Significance
New blood vessel formation is a physiological process seen in development, as well as in wound healing and tumorigenesis. Although the process of blood vasculature formation has been well documented, little is known about the molecular mechanisms that regulate endothelial migration during vascular network formation. In this study, we identified a critical role for Hippo effectors YAP and TAZ in the regulation of vascular network remodeling through controlling endothelial cell (EC) proliferation, filopodia formation, and cell migration. We found a striking cytoplasmic function of YAP in the regulation of EC migration through controlling the Rho family GTPase CDC42 activity. These findings identify a previously unrecognized YAP/TAZ function involved in the vascular network remodeling during angiogenesis.
Keywords: Hippo signaling, angiogenesis, cell migration, CDC42
Abstract
Angiogenesis and vascular remodeling are essential for the establishment of vascular networks during organogenesis. Here we show that the Hippo signaling pathway effectors YAP and TAZ are required, in a gene dosage-dependent manner, for the proliferation and migration of vascular endothelial cells (ECs) during retinal angiogenesis. Intriguingly, nuclear translocation of YAP and TAZ induced by Lats1/2-deletion blocked endothelial migration and phenocopied Yap/Taz-deficient mutants. Furthermore, overexpression of a cytoplasmic form of YAP (YAPS127D) partially rescued the migration defects caused by loss of YAP and TAZ function. Finally, we found that cytoplasmic YAP positively regulated the activity of the small GTPase CDC42, deletion of which caused severe defects in endothelial migration. These findings uncover a previously unrecognized role of cytoplasmic YAP/TAZ in promoting cell migration by activating CDC42 and provide insight into how Hippo signaling in ECs regulates angiogenesis.
Angiogenesis is a process of growth and remodeling in vascular networks that is essential for normal development. In adulthood, angiogenesis is activated as a reparative process, for example, during wound healing (1, 2). Aberrantly regulated angiogenesis can also be a component of disease (3) and can play a key role in tumor growth and metastasis (4), inflammatory diseases (5), diabetic retinopathy, and retinopathy of prematurity (6).
Retinal angiogenesis in mice begins at postnatal day 0 (P0). The retinal vasculature initiates its expansion from the optic nerve head and migrates outwards along a preexisting network of astrocytes (7, 8). This results in the formation of the superficial vascular plexus within the retinal ganglion cell layer during the first 8 d (9, 10). Endothelial cells (ECs) then migrate along nerve fibers to establish deep and intermediate vascular layers (9, 11). Cell proliferation and migration are essential for angiogenesis and these cell responses are regulated by many different signaling pathways, including the VEGF, Notch, Wnt, FGF, BMP, and integrin signaling responses (9, 12–16). VEGFA and CDC42 are known to regulate extension of the angiogenic front and filopodia formation in angiogenic tip cells (2, 17, 18).
The Hippo signaling pathway is an evolutionarily conserved, pivotal regulator of cell proliferation and organogenesis. YAP and TAZ are key components of the Hippo signaling pathway and function as transcription cofactors that regulate downstream gene expression via association with DNA binding proteins such as TEAD1–4 (19, 20). Loss of Hippo signaling can drive the expression of genes that regulate cell proliferation and survival (diap1, bantam, cyclin E, and E2F1), the Hippo pathway (Kibra, Crb, and Fj), and cell–cell interaction (E-Cadherin, Serrate, Wingless, and Vein) (20). The activity of YAP and TAZ is regulated by the LATS1 and LATS2 kinases. These kinases phosphorylate YAP and TAZ, thus preventing their nuclear translocation and regulating transcriptional activity. Although the function of YAP and TAZ in the nucleus has been subject to extensive studies (20, 21), the role of these proteins in the cytoplasm is not fully understood.
In the present study, we used the mouse postnatal retina as a model for investigating the function of YAP and TAZ during angiogenesis. We show that YAP and TAZ are required for vascular network formation by regulating endothelial cell proliferation and migration and that the influence of YAP and TAZ on angiogenesis is gene dosage dependent. Importantly, we show that cytoplasmic YAP, but not the nuclear form, is crucial for modulating endothelial cell migration by regulating the Rho family GTPase CDC42 activity. These findings identify a previously unrecognized role of cytoplasmic YAP in regulating angiogenesis via CDC42.
Results
YAP and TAZ Are Required for Vascular Development in the Retina.
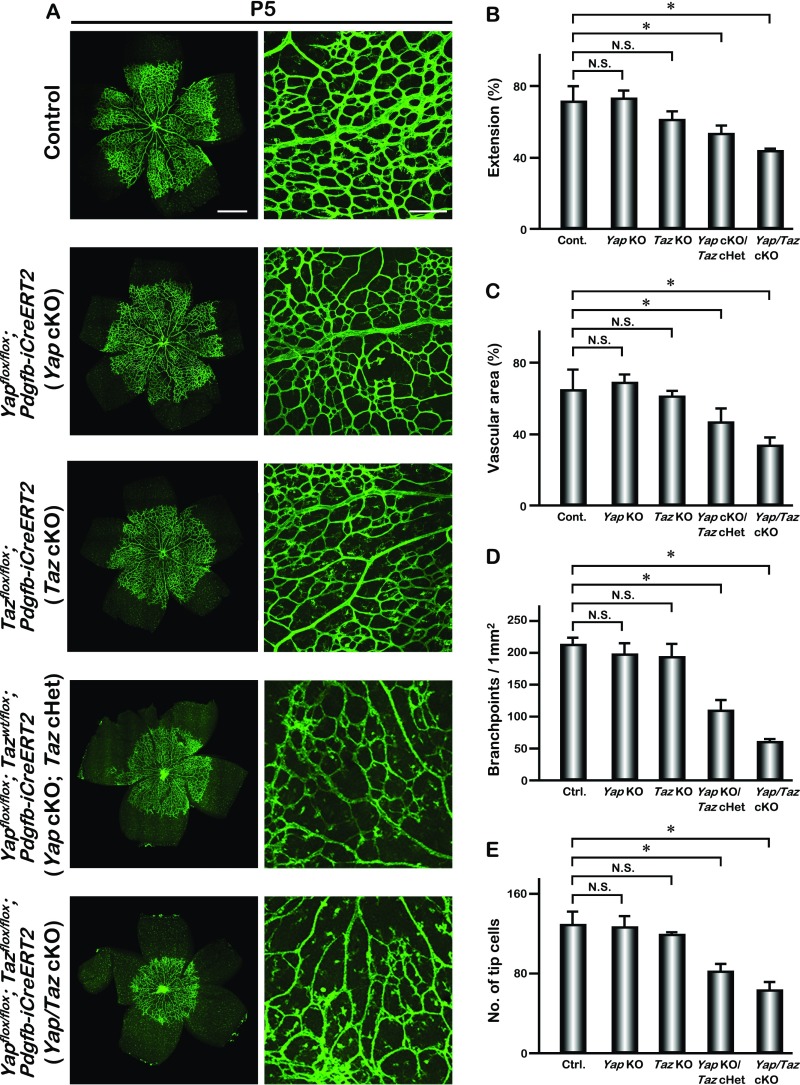
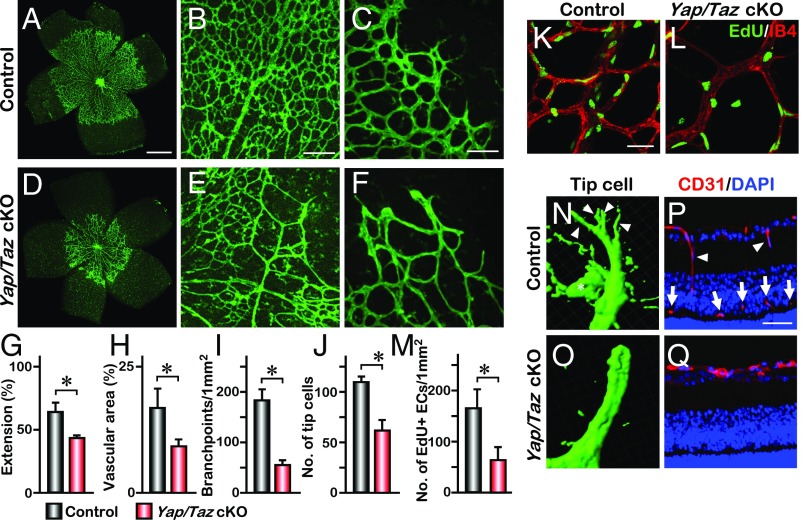
