286 |
| Difference | −13 (−29 to 3) | 712 (493 to 931) | 9878 (9532 to 10 224) | 10 572 (10 135 to 11 010) |
| LP | ||||
| Before | 415 | 2753 | 1271 | 4440 |
| After | 416 | 2584 | 10 856 | 13 856 |
| Difference | 0 (−46to 46) | −169 (−624 to 285) | 9585 (8663 to 10 507) | 9416 (8343 to 10 489) |
| RALP | ||||
| Before | 421 | 2724 | 1242 | 4392 |
| After | 392 | 2878 | 14 700 | 17 978 |
| Difference | −29 (−43 to −15) | 154 (−18 to 325) | 13 458 (13 057 to 13 859) | 13 586 (13 132 to 14 041) |
| Robot attributable costs | ||||
| Compared with OP | −16 (−37 to 5) | −558 (−832 to −284) | 3580 (3054 to 4107) | 3014 (2380 to 3648) |
| Compared with LP | −29 (−77 to 18) | 323 (−178 to 823) | 3873 (2865 to 4882) | 4170 (2986 to 5354) |
Values are mean costs (2014, €) per patient with 95% CI.
Before refers to the 12 months prior to the index surgery and after refers to the 12 months after the index surgery including the day of surgery.
LP, laparoscopic prostatectomy; OP, open prostatectomy; RALP, robot-assisted laparoscopic prostatectomy.
Figure 1 illustrates the cost patterns overtime. The process of getting referred by the general practitioner to the hospital for diagnosis and later treatment seems to be reflected as a rise of costs in the primary care sector, is followed by a rise in outpatient care and later in inpatient care at the time of the prostatectomy. Outpatient follow-up is clearly evident but is not set at a fixed time. No clear differences stood out except for higher inpatient costs of RALP at the time of the index prostatectomy. Both in the year prior to and after the prostatectomies included in this study the patterns are rather similar especially for OP and RALP while LP fluctuates more due to fewer patients having received this surgical technique.
Figure 1.
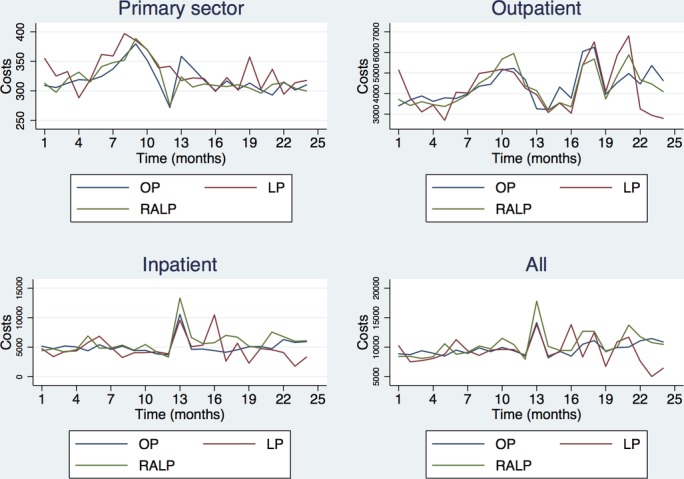
Time series graphics for the unadjusted mean costs (€). Month zero marks the time of prostatectomy, price year 2014. LP, laparoscopic prostatectomy; OP, open prostatectomy; RALP, robot-assisted laparoscopic prostatectomy.
Table 4 illustrates DID-estimates similar to those of table 3 except that multivariate modelling is used to adjust for eventual residual risk selection not handled by the DID-analytical strategy. Results support the unadjusted results as significant differences are revealed when RALP is held against OP and LP respectively. The adjusted costs attributable to RALP varied as RALP was associated with an extra €3860 (95% CI €559 to €7160) when held against LP and €2459 (95% CI €1377 to €3540) when compared with OP.
Table 4.
