Significance
In this study, we characterize the developmental mechanisms shaping body size in the solitary bee pollinator, Osmia lignaria. This study manipulates larval development in a solitary bee to understand how developmental mechanisms shape adult body size. We based our approach on the insect body size model that postulates a critical weight is necessary for normal metamorphosis. However, our study identified food absence as a cue for metamorphosis in this species, rather than a “critical weight.” These data directly challenge the ubiquity of critical weights and offer insights about context dependency of metamorphic regulation. Developmental mechanisms shape size variation in solitary bees, which will have strong effects on their capabilities as pollinators.
Keywords: insect body size model, critical weight, solitary bees, body size, pollinator
Abstract
Body size is an important phenotypic trait that correlates with performance and fitness. For determinate growing insects, body size variation is determined by growth rate and the mechanisms that stop growth at the end of juvenile growth. Endocrine mechanisms regulate growth cessation, and their relative timing along development shapes phenotypic variation in body size and development time. Larval insects are generally hypothesized to initiate metamorphosis once they attain a critical weight. However, the mechanisms underlying the critical weight have not been resolved even for well-studied insect species. More importantly, critical weights may or may not be generalizable across species. In this study, we characterized the developmental aspects of size regulation in the solitary bee, Osmia lignaria. We demonstrate that starvation cues metamorphosis in O. lignaria and that a critical weight does not exist in this species. Larvae initiated pupation <24 h after food was absent. However, even larvae fed ad libitum eventually underwent metamorphosis, suggesting that some secondary mechanism regulates metamorphosis when provisions are not completely consumed. We show that metamorphosis could be induced by precocene treatment in the presence of food, which suggests that this decision is regulated through juvenile hormone signaling. Removing food at different larval masses produced a 10-fold difference in mass between smallest and largest adults. We discuss the implications of body size variation for insect species that are provided with a fixed quantity of provisions, including many bees which have economic value as pollinators.
Body size is one of the most striking aspects of variation that occurs both within and among different species. Size correlates with performance and fitness, and their relationships are of central importance in life history theory (1), metabolic theory (2, 3), bioenergetics (4, 5), and ecological and evolutionary physiology (6, 7). For this reason, the developmental basis of size variation is of growing interest, especially common elements that cut across taxa (8). Theoretical and conceptual life history models have hypothesized that developmental thresholds shape patterns of adult size variation (9, 10), and the idea of a size-dependent basis of maturation is pervasive (9, 11).
The insect body size model integrates genetic, tissue signaling, and hormonal elements that regulate the developmental basis of adult size variation (12). In tandem with increasingly detailed identification and characterization of mechanisms shaping body size (12–15), there has been an effort to simplify this complexity for the purposes of generalization and predictability (8, 16). Three factors contribute to adult body size and serve as proxies for the endocrine regulation of metamorphosis: larval growth rate, the critical weight that induces metamorphosis, and the interval between the critical weight and cessation of growth (8, 16–19). The “critical weight” refers to a mass threshold at which the growing larvae becomes committed to metamorphosis physiologically, “growth cessation” occurs when the larva stops feeding and gut purges before pupation, and the interval between the critical weight and growth cessation is the “interval to the cessation of growth,” or “terminal growth period” (12, 17, 18, 20). Collectively, variation in these factors explains adult body size variation in Manduca sexta and Drosophila melanogaster, including variation in response to different environmental conditions (21–23) and selection under laboratory conditions (24–26). Of the three factors, the critical weight is the most important, representing the decision to commit to metamorphosis (8, 19, 20), and is thus a central component of understanding size variation.
The intrinsic or extrinsic cues that larvae use to sense that they have reached the critical weight remain unresolved. Seminal work with the kissing bug, Rhodnius prolixus, demonstrated that stretch receptors in the abdomen distend following a blood meal, inducing the molt to the adult form (27–29). While this early success inspired research to determine the intrinsic factor that initiates metamorphosis in other insects, the cue for metamorphosis at the critical weight remains elusive. A single cue has not been definitely identified, rather, multiple factors regulate size assessment at the critical weight in other insects. Research in M. sexta and D. melanogaster have demonstrated that oxygen sensing (23), growth of imaginal and endocrine tissues (30–34), and resource storage (35) contribute to intrinsic assessment of size. The critical weight can respond plastically to environmental factors including prior growth history (36), nutritional quality (21, 22), oxygen availability during growth (23, 37), and temperature (ref. 20, but see ref. 21). These environmental variables cannot induce metamorphosis in a size-independent manner, but they contribute to the critical weight decision. A priori, there are no reasons to assume that the cues driving metamorphic commitment are general across species or even within a species.
Although the insect size model attempts to generalize, there is growing consensus that the cues for metamorphosis are diverse, and the physiological mechanisms regulating metamorphic commitment can vary among different species. Some species undergo metamorphosis independent of a critical weight under certain conditions (11). For example, food removal induces metamorphosis in the scarab beetle, Onthophagus taurus, which initiates metamorphosis when food has been consumed or removed although the physiological basis for this response remains unexplored (38). Separately, metamorphosis can be initiated in M. sexta when the endocrine systems controlling the juvenile hormone (JH) biosynthesis are ablated before the terminal instar and when larvae consume food for a brief duration in the early terminal instar, suggesting that a critical weight acts as a checkpoint but is not necessary for metamorphosis (39). In other cases, metamorphic commitment is determined by entirely different physiological mechanisms. The insect size model is based on studies of M. sexta, in which JH biosynthesis regulates the critical weight, whereas metamorphic commitment appears to be regulated by ecdysone in D. melanogaster (40, 41). The overall complexity of metamorphic regulation among these species suggests that a detailed understanding of the mechanisms themselves is absolutely essential for modeling and predicting body size variation in different species of insects. Even more importantly, detailed studies of more species are necessary for building a greater comparative understanding of these important developmental mechanisms.
Insect pollinators are critical for the reproductive success of many plant communities—both natural and agricultural (42). For the reasons described above, bee size variation is expected to have a strong role in pollination efficacy of individuals and in the reproductive dynamics of pollinator populations. Many performance traits covary with body size and morphology, including better flight ability (43, 44), vision (45), and thermoregulation (46, 47). Larger bees typically gather more pollen in fewer trips to provision for their offspring, suggesting reproductive advantages. Furthermore, size predicts which flowers individual bee species prefer and affects fruit production (48). Adult body size variation responds to environmental conditions (49, 50), but the developmental mechanisms that are responsible for body size variation in bees are poorly described. Understanding the developmental mechanisms shaping size variation in these particular insects will deepen our understanding of their pollination abilities and population dynamics.