We examined the expression of YAP in retinal endothelial cells. YAP was detected mainly in the cytoplasm in most retinal vascular endothelial cells (VECs) (Fig. S1 A–D) and in both the nucleus and cytoplasm in some VECs (Fig. S1 C and D). Whole-mount retina staining also showed that YAP was mainly localized in the cytoplasm in both the migrating tip cells and the central region of retinal vessels (Fig. S1E). To determine the function of YAP in retinal VECs, we bred the conditional Yapflox/flox allele with the Pdgfb-iCreERT2 mouse line to delete Yap in endothelial cells in a temporally regulated manner. The expression of Pdgfb-iCreERT2 in the developing retinal VECs was confirmed by breeding with Rosa26-Loxp-STOP-Loxp-tdTomato reporter mice (22) (Fig. S2). Upon tamoxifen treatment from P1 to P3, Yapflox/flox; Pdgfb-iCreERT2 (referred to as Yap cKO) mice did not show overt abnormalities when examined at P5 (Fig. S3). To investigate whether the lack of phenotype in Yap cKO is due to a redundant function with TAZ (homolog of YAP in mammals), we generated endothelial-specific Taz knockout mice, Tazflox/flox; Pdgfb-iCreERT2 (referred to as Taz cKO). Similar to the Yap cKO mice, the Taz cKO mice appeared normal without an obvious vascular phenotype (Fig. S3). However, the deletion of both Yap alleles and one allele of Taz, Yapflox/flox; Tazwt/flox; Pdgfb-iCreERT2, (referred to as Yap cKO; Taz cHet) led to reduced vascular density (Fig. S3) and decreased extension of the retinal vascular field (vascular extension) (Fig. S3). Furthermore, deletion of both alleles of Yap and Taz in endothelial cells, Yapflox/flox; Tazflox/flox; Pdgfb-iCreERT2 (referred to as Yap/Taz cKO), caused a severe vascular phenotype with prominently impaired retinal vessel sprouting, vascular area, and reduced number of vascular branches (Fig. 1 A–I and Fig. S3). This severe vascular phenotype persisted until later developmental stages (Fig. S4), indicating that Yap and Taz are required for vessel morphogenesis in a gene dose-dependent manner. Quantitative PCR (Q-PCR) on RNA isolated from the brain VECs of Yap/Taz cKO mice confirmed a significantly lower level of each transcript as well as the expression of YAP target genes, Ctgf and Cyr61 (Fig. S5). Severe reduction of vascular density in Yap/Taz cKO mutants led us to investigate the possibility that endothelial cell proliferation was affected. The 5-ethynyl-2′-deoxyuridine (EdU) was delivered to P4 pups via i.p. injection 16 h before the analysis. We found that the number of proliferating endothelial cells was greatly reduced in the Yap/Taz cKO retinas compared with the littermate controls (Fig. 1 K–M), suggesting that YAP and TAZ are required for endothelial cell proliferation during angiogenesis.
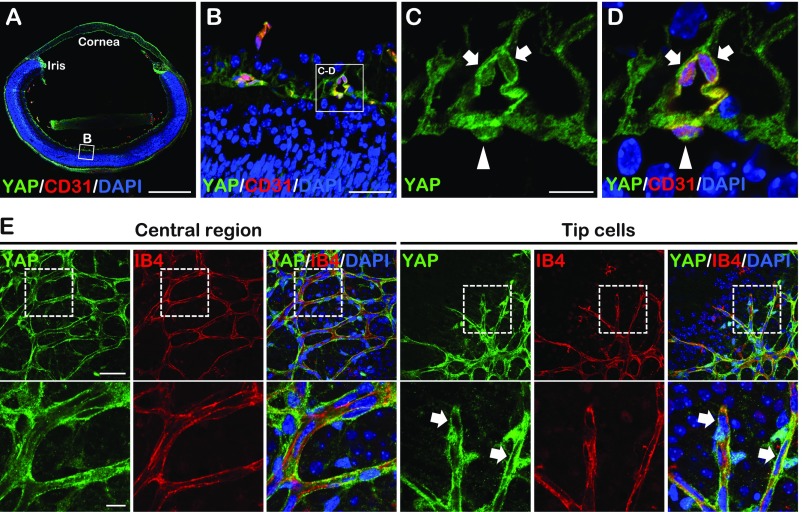
Fig. S1.
YAP expression in the developing retinal vasculature. (A–D) YAP staining images of cross-section of P5 wild-type eyes. High magnification images (C and D) showed extranuclear (arrows) and nuclear (arrowheads) YAP localization in retina VECs. (E) Whole-mount YAP staining of P5 wild-type retina. The higher magnification of the boxed areas (Upper) are shown (Lower). Tip cells showed extranuclear YAP localization (arrows). [Scale bars: 500 μm (A), 200 μm (E, Upper), 50 μm (B and E, Lower), and 12.5 μm (C).]
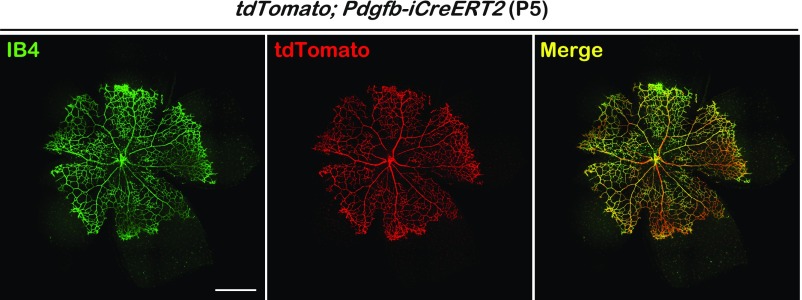
Fig. S2.
Pdgfb-iCreERT2 expression in the retinal vasculature endothelial cells. Whole-mount IB4 staining of retina collected from P5 Rosa26-tdTomato; Pdgfb-iCreERT2 mouse. iCreERT2 efficiency was visualized by tdTomato expression. [Scale bar: 500 μm (Left).]
Fig. S3.
Gene dosage requirement of Yap and Taz during retinal angiogenesis. (A) Whole-mount IB4 staining of P5 control, Yapflox/flox; Pdgfb-iCreERT2 (Yap cKO), Tazflox/flox; Pdgfb-iCreERT2 (Taz cKO), Yapflox/flox; Tazwt/flox; Pdgfb-iCreERT2 (Yap cKO; Taz cHet), and Yap/Taz cKO retina. (B–E) Quantification of the vascular extension (n = 6), vascular area (n = 3), number of branchpoints (n = 6), and tip cells (n = 4); mean ± SD, *P < 0.01 (Tukey–Kramer test). [Scale bars: 500 μm (Left) and 200 μm (Right).]
Fig. 1.
YAP/TAZ regulate vascular endothelial migration in the developing retina. (A–F) IB4 labeling of P5 retina vasculature from littermate Yapflox/flox; Tazflox/flox mice (control) (A) or with Pdgfb-iCreERT2 (Yap/Taz cKO) (D). Higher magnification images of the vascular plexus and front are shown in B, C, E, and F. (G–J) Quantification of the vascular extension (n = 6), vascular area (n = 3), number of branchpoints (n = 6), and tip cells (n = 4); mean ± SD, *P < 0.01. (K–M) Whole-mount EdU staining of P5 control and Yap/Taz cKO retina. Statistical analysis of the number of EdU-positive cells is shown in M (n = 4); mean ± SD, *P < 0.01. (N and O) Imaris image analysis of P5 retina tip cells. Arrowheads and asterisk in N indicate filopodia and a macrophage, respectively. (P and Q) Immunohistochemistry of retinal sections of P11 eyes. Control retina shows some migrating endothelial cells (arrowheads) and the deep vascular plexus (arrows). [Scale bars: 500 μm (A), 200 μm (B), 100 μm (C), and 50 μm (K and P).]
Fig. S4.
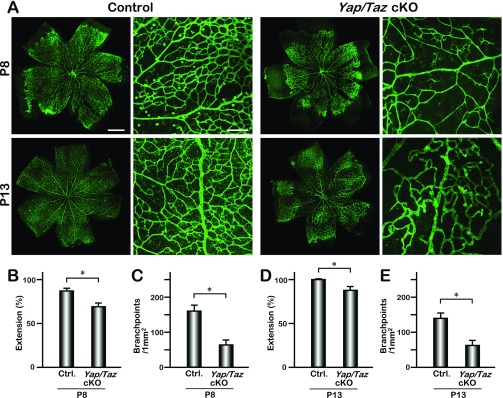
Retinal vascular defects in Yap/Taz cKO mice. (A) IB4-stained retinas of control and Yap/Taz cKO mice (P8 and P13). (B–E) Quantification of the vascular extension (P8; n = 6, P13; n = 6) and number of branchpoints (P8; n = 4, P13; n = 4); mean ± SD, *P < 0.01 (Student’s t test). [Scale bars: 500 μm (Left) and 200 μm (Right).]