Adjusted estimates of the costs attributable to robot-assisted laparoscopic prostatectomy (RALP): main model compared with extended model, which includes adjustment for tumour size and nodal involvement
| Feature | Main model | Extended model | ||
| Coefficient | p Value | Coefficient | p Value | |
| Treatment | ||||
| RALP (reference) | ||||
| OP | −2459 (−3540 to −1377) | 0.003 | −2756 (−3965 to −1548) | 0.003 |
| LP | −3860 (−7160 to −559) | 0.031 | −3990 (−7073 to −906) | 0.023 |
| Age | 14 (−43 to 71) | 0.541 | 7 (−66 to 80) | 0.815 |
| Region | ||||
| Central Denmark Region (reference) | ||||
| Capital Region of Denmark | 85 (−689 to 860) | 0.775 | 881 (−833 to 2594) | 0.227 |
| Region of Southern Denmark | 1907 (610 to 3204) | 0.015 | 1882 (−13 to 3777) | 0.051 |
| North Denmark Region | 241 (156 to 327) | 0.001 | 404 (−288 to 1096) | 0.181 |
| Organisation type | ||||
| Within-speciality (reference) | ||||
| Robotic centre | 1028 (460 to 1595) | 0.007 | 978 (−181 to 2136) | 0.079 |
| Year of surgery | ||||
| 2006 (reference) | ||||
| 2007 | 376 (−264 to 1016) | 0.178 | 304 (−253 to 861) | 0.204 |
| 2008 | 1386 (−41 to 2813) | 0.054 | 1222 (−51 to 2496) | 0.056 |
| 2009 | −688 (−1627 to 250) | 0.111 | −919 (−1870 to 32) | 0.055 |
| 2010 | 910 (−540 to 2361) | 0.156 | 668 (−734 to 2070) | 0.257 |
| 2011 | 1244 (–226 to 2714) | 0.079 | 971 (−552 to 2494) | 0.151 |
| 2012 | 1423 (205 to 2641) | 0.032 | 1371 (433 to 2309) | 0.015 |
| 2013 | 3036 (1338 to 4734) | 0.008 | 3058 (1591 to 4525) | 0.004 |
| Tumour size | ||||
| T0-T2 (reference) | ||||
| T3-T4 | 1172 (683 to 1660) | 0.003 | ||
| Missing data | 1599 (−1270 to 4469) | 0.197 | ||
| Nodal involvement | ||||
| N0 (reference) | ||||
| N1-N3 | −2676 (−5796 to 444) | 0.076 | ||
| Missing data | −1219 (−2102 to −335) | 0.019 | ||
| Constant | 10 803 (7643 to 13964) | 0.001 | 11 136 (7111 to 15 161) | 0.002 |
| n | 4309 | 4309 | ||
| R2 | 0.041 | 0.046 | ||
| Root mean SE | 10 232 | 10 213 | ||
Values are mean costs (2014, €) with 95% CI.
LP, laparoscopic prostatectomy; OP, open prostatectomy; RALP, robot-assisted laparoscopic prostatectomy.
Costs were significantly higher when patients were operated in Region of Southern Denmark or North Denmark Region (p<0.05), and when they were operated in hospitals with a robotic centre (p<0.05).
An extended model was applied to assess the role of informative missings on cancer severity. Adding cancer severity to the model did not substantially affect the cost attributable to RALP. Tumours categorised as T3–T4 were associated with significant additional costs for all surgical techniques and having missing data with respect to nodal involvement was associated with decreased costs, but there was no significant interaction between either tumour size or nodal involvement and surgical technique.
Restricting the main model to activity during the two most recent years (2012 and 2013) does not significantly alter the findings (the average attributable costs increases from €2459 to €3889 compared with OP and reduces from €3860 to €3359 compared with LP).
In order to directly analyse the contribution of the index admission versus the after-period for the costs attributable to RALP, sensitivity analyses restricting the costs to the after-period alone show comparable after-periods for LP and RALP whereas the after-period for OP is characterised by significantly more activity (€2332 (95% CI €1287 to €2777)).