Our aim was to begin characterization of developmental factors that regulate body size in the solitary bee, Osmia lignaria (Hymenoptera: Megachilidae). During the early spring, female O. lignaria construct nests in existing cavities. Individual females gather pollen and nectar that are rolled into distinct provision balls for each of her offspring. A single egg is deposited onto a provision ball, which the female then seals into a nest cell using clay. Nest cells are constructed in the cavity until the female caps the nest and begins searching for a new nest site. Larvae hatch and then grow while consuming provisions. Before metamorphosis, each larva wraps itself in a silk cocoon, which demarcates transition to the prepupal stage of development. Metamorphosis completes during the late summer or fall, and the adult overwinters until favorable temperatures are experienced the following spring.
Results
Food Absence Cues Metamorphosis—Not a Critical Weight—in O. lignaria.
Our initial experiment sought to determine the critical weight in O. lignaria, but instead resulted in an unexpected discovery—food absence was the cue for metamorphosis in this species. The critical weight for metamorphosis is traditionally determined by starving larvae at different sizes and measuring the delay from the time of starvation to pupation (20, 23, 35, 51, 52). The larval mass at which additional food is not required for a normal time course to pupation is defined as the critical weight, which coincides with the cessation of JH biosynthesis in M. sexta (20, 51, 52), although JH is not the accepted mechanistic basis in D. melanogaster (41, 53). We assigned fifth instar O. lignaria larvae of different masses to either a starved treatment (n = 96), in which food was removed, or a fed treatment (n = 96), in which provisions were provided ad libitum.
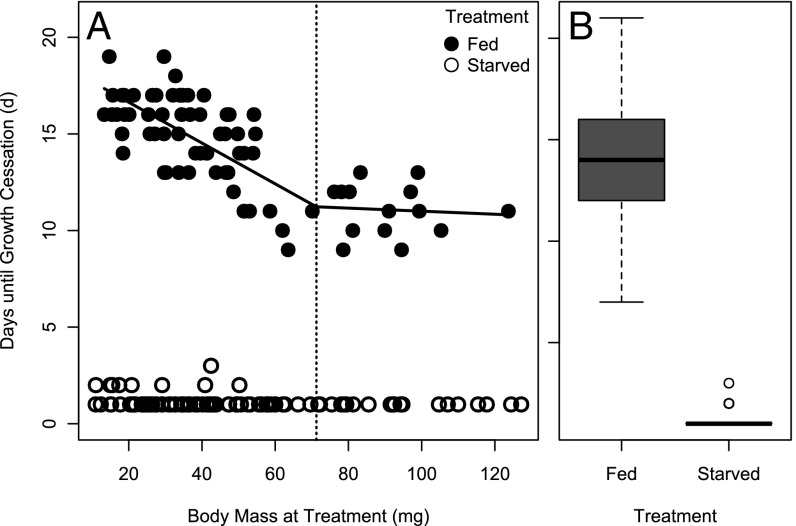
Contrary to expectations, starvation initiated the immediate onset (<24 h) of metamorphosis at all larval body masses tested for most individuals (Fig. 1). A few larvae took somewhat longer to begin metamorphosis, but still began spinning cocoons <72 h and far less than fed individuals (Fig. 1). This result is the complete opposite pattern from that observed in M. sexta and D. melanogaster, which delay metamorphosis if starved below the critical weight (20, 22, 23, 30, 35, 51, 52). While the effects of provision treatment (ANOVA, F1,185 = 2,928.34, P < 0.0001), body mass (ANOVA, F1,185 = 121.2, P < 0.0001), and their interaction (ANOVA, F1,185 = 30.1, P < 0.0001) all weighed heavily on the duration of growth until the prepupal stage, two key problems made the determination of critical weight in O. lignaria untenable. First, the development times of starved and fed individuals never converged, making it impossible to know whether starvation caused a delay in development relative to feeding (Fig. 1). We observed no larval body mass at which the timing of growth cessation became invariant with respect to food removal, as expected with other critical weights for metamorphosis (20, 22, 23, 30, 35, 51, 52). Second, instead of starvation delaying development, excess feeding delayed development (Fig. 1). These results were consistent across the full range of weights of larvae capable of metamorphosis. In sum, the size range of larvae tested would have been sufficient to capture the critical weight, if one had existed.
Fig. 1.
Days until growth cessation for larvae that were provided an excess of food provisions (Fed) or that had food provisions removed (Starved). (A) The relationship between days until growth cessation and larval mass. For the fed treatment, a bisegmented regression (lines) was performed to test for a switch in the response to excess feeding along larval growth. (B) Distribution of days until growth cessation relative to treatment.
Minimum Viable Weight and Upper Limits of Size.
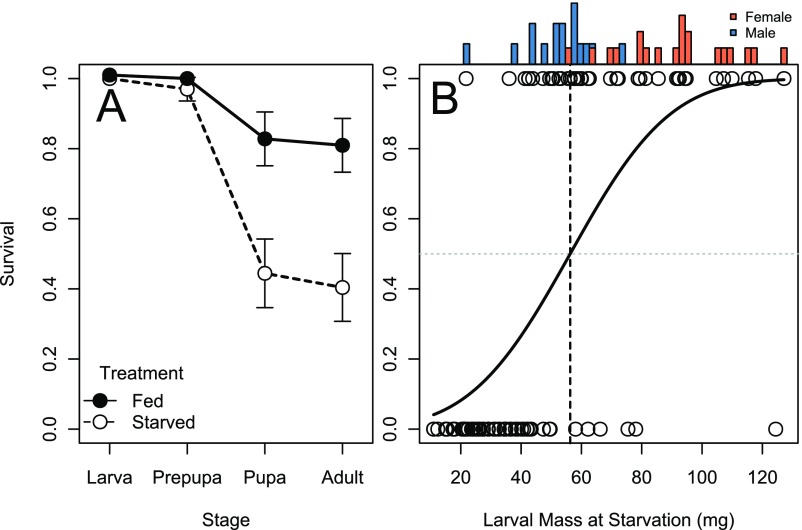
We calculated the minimum viable weight in O. lignaria to be 52.88 ± 4.68 mg (Fig. S1). Remarkably, some individuals as small as 25 mg still successfully reached adulthood, although survivors below the minimum viable weight were predominantly male (Fig. S1B). This suggests that there may be sex-specific differences in the minimum viable weight; however, this could not be confirmed or refuted because larvae, and pupae could not be reliably sexed. To determine the upper limit of body size, we used break-point analysis to calculate a size at which development time became invariant with respect to ad libitum feeding (7, 22). We found a significant shift in the relationship between development time and body size with respect to ad libitum feeding at 75.2 mg (Fig. 1; Davie’s test, P = 0.021), indicating a significant change in slope at this body size (Fig. 1A). However, this change in slope was not supported when each sex was considered separately (males, P = 0.2451; females, P = 0.169).