Fig. S5.
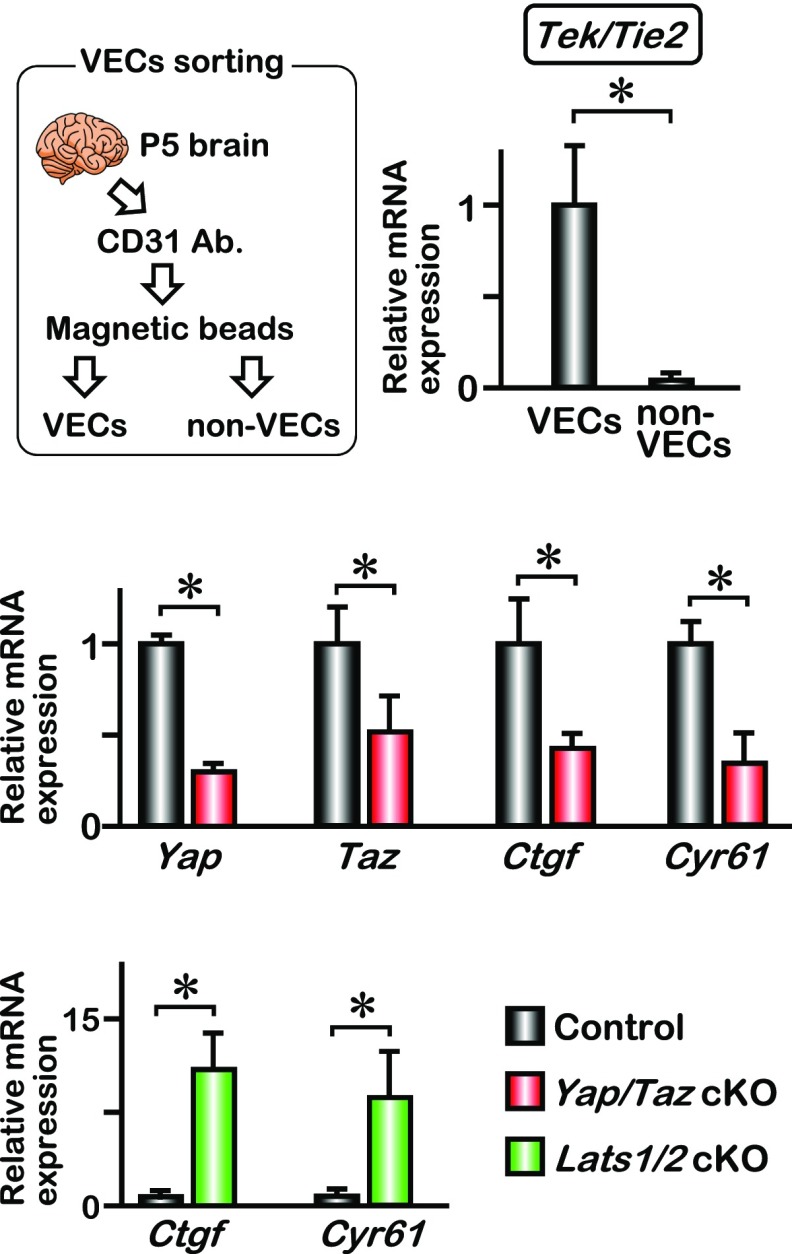
Isolation of brain VECs from neonatal mice. Brain VECs were isolated from P5 neonates as described in SI Materials and Methods. Quantitative PCR analysis for Tek/Tie2 expression indicated efficient separation of VECs and non-VECs; mean ± SD, *P < 0.01. The Yap and Taz mRNA expression was significantly down-regulated in Yap/Taz cKO brain VECs. The expression of YAP target genes (Ctgf and Cyr61) was down-regulated in Yap/Taz cKO and was up-regulated in Lats1/2 cKO brain VECs; mean ± SD, *P < 0.01.
Angiogenic sprouting is promoted by active filopodial protrusions and tip cell migration (23). To determine whether the vascular defect in Yap/Taz cKO mice involves tip cell migration, we examined the abundance and morphology of tip cells. The number of tip cells was significantly reduced in Yap/Taz cKO mice (Fig. 1 C, F, and J and Fig. S3E). Furthermore, tip cells in the double mutant mice exhibited only a few filopodia extending from vessel termini (Fig. 1 N and O). The reduced vascular extension and the morphology of the tip cells in Yap/Taz cKO mice led us to investigate whether YAP and TAZ are necessary for VEC migration. During retinal angiogenesis, vasculature expands from the optic stalk at P1 and reaches the periphery by about P8 (24). VECs then migrate downward into the regions where neurons reside to form the deep and intermediate vascular plexus by 3 wk of age. P11 retina sections showed that there were some migrating endothelial cells and an intermediate vascular plexus in the control, but not in the Yap/Taz cKO retinas (Fig. 1 P and Q). Whole-mount CD31 staining at P13 also indicated that endothelial-specific deletion of Yap and Taz prevented the migration that forms the deep and intermediate vascular layers (Movies S1 and S2). These data suggest that YAP and TAZ are required for endothelial cell proliferation and migration during vascular development.
Deletion of the Upstream Lats1/2 Results in Cell Migration Defect.
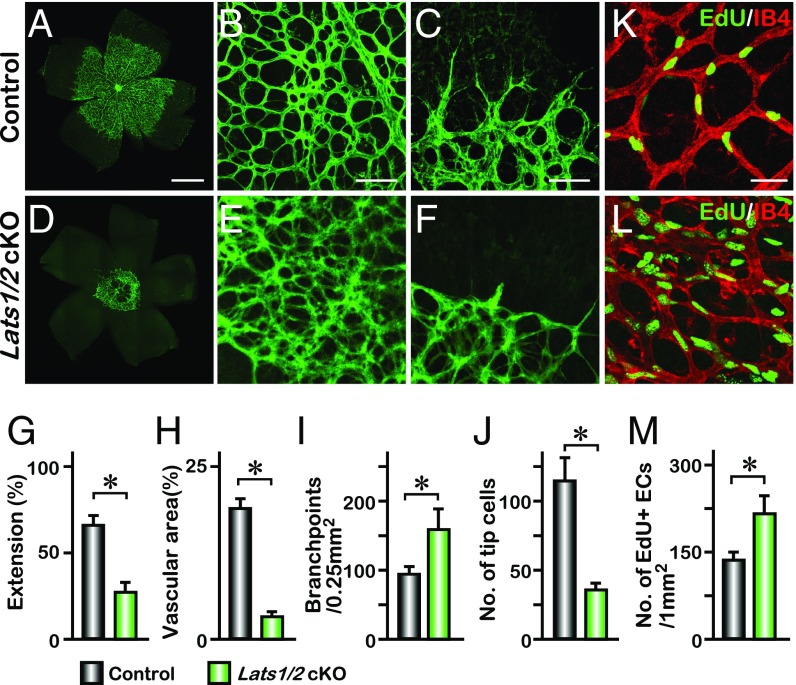
To investigate whether other components of the Hippo signaling pathway are involved in regulating cell proliferation and migration, we deleted the upstream kinases Lats1/2 by breeding Lats1flox/flox; Lats2flox/flox mice with Pdgfb-iCreERT2 to generate Lats1flox/flox; Lats2flox/flox; Pdgfb-iCreERT2 (referred to as Lats1/2 cKO). This eliminates LATS-dependent phosphorylation of YAP and TAZ in endothelial cells and prevents their phosphorylation-dependent sequestration in the cytoplasm (25, 26). The Lats1/2 cKO retinas exhibited a migration defect with reduced extension distance compared with the control mice (Fig. 2 A, D, and G). The angiogenic network in Lats1/2 cKO mice also displayed hyperplasia with increased vascular complexity evident by a 60% increase in branchpoints, reduced vascular area, and number of tip cells (Fig. 2 B, C, E, F, and H–J). The proliferation rate of VECs was significantly increased, whereas vascular area was reduced in Lats1/2 cKO retina (Fig. 2 K–M) and expression of YAP target genes was significantly increased in Lats1/2 cKO endothelial cells (Fig. S5). These results contrasted with the proliferation phenotype and gene expression in the Yap/Taz cKO retina (Fig. 1 K–M and Fig. S5). Although other effectors might be affected in the Lats1/2 cKO mice, these data suggest that nuclear YAP/TAZ might be mainly required for VEC proliferation, but not for cell migration.
Fig. 2.