Discussion
Practically all prostatectomies performed in Danish hospitals over a period of 8 years were included in this analysis, which focused on the broad healthcare sector consequences of using robot technology. The cost of RALP was found to be higher than the costs of both OP and LP due to the difference in DRG tariffs across these surgical techniques. No evidence was found of RALP impacting service use when compared with LP, however, some reduction in bed days in the after-period was found when compared with OP. Hence, the main contribution of this study is an important piece of evidence that, when considering a broad healthcare sector perspective and a longer time horizon than the index admission, the use of RALP does not seem to generate cost consequences that can outweigh the additional cost associated with the index surgery.
A recent study by Hughes et al estimated the resource use in the postoperative phase after prostatectomy in a hospital perspective and found that RALP led to costs savings, when the cost of the index surgery was excluded from the equation.22 This study is in many ways similar to the present in that it is based on a large sample and considers extra-index-surgery consequences of using robot technology. It has however a couple of weaknesses, that is, circumvented in the present study. First, it includes patients who were referred to centres not offering robot technology and who could have different profiles than those referred to centres offering robot technology. Second, the investigators did not analytically handle the fact that patients were selected into surgical technique. It thus remains unclear whether the difference between the present results of no cost saving and Hughes et al's finding of a cost saving is due to these weaknesses or whether they are simply do to differences between the British and the Danish context.
Previous studies have assessed the costs of robot technology in an analytical perspective restricted to hospital costs of the index surgery. Kim et al found that RALP, despite shorter hospital stays, was associated with higher operation costs than OP by an average that more or less corresponds to the difference in Danish DRG tariffs between surgical techniques (mean $11 932 vs $9390; p<0.001).23 Similarly, Bolenz et al found hospital costs to be higher for RALP compared with LP and OP, which was a bit lower but still within the level of the difference in the Danish DRG tariffs (median $6752 for RALP, $5687 LP and $4437 for OP; p<0.001).12 These studies were conducted in the USA, that is, not normally considered to be comparable as a setting due to different system structures and price levels.
The strengths of this study relates to the design where a cohort of consecutive patients are observed and where appropriate analytical effort is made into handling selection for surgical techniques. The hybrid DID-design in combination with regression-based adjustment for pretreatment covariates serves to minimise the effect of selection bias, which can be an important issue in observational designs that may have been chosen as the only option or in priority of external validity. This design has the ability to cleanse out exogenous factors, such as time and to isolate the costs related to the prostatectomy from the costs related to, for example, chronic comorbidities or other time invariant patient characteristics.24 The design is particularly powerful when combined with extra means for handling selection and multilevel multivariate regression was here used to adjust for hospital-level characteristics as well as patient-level characteristics that could have caused confounding. It should also be mentioned that we were able to validate the consecutiveness of data and the coding of surgical techniques by comparing register data to the independent clinical database UroLap, which supported that data were truly representing consecutive patients and which gave no reason to suspect misclassification.25
In the early stages of this work, we suspected that the cost implications of robot technology would be affected by centre volume and experience with the technology. We thus included variables in the regression model for these organisational-level covariates but they appeared to be insignificant contributors and were thus excluded from the reported main model. Also, we sought to assess whether there was any effect modification from point at the learning curve by including interaction terms between the dummies of year of surgery and the cost consequences of robot technology but again, these turned out to be insignificant and were thus left out in the main model. The geographical variations found could reflect patient heterogeneity caused by both cultural and structural variations such as different waiting times and referral practice.