Fig. S1.
The proportion of surviving individuals during larval, prepupal, pupal, and adult stage in response to ad libitum feeding and food removal (A). Binomial regression of survival in response to food removal at different larval masses and frequency histograms of males (blue) and females (coral) that survived to adulthood (B). The horizontal dashed gray line represents 50% survival, and the vertical dashed black line represents the calculated minimum viable weight.
Food Manipulation Alters Subsequent Development.
Larval feeding treatments had strong impacts on the duration of the larval growth stage, prepupal stage, and timing of pupation (Figs. 1 and 2). Ad libitum-fed larvae prolonged larval growth 14 d on average. Larvae below 75.2 mg showed delays that were inversely proportional to the body mass at which they were assigned to feeding treatment (Fig. 1A). Larvae above this mass delayed development ∼12 d, but the duration of larval growth was invariant with respect to body mass at treatment assignment (Fig. 1A).
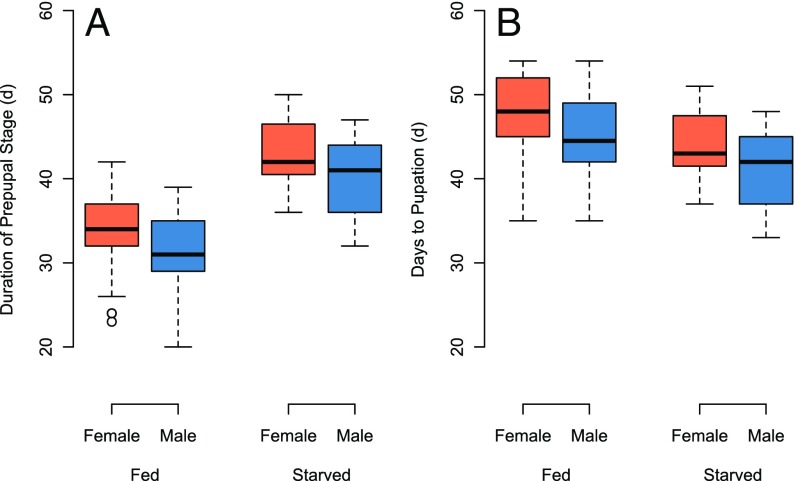
Fig. 2.
Boxplots of the duration of the prepupal period (A) and the days until pupation (B) of female and male O. lignaria that were fed ad libitum or starved at different masses during the larval stage.
Because ad libitum feeding prolonged larval growth by 2 wk, we predicted that subsequent developmental stages would also be delayed by an average of 14 d. However, the duration of the prepupal stage was 9.43 ± 1.26 d longer for starved larvae compared with those fed ad libitum (Fig. 2A; ANOVA, treatment, F1,106 = 97.339, P < 0.0001). Males had consistently shorter prepupal periods than females (Fig. 2A; ANOVA, sex, F1,106 = 8.500, P = 0.0043). As a consequence, slight differences in the time until pupation persisted (Fig. 2B; ANOVA, treatment, F1,106 = 11.484, P < 0.001), but the difference in the time until pupation was reduced to 4.07 ± 1.28 d (Fig. 2B). Males pupated earlier than females (Fig. 2B; ANOVA, sex, Fs = 15.894, P = 0.0001). Although differences in mean values were statistically significant, variances in the time to pupation among treatments and sexes overlapped graphically, suggesting that pupation times of individuals from fed and starved treatments converged despite an initial 14-d difference in the duration of the larval growth stage (Fig. 2B).
Feeding treatments did not cause differences in survival before growth cessation (Fig. S1A; Pearson’s χ2 Test, X2 = 1.232, P = 0.27). This is unsurprising, because food removal induced immediate transition to the prepupal stage in starved larvae. Survival declined during the prepupal stage for both fed and starved treatments (Fig. S1A), and this decline was more severe among individuals assigned to the starved treatment (Pearson’s χ2 Test, X2 = 28.641, P < 0.0001). Following pupation, there were minor declines in survivorship to adulthood for both fed and starved treatments (Fig. S1A), and differences in survivorship persisted through the pupal stage (Pearson’s χ2 Test, X2 = 34.443, P < 0.0001).
Manipulation of JH via Precocene.