Deletion of Lats1/2 disrupts retinal vascular extension and filopodia formation. (A–F) IB4 labeling of P5 retina vasculature from control and Lats1flox/flox; Lats2flox/flox; Pdgfb-iCreERT2 (Lats1/2 cKO) neonates. Higher magnification images of the vascular plexus and front are shown in B, C, E, and F. (G–J) Quantification of vascular extension (n = 6), vascular area (n = 4), number of branchpoints (n = 4), and tip cells (n = 4); mean ± SD, *P < 0.01. (K–M) Whole-mount EdU staining of P5 control and Lats1/2 cKO retina. Statistical analysis of the number of EdU-positive cells is shown in M (n = 4); mean ± SD, *P < 0.01. [Scale bars: 500 μm (A), 100 μm (B and C), and 50 μm (K).]
Loss of CDC42 Caused Abnormal Vessel Morphology and Migration Defect.
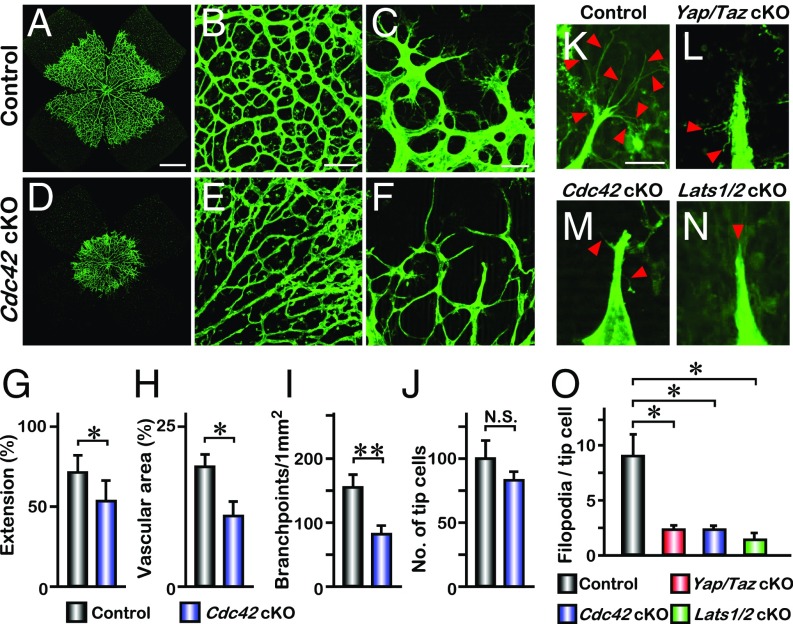
The Rho GTPase CDC42 has been shown to be required for blood vessel formation during vasculogenesis by promoting filopodia formation in endothelial tip cells (17, 18, 27, 28). To confirm the activity of CDC42 in the formation of filopodia, we combined the Cdc42flox/flox allele (29) with Pdgfb-iCreERT2 mice to generate an endothelial-specific deletion of Cdc42, Cdc42flox/flox; Pdgfb-iCreERT2 (referred to as Cdc42 cKO). Cdc42 cKO retinas exhibited reduced radical extension of vasculature at P5 (Fig. 3 A, D, and G). The vascular density was also reduced in Cdc42 cKO mice (Fig. 3 B, E, and I). The number of tip cells at the sprouting front did not show a significant difference between Cdc42 cKO and the littermate controls (Fig. 3 C, F, and J).
Fig. 3.
Phenocopy of Cdc42 cKO filopodial loss in Yap/Taz and Lats1/2 cKO. (A–F) Whole-mount IB4 staining of P5 retina from control (A) and Cdc42flox/flox; Pdgfb-iCreERT2 (Cdc42 cKO) neonates (D). Higher magnification images of the vascular plexus and front are shown in B, C, E, and F. (G–J) Quantification of vascular extension (n = 4), vascular area (n = 3), number of branchpoints (n = 6), and tip cells (n = 3); mean ± SD, *P < 0.05, **P < 0.01, N.S., not significant. (K–N) Comparison of filopodia formation of each genotype. Tip cells are labeled by IB4. Red arrowheads indicate filopodia. (O) Quantification of the number of filopodia per tip cell (n = 4); mean ± SD, *P < 0.01. [Scale bars: 500 μm (A), 200 μm (B), 50 μm (C), and 10 μm (K).]
Cdc42 cKO retina tip cells had few filopodia (Fig. 3M), and lacking of filopodia in tip cells was also observed in Yap/Taz cKO and Lats1/2 cKO retinas (Fig. 3 L and N). Quantitative analysis showed a significant decrease in filopodia density in these mice (Fig. 3O). The converging phenotype of endothelial-specific deletion of Yap/Taz, Cdc42, or Lats1/2 in the filopodia-mediated vascular sprouting and branching in the retina suggests that these molecules might operate in a common pathway in angiogenic tip cell development.
YAP and TAZ Regulate CDC42 Activity in Migrating Endothelial Cells.
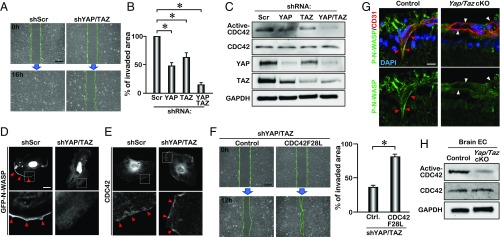
The similar tip cell phenotype in Yap/Taz cKO and Cdc42 cKO mice lead us to examine how YAP/TAZ regulates endothelial cell migration and whether YAP and TAZ regulate CDC42 activity and its cellular localization in endothelial cells. We depleted YAP and TAZ in vitro in human umbilical vein endothelial cells (HUVECs), using lentiviruses expressing short hairpin RNAs targeting human YAP and TAZ, and assessed HUVECs migration by a wound healing scratch assay. To distinguish the effect of proliferation from cell migration, HUVECs were treated with hydroxyurea for 4 h before the migration assay. Quantification analysis of the invaded area 16 h after the scratch demonstrated that knocking down YAP and TAZ in HUVECs inhibited cell migration (Fig. 4 A and B). The cell migration defect was exacerbated when both YAP and TAZ were knocked down, consistent with the mouse in vivo data showing that YAP and TAZ are required for cell migration and that this requirement is gene dosage dependent.
Fig. 4.
Down-regulation of CDC42 activity in YAP/TAZ-deficient endothelial cells. (A) Scratch assay of Scrambled-shRNA (shScr) and YAP/TAZ-shRNA (shYAP/TAZ)-infected HUVECs. Images were taken at 0 and 16 h after cell scratch. (B) Quantification of invaded area within the scratched region of YAP/TAZ knockdown HUVECs (n = 3); mean ± SD, *P < 0.01. (C) Active-CDC42 pull-down assay and Western blot analysis of YAP/TAZ knockdown HUVECs. (D and E) GFP-N-WASP expression (D) and endogenous CDC42 expression (E) in YAP/TAZ knockdown HUVECs. The boxed areas are enlarged (Lower). Arrowheads indicate GFP-N-WASP and endogenous CDC42 expression at the edge of the cell. (F) Scratch assay of YAP/TAZ knockdown HUVECs expressing CDC42F28L. Images were taken at 0 and 12 h after the scratch. Quantification of invaded area within the scratched region (n = 3); mean ± SD, *P < 0.01. (G) Phosphorylated N-WASP expression in the retinal vascular endothelial cells (VECs) of P11 eyes. Arrowheads indicate the VECs. (H) Active-CDC42 pull-down assay of P5 brain VECs. [Scale bars: 50 μm (A and F) and 10 μm (D).]
We found that, while the total CDC42 level did not change, the level of active CDC42 was greatly reduced upon knockdown of YAP and TAZ (Fig. 4C). For better visualization of active CDC42 in a single cell, we transfected HUVECs with a GFP-tagged CDC42/RAC interactive binding domain of neural Wiskott Aldrich syndrome protein (GFP-N-WASP) (30), which binds to endogenous active CDC42. The active CDC42 was located at the lamellipodial edge of the control HUVECs (Fig. 4D). In YAP/TAZ knockdown HUVECs, only active CDC42 was diminished in the protruding edge (Fig. 4D), while CDC42 localization was not disrupted (Fig. 4E), suggesting that YAP and TAZ regulate CDC42 activation rather than its cellular localization in HUVECs. The migration defect in YAP/TAZ knockdown HUVECs can be rescued by a constitutively active form of CDC42 (CDC42F28L) (Fig. 4F), which is capable of spontaneously exchanging GDP for GTP (31, 32), suggesting that YAP/TAZ regulation of the HUVEC migration at least in part channels through CDC42 activity. To confirm the effect of YAP/TAZ in vivo, we examined the expression of phosphorylated-N-WASP, an effector of CDC42 (33), in the developing mouse retinal vasculature. Phosphorylated-N-WASP was detected in the migrating endothelial cells in control retinas at P11; however, the level of phosphorylated-N-WASP was greatly reduced in the Yap/Taz cKO retinal VECs (Fig. 4G). These data suggest that YAP/TAZ regulate cell migration through activating the CDC42-mediated N-WASP pathway in vivo. Moreover, CDC42 activity was down-regulated in the brain endothelial cells from Yap/Taz cKO mice. (Fig. 4H). Collectively, these observations indicate that the endothelial migration defect in the Yap/Taz cKO retinas is at least partially due to the down-regulation of CDC42 activity.