The main weakness of this study lies in the premises of basing it on registry data, where severity and other clinical details are not routinely recorded. One variable of relevance to choice of surgical technique would be body mass index (BMI).26 Another weakness concerns the missing values on cancer stage, as it appeared that doctors are not routinely registering TNM status in relation to prostatectomy. Tumour size was registered for about 50% of patients while nodal involvement and metastasis were registered for around 25% of patients only. Whether this reflects irrelevance of registration in relation to the choice of surgical technique and expected outcome or other reasons is unclear, but conducting parallel analysis with and without TNM status did not substantially affect results. And more importantly, patients with missing values on the TNM status did not seem to be different from patients with complete data. A number of sensitivity analyses were undertaken to address limitations of the study. First, the use of national tariffs as an expression for the patient-level cost of hospital service ignores patient-level and hospital-level variation. For example, differences in coefficient of usage are not reflected in the tariffs. A sensitivity analysis where the number of bed days was included in the model was therefore undertaken and confirmed that variation captured in bed days had no influence on the main result. This analysis is, however, no full compensation for the lack of patient-level variation and this limits the interpretation of the analysis to the broad-sector consequences of using robot technology as opposed to the technical efficiency or productivity that characterises the operation of the robot technology. Also, it should be noted that time dummies were included in the base-case model in order to take out variation that was due to changes in the DRG tariffs overtime. If centres in the future administer the robot technology (and other surgical techniques for that sake) in a more of less efficient way, for example, by operating more patients per robot this will affect the cost of index surgery (and should lead to an adjustment of the DRG tariff) whereas the main focus of this analysis, the broader-sector cost consequences, should be unaffected if the quality level is kept.
Further research seems warranted as RALP is here found to be overall more costly than its alternatives while there appears to be limited evidence for a clinical benefit to the patients. At best, a randomised controlled trial comparing RALP to both LP and OP should be conducted and followed by a cost-effectiveness evaluation. LP is a relatively rare choice of surgical approach in Denmark although it has been found to create health outcomes and functional outcomes comparable to those of RALP.3 9 27 However, there is evidence that RALP is a superior choice with regards to the risk of erectile dysfunction.28 If this was also the case in the present cohort, it was not reflected in the number of visits to neither hospitals nor the primary healthcare sector.
Conclusions
In this study from the Danish context, the use of RALP generates a factor 1.3 additional cost when compared with OP and a factor 1.6 additional cost when compared with LP, on average, based on 12 months follow-up. The policy interpretation is that the use of robots for prostatectomy should be driven by clinical superiority and that formal effectiveness analysis is required to determine whether the current and eventual new purchasing of robot capacity is best used for prostatectomy.
Supplementary Material
Acknowledgments
The authors would like to thank Line Stjernholm Tipsmark (LST) for assistance in requisitioning data and for performing preliminary analyses.
Footnotes
Contributors: Drafting the manuscript: VBH. Analysis and interpretation: VBH, RS. Statistical analysis: VBH, KRL. Concept and design: JP, RS. Acquisition of data: RS, KRL, LST. Critical revision of manuscript: JP, RS, KRL, VBH. Supervision: RS.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Unfortunately no additional data can be made available due to legal restrictions.
References
- 1. De Carlo F, Celestino F, Verri C, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: surgical, oncological, and functional outcomes: a systematic review. Urol Int 2014;93:373–83. 10.1159/000366008 [DOI] [PubMed] [Google Scholar]
- 2. Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 2009;55:1037–63. 10.1016/j.eururo.2009.01.036 [DOI] [PubMed] [Google Scholar]
- 3. Close A, Robertson C, Rushton S, et al. Comparative cost-effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of men with localised prostate cancer: a health technology assessment from the perspective of the UK National Health Service. Eur Urol 2013;64:361–9. 10.1016/j.eururo.2013.02.040 [DOI] [PubMed] [Google Scholar]