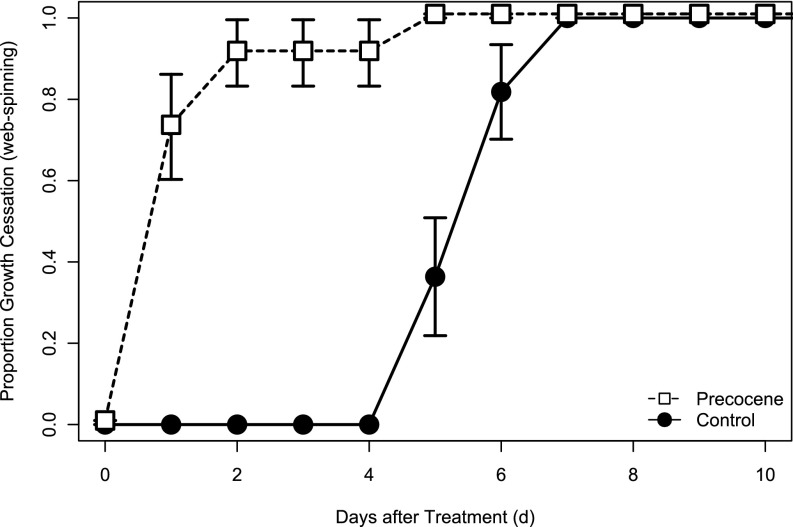
To provide deeper insights into the physiological determinants of body size variation in O. lignaria, we wanted to understand how food removal related to the underlying hormonal dynamics of metamorphic induction. To this end, we conducted a study to test the hypothesis that food removal induced a decline in JH biosynthesis in O. lignaria, thus committing the larvae to growth cessation at the next ecdysone signaling event. We experimentally inhibited JH biosynthesis by applying precocene, a chemical that ablates corpora allata cells that produce JH (29). Our prediction was that precocene application would mimic starvation, with larvae spinning cocoons within 24 h of precocene application. Larvae were reared on their maternal provisions, and a 1.5-µg dose of precocene dissolved in acetone or acetone control was applied topically. Treatment with precocene induced growth cessation and cocoon-spinning behavior within 48 h, whereas individuals treated with acetone alone continued larval feeding unabated until food was consumed entirely (Fig. 3, treatment, F1,20 = 83.221, P < 0.0001). In summary, precocene-mediated knockdown of JH biosynthesis induced growth cessation and cocooning-spinning responses that were directly analogous with removal of food provisions, suggesting that an absence of JH biosynthesis is responsible for metamorphic commitment in O. lignaria.
Fig. 3.
Proportion of individuals that underwent growth cessation at different times following treatment with either 1.5 µg of precocene or an acetone control. Larvae were provisioned with their natural provision.
Adult Body Size Variation.
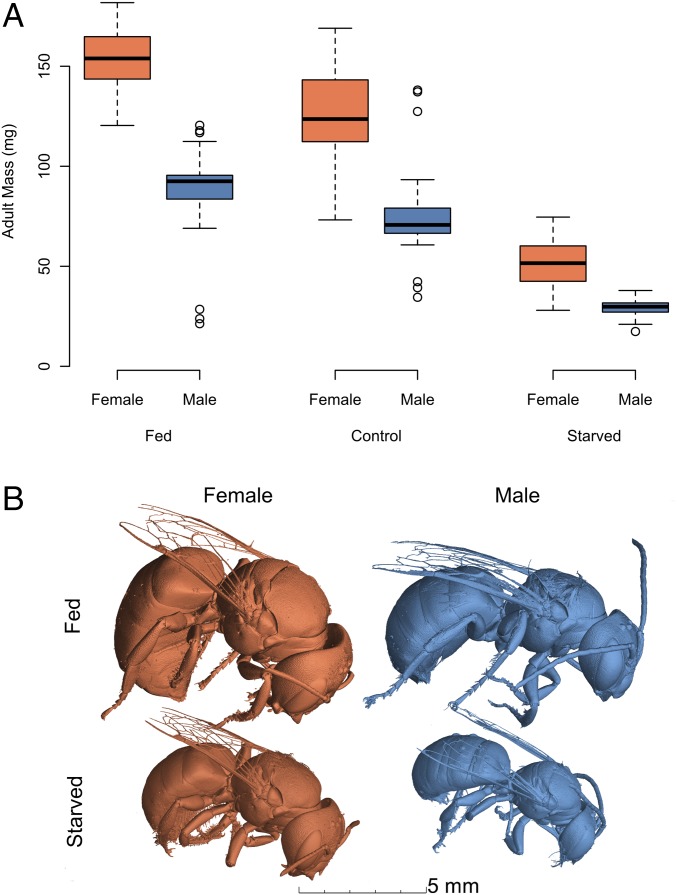
The quantity of consumed larval provisions had significant effects on adult body mass (Fig. 4). Fed individuals were larger than larvae that were provisioned naturally, whereas starved individuals were smaller (Fig. 4: ANOVA, treatment, F2,184 = 215.69, P < 0.0001). As expected for a dimorphic species, females were larger than males (ANOVA, sex, F1,184 = 383.36, P < 0.0001) among different feeding treatments, although the degree to which this dimorphism manifested depended on treatment (Fig. 4: ANOVA, treatment × sex, F1,184 = 14.74, P < 0.0001). In the ad libitum feeding treatment, females were 20% larger than females reared on natural provision quantities; males were 17% larger. In the starved treatment, females were 40% smaller than those from the natural population, and males were 38% smaller. Across all treatments, food manipulation treatments induced nearly twice the variation observed in the natural population, and there was a 10-fold difference in mass between smallest and largest individuals in the study.
Fig. 4.
Boxplots of female and male adult mass following ad libitum (fed), natural (control), and food removal (starved) treatments (A). For each treatment, one individual was scanned using micro-computed tomography to illustrate the size differences between males and females that had food removed early or were fed ad libitum (B).
Discussion
Larval Developmental Mechanisms and Their Relationship to Feeding Ecology in O. lignaria.
Our data suggest that we have elucidated an extrinsic cue for metamorphosis in the solitary bee, O. lignaria. Larvae initiated pupation when we starved O. lignaria larvae at a wide range of body sizes (Fig. 1). These results suggest that complete consumption of the larval provision is an extrinsic cue that initiates metamorphosis under natural conditions. Starvation following food absence may trigger metamorphosis through the conserved hormonal cascade that is present in all insects—specifically JH biosynthesis. Chemical inhibition of the JH synthesis in O. lignaria larvae with copious provisioning induced metamorphosis in an identical manner to food removal. These results strongly support the hypothesis that food absence, independent of a critical weight, is the cue for metamorphic commitment in solitary bees.
This interpretation challenges the insect body size model by questioning the critical weight as a generalizable mechanism of body size regulation for many holometabolous insects. O. lignaria were able to initiate metamorphosis over a wide range of body sizes that exceed those observed in nature, suggesting that a critical weight does not exist, as understood in M. sexta and D. melanogaster. A very similar type of metamorphosis has been documented in some beetle species (38, 54, 55), which can respond to food removal rather than a mass-threshold critical weight. This response has been called a “bailout response,” because it is presumed that the larva speeds its developmental time course in a deteriorating environment. For example, D. melanogaster shortens development when food provisions are absent postcritical weight, but will delay metamorphosis if precritical weight (11). This is not the case for O. lignaria, where metamorphosis simply occurs when provisions are removed and not in response to a developmental threshold. Moreover, this study provides evidence that the responses observed were mediated through JH signaling, and to our knowledge there is no other empirical evidence of what may regulate size-independent metamorphosis. Further characterization of hormonal dynamics are required to test whether a decline in JH occurs at starvation under normal circumstances. Taken together, however, multiple species have now shown metamorphosis may be induced without body size checkpoints.