Cytoplasmic YAP Promotes Endothelial Cell Migration.
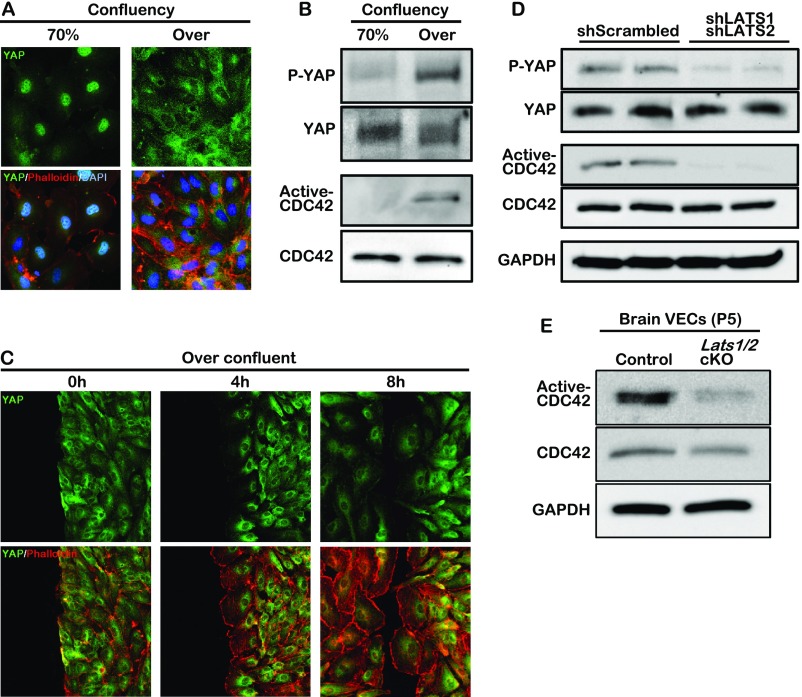
YAP is a mechanical sensor whose cellular localization changes in response to various environmental stimuli including cell–cell interaction and alterations of cytoskeletal dynamics (34, 35). We assessed whether YAP cellular localization affected CDC42 activity. When HUVECs were at low density, YAP was localized in the nucleus and translocated to the cytoplasm when cells reached confluency (Fig. S6A). Consequently, the level of phosphorylated YAP was greatly up-regulated in the overconfluent cells (Fig. S6B). The active CDC42 level also increased dramatically in the overconfluent cells compared with the cells at low density (Fig. S6B). In the wound scratch assay on overconfluent HUVECs, YAP remained in the cytoplasm while the cells migrated (Fig. S6C), suggesting a promigratory role of the cytoplasmic YAP. The decrease of CDC42 activity in LATS1/2 knockdown HUVECs and Lats1/2 cKO brain endothelial cells (Fig. S6 D and E) further supports the hypothesis that the cytoplasmic YAP regulates the migration of endothelial cells.
Fig. S6.
Correlation between cytoplasmic YAP (phospho-YAP) and CDC42 activity in HUVECs. (A) Endogenous YAP localization in 70% or overconfluent HUVECs. (B) Phospho-YAP (P-YAP) and GTP-bound CDC42 (active-CDC42) expression in different cell densities are determined by immunoblotting. Active-CDC42 pull-down assay was performed as described in Materials and Methods. (C) Endogenous YAP localization in migrating HUVECs. Overconfluent HUVECs are scratched, incubated for 0, 4, or 8 h, and then stained with YAP antibody (green) and phalloidin (red). (D and E) Western blot analysis for phosphorylated-YAP and active-CDC42 pull-down assay using LATS1/2 knockdown HUVECs and brain endothelial cells isolated from P5 Lats1/2 cKO neonates.
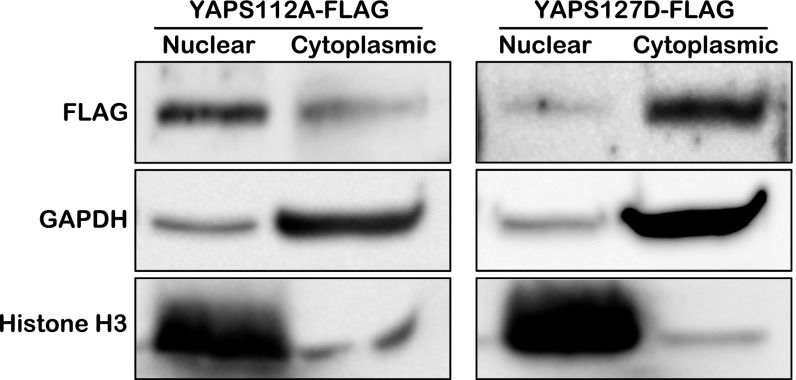
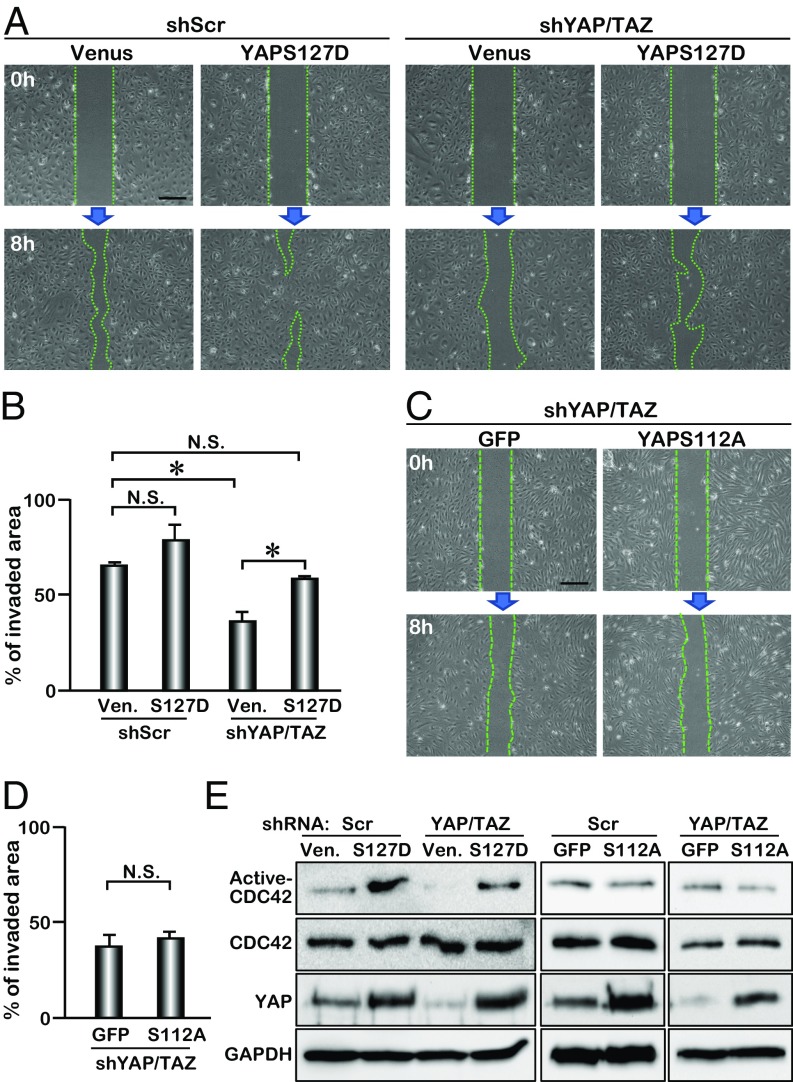
To further investigate whether cytoplasmic YAP promotes cell migration and activates CDC42, we transduced HUVECs with a lentivirus expressing YAPS127D. Substitution of Ser127 with Asp (S127D) generates a human YAP protein that is sequestered in the cytoplasm mimicking phospho-YAP (Fig. S7). HUVECs treated with lenti-YAPS127D migrated with a trend faster than cells treated with the control lentivirus (Venus), although the difference in migration did not reach significance (Fig. 5 A and B). YAPS127D did partially rescue the migration defects caused by shRNA-mediated YAP/TAZ knockdown, suggesting that phospho-YAP promotes endothelial cell migration (Fig. 5 A and B). We further examined the effect of nuclear YAP, using a constitutively active form of murine YAP (YAPS112A, in which Ser112 is mutated to Ala) in retinal angiogenesis, by breeding Pdgfb-iCreERT2 with transgenic mice harboring a YAPS112A transgene under the control of CAG-LoxP-CAT-Stop-Loxp cassette. No substantial effect on retinal angiogenesis was detected in Tg-YAPS112A (Fig. S8), suggesting that nuclear YAPS112A overexpression does not alter neovasculature formation in the retina. Unlike the Lats1/2 cKO phenotype, VEC proliferation was not up-regulated by YAPS112A, although YAP targets genes (Ctgf and Cyr61) were up-regulated in VECs (Fig. S8). Notably, YAPS112A did not rescue the migration defect in YAP/TAZ knockdown HUVECs (Fig. 5 C and D). To examine whether this promigration function of YAP is through activation of CDC42, we overexpressed YAPS127D in HUVECs and found that the level of active CDC42 was greatly increased (Fig. 5E). The reduction of active CDC42 with shRNA-mediated YAP/TAZ knockdown was also rescued by YAPS127D, but not by YAPS112A expression (Fig. 5E). These results indicate an important role of cytoplasmic YAP in promoting cell migration by activating CDC42 (Fig. S9). The partial rescue of the cell migration and CDC42 activity with YAPS127D could be due to the fact that only the phospho-YAP mimic is overexpressed in the HUVECs in which both YAP and TAZ are knocked down. Although YAP and TAZ play redundant roles in regulating retinal angiogenesis, they may have distinct functions in interacting with different proteins in the cytoplasm to regulate cell migration.