- 4. Bolenz C, Freedland SJ, Hollenbeck BK, et al. Costs of radical prostatectomy for prostate cancer: a systematic review. Eur Urol 2014;65:316–24. 10.1016/j.eururo.2012.08.059 [DOI] [PubMed] [Google Scholar]
- 5. Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol 2012;61:679–85. 10.1016/j.eururo.2011.12.027 [DOI] [PubMed] [Google Scholar]
- 6. Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology 2010;75:1092–7. 10.1016/j.urology.2009.09.075 [DOI] [PubMed] [Google Scholar]
- 7. Davis JW, Kreaden US, Gabbert J, et al. Learning curve assessment of robot-assisted radical prostatectomy compared with open-surgery controls from the premier perspective database. J Endourol 2014;28:560–6. 10.1089/end.2013.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hofer MD, Meeks JJ, Cashy J, et al. Impact of increasing prevalence of minimally invasive prostatectomy on open prostatectomy observed in the national inpatient sample and national surgical quality improvement program. J Endourol 2013;27:102–7. 10.1089/end.2012.0315 [DOI] [PubMed] [Google Scholar]
- 9. Berge V, Berg RE, Hoff JR, et al. A prospective study of transition from laparoscopic to robot-assisted radical prostatectomy: quality of life outcomes after 36-month follow-up. Urology 2013;81:781–6. 10.1016/j.urology.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 10. Busch J, Gonzalgo ML, Leva N, et al. Matched comparison of robot-assisted, laparoscopic and open radical prostatectomy regarding pathologic and oncologic outcomes in obese patients. World J Urol 2015;33:397–402. 10.1007/s00345-014-1326-1 [DOI] [PubMed] [Google Scholar]
- 11. Hohwü L, Borre M, Ehlers L, et al. A short-term cost-effectiveness study comparing robot-assisted laparoscopic and open retropubic radical prostatectomy. J Med Econ 2011;14:403–9. 10.3111/13696998.2011.586621 [DOI] [PubMed] [Google Scholar]
- 12. Bolenz C, Gupta A, Hotze T, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol 2010;57:453–8. 10.1016/j.eururo.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 13. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA 2014;312:2401–2. 10.1001/jama.2014.16153 [DOI] [PubMed] [Google Scholar]
- 14. Abadie A. Semiparametric difference-in-differences estimators. Rev Econ Stud 2005;72:1–19. 10.1111/0034-6527.00321 [DOI] [Google Scholar]
- 15. CFK Folkesundhed & Kvalitetsudvikling Region Midtjylland. Medicinsk teknologivurdering af robotassisteret kirurgi (HTA of robot-assisted surgery). Aarhus, 2015. [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39:30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 17. CPR-Kontoret. Udtræksvejledning for offentlige brugere. version 10. Denmark, 2015:1–98. [Google Scholar]
- 18. Andersen JS, Olivarius NF, Krasnik A. The Danish National Health Service Register. Scand J Public Health 2011;39:34–7. 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 19. Sundhedsstyrelsen. Takstberegning for sygehusene. version 2.0. Copenhagen, 2009:12. [Google Scholar]
- 20. NIH National Cancer Institute. Cancer staging [Internet]. http://www.cancer.gov/about-cancer/diagnosis-staging/staging/staging-fact-sheet (accessed 2 Oct 2015).
- 21. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22. Hughes D, Camp C, O'Hara J, et al. Health resource use after robot-assisted surgery vs open and conventional laparoscopic techniques in oncology: analysis of English secondary care data for radical prostatectomy and partial nephrectomy. BJU Int 2016;117:940–7. 10.1111/bju.13401 [DOI] [PubMed] [Google Scholar]
- 23. Kim SP, Shah ND, Karnes RJ, et al. Hospitalization costs for radical prostatectomy attributable to robotic surgery. Eur Urol 2013;64:11–16. 10.1016/j.eururo.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 24. Imbens GW, Wooldridge JM. Recent developments in the econometrics of program evaluation. J Econ Lit 2009;47:5–86. 10.1257/jel.47.1.5 [DOI] [Google Scholar]
- 25. Dansk Urologisk Selskab. UroLap. København: Årsrapport 2013, 2014. [Google Scholar]
- 26. Ramsay C, Pickard R, Robertson C, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess 2012;16:1-313 10.3310/hta16410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kasraeian A, Barret E, Chan J, et al. Comparison of the rate, location and size of positive surgical margins after laparoscopic and robot-assisted laparoscopic radical prostatectomy. BJU Int 2011;108:1174–8. 10.1111/j.1464-410X.2010.10077.x [DOI] [PubMed] [Google Scholar]
- 28. Asimakopoulos AD, Pereira Fraga CT, Annino F, et al. Randomized comparison between laparoscopic and robot-assisted nerve-sparing radical prostatectomy. J Sex Med 2011;8:1503–12. 10.1111/j.1743-6109.2011.02215.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015580supp001.pdf (37.4KB, pdf)