O. lignaria larvae displayed an unusual response to overfeeding compared with insect species with a critical weight. Extension of the growth period decreased with larval body size (Fig. 1), up to a mass of ≈75 mg. Above this mass threshold, the degree to which growth was prolonged became invariant with respect to the size of the larvae (Fig. 1). For other species of insects, the critical weight is typically measured as the weight at which food removal no longer delays growth cessation (20, 22, 30, 35, 51, 52); however, we observed that O. lignaria have a body mass at which growth duration becomes invariant with respect to additional feeding. This observation raises an important comparative question: What stops growth in the absence of food removal in O. lignaria? Perhaps, food absence serves as a strong primary cue for metamorphic induction in O. lignaria, but secondary cues trigger the endocrine control of metamorphosis as the larva grows to larger sizes. This suggests metamorphic induction may have multiple cues that change in relevance as the larva grows, rather than one all-inclusive cue as conceptualized in the traditional insect body size model (i.e., a single critical weight with a single cue). Environmental conditions and/or the evolved natural context of each species may change the manner in which metamorphosis is induced, but also the relative importance of different cues may change along a single individual’s growth trajectory.
In summary, the range of potential body sizes was bounded by the minimum viable weight on the low end and an undefined constraint on the upper end. The minimum viable weight is the body size necessary for 50% of individuals to reach adulthood and is distinct from the critical weight for metamorphosis (18, 20, 30). Individuals below this weight tend to experience disproportionately high mortality when food is removed, although they do try to spin cocoons. Many did not survive the prepupal phase (Fig. 2), which suggests they did not have the metabolic reserves to complete development. When fed ad libitum, larvae underwent metamorphosis after a long delay. This suggests that there may be constraints or factors limiting larval growth when larvae do not run out of provisions.
The Effects of Provision Size on Progression of Metamorphosis.
Duration of metamorphic stages varies among distinct geographically isolated populations of O. lignaria (56, 57). Interestingly, the prepupal period shows the most significant variation among populations (57), and population variance during the prepupal stage is responsible for synchronizing development among individuals within populations and variation among different populations (56). We observed that removing food early or providing excess food altered the duration of larval and prepupal stages. Prepupa that had food removed early showed disproportionately longer prepupal stages compared with individuals that had been provided food ad libitum. Thus, the duration of larval growth and prepupal development counterbalanced one another such that there were reduced differences in the timing of pupation with respect to feeding treatment (Fig. 3). These counterbalancing interactions may serve to synchronize individuals in a common population that have been provisioned differently or at different times (56).
The prepupal stage of O. lignaria is unusual among solitary bees in the Megachilidae family because there is a hypothesized dormancy period during the summer prepupal stage (57, 58), followed by a second overwintering dormancy in the adult. During the prepupal dormancy, metabolic rate decreases substantially in a manner similar to diapause, metabolic resources are retained, and there is an overall resilience to warm summer temperatures (57, 58). In this study, individuals that were provisioned less in the larval growth period may have prolonged the duration of this dormancy to preserve metabolic resources for subsequent development and overwintering. Individuals that have maximal metabolic resources (i.e., were fed ad libitum) may shorten or even skip this dormancy because preservation of resources is less of an issue, especially if there is a risk of incomplete adult development before winter conditions (57). This may explain the differences in prepupal stage duration that resulted from manipulation of food provisions.
Consequences and Developmental Implications of Size Variation for Bee Pollinators.
Body size in O. lignaria can vary substantially beyond the size range commonly seen in nature with a 10-fold mass difference potential between the smallest and largest adult bees. Thus, the range of sizes that are developmentally possible far exceeds the range observed in the natural population from which O. lignaria in this study were derived. Body size has very low heritability in Osmia spp. (59), which suggest that body size is constrained by ecological and behavioral factors over relatively short time frames, i.e., within a single generation. Tradeoffs inherent to parent-offspring provisioning (60–64) and different ecological conditions (65, 66) are likely to play a predominant role in natural populations. This high degree of plasticity ensures that females can produce viable offspring in a wide variety of contexts. Although measuring body size consequences of experimentally induced size variation is far removed from knowing how well large or small bees perform in the field as pollinators, the responses documented here serve as an important basis for understanding links between larval development and adult phenotype.
We hypothesize that food provisioning as a cue for developmental regulation of metamorphosis may widely occur among Hymenoptera. Almost all solitary bees species construct nests with cells and provision their individual offspring with a fixed quantity of food resources (67). Even for predatory and parasitoid species of Hymenoptera, food resources that are available for offspring are discretely limited, and provision absence may induce metamorphosis in these species, as well. Evolutionarily, the developmental basis of size variation in solitary hymenopterans is ancestral to eusocial species, which frequently show distinct size variations associated with caste. Critical size thresholds shape both intraspecific and interspecific variation in body size among ant castes (68, 69) and infrequent feeding contributes to timing of metamorphosis in bumblebees (70); however, empirical studies have not been conducted which can confirm or refute this hypothesis directly for these species.
The feeding ecology of insects falls along a continuum: species that consume potentially limitless food resources and species that have a discrete quantity of a resources (71). Many hymenopterans, including solitary bees such as O. lignaria, fit into the discrete-resource category, because larvae feed exclusively on a discrete quantity of food (67). Other insects that fall into the discrete-resource category include parasitic, seed-eating, and dung-feeding insects (71). Insects with discrete resources are likely to have different metamorphic responses than M. sexta and D. melanogaster, because once larvae consume their provisions, there is no possibility of obtaining more.