Fig. S7.
Cell fractionation of YAPS112A- and YAPS127D-expressing cells. Fractionation assay of NIH 3T3 cells transfected with FLAG-YAPS112A or FLAG-YAPS127D expression construct. YAPS112A and YAPS127D are mainly expressed in nuclear and cytoplasmic fractions, respectively.
Fig. 5.
Cytoplasmic YAP regulates CDC42 activity in HUVEC. (A) Scratch assay of YAP/TAZ knockdown HUVECs expressing Venus or YAPS127D. (B) Quantification of invaded area within the scratched region (n = 3); mean ± SD, *P < 0.01, N.S., not significant; S127D, YAPS127D; Ven, venus. (C) Scratch assay of YAP/TAZ knockdown HUVECs expressing YAPS112A. (D) Quantification of invaded area within the scratched region (n = 3); mean ± SD, N.S., not significant; S112A, YAPS112A. (E) Active-CDC42 pull-down assay of YAPS127D lentivirus-infected (S127D) and YAPS112A adenovirus-infected (S112A) HUVECs. CDC42, YAP, and GAPDH expression levels are detected by Western blot analysis. [Scale bar: 50 μm (A and C).]
Fig. S8.
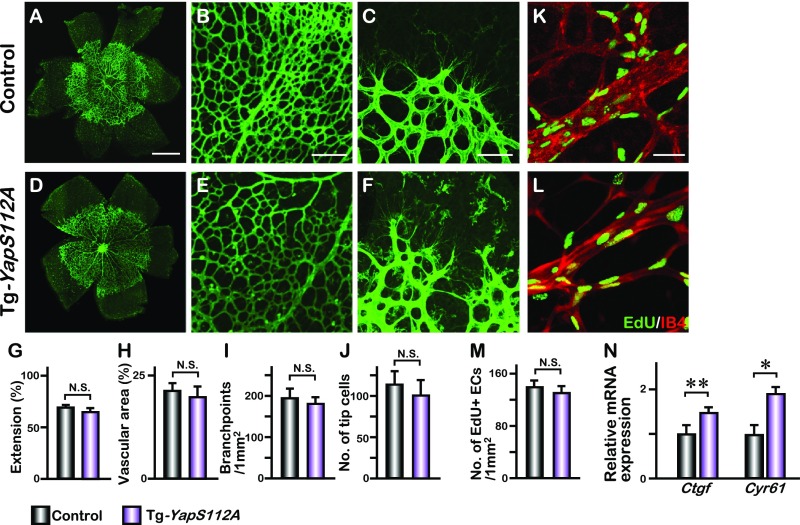
Retinal angiogenesis in endothelial YAPS112A transgenic mice. Endothelial-specific overexpression of a constitutively active YAPS112A, in which serine 112 is mutated to alanine, was achieved by crossing Pdgfb-iCreERT2 with transgenic mice harboring a YAP112A transgene under the control of CAG-LoxP-CAT-Stop-LoxP cassette (CAG-loxP-CAT-Stop-loxP-YapS112A, referred to as Tg-YapS112A). (A–F) P5 control and YAPS112A-expressed retinas are stained with IB4. Higher magnification images of the vascular plexus and front are shown in B, C, E, and F. (G–J) Quantification of the vascular extension (n = 6), vascular area (n = 3), number of branchpoints (n = 7), and tip cells (n = 3) at P5 control and Tg-YapS112A retinas; mean ± SD, N.S., not significant. (K and L) Whole-mount EdU staining of P5 control and Tg-YapS112A retinas. (M) Statistical analysis of the number of EdU-positive cells of the respective genotypes (n = 4); mean ± SD, N.S., not significant. (N) Quantitative PCR analysis of Ctgf and Cyr61 expression in the control and Tg-YapS112A brain endothelial cells. Mean ± SD, *P < 0.01, **P < 0.05. [Scale bars: 500 μm (A), 200 μm (B), 100 μm (C), and 50 μm (K).]
Fig. S9.
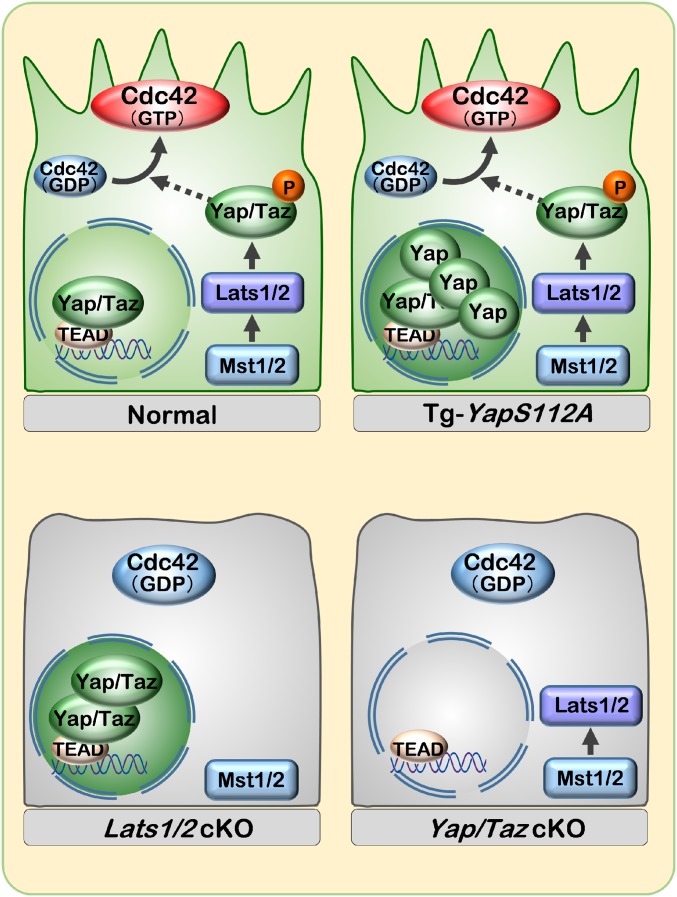
Schematic representation of cytoplasmic YAP during vascular migration. Phosphorylated-YAP is localized in the cytoplasm and up-regulates CDC42 activity in the migrating endothelial cells.
Discussion
Angiogenesis is a highly regulated process. This reflects the potentially detrimental consequences of a deficiency or an excess of blood vessels. The Hippo signaling pathway has been implicated in vascular development (36–38) but the underlying mechanisms have not been fully described. In this study, we found that the cytoplasmically localized phospho-YAP, which is not involved in transcription, plays an important role in promoting cell migration via activating CDC42.
Cell Autonomous Function of YAP/TAZ Vascular Development in Vivo.
A migration defect in endothelial–mesenchymal transition (EMT) during cardiac cushion formation causes early embryonic lethality instigated by deletion of a floxed Yap allele using Tie2-Cre. This made it difficult to study YAP function in the developing vasculature (37). Global knockdown of Yap via siRNA injection in mice revealed that YAP is important for mediating the stability of endothelial cell junction and vascular remodeling (36); however, the cell autonomous function of YAP could not be addressed due to the systemic distribution of the siRNA. We report here that deletion of Yap using endothelial cell-expressing Pdgfb-iCreERT2 allows for assessment of postnatal retinal vascular development. Combined endothelial deletion of Yap and Taz in mice revealed gene dosage-dependent effects on retinal vascular sprouting, endothelial cell proliferation, and migration.