In conclusion, solitary bees incorporate a different perspective for the developmental basis of size variation in insects because their larval ecology—and corresponding physiology—contrasts with traditionally studied models. Understanding the developmental mechanisms shaping body size in bees is critical given their central role in pollination. O. lignaria shares a similar feeding ecology with many insects, and we predict this extrinsic cue to metamorphosis would apply across many insect groups. Our finding that food absence serves as a critical factor driving the physiology of metamorphosis is applicable across many species of bees, and any insect feeding on a discrete food source. Thus, ecology may drive the evolution of metamorphic cues, resulting in diverse mechanisms that are reliable and resilient in different life history contexts among the Insecta.
Materials and Methods
Food Manipulation Experiment.
Bamboo nesting reeds were obtained from Crown Bees in May 2014. Freshly capped nests were mailed overnight from Woodinville, Washington, to Fargo, North Dakota, and then placed immediately into a 25 °C environmental chamber. Final instar larvae were removed from nesting reeds, weighed (UMT2; Mettler Toledo), and placed into 24-well plates. Larvae were randomly starved (n = 96) or fed an ad libitum amount of food (n = 96). In the starved treatment, food provisions were immediately removed from larvae following weighing. In the ad libitum treatment, provisions mixed from multiple brood cells were then provided on a continuous basis. Starved and fed larvae were alternated into adjacent wells of 24-well plates, which were then placed into water-tight rearing containers. Larvae were kept in darkness at 25 °C and 75% humidity—maintained by placing a saturated NaCl solution into rearing containers—in an incubator, except when removed for daily observations. Larvae were monitored daily for survival, cocoon spinning, and pupation.
Comparison of Adult Sizes.
All surviving larvae in the fed and starvation treatments were permitted to complete metamorphosis to the adult stage and were then weighed. So that feeding manipulations could be compared with natural body size variation, a separate set of larvae were reared in bamboo nesting reeds without food manipulation (n = 80). These adults were extracted from nest reeds, weighed, and compared with starved and fed treatments.
Precocene Treatment.
To test the hypothesis that developmental responses to starvation were physiologically analogous with the critical weight, we topically applied 1.5 µg of the JH antagonist precocene dissolved in 1 µL of acetone (n = 24). Larvae were monitored daily following treatment, and indicators of metamorphic commitment (described above) were scored. The number of days to cocoon spinning was recorded and compared with control individuals that were treated with 1 µL of acetone (n = 24).
Statistical Analysis.
All statistical analyses were performed using R version 3.2.0. Time to growth cessation for starved and fed treatments were compared by using analysis of variance and post hoc tests. Broken lines regression was performed on time to growth cessation using the “segmented” package for R. Following metamorphosis, adult masses were compared using analysis of variance and post hoc tests. Stage-specific survival was compared using Pearson’s χ2 Test with Yate’s continuity correction. A minimum viable weight was calculated as the body mass at which 50% of individuals survive without additional food. This was determined by modeling survival as a logistic regression with larval mass at the time of food removal as the independent variable. The 50% value and SEs were calculated using the dose.p function from the “MASS” package. For precocene experiments, the proportion of individuals that underwent growth cessation in the days following precocene treatment was compared with control using a generalized linear model with probit link function.
Acknowledgments
We thank J. Bosch, W. Kemp, G. Davidowitz, F. Nijhout, Y. Suzuki, B. Heidinger, N. Dochtermann, R. Royaute, and R. Mallinger for providing intellectual feedback that shaped the outcomes of this study. M. Larson and A. Rajamohan helped develop experimental procedures. D. Hunter of Crown Bees provided larval O. lignaria for this study. This manuscript was significantly improved by the critiques and suggestions of two anonymous reviewers. This work was supported by National Science Foundation Grant IOS 155794, funding and resources from the US Department of Agriculture Agricultural Research Service Insect Genetics and Biochemistry, and funding from the Department of Biological Sciences at North Dakota State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703008114/-/DCSupplemental.
References
- 1.Calder WA. Size, Function, and Life History. 2nd Ed Dover Publications; Mineola, NY: 1996. [Google Scholar]
- 2.Brown JH, West GB, Enquist BJ. Scaling in biology: Patterns and processes, causes and consequences. In: Brown JH, West GB, editors. Scaling in Biology. Oxford Univ Press; New York: 2000. pp. 1–24. [Google Scholar]
- 3.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 4.Kooijman SALM. Quantitative aspects of metabolic organization: A discussion of concepts. Philos Trans R Soc Lond B Biol Sci. 2001;356:331–349. doi: 10.1098/rstb.2000.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooijman SALM. Dynamic Energy Budget Theory. 3rd Ed Cambridge Univ Press; New York: 2010. [Google Scholar]
- 6.Chown SL, Gaston KJ. Body size variation in insects: A macroecological perspective. Biol Rev Camb Philos Soc. 2010;85:139–169. doi: 10.1111/j.1469-185X.2009.00097.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Nielsen K. Scaling: Why Is Animal Size so Important? Cambridge Univ Press; New York: 1984. [Google Scholar]
- 8.Davidowitz G, Helm BR. A common framework for the regulation of growth and size: Stepping away from the trees to see the forest. In: Martin LB, Ghalambor CK, Woods HA, editors. Integrative Organismal Biology. Wiley-Blackwell; Hoboken, NJ: 2015. pp. 207–218. [Google Scholar]
- 9.Bernardo J. Determinants of maturation in animals. Trends Ecol Evol. 1993;8:166–173. doi: 10.1016/0169-5347(93)90142-C. [DOI] [PubMed] [Google Scholar]
- 10.Day T, Rowe L. Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am Nat. 2002;159:338–350. doi: 10.1086/338989. [DOI] [PubMed] [Google Scholar]
- 11.Callier V, Nijhout HF. Body size determination in insects: A review and synthesis of size- and brain-dependent and independent mechanisms. Biol Rev Camb Philos Soc. 2013;88:944–954. doi: 10.1111/brv.12033. [DOI] [PubMed] [Google Scholar]
- 12.Nijhout HF, et al. The developmental control of size in insects. Wiley Interdiscip Rev Dev Biol. 2014;3:113–134. doi: 10.1002/wdev.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokhale RH, Shingleton AW. Size control: The developmental physiology of body and organ size regulation. Wiley Interdiscip Rev Dev Biol. 2015;4:335–356. doi: 10.1002/wdev.181. [DOI] [PubMed] [Google Scholar]
- 14.Jindra M, Bellés X, Shinoda T. Molecular basis of juvenile hormone signaling. Curr Opin Insect Sci. 2015;11:39–46. doi: 10.1016/j.cois.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 16.Davidowitz G. Endocrine proxies can simplify endocrine complexity to enable evolutionary prediction. Integr Comp Biol. 2016;56:198–206. doi: 10.1093/icb/icw021. [DOI] [PubMed] [Google Scholar]
- 17.Nijhout HF, Davidowitz G, Roff DA. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J Biol. 2006;5:16. doi: 10.1186/jbiol43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shingleton AW. Evolution and the regulation of growth and body size. In: Flatt T, Heyland A, editors. Mechanisms of Life History Evolution. 1st Ed. Oxford Univ Press; New York: 2011. pp. 43–55. [Google Scholar]
- 19.Nijhout HF, Callier V. Developmental mechanisms of body size and wing-body scaling in insects. Annu Rev Entomol. 2015;60:141–156. doi: 10.1146/annurev-ento-010814-020841. [DOI] [PubMed] [Google Scholar]
- 20.Davidowitz G, D’Amico LJ, Nijhout HF. Critical weight in the development of insect body size. Evol Dev. 2003;5:188–197. doi: 10.1046/j.1525-142x.2003.03026.x. [DOI] [PubMed] [Google Scholar]
- 21.Davidowitz G, D’Amico L, Nijhout H. The effects of environmental variation on a mechanism that controls insect body size. Evol Ecol Res. 2004;6:49–62. [Google Scholar]
- 22.Ghosh SM, Testa ND, Shingleton AW. Temperature-size rule is mediated by thermal plasticity of critical size in Drosophila melanogaster. Proc Biol Sci. 2013;280:20130174. doi: 10.1098/rspb.2013.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callier V, Nijhout HF. Control of body size by oxygen supply reveals size-dependent and size-independent mechanisms of molting and metamorphosis. Proc Natl Acad Sci USA. 2011;108:14664–14669. doi: 10.1073/pnas.1106556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Amico LJ, Davidowitz G, Nijhout HF. The developmental and physiological basis of body size evolution in an insect. Proc Biol Sci. 2001;268:1589–1593. doi: 10.1098/rspb.2001.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidowitz G, Roff DA, Nijhout HF. A physiological perspective on the response of body size and development time to simultaneous directional selection. Integr Comp Biol. 2005;45:525–531. doi: 10.1093/icb/45.3.525. [DOI] [PubMed] [Google Scholar]
- 26.Davidowitz G, Roff D, Nijhout HF. Synergism and antagonism of proximate mechanisms enable and constrain the response to simultaneous selection on body size and development time: An empirical test using experimental evolution. Am Nat. 2016;188:499–520. doi: 10.1086/688653. [DOI] [PubMed] [Google Scholar]
- 27.Wigglesworth V. Hormone balance and the control of metamorphosis in Rhodnius prolixus (Hemiptera) J Exp Biol. 1952;29:620–631. [Google Scholar]
- 28.Anwyl R. The structure and properties of an abdominal stretch receptor in Rhodnius prolixus. J Insect Physiol. 1972;18:2143–2153. [Google Scholar]
- 29.Wigglesworth V. The function of the corpus allatum in the growth and reproduction of Rhodnius prolixus (Hemiptera) Q J Microsc Sci. 1936;79:91–123. [Google Scholar]
- 30.Stieper BC, Kupershtok M, Driscoll MV, Shingleton AW. Imaginal discs regulate developmental timing in Drosophila melanogaster. Dev Biol. 2008;321:18–26. doi: 10.1016/j.ydbio.2008.05.556. [DOI] [PubMed] [Google Scholar]
- 31.Mirth CK, Riddiford LM. Size assessment and growth control: How adult size is determined in insects. BioEssays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- 32.Mirth C, Truman JW, Riddiford LML. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Hatem NE, Wang Z, Nave KB, Koyama T, Suzuki Y. The role of juvenile hormone and insulin/TOR signaling in the growth of Manduca sexta. BMC Biol. 2015;13:44. doi: 10.1186/s12915-015-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohhara Y, Kobayashi S, Yamanaka N. Nutrient-dependent endocycling in steroidogenic tissue dictates timing of metamorphosis in Drosophila melanogaster. PLoS Genet. 2017;13:e1006583. doi: 10.1371/journal.pgen.1006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helm BR, Davidowitz G. Evidence of a hemolymph-born factor that induces onset of maturation in Manduca sexta larvae. J Insect Physiol. 2015;78:78–86. doi: 10.1016/j.jinsphys.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Nijhout HF. A threshold size for metamorphosis in the tobacco hornworm, Manduca sexta (L.) Biol Bull. 1975;149:214–225. doi: 10.2307/1540491. [DOI] [PubMed] [Google Scholar]
- 37.Callier V, et al. The role of reduced oxygen in the developmental physiology of growth and metamorphosis initiation in Drosophila melanogaster. J Exp Biol. 2013;216:4334–4340. doi: 10.1242/jeb.093120. [DOI] [PubMed] [Google Scholar]
- 38.Shafiei M, Moczek AP, Nijhout HF. Food availability controls the onset of metamorphosis in the dung beetle Onthophagus taurus (Coleoptera: Scarabaeidae) Physiol Entomol. 2001;26:173–180. [Google Scholar]