We found that YAP target genes such as Ctgf and Cyr61 are down-regulated in Yap/Taz cKO brain endothelial cells, while they are up-regulated in Lats1/2 cKO endothelial cells, suggesting that transcriptional activity of YAP/TAZ might contribute to the regulation of proliferation in endothelial cells. In contrast to Yap/Taz cKO and Lats1/2 cKO, YAPS112A overexpression alone is insufficient for retinal VEC proliferation, and the level or strength of YAP activity or additional factors such as TAZ may control EC proliferation. Furthermore, nuclear YAPS112A overexpression in transgenic mice does not alter angiogenesis in the retina as opposed to the vascular defects in Lats1/2 cKO mice, suggesting that LATS1/2 could regulate other effectors, in addition to the subcellular localization of YAP/TAZ for vascular morphogenesis.
The majority of studies of YAP and TAZ report their transcriptional activity in the nucleus via association with TEAD transcription factors and that phosphorylation of YAP and TAZ by the upstream kinases induces their cytoplasmic retention and degradation (20). Studies have revealed that phosphorylated YAP and TAZ are associated with 14-3-3 to bind to proteins in the cytoplasmic and tight junctions (39, 40). In kidney cells, it has been reported that cytoplasmic YAP and TAZ interact with angiomotin (AMOT) to facilitate the localization of YAP and TAZ to tight junctions and to promote phosphorylation by the upstream kinases in the Hippo pathway (41). In addition, cytoplasmically localized phospho-YAP and -TAZ have been shown to interact with DVL to inhibit Wnt/β-catenin and SMAD signaling (42, 43). Expression of YAPS112A in YAP/TAZ knockdown HUVECs cannot rescue the migration defect while the cytoplasmic mutant YAPS127D can, suggesting that cytoplasmic YAP but not nuclear YAP is required for cell migration. Hence, our data reveal a previously unrecognized function of cytoplasmic YAP/TAZ in the regulation of endothelial cell migration.
The Crosstalk Between Hippo Signaling and CDC42.
The small Rho family GTPase CDC42 is required for lumen formation during vasculogenesis and filopodia formation in endothelial cells (17, 27, 44). When Cdc42 was deleted in endothelial cells using Cdh5(PAC)-CreERT2 (17), vascular extension was not significantly changed between the Cdc42 mutant and littermate controls. In our study, we observed a reduced vascular extension phenotype using Pdgfb-iCreERT2 to delete the floxed Cdc42 allele. The difference in the phenotype between these two studies could be due to the distinct Cre line used and the timing of tamoxifen administration. A previous study reported that deletion of Cdc42 in kidney progenitor cells resulted in reduced YAP nuclear localization and target gene expression, suggesting that CDC42 acts upstream of YAP in mouse kidney development (45). Our study demonstrated that cytoplasmic YAP promoted CDC42 activation, providing a complementary mechanism of crosstalk between the Hippo pathway and CDC42. How cytoplasmic YAP regulates CDC42 activity in endothelial cell migration remains to be defined. A recent study indicates that YAP regulates RhoA activity through the controlling the expression of ARHGAP29 (Rho GTPase activating protein) (46). While CDC42 is not able to be directly activated by YAPS127D, there is a possibility that cytoplasmic YAP regulates CDC42-GEF or CDC42-GAP activity in migrating endothelial cells. The result of the rescue experiment using CDC42F28L, which can bind to GTP in the absence of GEF, supports this hypothesis.
YAP and TAZ join a collection of cellular factors and signaling molecules with the known ability to promote vascular sprouting and angiogenesis. Although our findings clearly demonstrate that YAP can activate CDC42 to promote endothelial cell migration, multiple mechanisms likely contribute. More evidence continues to demonstrate crosstalk between different signaling pathways to control vascular development. The Notch, VEGF, and BMP signaling pathways have been shown to play important roles in regulating vascular sprouting and tip cell formation during angiogenesis (9, 12–14). One report showed that BMP9 crosstalks with the Hippo pathway by repressing YAP target genes in endothelial cells (47). It seems possible that, in turn, YAP and TAZ could regulate BMP, Notch, and other pathways to control vascular development. Future investigations would need to identify the cellular mechanism underlying how cytoplasmic YAP activates CDC42 and to test the potential synergistic activities between YAP and regulators in other signaling pathways. The findings of cytoplasmic YAP activity may help to develop pharmacologic and genetic strategies to further enhance the proangiogenic potential for treating patients suffering from ischemic diseases.
Materials and Methods
Animals.
All animal experiments were performed with the approval of the institutional animal care and use committee of Cincinnati Children’s Hospital Medical Center. Please see SI Materials and Methods for origins of knockout and transgenic mice.
Cell Culture.
HUVECs were maintained in EGM-2 medium (Lonza) and were infected with an adenovirus, retrovirus, and lentiviruses. Plasmid transfection was performed using PolyJet DNA In Vitro Transfection Reagent (SignaGen Laboratories). Additional details can be found in SI Materials and Methods.
Immunostaining and EdU Labeling.
Eyes were fixed with 4% paraformaldehyde (PFA) for 1 h, and then retinas were incubated with IB4-FITC (Molecular Probes) overnight. For EdU studies, P4 neonates were administered an i.p. (IP) injection of EdU (5 μg/g of mouse body weight). EdU incorporation was assessed using Click-IT EdU system (Invitrogen). Detailed information is described in SI Materials and Methods.
Active CDC42 Assay.
CDC42 activity was performed as previously described (48). Additional details can be found in SI Materials and Methods.
Statistics.
All datasets were taken from n ≥ 3 biological replicates. Data are presented as mean ± SD. We calculated P values with an unpaired Student’s t test or Tukey–Kramer test with Excel (Microsoft Office); P < 0.05 was considered significant.
SI Materials and Methods
Animals.
Mouse lines harboring the Yap, Taz, and Cdc42 floxed alleles and Pdgfb-iCreERT2 have been described previously (22, 29, 49, 50). Lats1 and Lats2 floxed mice were a kind gift from Randy L. Johnson, University of Texas MD Anderson Cancer Center, Houston. The YapS112A transgenic expression construct was generated by using the ubiquitously expressed CAG promoter followed by a LoxP-CAT-STOP-LoxP cassette fused to murine YAP cDNA that has alanine 112 substituted for serine, resulting in a constitutively active nuclear YAP. Tamoxifen (Sigma) was dissolved in sunflower oil (Sigma) at 2 mg/mL, and 0.1 mg tamoxifen was administered at P1, P2, and P3 via i.p. injection. All mouse lines were maintained on a mixed background. Birth was defined as postnatal day 0 (P0).
Plasmids.
The expression vector encoding the CRIB domain of N-WASP with an N-terminal GFP tag (GFP-N-WASP) was provided by Fukuhara and colleagues (30). The constitutively phosphorylated YAP (p2xFLAG CMV2-YAP2-S127D) plasmid (Addgene, plasmid 19051) was a gift from Marius Sudol, National University of Singapore, Singapore, Republic of Singapore (51). Lentiviral vector MISSION pLKO.1-shRNA-puro constructs targeting human YAP and TAZ were obtained from Sigma-Aldrich.
Lentivirus, Adenovirus, and Retrovirus.
To generate YAP and TAZ knockdown cells, HUVECs were infected with a lentivirus containing shRNA targeting YAP and TAZ. A lentiviral vector (pLKO.1), packaging vector (psPAX2), and envelope vector (pMD2.G) were cotransfected into HEK293-FT cells for virus production. HUVECs were selected in culture medium containing 2 μg/mL puromycin. To make the YAPS127D lentivirus, we used the lentivirus vector (CSII-EF-MCS-IRES2-Venus) provided by H. Miyoshi, Keio University, Tokyo. YAPS127D was subcloned into the lentivirus vector and was transfected into HEK293-FT cells with the packaging vector (pCAG-HIVgp) and envelope vector (pCMV-VSV-G-RSV-Rev). The adenovirus (YAPS112A) and retrovirus (CDC42F28L) have been described previously (31, 49).
Cell Culture and Scratch Assay.
HUVECs were maintained in EGM-2 medium (Lonza). The HUVEC monolayers were scratched with a P200 pipette tip. Cells were photographed at 0, 8, and 16 h after the scratch. Invaded area was measured by digital imaging using ImageJ software.