- 39.Suzuki Y, Koyama T, Hiruma K, Riddiford LM, Truman JW. A molt timer is involved in the metamorphic molt in Manduca sexta larvae. Proc Natl Acad Sci USA. 2013;110:12518–12525. doi: 10.1073/pnas.1311405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirth CK, et al. Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. Proc Natl Acad Sci USA. 2014;111:7018–7023. doi: 10.1073/pnas.1313058111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koyama T, Rodrigues MA, Athanasiadis A, Shingleton AW, Mirth CK. Nutritional control of body size through FoxO-Ultraspiracle mediated ecdysone biosynthesis. Elife. 2014;3:e03091. doi: 10.7554/eLife.03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winfree R, Bartomeus I, Cariveau DP. Native pollinators in anthropogenic habitats. Annu Rev Ecol Evol Syst. 2011;42:1–22. [Google Scholar]
- 43.Greenleaf SS, Williams NM, Winfree R, Kremen C. Bee foraging ranges and their relationship to body size. Oecologia. 2007;153:589–596. doi: 10.1007/s00442-007-0752-9. [DOI] [PubMed] [Google Scholar]
- 44.Kapustjanskij A, Streinzer M, Paulus HF, Spaethe J. Bigger is better: Implications of body size for flight ability under different light conditions and the evolution of alloethism in bumblebees. Funct Ecol. 2007;21:1130–1136. [Google Scholar]
- 45.Streinzer M, Huber W, Spaethe J. Body size limits dim-light foraging activity in stingless bees (Apidae: Meliponini) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2016;202:643–655. doi: 10.1007/s00359-016-1118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone GN, Willmer PG. Warm-up rates and body temperatures in bees: The importance of body size, thermal regime and phylogeny. J Exp Biol. 1989;147:303–328. [Google Scholar]
- 47.Bishop JA, Armbruster WS. Thermoregulatory abilities of Alaskan bees: Effects of size, phylogeny and ecology. Funct Ecol. 1999;13:711–724. [Google Scholar]
- 48.Garibaldi LA, et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339:1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- 49.Torné-Noguera A, et al. Determinants of spatial distribution in a bee community: Nesting resources, flower resources, and body size. PLoS One. 2014;9:e97255. doi: 10.1371/journal.pone.0097255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warzecha D, Diekötter T, Wolters V, Jauker F. Intraspecific body size increases with habitat fragmentation in wild bee pollinators. Landsc Ecol. 2016;31:1449–1455. [Google Scholar]
- 51.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Growth of the last-instar larva and the decision to pupate. J Exp Biol. 1974;61:481–491. doi: 10.1242/jeb.61.2.481. [DOI] [PubMed] [Google Scholar]
- 52.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol. 1974;61:493–501. doi: 10.1242/jeb.61.2.493. [DOI] [PubMed] [Google Scholar]
- 53.Mirth CK, Shingleton AW. The roles of juvenile hormone, insulin/target of rapamycin, and ecydsone signaling in regulating body size in Drosophila. Commun Integr Biol. 2014;7:1–3. doi: 10.4161/cib.29240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato T, Suzuki A. Effect of starvation and feeding of larvae during 4th stadia on pupation and adult size in Dacne picta (Coleoptera: Erotylidae) Appl Entomol Zool. 2001;36:189–197. [Google Scholar]
- 55.Terao M, Hirose Y, Shintani Y. Food-availability dependent premature metamorphosis in the bean blister beetle Epicauta gorhami (Coleoptera: Meloidae), a hypermetamorphic insect that feeds on grasshopper eggs in the larval stage. Entomol Sci. 2015;18:85–93. [Google Scholar]
- 56.Sgolastra F, et al. The long summer: Pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. J Insect Physiol. 2011;57:1651–1659. doi: 10.1016/j.jinsphys.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Sgolastra F, Kemp WP, Maini S, Bosch J. Duration of prepupal summer dormancy regulates synchronization of adult diapause with winter temperatures in bees of the genus Osmia. J Insect Physiol. 2012;58:924–933. doi: 10.1016/j.jinsphys.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Kemp WP, Bosch J, Dennis B. Oxygen consumption during the life cycles of the prepupa-wintering bee Megachile rotundata and the adult-wintering bee Osmia lignaria (Hymenoptera: Megachilidae) Ann Entomol Soc. 2004;97:161–170. [Google Scholar]
- 59.Tepedino V, Thompson R, Torchio P. Heritability for size in the megachilid bee Osmia lignaria propinqua Cresson. Apidologie. 1984;15:83–87. [Google Scholar]
- 60.Torchio PF, Tepedino VJ. Sex ratio, body size and seasonality in a solitary bee, Osmia lignaria propinqua Cresson (Hymenoptera: Megachilidae) Evolution. 1980;34:993–1003. doi: 10.1111/j.1558-5646.1980.tb04037.x. [DOI] [PubMed] [Google Scholar]
- 61.Tepedino V, Torchio P. Phenotypic variability in nesting success among Osmia lignaria propinqua females in a glasshouse experiment. Ecol Entomol. 1982;7:453–462. [Google Scholar]
- 62.Seidelmann K. The race for females: The mating system of the red mason bee, Osmia rufa (L.) (Hymenoptera : Megachilidae) J Insect Behav. 1999;12:13–25. [Google Scholar]
- 63.Seidelmann K, Ulbrich K, Mielenz N. Conditional sex allocation in the red mason bee, Osmia rufa. Behav Ecol Sociobiol. 2009;64:337–347. [Google Scholar]
- 64.Seidelmann K. Optimal progeny body size in a solitary bee, Osmia bicornis (Apoidea: Megachilidae) Ecol Entomol. 2014;39:656–663. [Google Scholar]
- 65.Pitts-Singer TL, Bosch J. Nest establishment, pollination efficiency, and reproductive success of Megachile rotundata (Hymenoptera: Megachilidae) in relation to resource availability in field enclosures. Environ Entomol. 2010;39:149–158. doi: 10.1603/EN09077. [DOI] [PubMed] [Google Scholar]
- 66.Bosch J, Vicens N. Body size as an estimator of production costs in a solitary bee. Ecol Entomol. 2002;27:129–137. [Google Scholar]
- 67.Neff JL. Components of nest provisioning behavior in solitary bees (Hymenoptera : Apoidea) Apidologie. 2008;39:30–45. [Google Scholar]
- 68.Wheeler DE. The developmental basis of worker caste polymorphism in ants. Am Nat. 1991;138:1218–1238. [Google Scholar]
- 69.Wheeler DE, Nijhout HF. Soldier determination in ants : New role for juvenile hormone. Science. 1981;213:361–363. doi: 10.1126/science.213.4505.361. [DOI] [PubMed] [Google Scholar]
- 70.Plowright RC, Jay SC. On the size determination of bumble bee castes (Hymenoptera: Apidae) Can J Zool. 1977;55:1133–1138. [Google Scholar]
- 71.Teder T, Vellau H, Tammaru T. Age and size at maturity: A quantitative review of diet-induced reaction norms in insects. Evolution. 2014;68:3217–3228. doi: 10.1111/evo.12518. [DOI] [PubMed] [Google Scholar]