Whole-Mount Immunostaining of Retinas.
Eyes were fixed with 4% paraformaldehyde (PFA) for 1 h at room temperature and washed three times with phosphate buffer saline (PBS). After removing the cornea, lens, and sclera, four or five radial incisions were made in the retina. Collected retinas were blocked with 1% BSA in PBS including 0.1% Tween 20 (PBS-T) overnight. Then the retinas were incubated with IB4-FITC (Molecular Probes) overnight and washed with PBS-T before being coverslipped with mounting media. Quantification of the number of branchpoints was conducted on a 1 mm × 1 mm area (on 500 μm × 500 μm area for Lats1/2 cKO retina) in scanned images. For quantification of the number of tip cells, blind-ended endothelial protrusions with associated filopodia were scored as tip cells.
Immunohistochemistry.
Eyes were fixed with 4% PFA overnight at 4 °C and washed three times with PBS. The eyes were treated with 30% sucrose before embedding in OCT compound and sectioned at a thickness of 8 μm. Sections were blocked with 1% BSA/PBS-T for 30 min, incubated with primary antibodies against YAP (Cell Signaling) and CD31 (BD Bioscience), and were further incubated with Alexa Fluor-conjugated antibodies. Fluorescence images were captured using Eclipse Ti confocal microscopy with a C2 laser-scanning head (Nikon).
Western Blotting.
HUVECs were lysed with 2× sample buffer (Bio-Rad) containing 2-mercaptoethanol and heated for 5 min at 95 °C. Equal amounts of protein were run on SDS-polyacrylamide gels and transferred to PVDF membranes. Membranes were incubated with anti-YAP (Novus), anti-YAP/TAZ (Cell Signaling), and anti-GAPDH (Cell Signaling) antibodies at 4 °C overnight. Anti-rabbit horseradish peroxidase (GE Healthcare) was used as secondary antibody, followed by detection with SuperSignal West Pico chemiluminescent substrate (Thermo).
Active CDC42 Assay.
CDC42 activity was examined by an effector domain, GST-fusion pull-down protocol, as previously described (48). HUVECs were lysed in a lysis buffer containing 1% Triton X-100 and incubated with the glutathione bead-bound GST-PAK1. The bead-immobilized GTP-bound CDC42 and total CDC42 in the lysates were probed by immunoblotting with anti-CDC42 antibody (Cell Signaling).
Endothelial Cell Isolation.
The method of endothelial cell (EC) isolation from P5 or P7 mouse brains was described previously (52).
Reverse Transcription PCR Analysis.
Total RNA from isolated-brain ECs was extracted using the Nucleospin RNA II kit (Machery-Nagel). cDNA was synthesized with 500 ng of total RNA using PrimeScript RT Master Mix (TaKaRa). Quantification of the RNA levels was carried out using real-time PCR (KAPA SYBR FAST qPCR kit) (KAPA) and normalized to the level of 18s ribosomal RNA expression. Sequences of PCR primers are as follows: Yap, 5′-ACCCTCGTTTTGCCATGAAC-3′ and 5′-TGTGCTGGGATTGATATTCCGTA-3′; Taz, 5′-CATGGCGGAAAAAGATCCTCC-3′ and 5′-GTCGGTCACGTCATAGGACTG-3′; and 18s ribosomal RNA, 5′-GTCTGTGATGCCCTTAGATG-3′ and 5′-AGCTTATGACCCGCACTTAC-3′.
Fractionation Assay.
The fractionation assay was performed as previously described (53). For the cytoplasmic extract, 3T3 cells were lysed in hypotonic buffer [10 mM Hepes (pH 8), 1.5 mM MgCl2, 10 mM KCl, and 1 mM DTT]. Nuclei were then resuspended in hypotonic buffer with 420 mM NaCl, 0.2 mM EDTA, 25% glycerol, 1% Nonidet P-40, and 1 mM PMSF.
Supplementary Material
Acknowledgments
We thank E. N. Olson for providing the inducible Tg-YapS112A mouse line; R. L. Johnson for providing the Lats1/2flox/flox mouse lines; S. Fukuhara and H. Miyoshi for providing the GFP-N-WASP and lentiviral vectors; M. Sudol for the YAPS127D plasmid; and E. Boscolo, N. Gowri, and other M.X. laboratory members for discussion and technical assistance. Work in the laboratory of M.X. was supported by the National Institutes of Health (HL132211-01) and Cincinnati Children’s Hospital.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704030114/-/DCSupplemental.
References
- 1.Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: Angiogenic and cell therapies. Circ Res. 2015;116:1561–1578. doi: 10.1161/CIRCRESAHA.115.303565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 4.Mazzone M, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tas SW, Maracle CX, Balogh E, Szekanecz Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat Rev Rheumatol. 2016;12:111–122. doi: 10.1038/nrrheum.2015.164. [DOI] [PubMed] [Google Scholar]
- 6.Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood. 2012;120:2182–2194. doi: 10.1182/blood-2012-04-396846. [DOI] [PubMed] [Google Scholar]
- 7.West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005;132:1855–1862. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- 8.Fruttiger M, et al. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron. 1996;17:1117–1131. doi: 10.1016/s0896-6273(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao H, et al. The development of blood-retinal barrier during the interaction of astrocytes with vascular wall cells. Neural Regen Res. 2014;9:1047–1054. doi: 10.4103/1673-5374.133169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med. 2010;16:417–425. doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellström M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 13.Benedito R, et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 14.Ricard N, et al. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kano MR, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118:3759–3768. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- 16.Zovein AC, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry DM, et al. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development. 2015;142:3058–3070. doi: 10.1242/dev.125260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantin A, et al. NRP1 regulates CDC42 activation to promote filopodia formation in endothelial tip cells. Cell Rep. 2015;11:1577–1590. doi: 10.1016/j.celrep.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claxton S, et al. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 23.De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 24.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 2015;29:1285–1297. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DH, et al. LATS-YAP/TAZ controls lineage specification by regulating TGFβ signaling and Hnf4α expression during liver development. Nat Commun. 2016;7:11961. doi: 10.1038/ncomms11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y, et al. Deletion of Cdc42 enhances ADAM17-mediated vascular endothelial growth factor receptor 2 shedding and impairs vascular endothelial cell survival and vasculogenesis. Mol Cell Biol. 2013;33:4181–4197. doi: 10.1128/MCB.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakayama Y, Fukuhara S, Ando K, Matsuda M, Mochizuki N. Cdc42 mediates Bmp-induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev Cell. 2015;32:109–122. doi: 10.1016/j.devcel.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci USA. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando K, et al. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol. 2013;202:901–916. doi: 10.1083/jcb.201301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo F, Zheng Y. Involvement of Rho family GTPases in p19Arf- and p53-mediated proliferation of primary mouse embryonic fibroblasts. Mol Cell Biol. 2004;24:1426–1438. doi: 10.1128/MCB.24.3.1426-1438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu WJ, Erickson JW, Lin R, Cerione RA. The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature. 2000;405:800–804. doi: 10.1038/35015585. [DOI] [PubMed] [Google Scholar]
- 33.Torres E, Rosen MK. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J Biol Chem. 2006;281:3513–3520. doi: 10.1074/jbc.M509416200. [DOI] [PubMed] [Google Scholar]
- 34.Wang KC, et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci USA. 2016;113:11525–11530. doi: 10.1073/pnas.1613121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 36.Choi HJ, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, et al. Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J Biol Chem. 2014;289:18681–18692. doi: 10.1074/jbc.M114.554584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh A, et al. Hippo signaling mediators Yap and Taz are required in the epicardium for coronary vasculature eevelopment. Cell Rep. 2016;15:1384–1393. doi: 10.1016/j.celrep.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varelas X, Wrana JL. Coordinating developmental signaling: Novel roles for the Hippo pathway. Trends Cell Biol. 2012;22:88–96. doi: 10.1016/j.tcb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Morrison DK. The 14-3-3 proteins: Integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varelas X, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115:1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 45.Reginensi A, et al. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao Y, et al. YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. 2017;19:1495–1502. doi: 10.1016/j.celrep.2017.04.075. [DOI] [PubMed] [Google Scholar]
- 47.Young K, et al. BMP9 crosstalk with the hippo pathway regulates endothelial cell matricellular and chemokine responses. PLoS One. 2015;10:e0122892. doi: 10.1371/journal.pone.0122892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang HR, et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 49.Xin M, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xin M, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP) J Biol Chem. 2008;283:27534–27546. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- 52.Kumar TP, Vasudevan A. Isolation and culture of endothelial cells from the embryonic forebrain. J Vis Exp. 2014:e51021. doi: 10.3791/51021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.